Similar presentations:
Radical reactions. Decarboxylative alkenylations and alkynylations
1. Radical reactions. Decarboxylative alkenylations and alkynylations
Dr. Vadim Korotkov, St.-Petersburg, 03.01.20202.
Methods of C–C – Bond Formation-
Reactions of nucleophiles with electrophiles
-
Pericyclic reactions
-
Radical reactions
-
Other reactions (metathesis)
3.
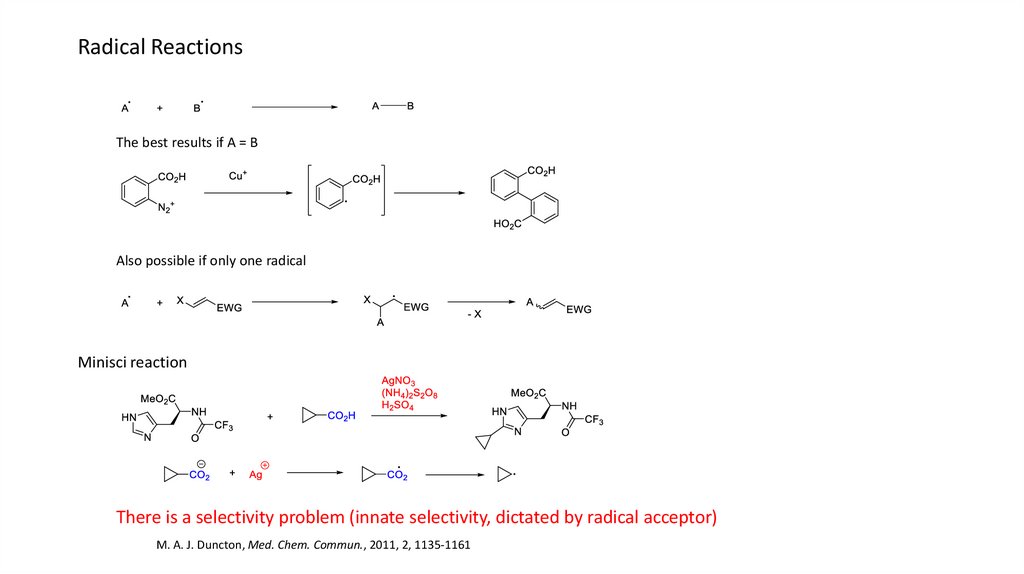
Radical ReactionsThe best results if A = B
Also possible if only one radical
Minisci reaction
There is a selectivity problem (innate selectivity, dictated by radical acceptor)
M. A. J. Duncton, Med. Chem. Commun., 2011, 2, 1135-1161
4.
Radical ReactionsGiese Reaction
N. P. Ramirez,J. C. Gonzalez-Gomez, Eur. J. Org. Chem. 2017, 2154–2163
I. Ryu, S. Uehara, H. Hirao, T. Fukuyama, Org. Lett., 2008, 10, 1005-1008.
Barton Decarboxylation
K. Okada, K. Okamoto, M. Oda, J. Am. Chem. Soc., 1988, 110 (26), pp 8736–8738
5.
Radical ReactionsModification of Giese reaction
T. Qin, L. R. Malins, J. T. Edwards, R. R. Merchant, A. J. E. Novak, J. Z. Zhong, R. B. Mills, M. Yan, C. Yuan, M. D. Eastgate, and P. S. Baran, Angew.
Chem. Int. Ed. 2017, 56, 260 –265
6.
7.
Radical ReactionsIs there an alternative for innate radical cross couplings (RCC)?
Programmed radical cross-coupling (RCC)!
The radical binds to a metal ion and then selectively (!!!) reacts with the determined partner
8.
Radical ReactionsKey steps of RCC
J. M. Smith, S. J. Harwood, P. S. Baran, Acc. Chem. Res. 2018, 51, 1807-1817.
9.
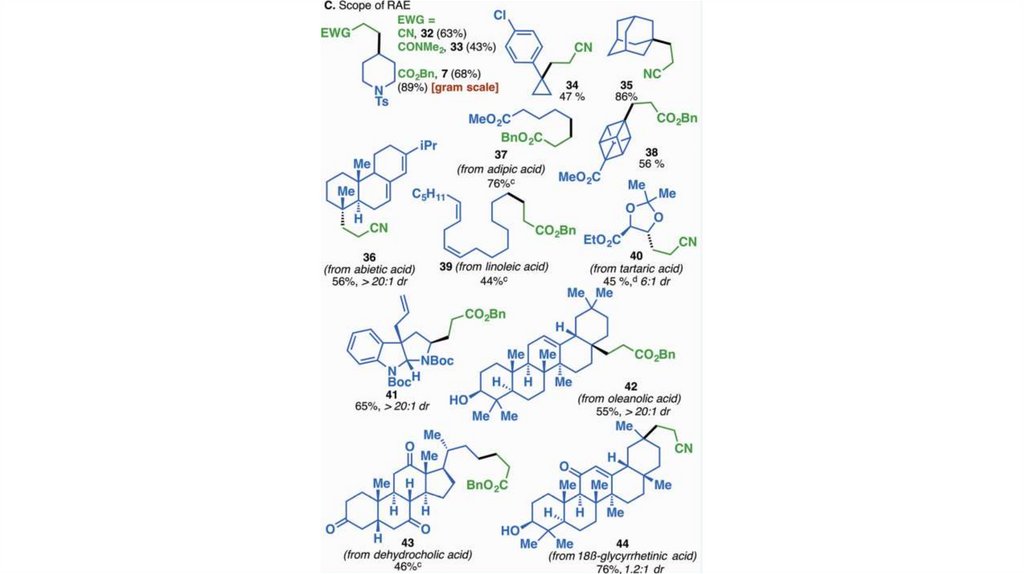
Decarboxylative Alkenylations-
Primary, secondary and tertiary acids can be used
-
Usual as well as α, β – unsaturated alkenylzinc reagents can be used
-
Many functional groups are tolerated
J. T. Edwards, R. R. Merchant, K. S. McClymont, K. W. Knouse, T. Qin, L. R. Malins, B. Vokits, S. A. Shaw, D.-H.Bao, F.-L. Wei, T. Zhou, M. D.
Eastgate, P. S. Baran, Nature, 545, 213–218
X.-G. Liu, C.-J. Zhou, E. Lin, X.-L. Han, S.-S. Zhang, Q. Li, H. Wang, , Angew. Chem. Int. Ed. 2018, 57, 13096-13100
10.
11.
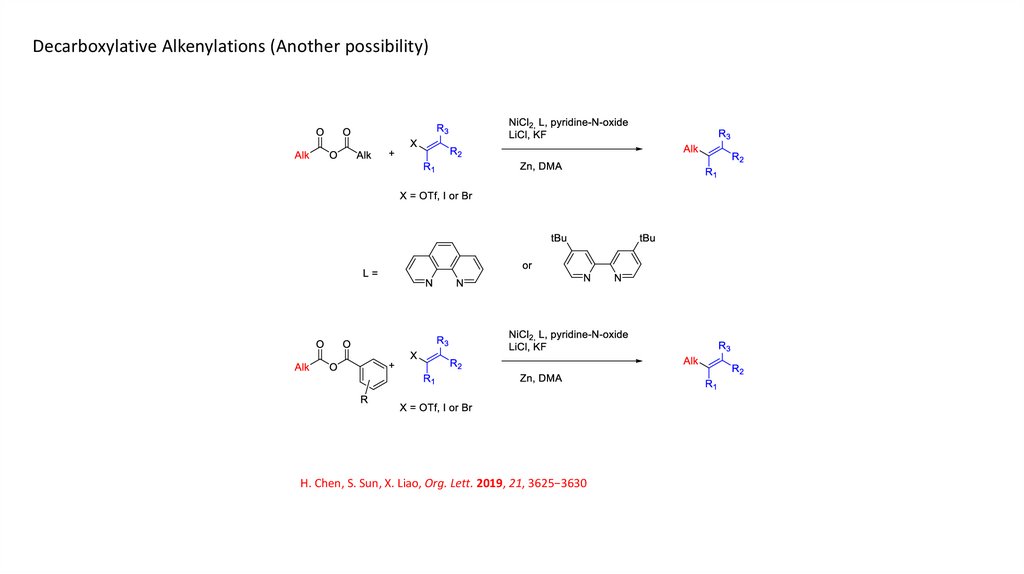
Decarboxylative Alkenylations (Another possibility)H. Chen, S. Sun, X. Liao, Org. Lett. 2019, 21, 3625−3630
12.
Decarboxylative (Hetero)ArylationsAlso ArB(OH)2 can be used (sometimes) instead of ArZnCl!
F. Sandfort, M. J. O’Neill, J.Cornella, L. Wimmer, P. S. Baran, Angew. Chem. Int. Ed. 2017, 56, 3319 –3323
T.-G. Chen, H. Zhang, P. K. Mykhailiuk, R. R. Merchant, C. A. Smith, T. Qin, P. S. Baran, 2Angew. Chem. Int. Ed. 2019, 58, 2454 –2458
X.-G. Liu, C.-J. Zhou, E. Lin, X.-L. Han, S.-S. Zhang, Q. Li, H. Wang, , Angew. Chem. Int. Ed. 2018, 57, 13096-13100
13.
14.
Another Possibility of (Hetero)ArylationsSynthesis of the sulfones is very simple
Particiularly interesting for the synthesis of fluorinated compounds
Merchant, R. R.; Edwards, J. T.; Qin, T.; Kruszyk, M. M.; Bi, C.; Che, G.; Bao D-H.; Qiao, W.; Sun, L.; Collins, M. R.; Gallego, G. M.; Mousseau, J. J.; Nuhant, P.; Baran, P. S., Science,
2018, 360, 75-80.
15.
Decarboxylative AlkynylationsSummary:
-
two variants of decarboxylative alkynylation (Ni and Fe): one for
monosubstituted alkynes (Ni), one for disubstituted alkynes (Fe)
terminal alkynes can be synthesized using BOTH variants
nearly all functional groups in acids are tolerated
very mild conditions
there are some limitations
Smith, J. M.; Qin, T.; Merchant, R. R.; Edwards, J. T.; Malins, L. R.; Liu, Z.; Che, G.; Shen, Z.; Shaw, S. A.; Eastgate, M. D.; Baran, P. S., Angew. Chem. Int. Ed. 2017, 56, 11906-11910
16.
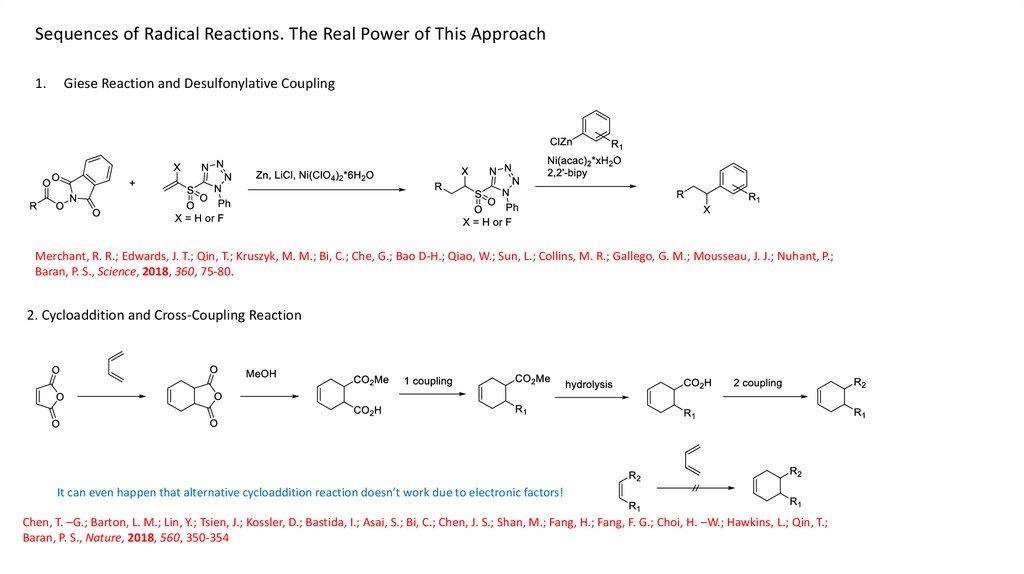
Sequences of Radical Reactions. The Real Power of This Approach1.
Giese Reaction and Desulfonylative Coupling
Merchant, R. R.; Edwards, J. T.; Qin, T.; Kruszyk, M. M.; Bi, C.; Che, G.; Bao D-H.; Qiao, W.; Sun, L.; Collins, M. R.; Gallego, G. M.; Mousseau, J. J.; Nuhant, P.;
Baran, P. S., Science, 2018, 360, 75-80.
2. Cycloaddition and Cross-Coupling Reaction
It can even happen that alternative cycloaddition reaction doesn’t work due to electronic factors!
Chen, T. –G.; Barton, L. M.; Lin, Y.; Tsien, J.; Kossler, D.; Bastida, I.; Asai, S.; Bi, C.; Chen, J. S.; Shan, M.; Fang, H.; Fang, F. G.; Choi, H. –W.; Hawkins, L.; Qin, T.;
Baran, P. S., Nature, 2018, 560, 350-354
17.
Synthesis of Amino Acids-
Sulfinimines are chiral. They can be used as chiral auxiliaries
Products are enantiomerically pure
The reaction works also without protecting gas
For Bn-Substituents doesn’t work
Ni, S.; Garrido-Castro, A. F.; Merchant, R. R.; deGruyter, J. N.; Schmitt, D. C.; Mousseau, J. J.; Gallego, G. M.; Yang, S.; Collins, M. R.; Qiao, J. X.; Yeung, K.; Langley, D. R.;
Poss, M. A.; Scola, P. M.; Qin, T.; Baran, P. S., Angew. Chem. Int. Ed. 2018, 57, 14560














 chemistry
chemistry








