Similar presentations:
Orbit quantization rule. Lecture №3
1. Orbit quantization rule Lecture №3
2.
-Isolated atoms in the form ofrarefied gas or metal vapors emit a
spectrum consisting of separate
spectral lines (line spectrum).
-Lines in the spectra are not
randomly distributed, they located in
series.
-The distance between the lines in
the series
decreases as the
transition from long waves to short
waves.
3.
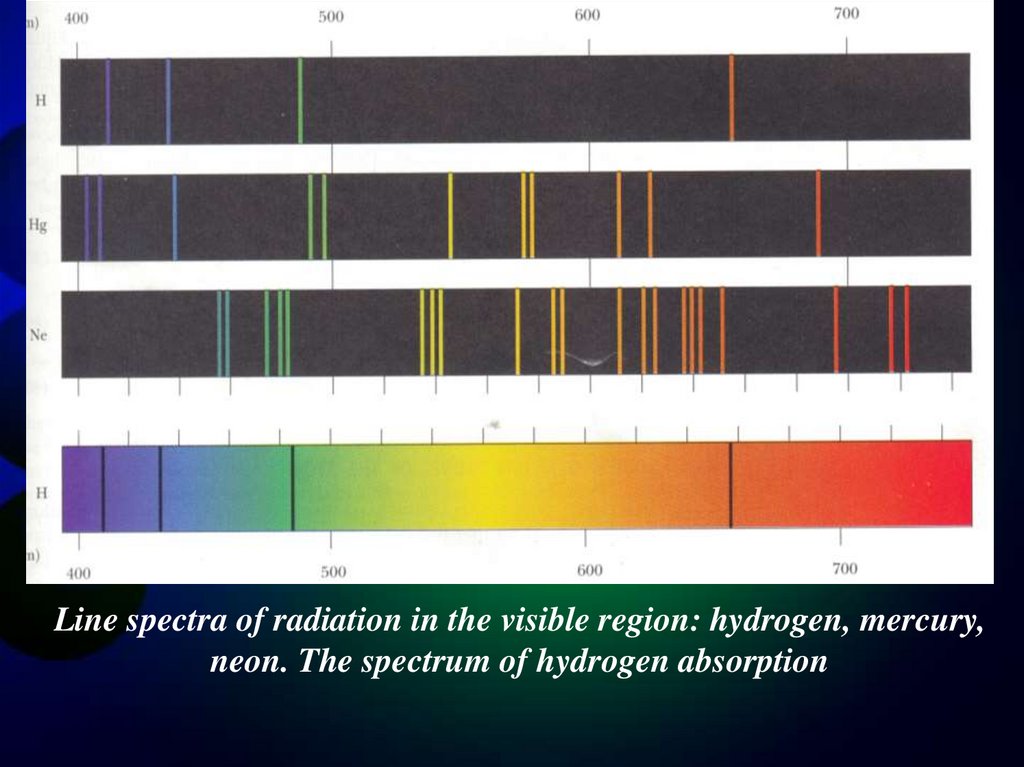
Line spectra of radiation in the visible region: hydrogen, mercury,neon. The spectrum of hydrogen absorption
4.
5.
R =1,09·107m-1 – Rydberg constant.R = R ·с.
R = 3,29·1015 s-1
.
6.
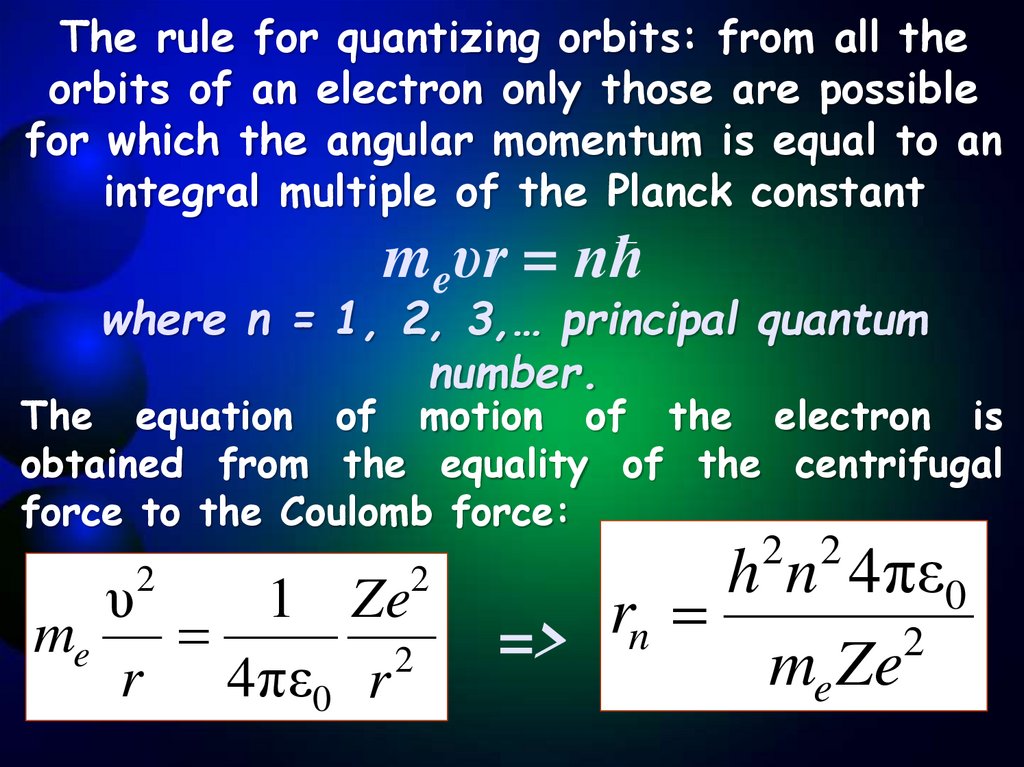
The rule for quantizing orbits: from all theorbits of an electron only those are possible
for which the angular momentum is equal to an
integral multiple of the Planck constant
meυr = nħ
where n = 1, 2, 3,… principal quantum
number.
The equation of motion of the electron is
obtained from the equality of the centrifugal
force to the Coulomb force:
2 2
2
2
0
υ
1 Ze
n
me
2
=>
2
r 4πε0 r
e
h n 4πε
r
m Ze
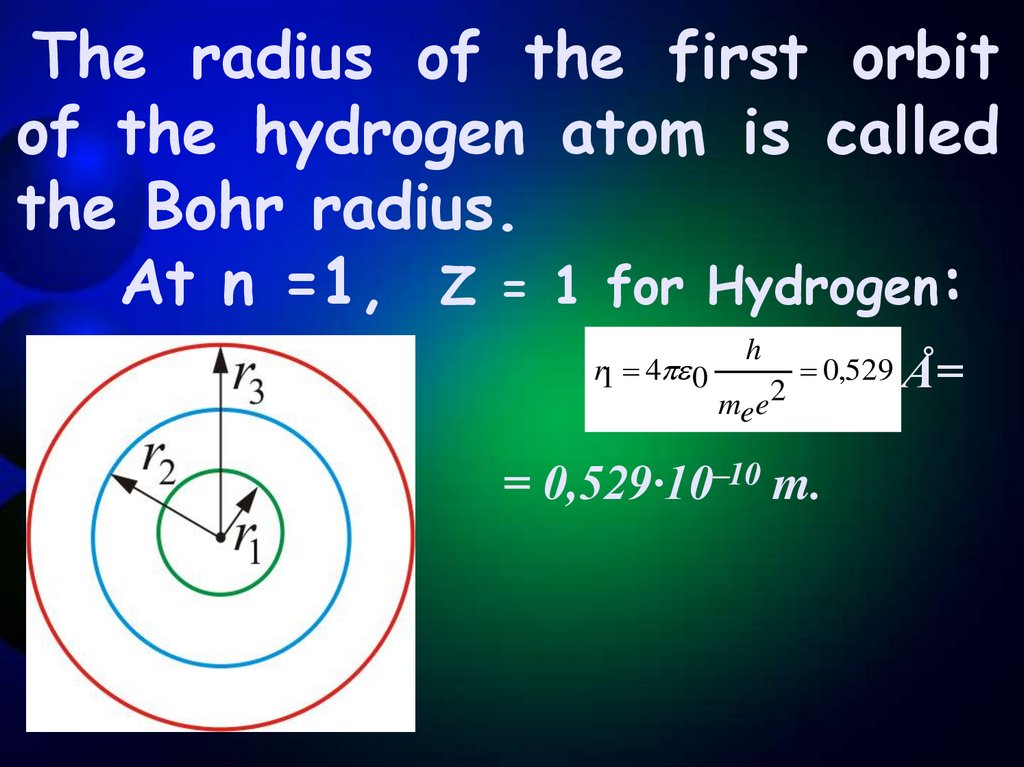
7.
The radius of the first orbitof the hydrogen atom is called
the Bohr radius.
At n =1, Z = 1 for Hydrogen:
r1 4 0
h
mee 2
0,529 Å=
= 0,529·10–10 m.
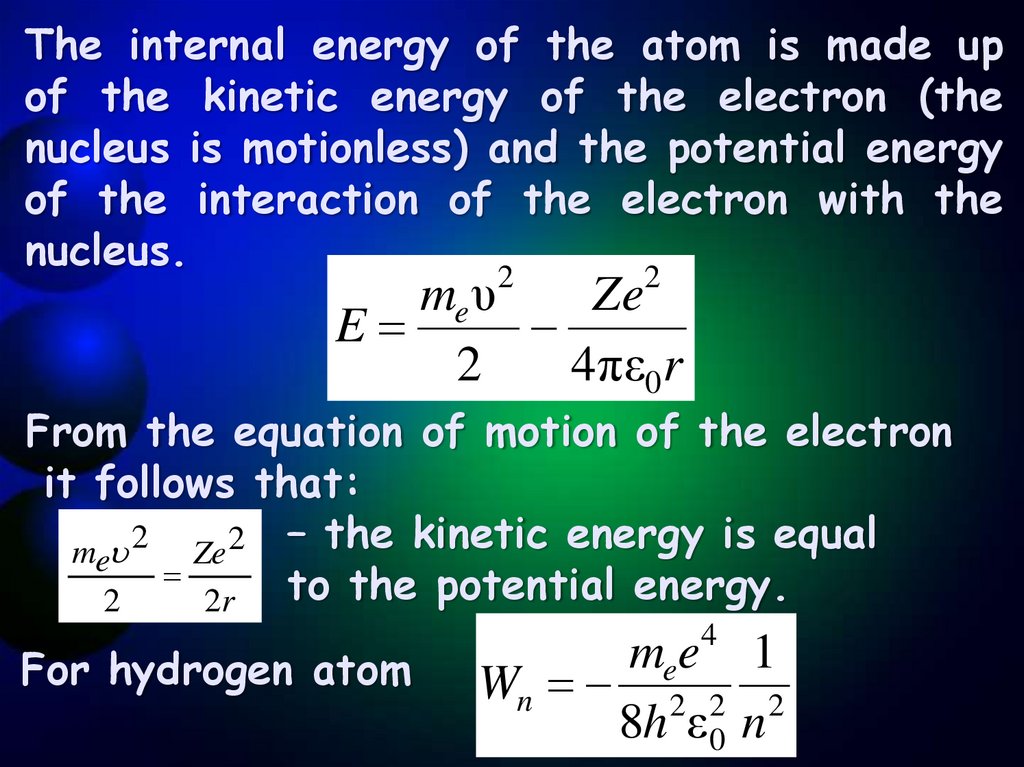
8.
The internal energy of the atom is made upof the kinetic energy of the electron (the
nucleus is motionless) and the potential energy
of the interaction of the electron with the
nucleus.
me υ
Ze
E
2
4πε0r
2
2
From the equation of motion of the electron
it follows that:
me 2 Ze 2 – the kinetic energy is equal
to the potential energy.
2
2r
For hydrogen atom
4
mee 1
Wn 2 2 2
8h ε 0 n
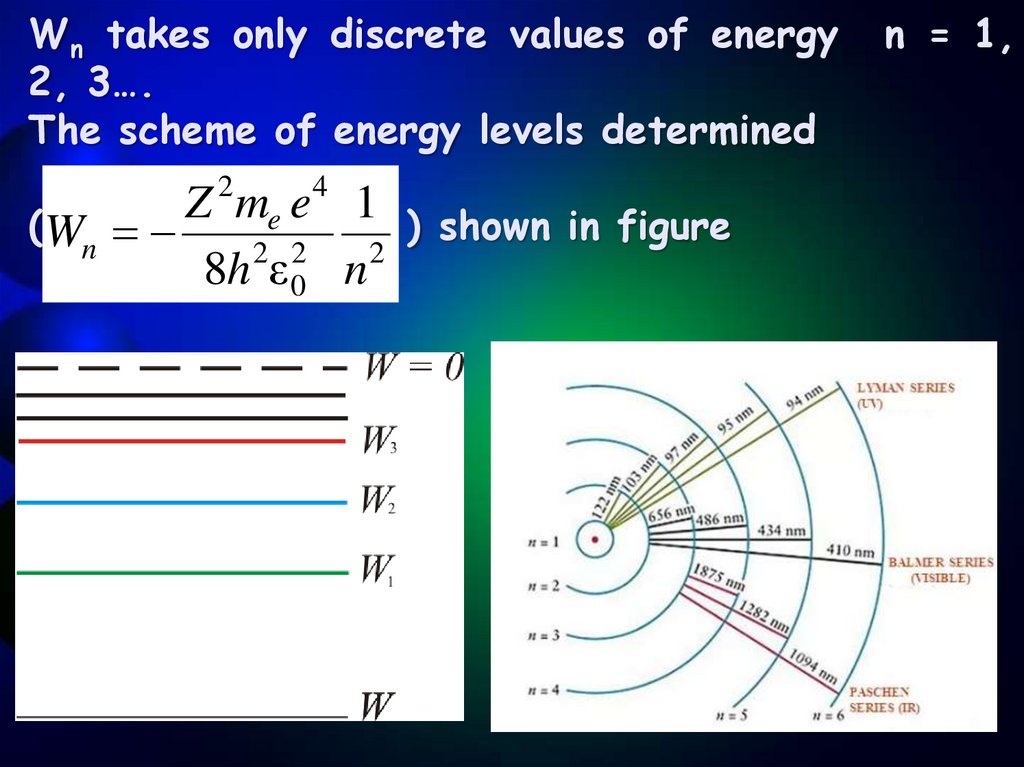
9.
Wn takes only discrete values of energy2, 3….
The scheme of energy levels determined
2
4
Z
m
e
1
e
(Wn
) shown in figure
2 2
2
8h ε 0 n
n = 1,
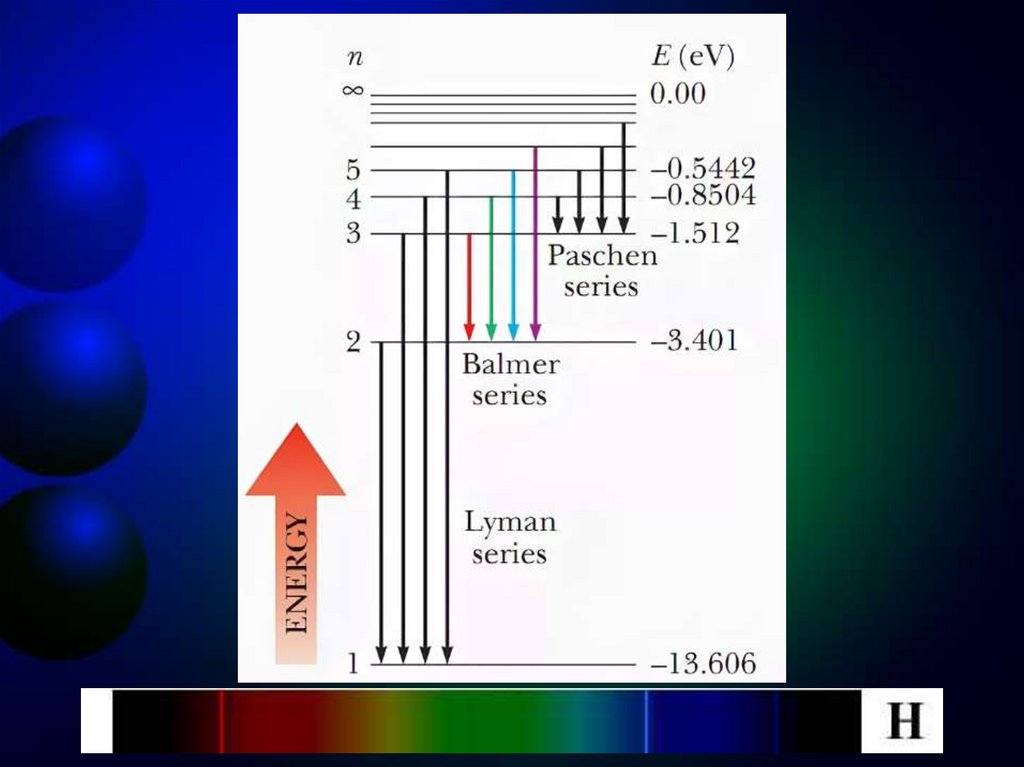
10.
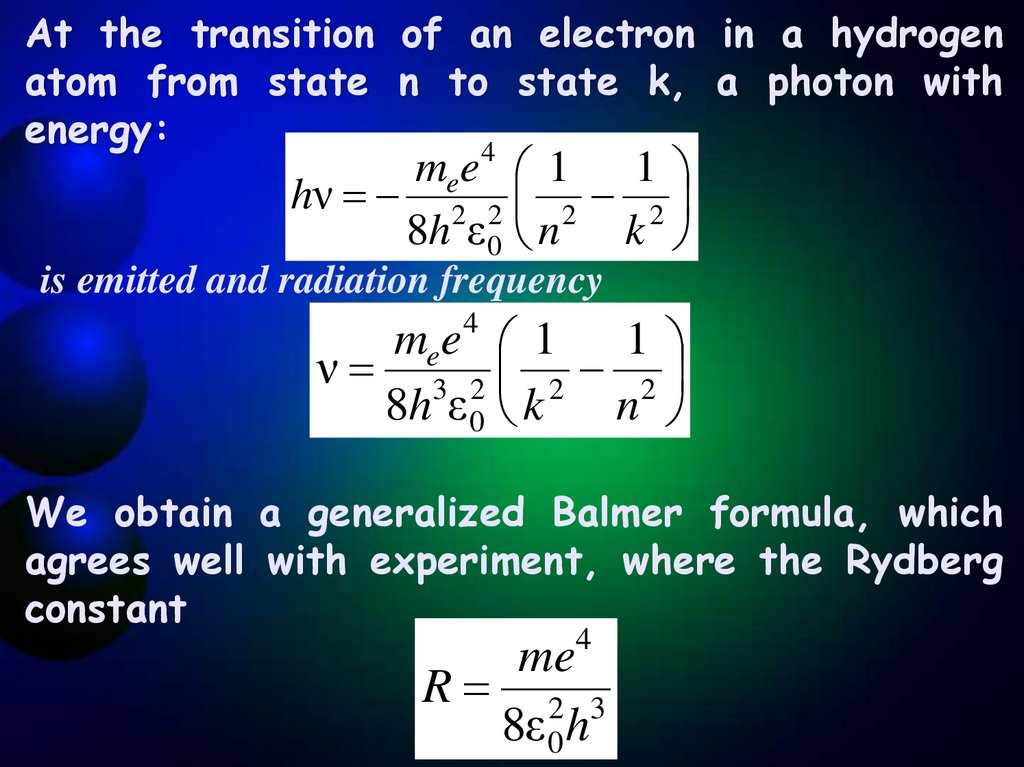
At the transition of an electron in a hydrogenatom from state n to state k, a photon with
energy:
4
mee 1 1
hν 2 2 2 2
8h ε 0 n k
is emitted and radiation frequency
mee 1 1
ν 3 2 2 2
8h ε 0 k
n
4
We obtain a generalized Balmer formula, which
agrees well with experiment, where the Rydberg
constant
me4
R 2 3
8ε 0 h
11.
The success of Bohr'stheory:
-calculation of the Rydberg constant
for hydrogen-like systems;
-explanation of the structure of their
line spectra.
.
12.
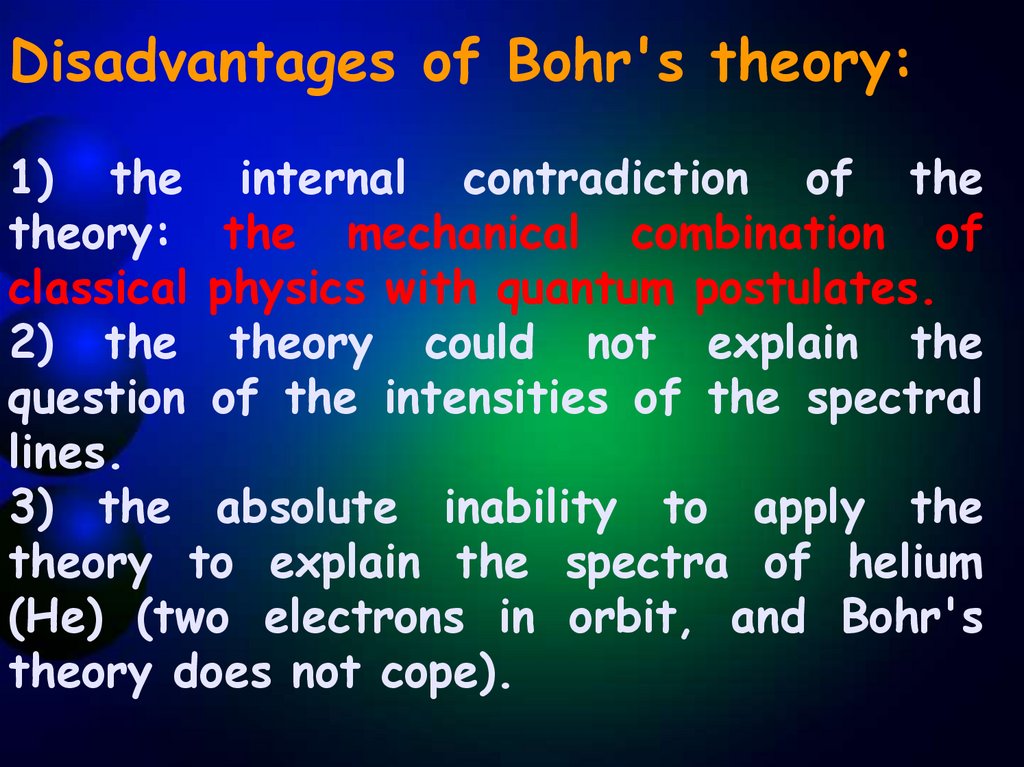
Disadvantages of Bohr's theory:1) the internal contradiction of the
theory: the mechanical combination of
classical physics with quantum postulates.
2) the theory could not explain the
question of the intensities of the spectral
lines.
3) the absolute inability to apply the
theory to explain the spectra of helium
(He) (two electrons in orbit, and Bohr's
theory does not cope).


 physics
physics








