Similar presentations:
Emission spectrum of H
1. Emission spectrum of H
Any DE ispossible
“Quantized” spectrum
使量子化
DE
DE
“Continuous” spectrum
Only certain DE
are
allowed
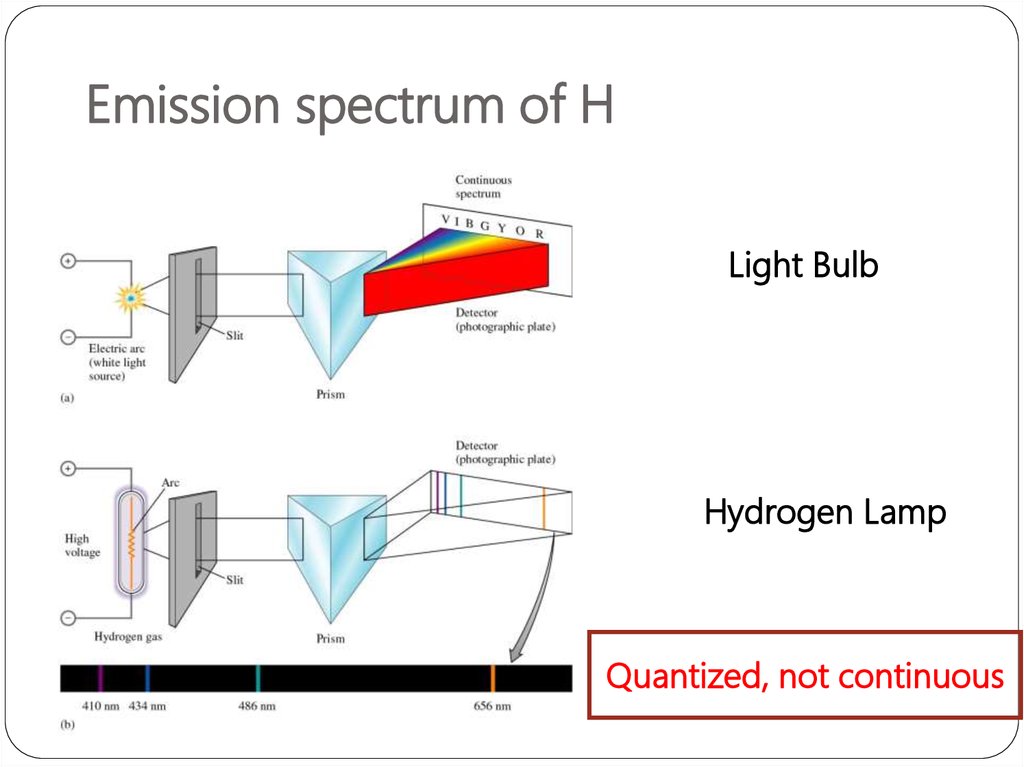
2. Emission spectrum of H
Light BulbHydrogen Lamp
Quantized, not continuous
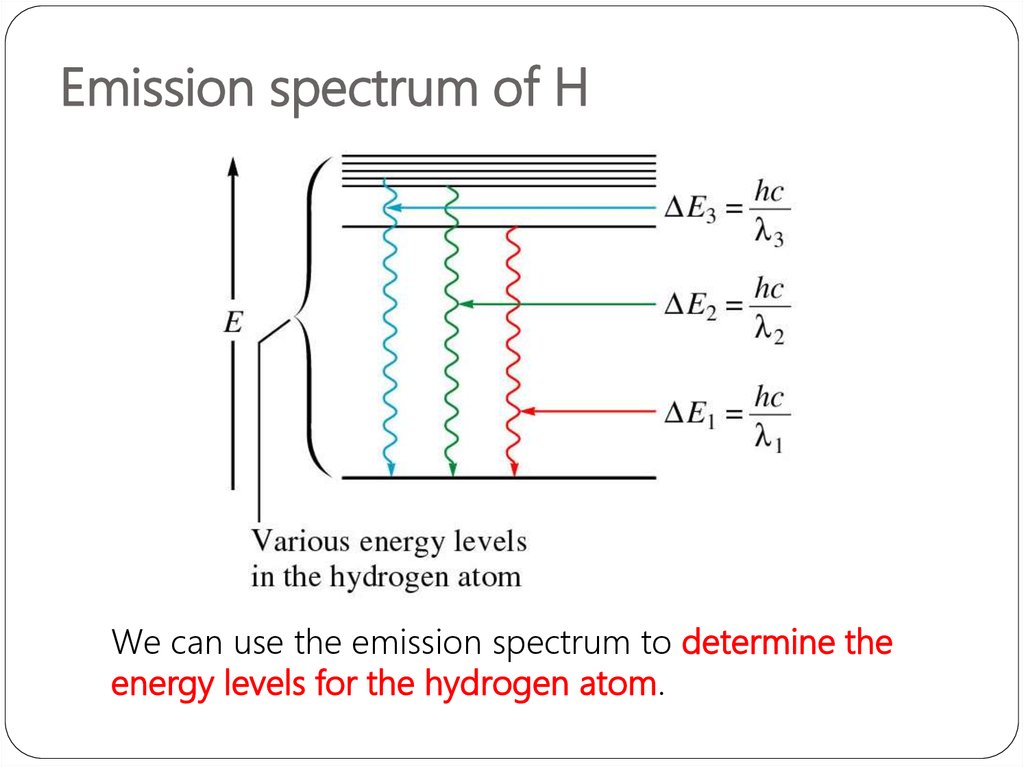
3. Emission spectrum of H
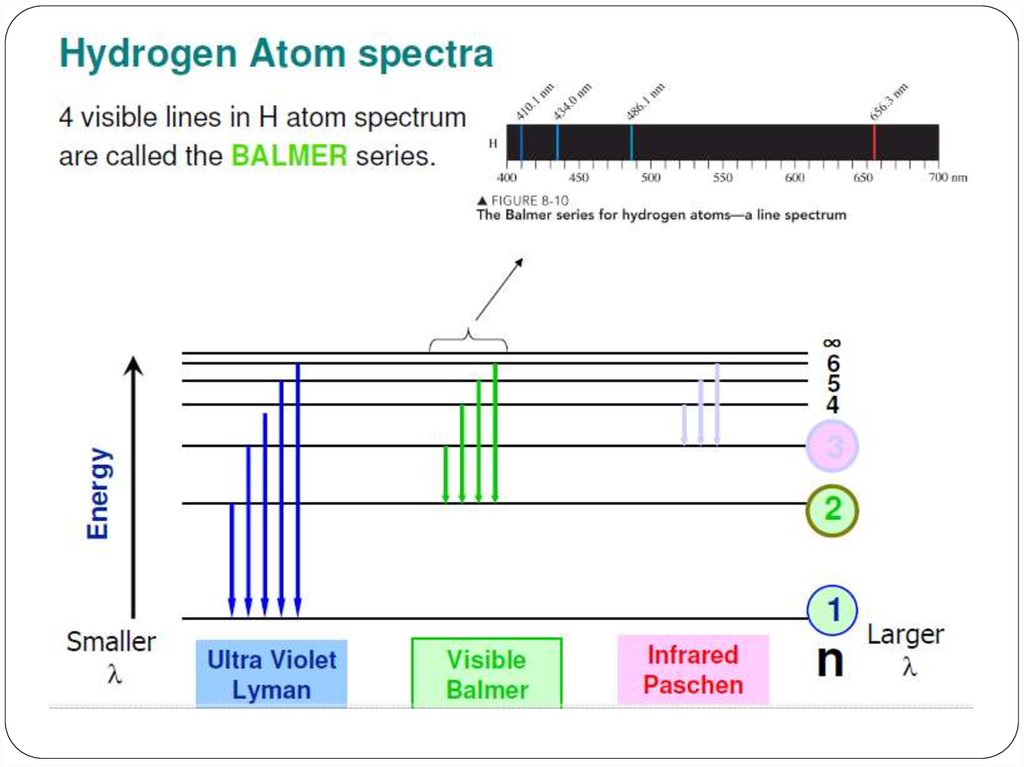
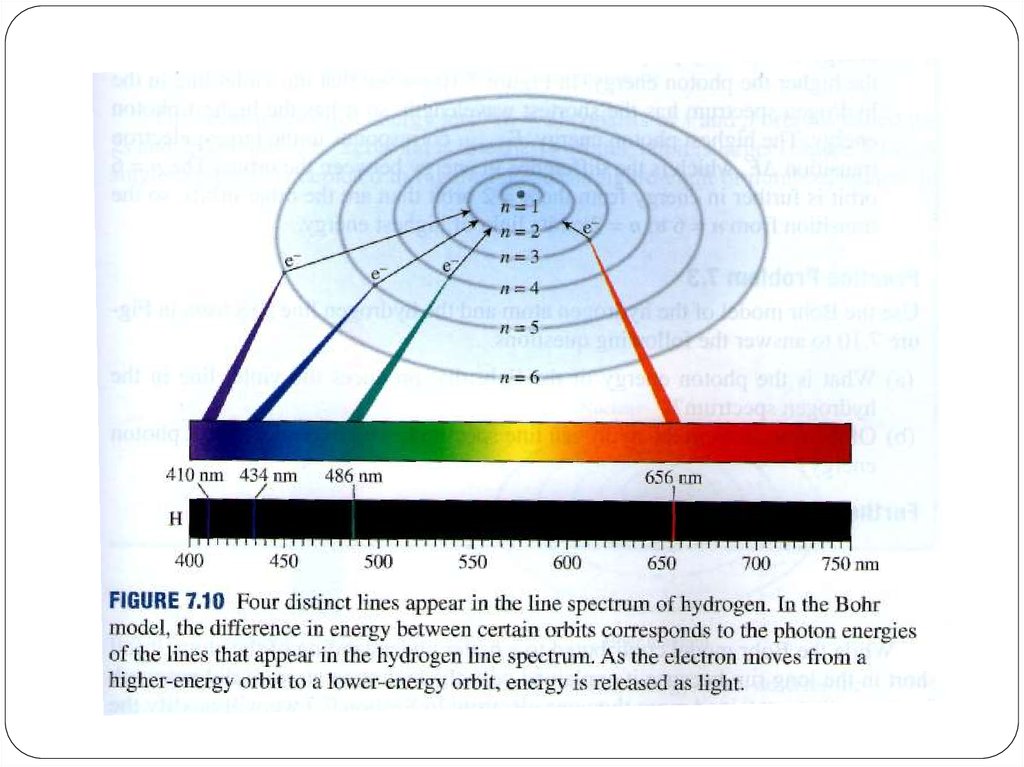
We can use the emission spectrum to determine theenergy levels for the hydrogen atom.
4.
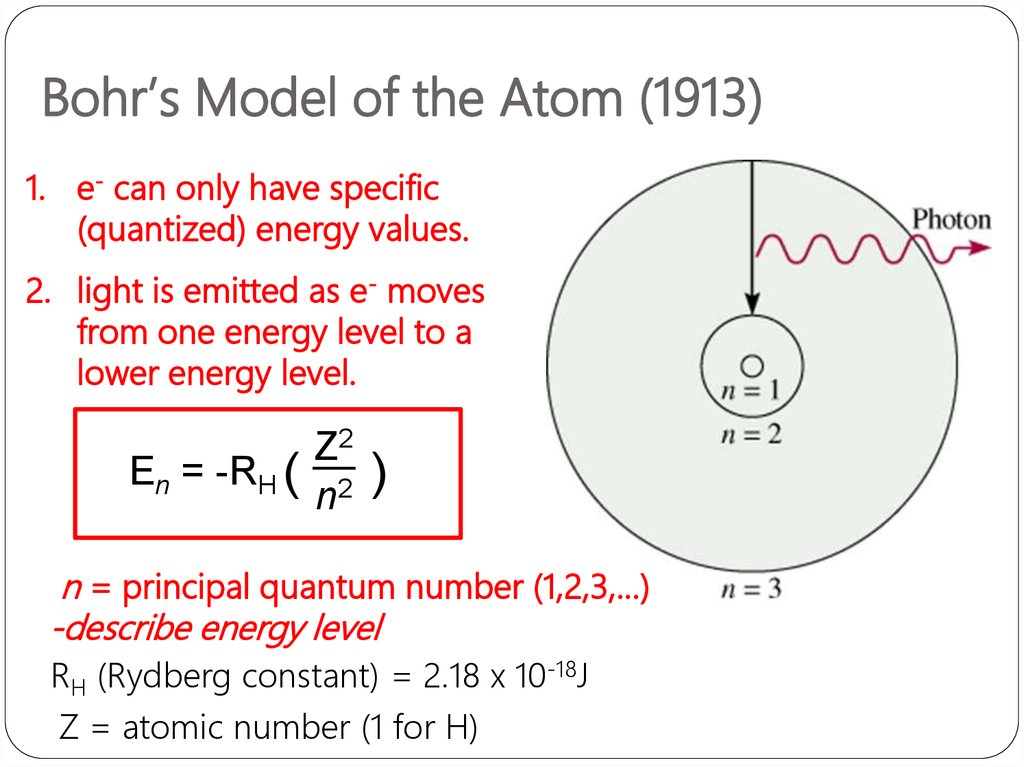
Bohr’s Model of the Atom (1913)1. e- can only have specific
(quantized) energy values.
2. light is emitted as e- moves
from one energy level to a
lower energy level.
En = -RH (
Z2
n2
)
n = principal quantum number (1,2,3,…)
-describe energy level
RH (Rydberg constant) = 2.18 x 10-18J
Z = atomic number (1 for H)
5.
1.Moving from higher tolower energy levels
emission of energy (e.g.
radiation as light)
2. Moving from lower to
higher energy levels
absorption of
energy (e.g. external
stimulation)
E = hn
E = hn
• Energy levels get closer together as n
increases
• at n = infinity, E = 0
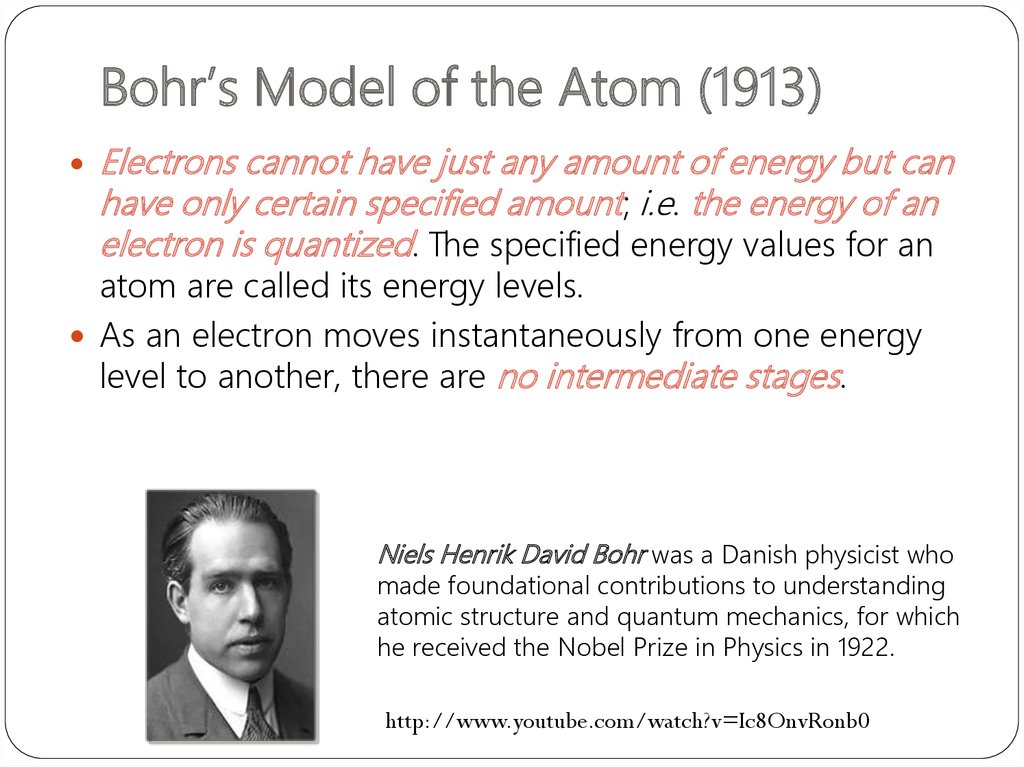
6. Bohr’s Model of the Atom (1913)
Electrons cannot have just any amount of energy but canhave only certain specified amount; i.e. the energy of an
electron is quantized. The specified energy values for an
atom are called its energy levels.
As an electron moves instantaneously from one energy
level to another, there are no intermediate stages.
Niels Henrik David Bohr was a Danish physicist who
made foundational contributions to understanding
atomic structure and quantum mechanics, for which
he received the Nobel Prize in Physics in 1922.
http://www.youtube.com/watch?v=Ic8OnvRonb0
7.
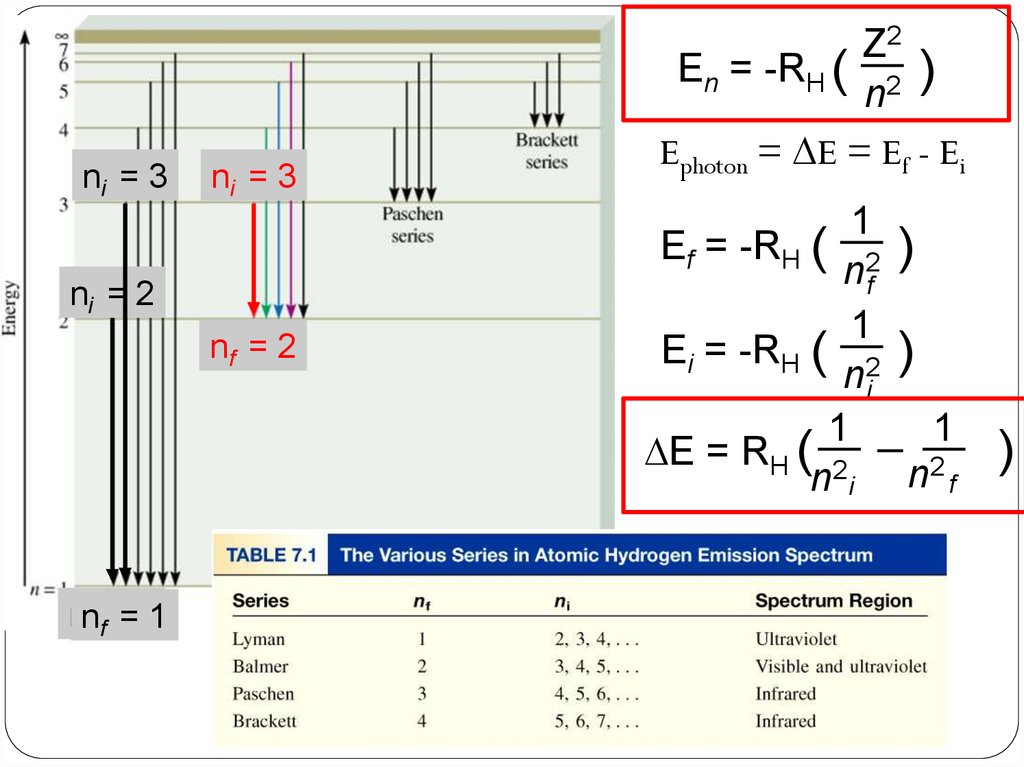
ni = 3ni = 3
ni = 2
nf = 2
nnf f==11
Z2
En = -RH ( 2 )
n
Ephoton = DE = Ef - Ei
1
Ef = -RH ( 2 )
nf
1
Ei = -RH ( 2 )
ni
1
1
DE = RH ( 2
n i n2 f
)





 physics
physics








