Similar presentations:
Hypertrophic cardiomyopathy
1.
HYPERTROPHICCARDIOMYOPATHY
(HCM)
Dr. Michael Kapeliovich MD, PhD
11.11.2021
2.
Elliott et al. Eur Heart J 2008; 29:2703.
CARDIOMYOPATHIESHypertrophic cardiomyopathy (HCM)
Dilated cardiomyopathy (DCM)
Restrictive cardiomyopathy
Arrhythmogenic right ventricular dysplasia
Unclassified
Elliott et al. Eur Heart J 2008; 29:270
4.
Circulation 2020; 142: e558-e6315.
Eur Heart J 2014;35:2733-796.
HCM - DefinitionHCM – a disease state in which morphologic
expression is confined solely to the heart.
It is characterized predominantly by LVH in the
absence of another cardiac , systemic or
metabolic disease capable of producing
hypertrophy in a given patient .
For this patient a disease-causing sarcomere (or
sarcomere-related) variant is identified, or
genetic etiology remains unresolved.
7.
SarcomereTolkatchev et al. Progr Molec Biol and Translational Science 2019
8.
HCM - Diagnosis• In adult pt is established by imaging (Echo, CMR)
showing a maximal end diastolic wall thickness >15
mm anywhere in left ventricle, in the absence of
another cause of hypertrophy.
• Limited hypertrophy (13-14 mm) can be diagnostic
when present in family members of a pt with HCM or
in conjunction with positive genetic test.
• For children there is a need to adjustment for body
size and growth.
9.
HCM : differential diagnosis• Systemic disorders including various metabolic and multiorgan
syndromes:
RASopathies*, mitochondrial myopathies, glycogen/lysosomal
storage disease, Fabry, amyloid, sarcoid, hemochromatosis,
Danon cardiomyopathy
• Hypertension
• CAD
• Athletes heart
• Valvular and subvalvular aortic stenosis
--------------------* A group of rare genetic conditions caused by mutations in genes of the
Ras-MAPK pathway
10.
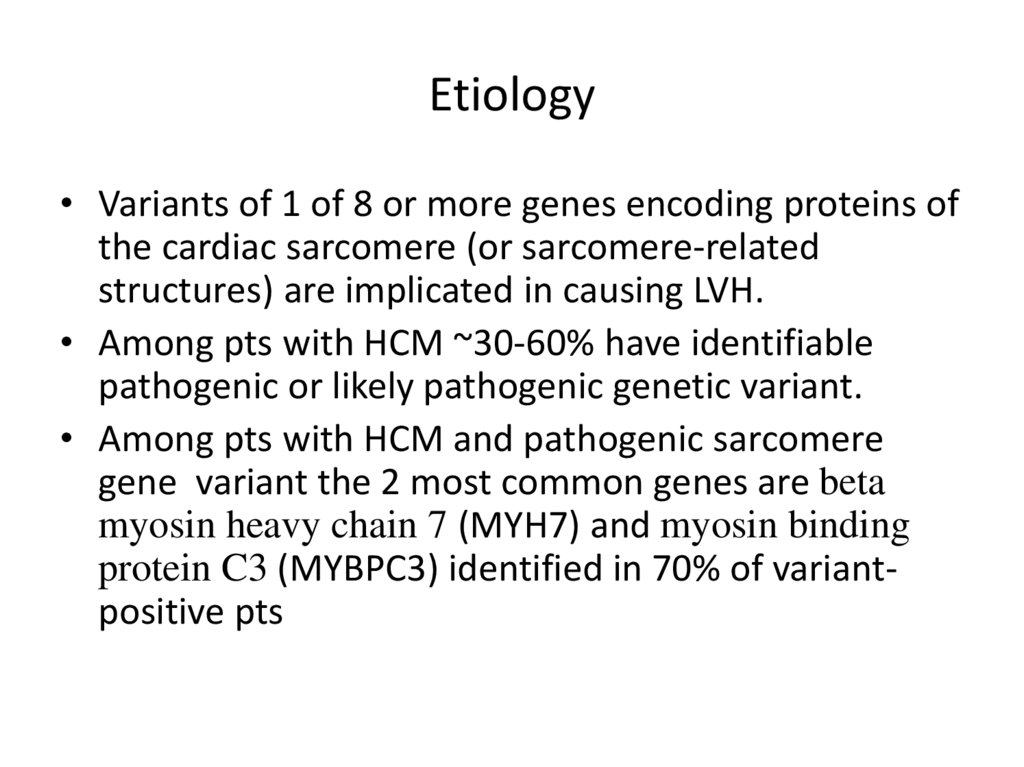
Etiology• Variants of 1 of 8 or more genes encoding proteins of
the cardiac sarcomere (or sarcomere-related
structures) are implicated in causing LVH.
• Among pts with HCM ~30-60% have identifiable
pathogenic or likely pathogenic genetic variant.
• Among pts with HCM and pathogenic sarcomere
gene variant the 2 most common genes are beta
myosin heavy chain 7 (MYH7) and myosin binding
protein C3 (MYBPC3) identified in 70% of variantpositive pts
11.
Etiology• Other genes (TNNI3, TNN2, TPM1, MYL2,
MYL3, ACTC1) each account for a small
proportion of pts (1-5%).
• Within these genes >1500 variants are
recognized.
• Each offspring of an affected family member
has a 50% chance of inheriting the variant.
12.
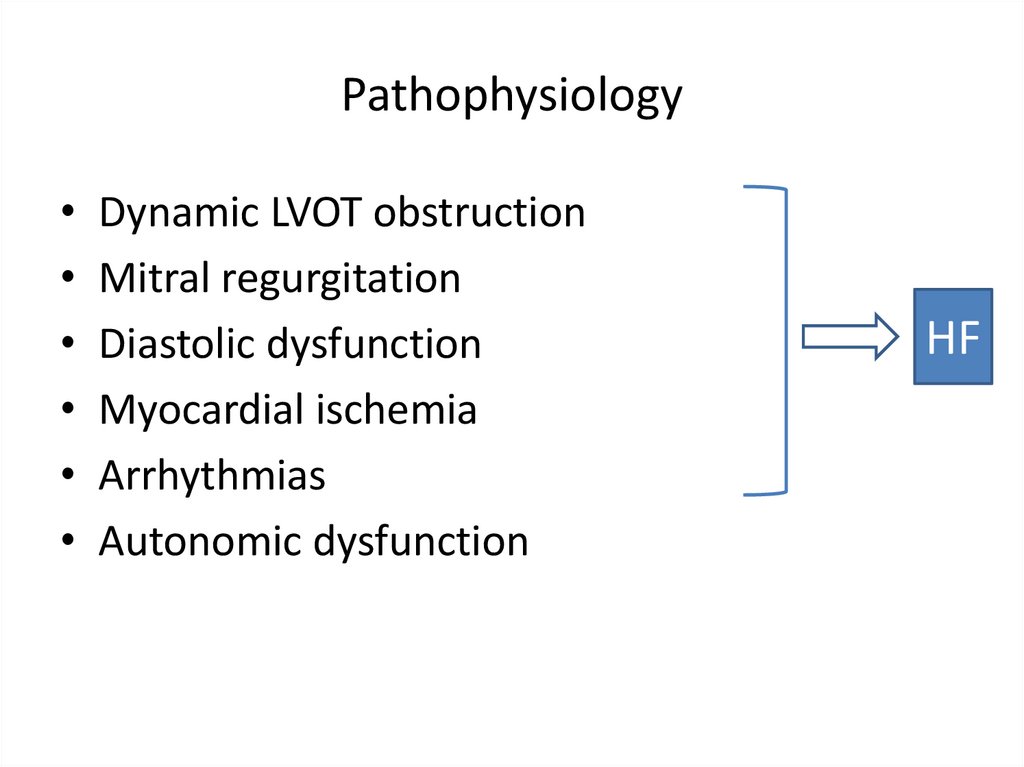
PathophysiologyDynamic LVOT obstruction
Mitral regurgitation
Diastolic dysfunction
Myocardial ischemia
Arrhythmias
Autonomic dysfunction
HF
13.
Diagnostic methods• Cardiac anamnesis and family history including
3 generations
• Physical examination
• Echocardiography
• CMR
• Cardiac CT
• Heart rhythm assessment
• Angiography and invasive hemodynamic assessment
• Exercise stress testing
14.
Dynamic LVOT obstruction15.
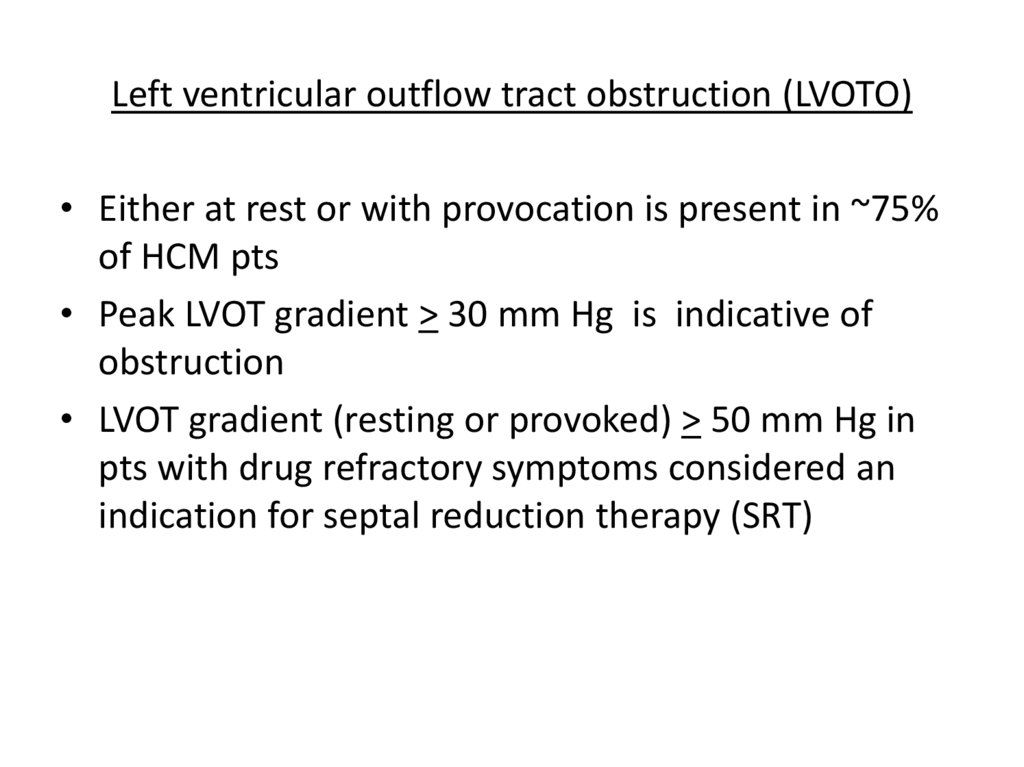
Left ventricular outflow tract obstruction (LVOTO)• Either at rest or with provocation is present in ~75%
of HCM pts
• Peak LVOT gradient > 30 mm Hg is indicative of
obstruction
• LVOT gradient (resting or provoked) > 50 mm Hg in
pts with drug refractory symptoms considered an
indication for septal reduction therapy (SRT)
16.
Left ventricular outflow tract obstruction (LVOTO)• Provocative maneuvers :
- standing
- Valsalva
- amyl nitrite inhalation
- exercise
17.
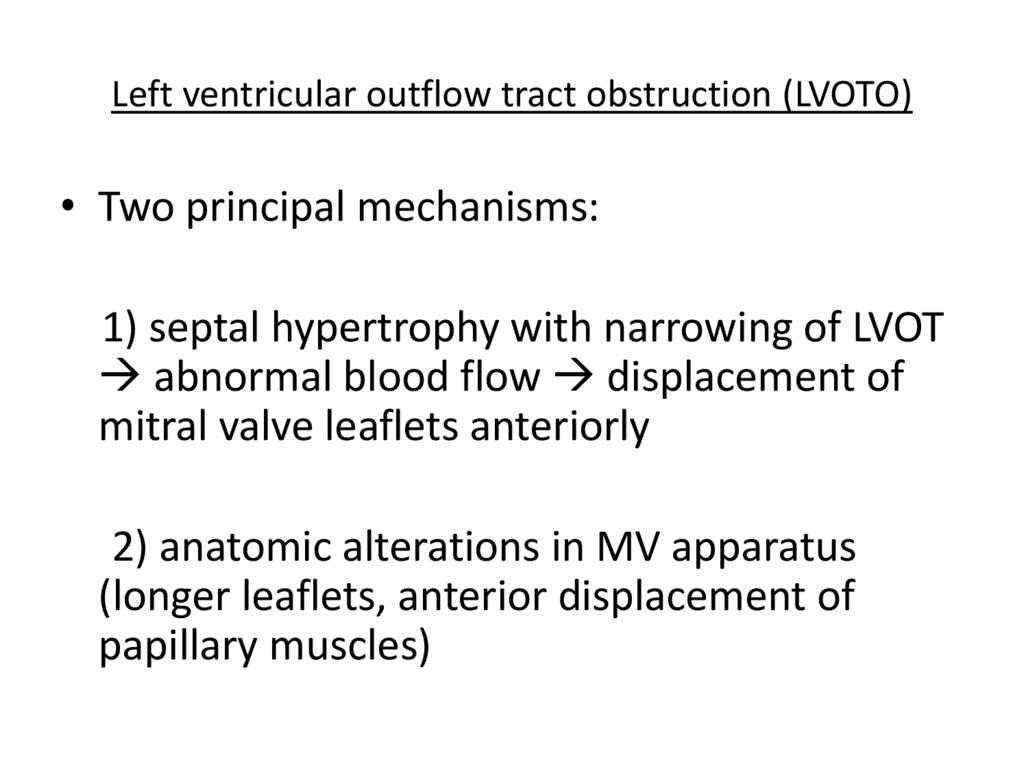
Left ventricular outflow tract obstruction (LVOTO)• Two principal mechanisms:
1) septal hypertrophy with narrowing of LVOT
abnormal blood flow displacement of
mitral valve leaflets anteriorly
2) anatomic alterations in MV apparatus
(longer leaflets, anterior displacement of
papillary muscles)
18.
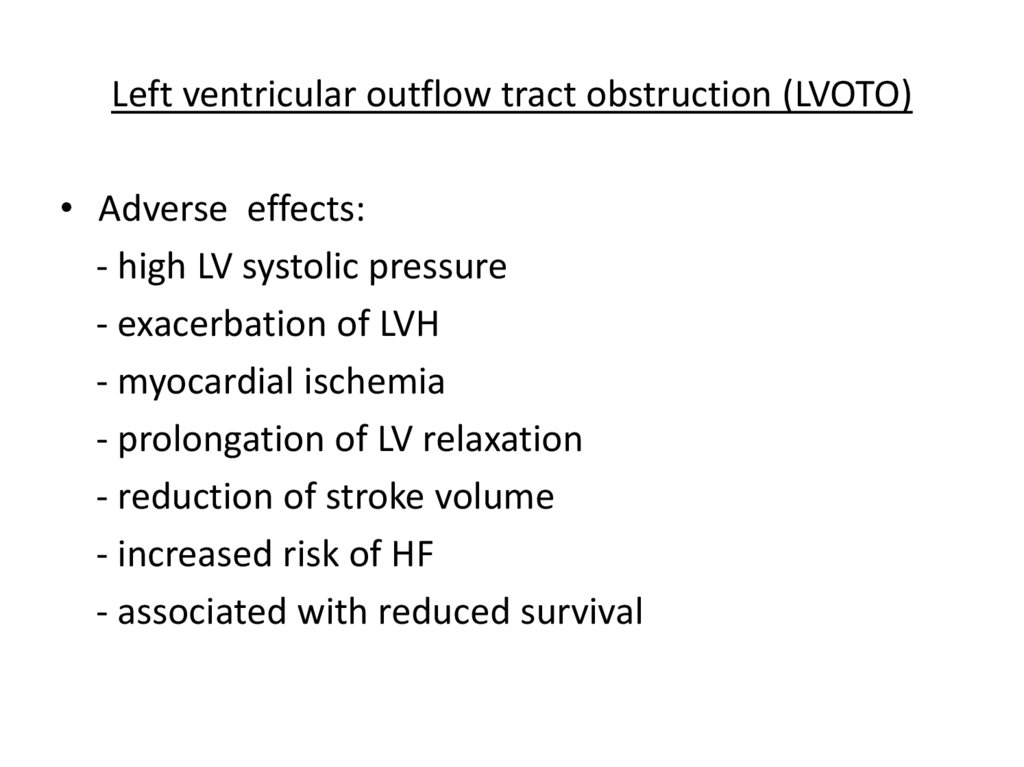
Left ventricular outflow tract obstruction (LVOTO)• Adverse effects:
- high LV systolic pressure
- exacerbation of LVH
- myocardial ischemia
- prolongation of LV relaxation
- reduction of stroke volume
- increased risk of HF
- associated with reduced survival
19.
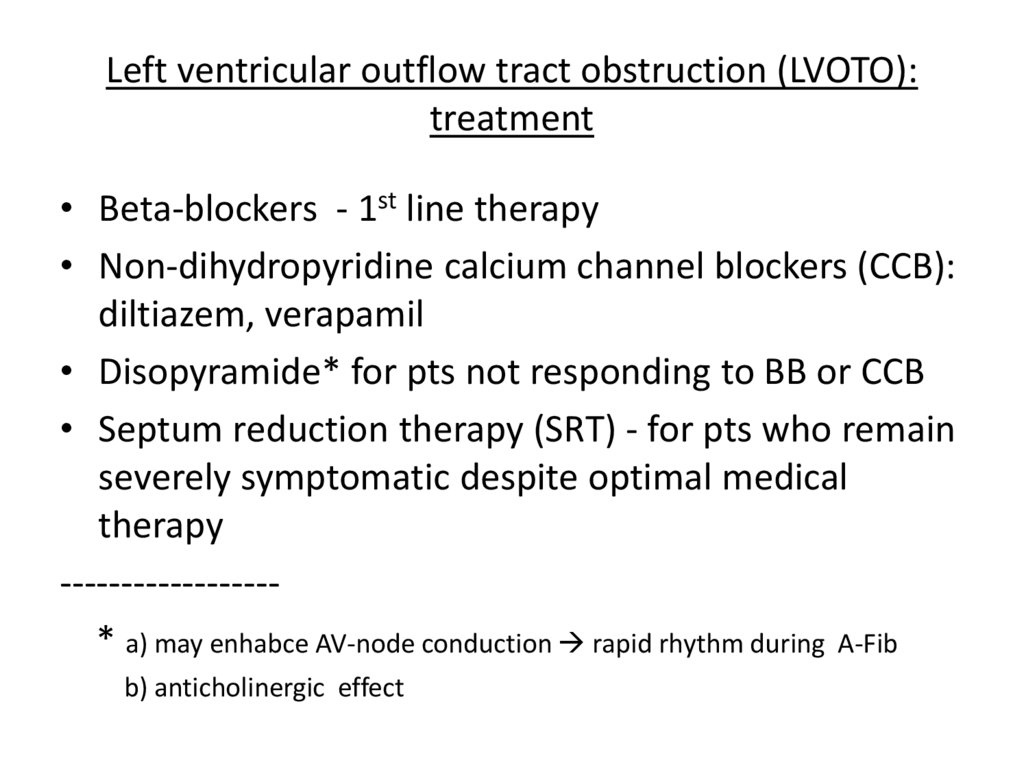
Left ventricular outflow tract obstruction (LVOTO):treatment
• Beta-blockers - 1st line therapy
• Non-dihydropyridine calcium channel blockers (CCB):
diltiazem, verapamil
• Disopyramide* for pts not responding to BB or CCB
• Septum reduction therapy (SRT) - for pts who remain
severely symptomatic despite optimal medical
therapy
-----------------* a) may enhabce AV-node conduction rapid rhythm during A-Fib
b) anticholinergic effect
20.
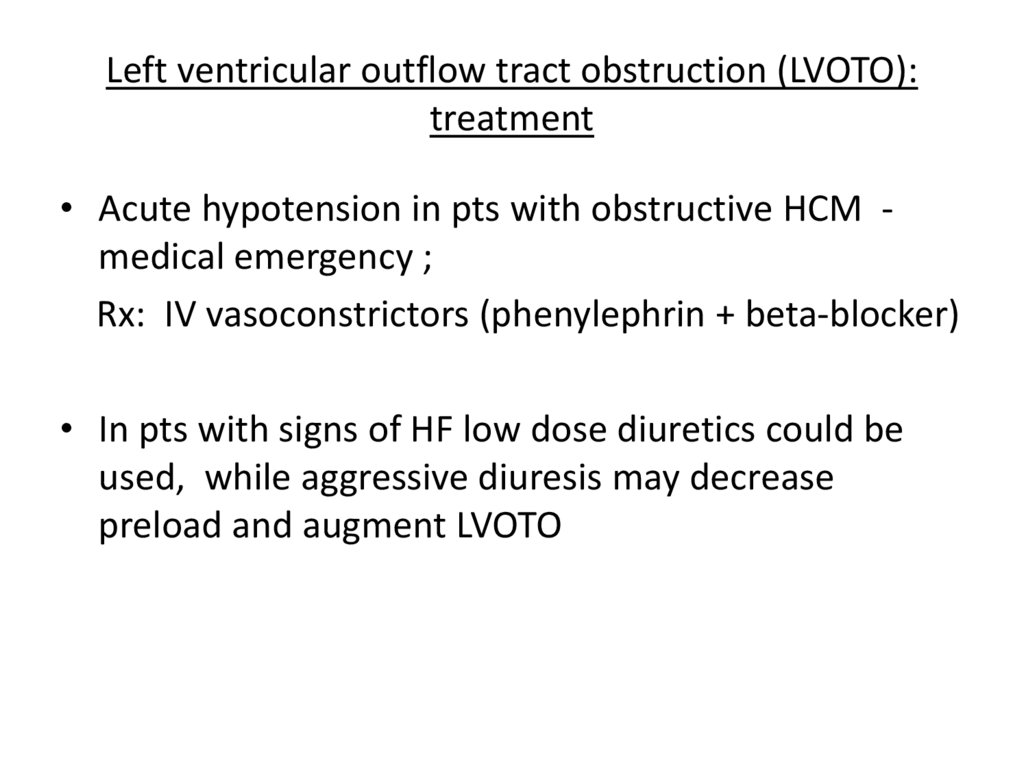
Left ventricular outflow tract obstruction (LVOTO):treatment
• Acute hypotension in pts with obstructive HCM medical emergency ;
Rx: IV vasoconstrictors (phenylephrin + beta-blocker)
• In pts with signs of HF low dose diuretics could be
used, while aggressive diuresis may decrease
preload and augment LVOTO
21.
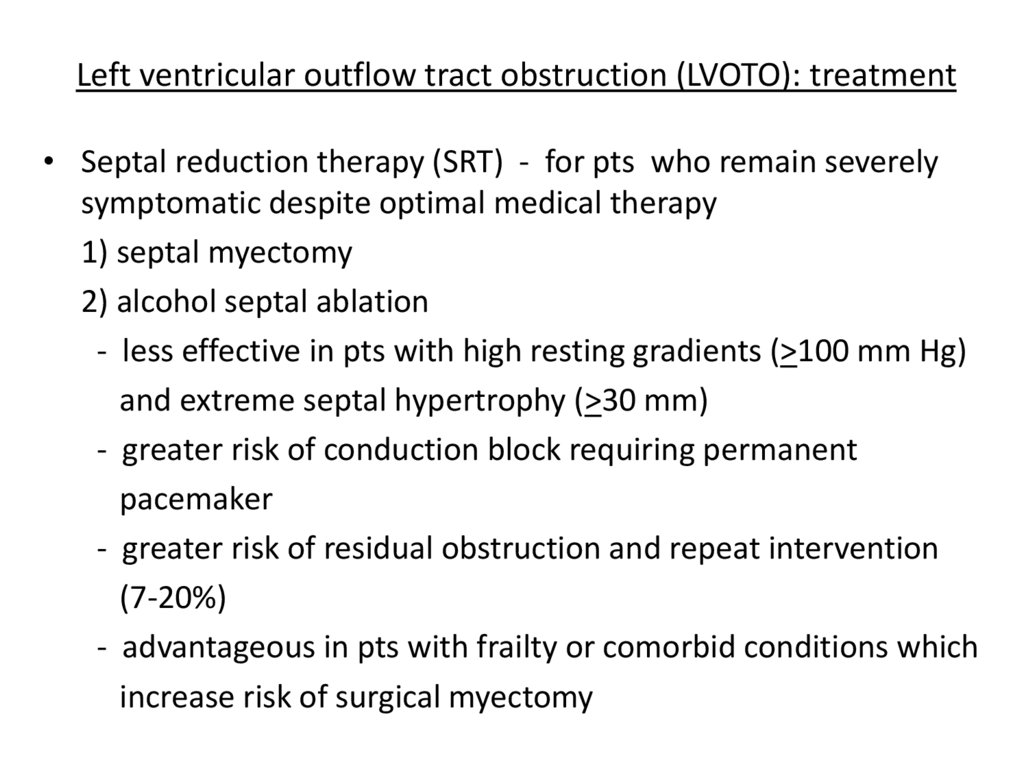
Left ventricular outflow tract obstruction (LVOTO): treatment• Septal reduction therapy (SRT) - for pts who remain severely
symptomatic despite optimal medical therapy
1) septal myectomy
2) alcohol septal ablation
- less effective in pts with high resting gradients (>100 mm Hg)
and extreme septal hypertrophy (>30 mm)
- greater risk of conduction block requiring permanent
pacemaker
- greater risk of residual obstruction and repeat intervention
(7-20%)
- advantageous in pts with frailty or comorbid conditions which
increase risk of surgical myectomy
22.
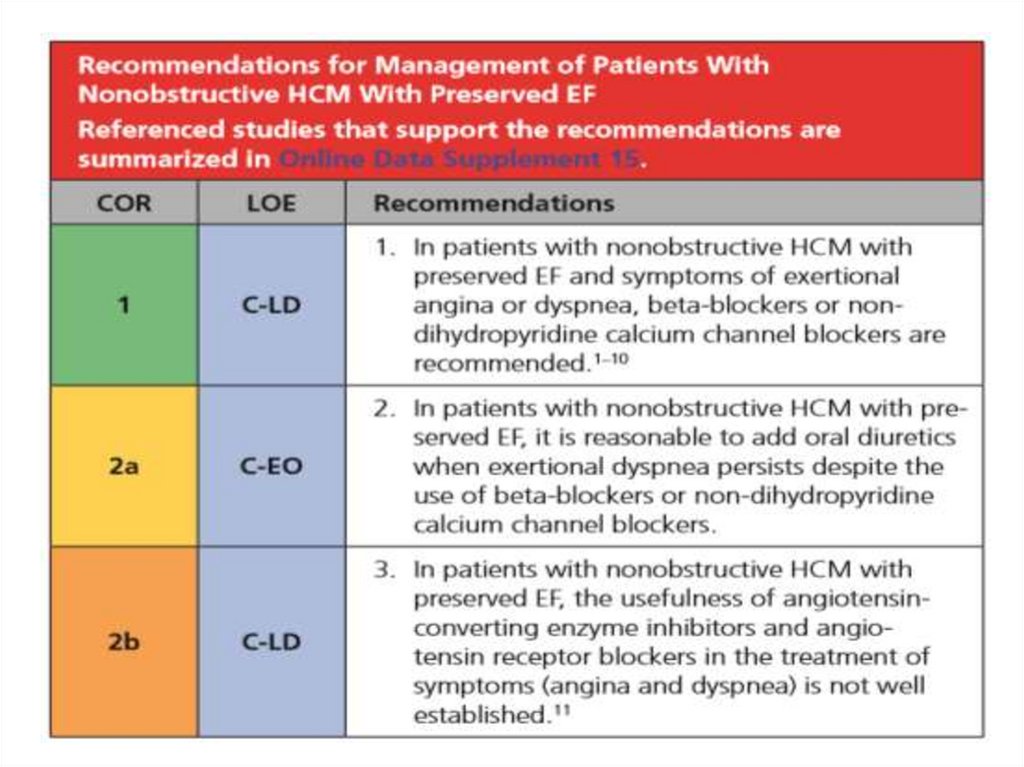
Nonobstructive HCM23.
24.
25.
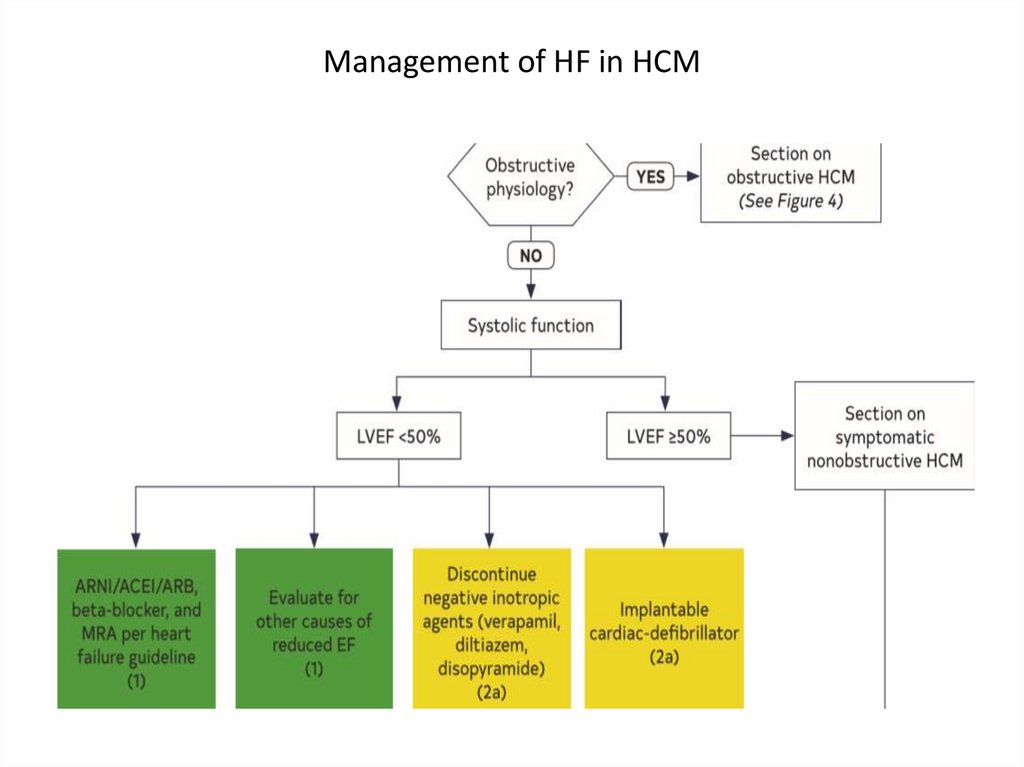
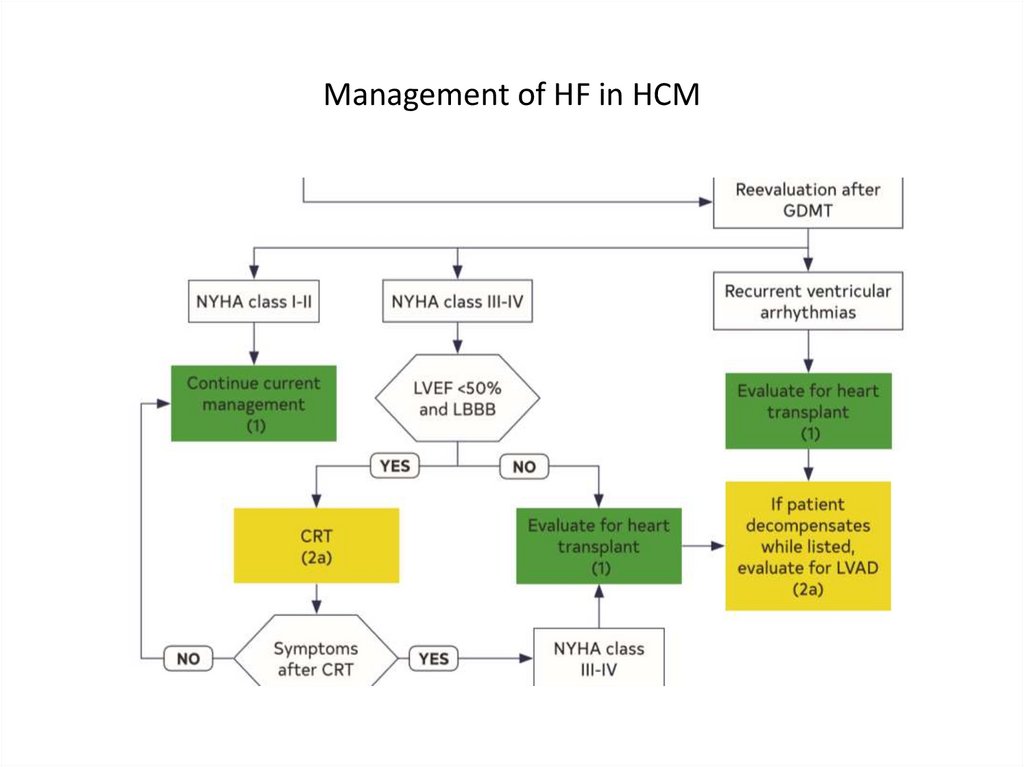
Management of HF in HCM26.
Management of HF in HCM27.
Management of HF in HCM28.
Myocardial ischemia• Mismatch between myocardial oxygen supply and
demand
- myocardial hypertrophy
- microvascular dysfunction
- impaired coronary flow reserve
- medial hypertrophy of intramural arterioles
- hyperdynamic systolic function
- LVOTO with high intracoronary pressure
- myocardial bridging
29.
Arrhythmias and SCD30.
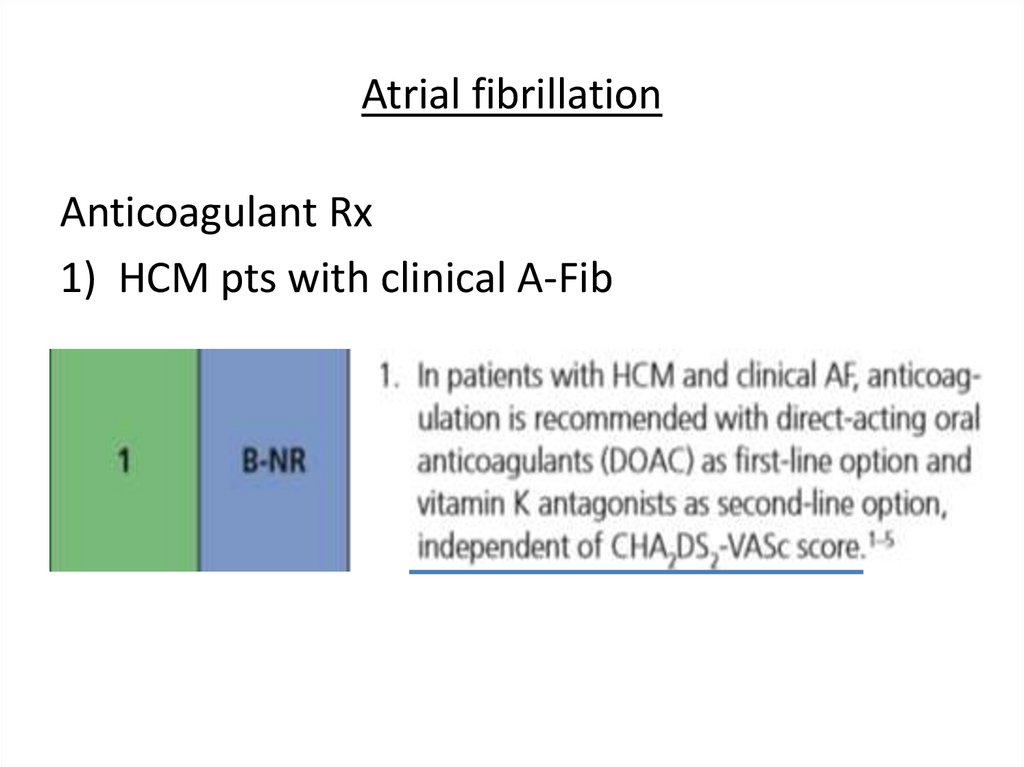
Atrial fibrillationAnticoagulant Rx
1) HCM pts with clinical A-Fib
31.
Atrial fibrillationAnticoagulant Rx
2) HCM pts with subclinical A-Fib >24 hours
32.
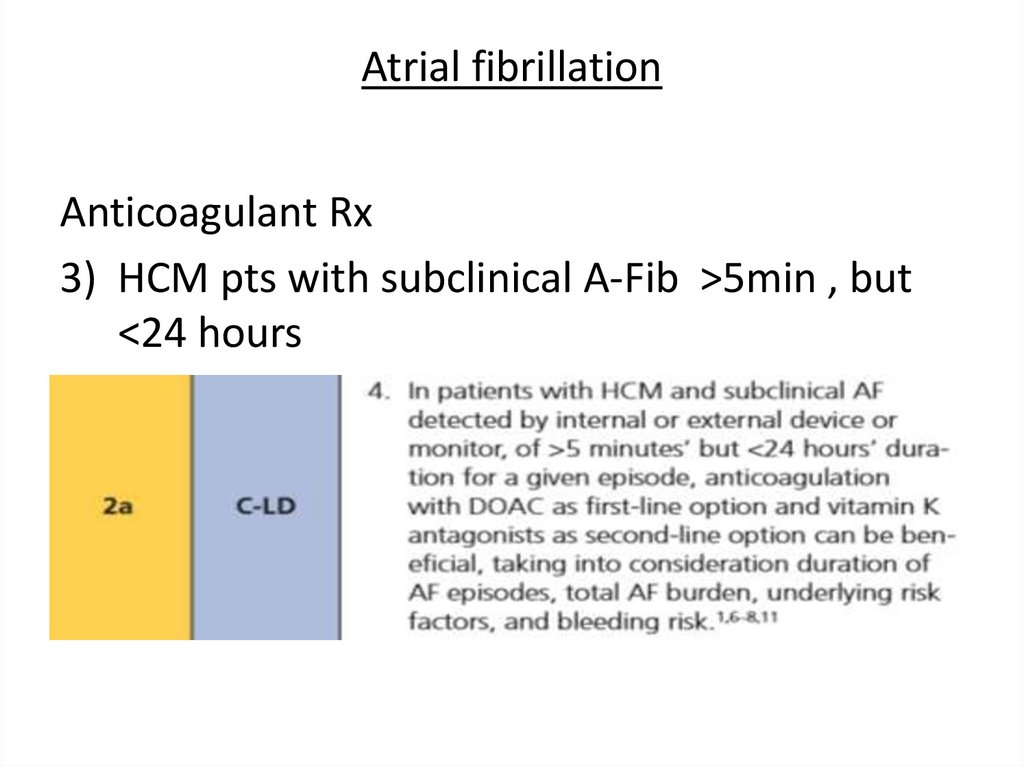
Atrial fibrillationAnticoagulant Rx
3) HCM pts with subclinical A-Fib >5min , but
<24 hours
33.
Atrial fibrillationRate control
34.
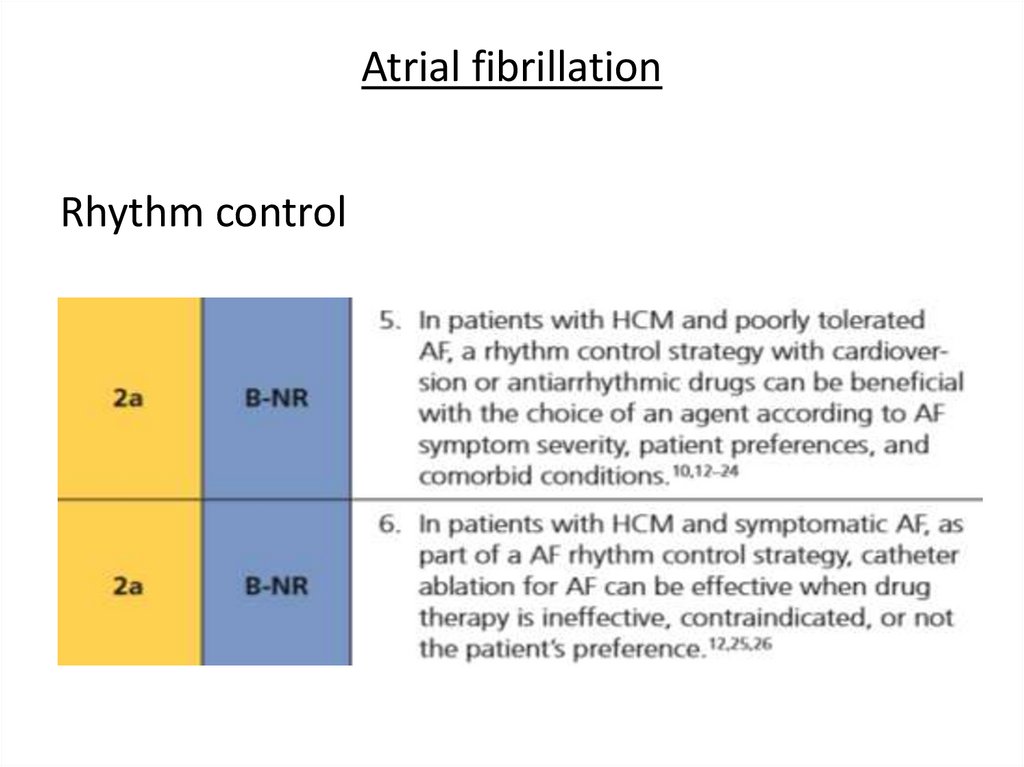
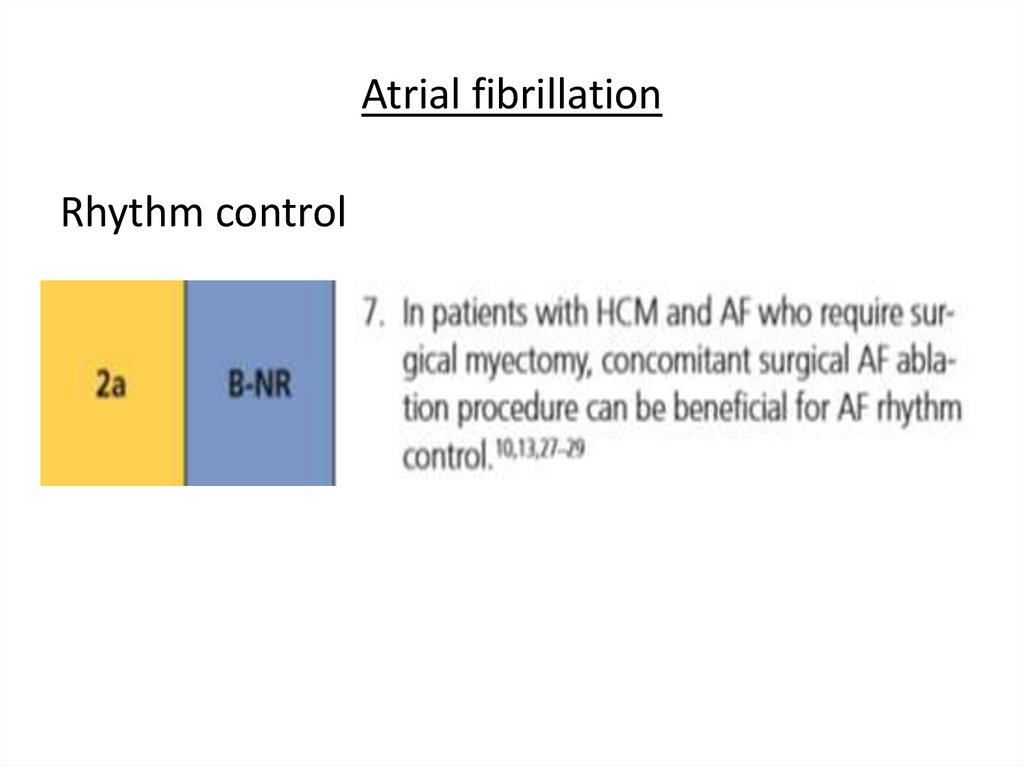
Atrial fibrillationRhythm control
35.
Atrial fibrillationDrugs for rhythm control
- sotalol
- amiodarone
- disopyramide (efficacy in A-Fib is not well established)
- class 1c (propafenon, flecainide) not recommended in
pts with structural disease, but is safe in pts with ICD
- dofetilide
36.
Atrial fibrillationRhythm control
37.
Ventricular arrhythmias38.
Ventricular arrhythmias39.
Ventricular arrhythmias40.
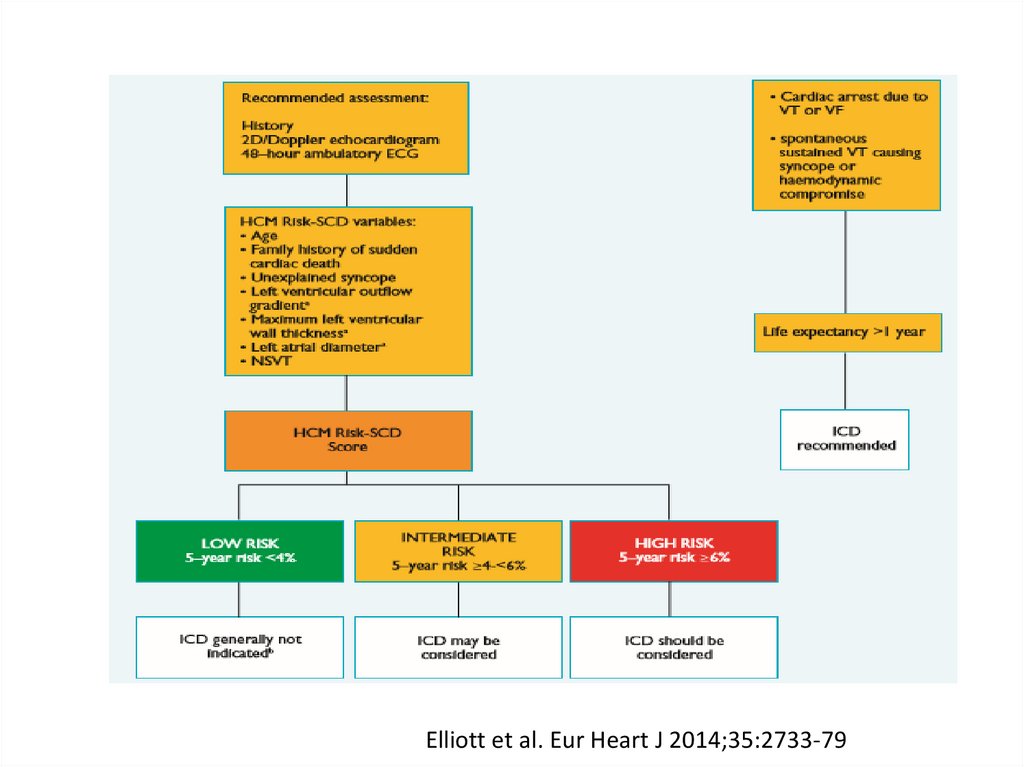
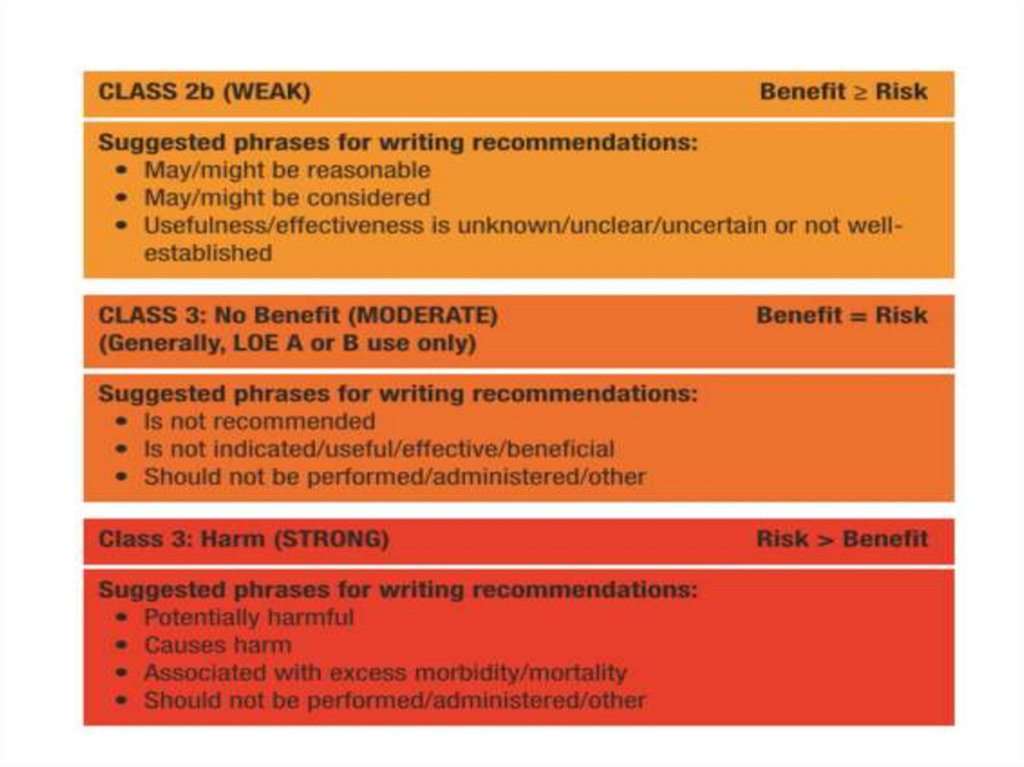
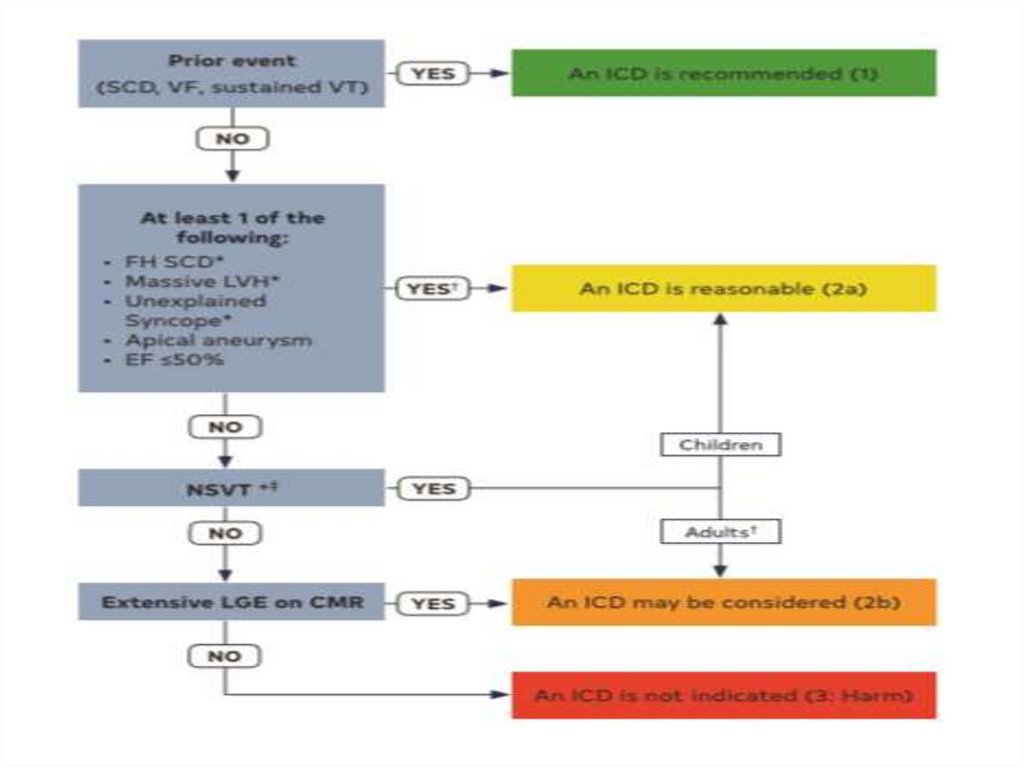
SCD: risk assessment and prevention• HCM is the most common cause of SCD in young
people in North America
• Among pts with HCM younger at higher risk for SCD
than older pts
• Major clinical risk factors help to stratify pts
according to level of risk and to identify those most
likely to benefit from primary ICD therapy
• Risk score is available , but it’s performance is not
very good
• SCD assessment at initial visit and repeated every 1-2
years is recommended
41.
HCM sudden cardiac death risk stratification: clinical riskfactors
42.
HCM sudden cardiac death risk stratification• Family Hx of sudden death from HCM
- < 50 years old
- 1st degree relative
- other relative (generally 2nd degree, but multiple
SCDs in tertiary relatives should be also consider
relevant
43.
HCM sudden cardiac death risk stratification• Massive LVH
- wall thickness > 30 mm in any segment within
chamber by Echo or CMR imaging
- > 28 mm in individual pts
- for pediatric pts with HCM threshold for wall
thickness is not well established: max wall
corresponds to z-score > 20 and > 10 mm seems
reasonable
44.
HCM sudden cardiac death risk stratification• Unexplained syncope
- > 1 unexplained episodes of acute transient loss of
consciousness, unlikely to be of neurogenic
(vasovagal) etiology
- not attributable to LVOTO
- especially occurring within 6 months of evaluation;
events beyond 5 years in the past have little
significance
45.
HCM sudden cardiac death risk stratification• HCM with LV dysfunction
- systolic LV dysfunction with EF < 50% by Echo or
CMR imaging
46.
HCM sudden cardiac death risk stratification• LV apical aneurysm
- apical aneurysm defined as a discrete
thin-walled dyskinetic or akinetic segment of
the most distal portion of the LV chamber,
independent of size
47.
HCM sudden cardiac death risk stratification• Extensive LGE on MRI imaging
- diffuse and extensive LGE, representing fibrosis,
either quantified or estimated by visual inspection
- comprising > 15% of LV mass
- not established in children
48.
HCM sudden cardiac death risk stratification• NSVT on ambulatory monitoring
- runs are frequent (> 3)
greater weight
- long (> 10 beats)
- fast (> 200 bpm)
- occurring over 24-48 hr of monitoring
- for pediatric pts a VT rate that exceeds the
baseline sinus rate by 20% is considered significant































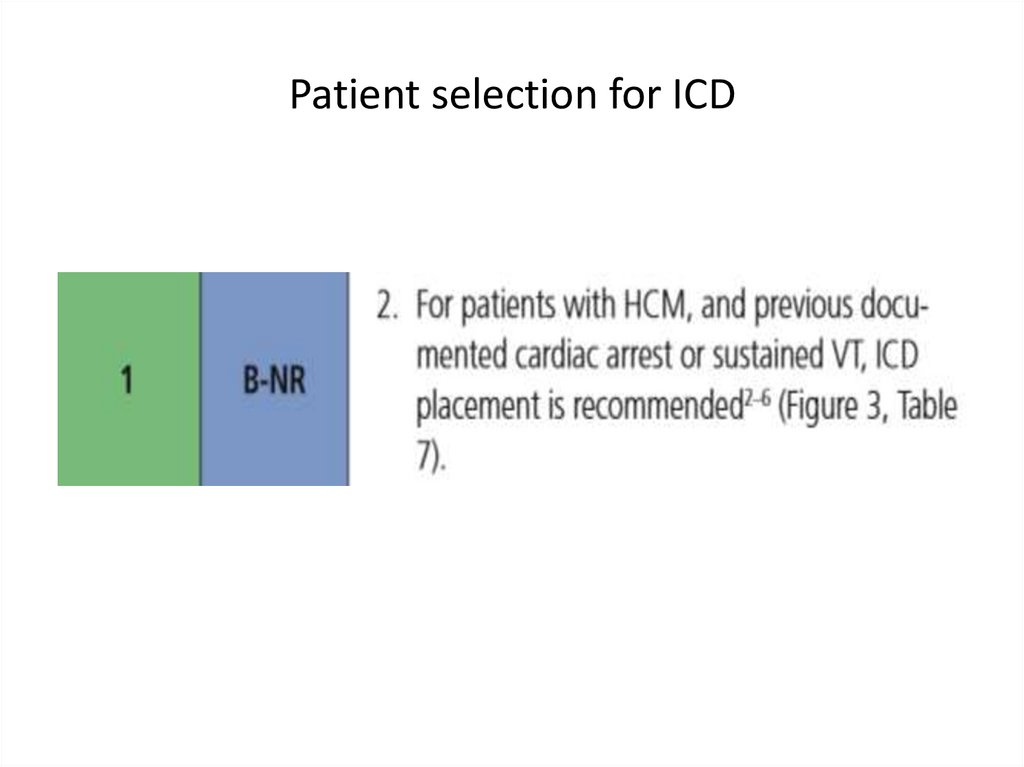
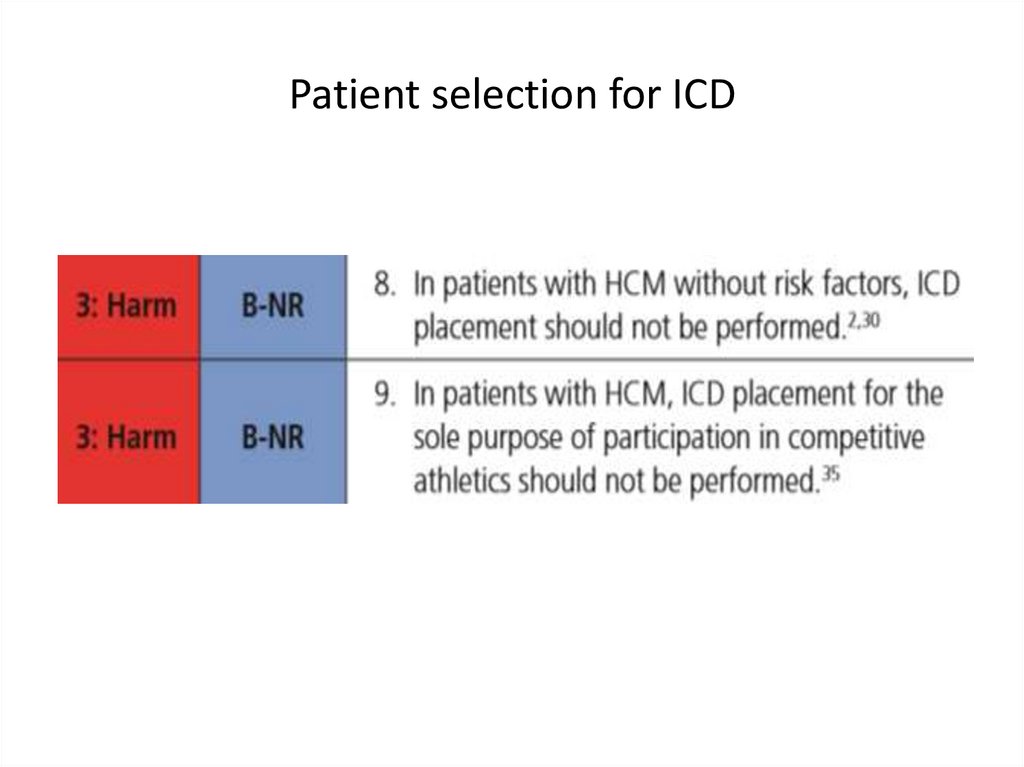
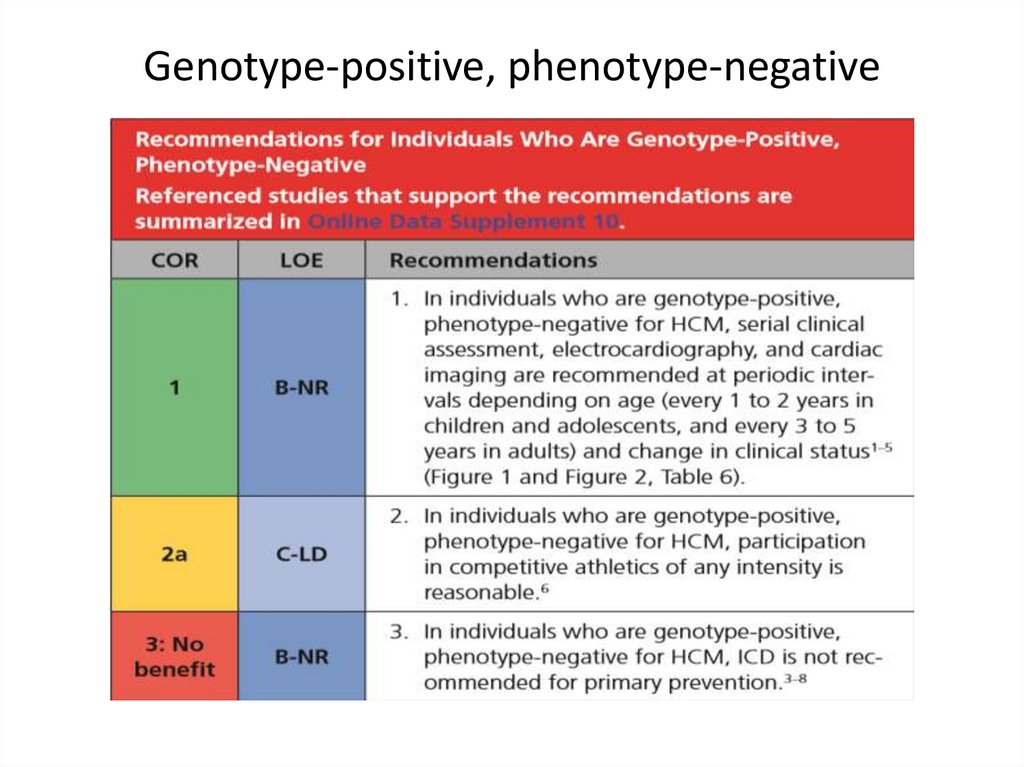





 medicine
medicine