Similar presentations:
Free Radical Polymerization
1.
Chapter 6. Free Radical Polymerization6.1 Introduction
6.2 Free Radical Initiators.
6.3 Techniques of Free Radical Polymerization.
6.4 Kinetic and Mechanism of polymerization.
6.5 Stereochemistry of polymerization.
6.6 Polymerization of Dienes
6.7 Monomer Reactivity
6.8 Copolymerization.
POLYMER CHEMISTRY
2.
6. 1 IntroductionA. Type of polymerization.
polymerization
Addition polymerization
Condensation polymerization
1. Free-radical polymerization
2. Ionic polymerization
3. Complex coordination polymerization
POLYMER CHEMISTRY
3.
B. Commercialized free-radical polymerization.4.
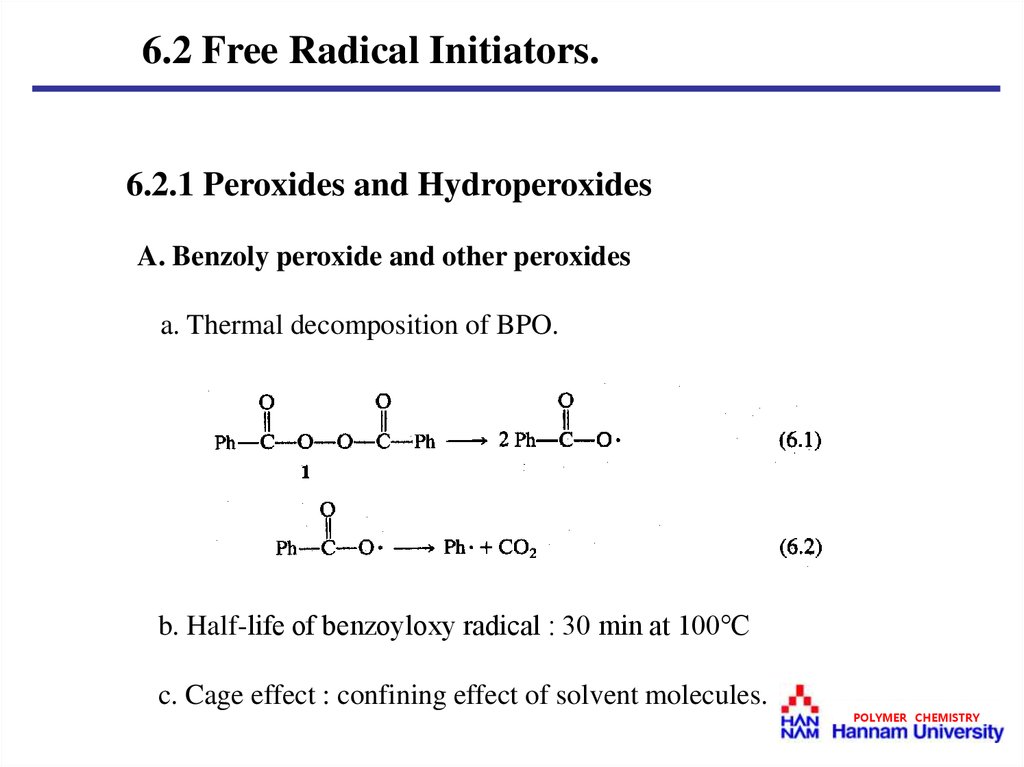
6.2 Free Radical Initiators.6.2.1 Peroxides and Hydroperoxides
A. Benzoly peroxide and other peroxides
a. Thermal decomposition of BPO.
b. Half-life of benzoyloxy radical : 30 min at 100℃
c. Cage effect : confining effect of solvent molecules.
POLYMER CHEMISTRY
5.
6.2.1 Peroxides and HydroperoxidesO
Ph
C
O
O
O
C
Ph
+
Ph
·
(6.5)
O
Ph
·
d. Other peroxides.
Ph
C
O
O
O
O
C
Diacetyl peroxide
Diacetyl peroxide
Ph
Ph
C
O
O
·
Ph + HO
Di-t-butyl peroxide
Di-t-butyl peroxide
(half-life:10hours at 120℃)
C
Ph
POLYMER CHEMISTRY
6.
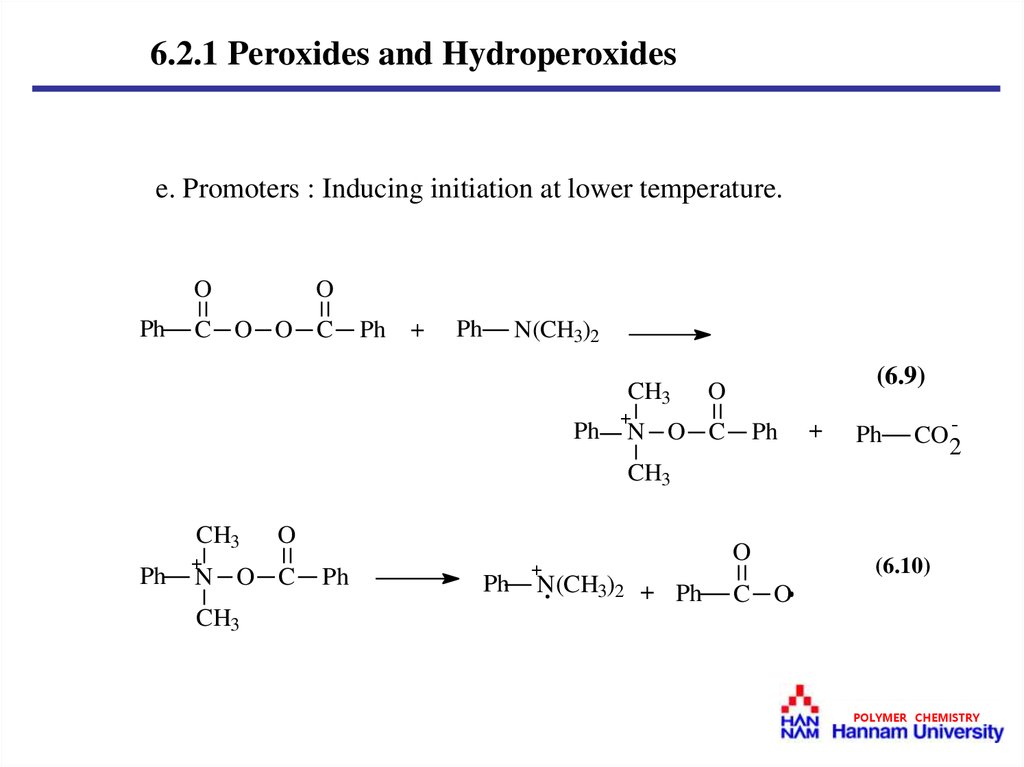
6.2.1 Peroxides and Hydroperoxidese. Promoters : Inducing initiation at lower temperature.
O
Ph
O
C O O C
Ph
+
Ph
N(CH3)2
CH3
Ph
+
(6.9)
O
N O C
Ph
+
Ph
CO2-
CH3
CH3
Ph
+
O
N O C
Ph
Ph
+
N(CH3)2 + Ph
O
(6.10)
C O
CH3
POLYMER CHEMISTRY
7.
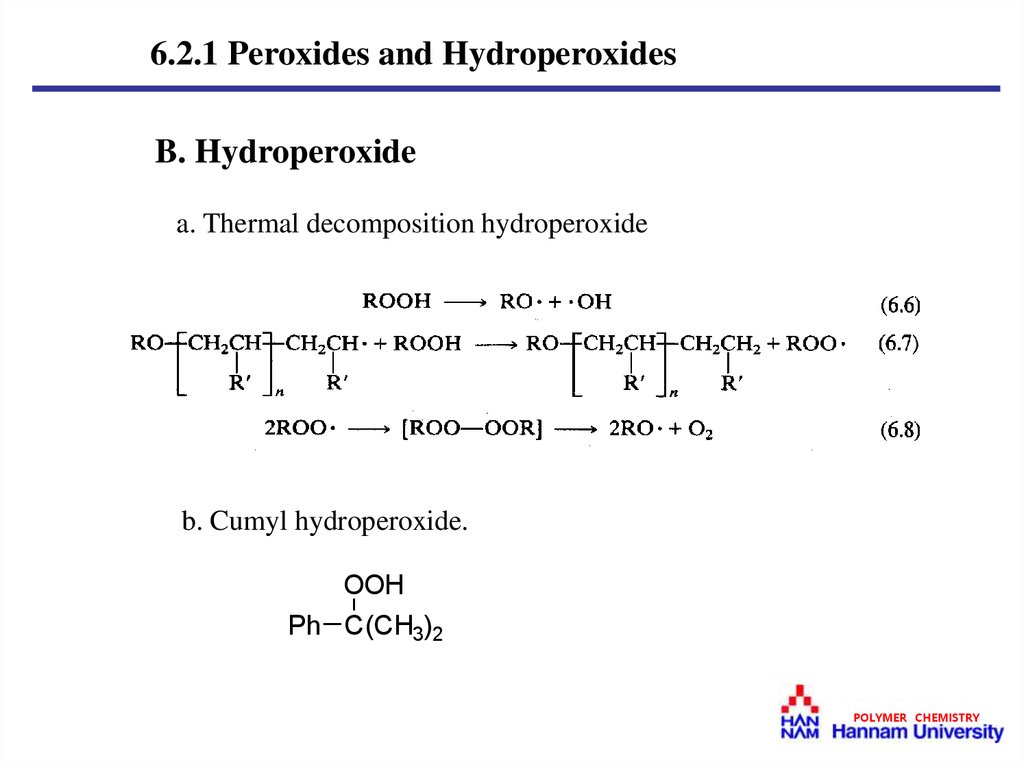
6.2.1 Peroxides and HydroperoxidesB. Hydroperoxide
a. Thermal decomposition hydroperoxide
b. Cumyl hydroperoxide.
OOH
Ph C(CH3)2
POLYMER CHEMISTRY
8.
6.2.2 Azo Compounds.A. α,α'-Azobis(isobutyronitrile) (AIBN).
a. Decomposition of AIBN.
b. Half-life of isobutyronitrile radical : 1.3 hours at 80℃.
POLYMER CHEMISTRY
9.
6.2.2 Azo Compounds.B. Side reaction : Cage effect.
a. Tetramethylsuccinonitrile
b. Ketenimine
POLYMER CHEMISTRY
10.
6.2.3 Redox Initiators.A. One electron transfer reaction.
a. Making free radical by one electron transfer by redox reaction.
b. Low-temperature reaction.
c. Emulsion polymerization.
B. Example of redox system.
POLYMER CHEMISTRY
11.
6.2.4 PhotoinitiatorA. Peroxide and Azo compound.
Photolysis and thermalysis.
B. Photolabile initiator.
POLYMER CHEMISTRY
12.
6.2.5 Thermal Polymerization.A. Polymerization without initiators.
a. Dimer formation by Diels-Alder reation.
2CH2
CHPh
H
Ph
10
b. Radical formation from dimer.
10 + CH2
CHPh
·
·
CH3CHPh +
Ph
11
12
POLYMER CHEMISTRY
13.
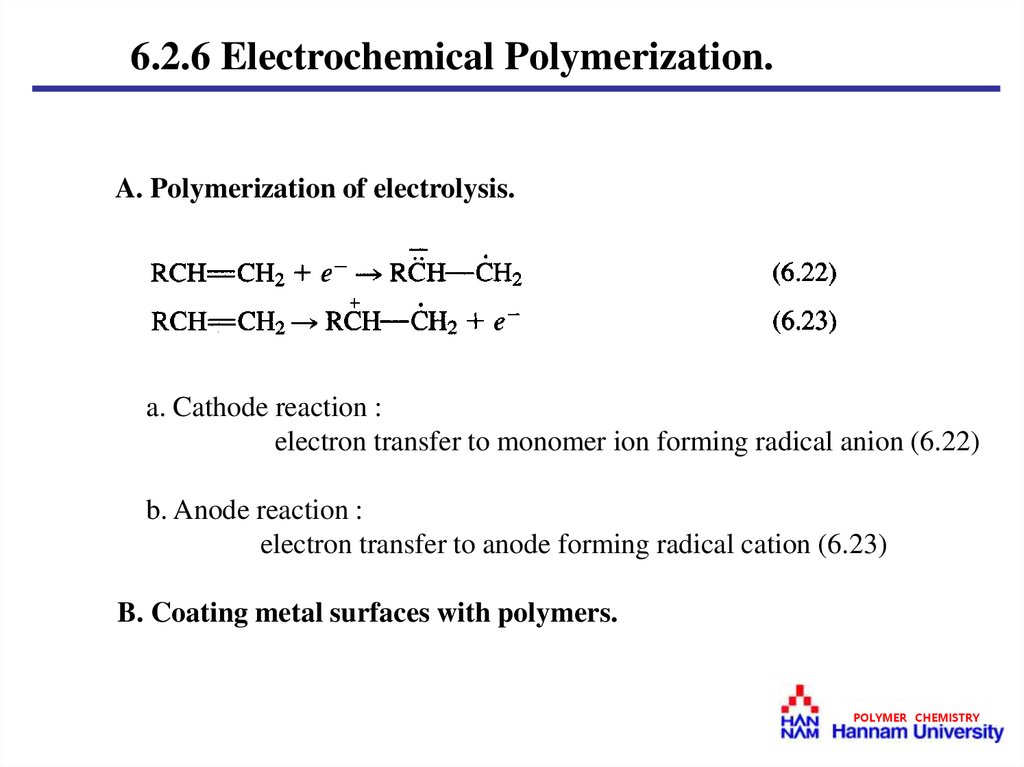
6.2.6 Electrochemical Polymerization.A. Polymerization of electrolysis.
a. Cathode reaction :
electron transfer to monomer ion forming radical anion (6.22)
b. Anode reaction :
electron transfer to anode forming radical cation (6.23)
B. Coating metal surfaces with polymers.
POLYMER CHEMISTRY
14.
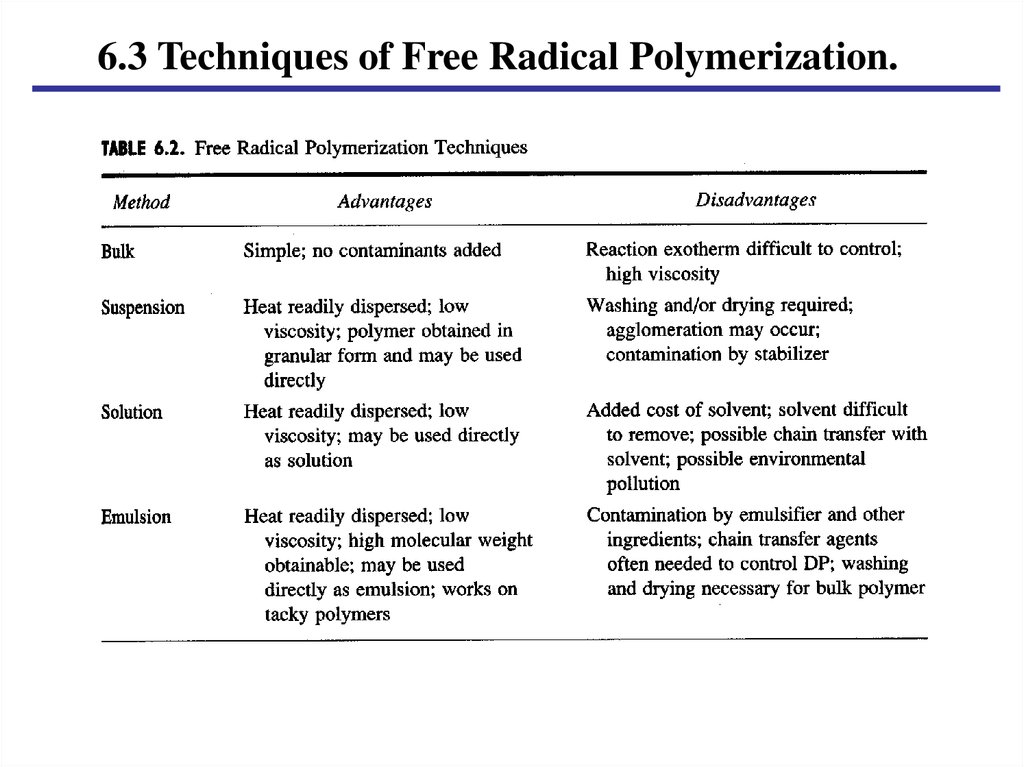
6.3 Techniques of Free Radical Polymerization.15.
6.3 Techniques of Free Radical Polymerization.6.3.1 Bulk
A. Reactor charges.
a. Monomer.
b. Initiator (soluble in monomer).
B. Problems.
a. Heat transfer.
b. Viscosity.
c. Auto-acceleration.
POLYMER CHEMISTRY
16.
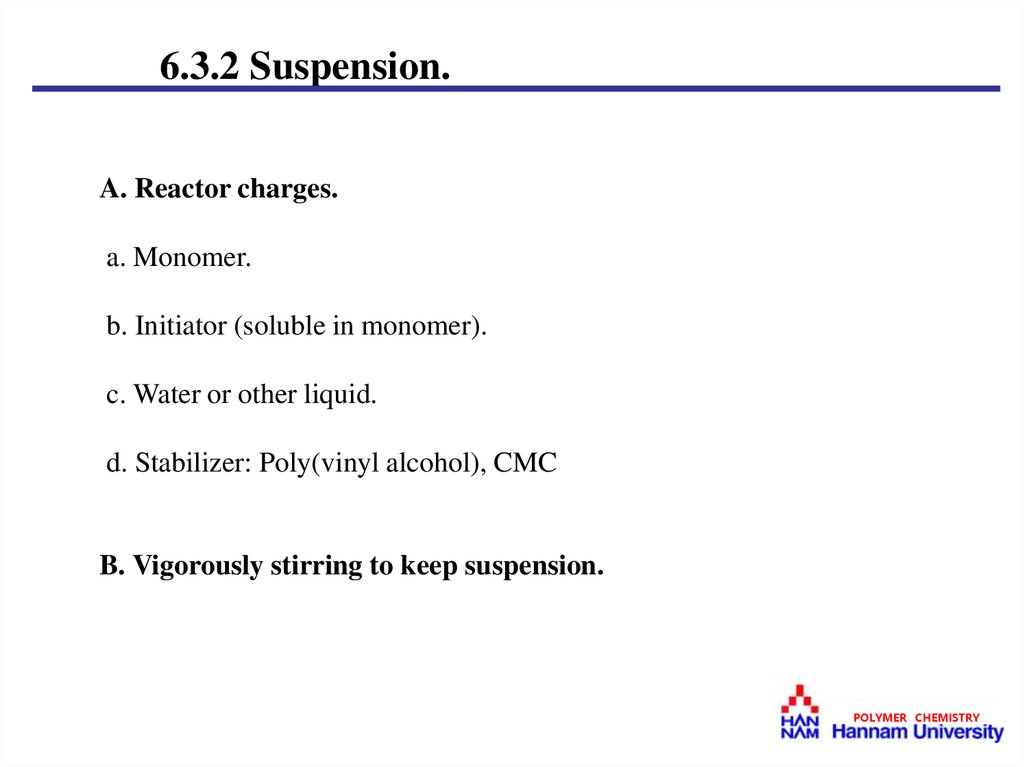
6.3.2 Suspension.A. Reactor charges.
a. Monomer.
b. Initiator (soluble in monomer).
c. Water or other liquid.
d. Stabilizer: Poly(vinyl alcohol), CMC
B. Vigorously stirring to keep suspension.
POLYMER CHEMISTRY
17.
6.3.3 Solution.A. Reactor charges.
a. Monomer (soluble in solvent).
b. Initiator (soluble in solvent).
c. Solvent.
B. Refluxing solution.
POLYMER CHEMISTRY
18.
6.3.4 Emulsion.A. Reactor charges.
a. Monomer.
b. Redox initiator
c. Soap or emulsifier.
d. Water.
e. Others (cf. Table 6.3).
B. Polymerization in swollen micelle.
Latex products.
POLYMER CHEMISTRY
19.
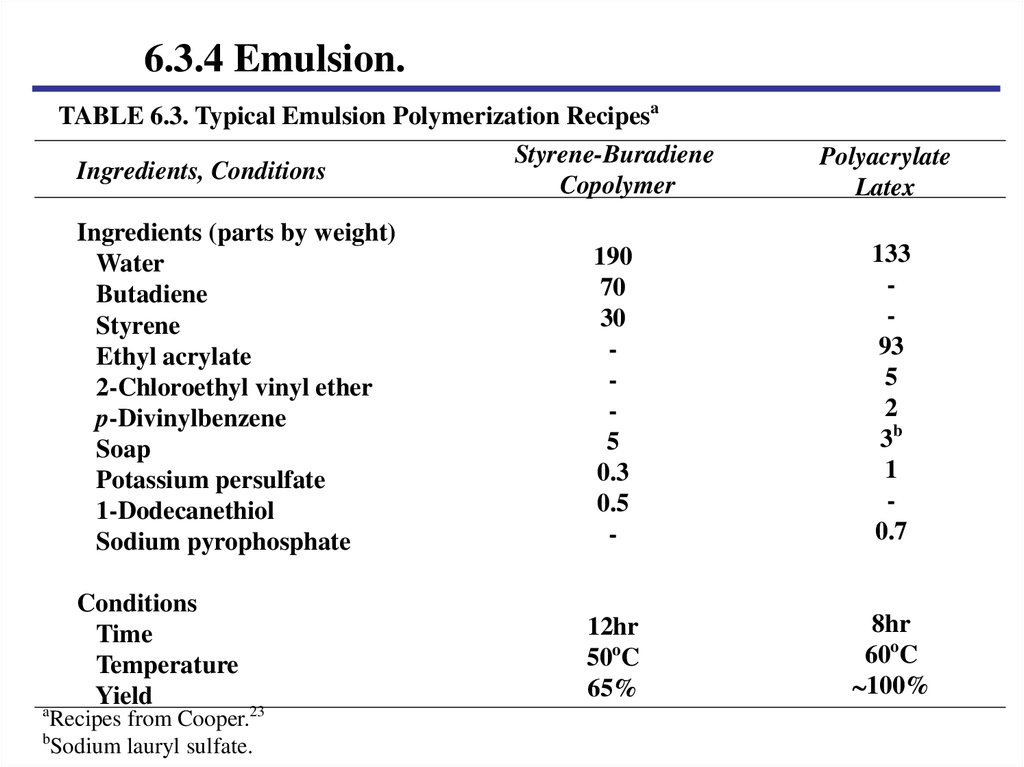
6.3.4 Emulsion.TABLE 6.3. Typical Emulsion Polymerization Recipesa
Styrene-Buradiene
Ingredients, Conditions
Copolymer
a
Polyacrylate
Latex
Ingredients (parts by weight)
Water
Butadiene
Styrene
Ethyl acrylate
2-Chloroethyl vinyl ether
p-Divinylbenzene
Soap
Potassium persulfate
1-Dodecanethiol
Sodium pyrophosphate
190
70
30
5
0.3
0.5
-
133
93
5
2
3b
1
0.7
Conditions
Time
Temperature
Yield
12hr
50oC
65%
8hr
60oC
100%
Recipes from Cooper.23
b
Sodium lauryl sulfate.
20.
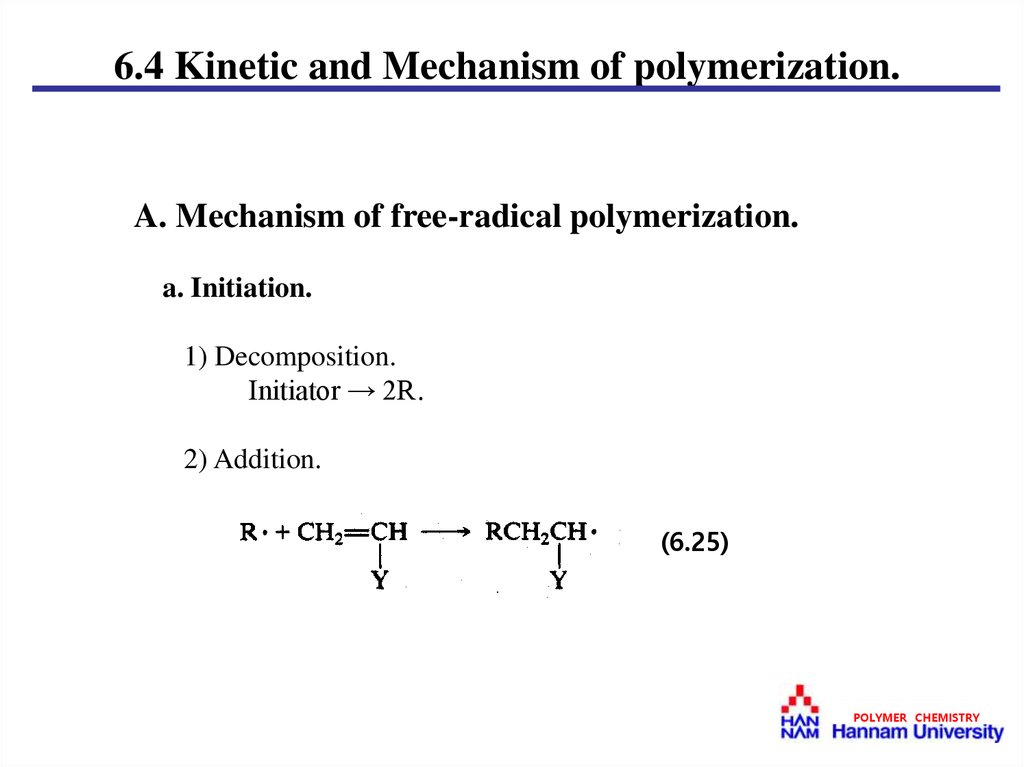
6.4 Kinetic and Mechanism of polymerization.A. Mechanism of free-radical polymerization.
a. Initiation.
1) Decomposition.
Initiator → 2R․
2) Addition.
(6.25)
POLYMER CHEMISTRY
21.
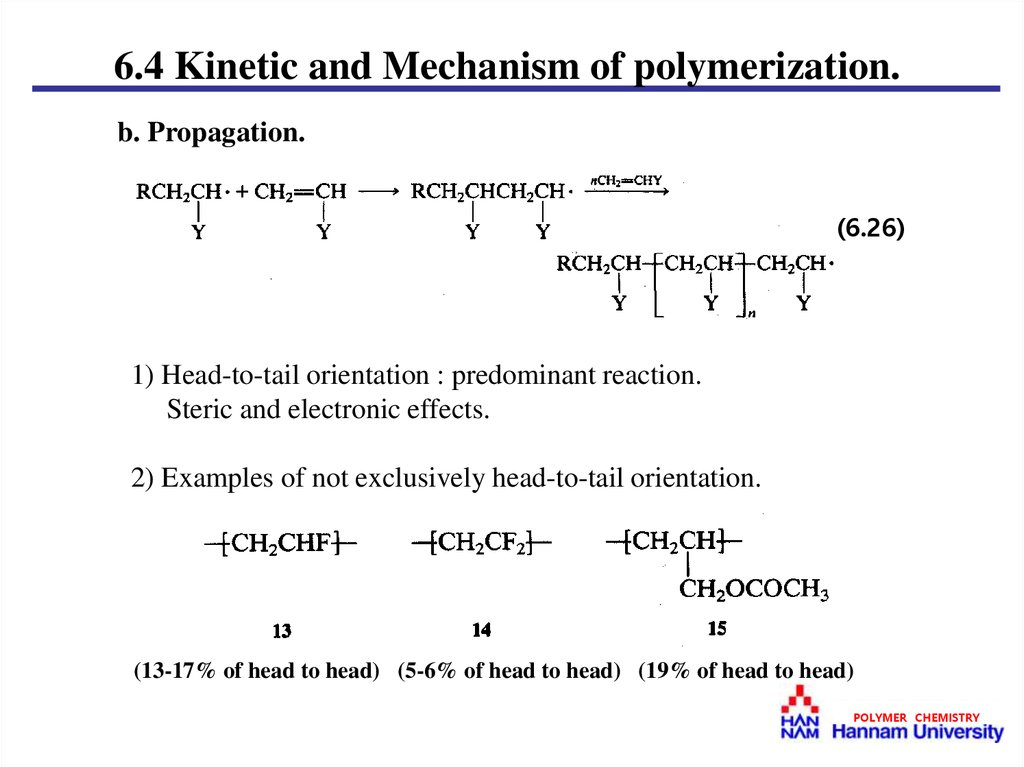
6.4 Kinetic and Mechanism of polymerization.b. Propagation.
(6.26)
1) Head-to-tail orientation : predominant reaction.
Steric and electronic effects.
2) Examples of not exclusively head-to-tail orientation.
(13-17% of head to head) (5-6% of head to head) (19% of head to head)
POLYMER CHEMISTRY
22.
6.4 Kinetic and Mechanism of polymerization.c. Termination.
1) Combination.
(6.27)
Polystyrene radical.
(6.29)
POLYMER CHEMISTRY
23.
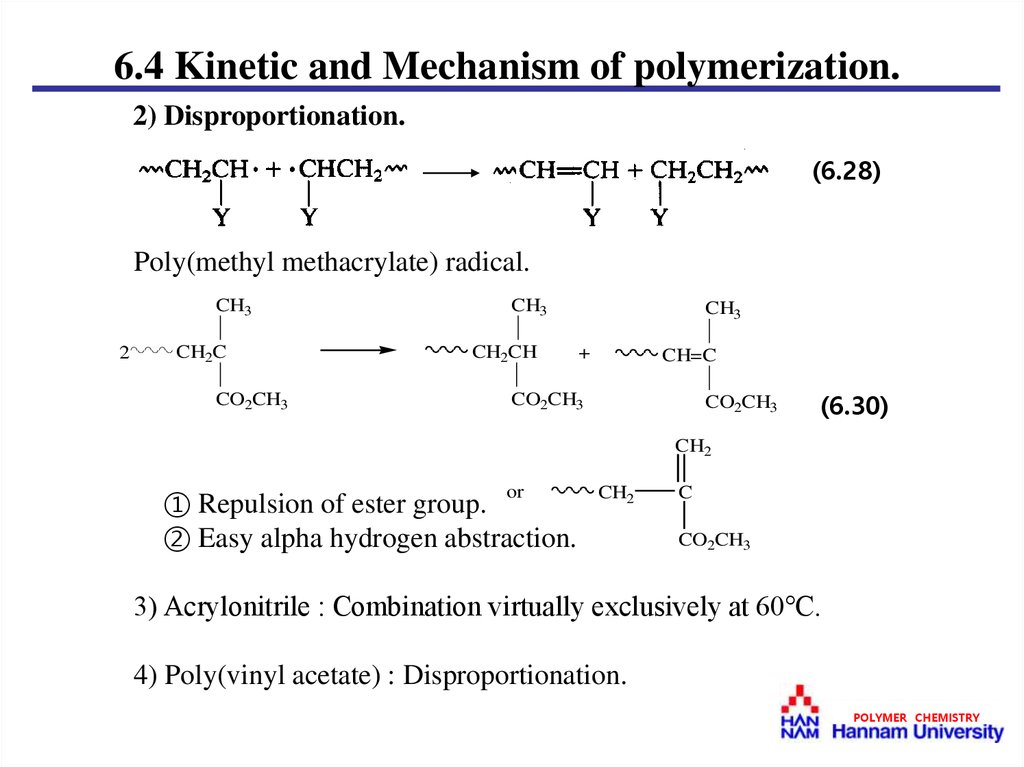
6.4 Kinetic and Mechanism of polymerization.2) Disproportionation.
(6.28)
Poly(methyl methacrylate) radical.
CH3
2
CH2C
CO2CH3
CH3
CH2CH
CH3
+
CH=C
CO2CH3
CO2CH3
(6.30)
CH2
① Repulsion of ester group.
② Easy alpha hydrogen abstraction.
or
CH2
C
CO2CH3
3) Acrylonitrile : Combination virtually exclusively at 60℃.
4) Poly(vinyl acetate) : Disproportionation.
POLYMER CHEMISTRY
24.
B. Kinetic of free radical polymerization.a. Assumption.
1) The rates of initiation, propagation, and termination are all different.
2) Independent of chain length.
3) Negligible end group.
4) At steady state, constant radical concentration.
(steady state assumption)
b. Initiation (Ri)
Ri =
-d[M ·]
dt
= 2fkd[I]
f : Initiator efficiency.
f=
radicals that initiate a polymer chain
radicals formed from initiator
kd : Decomposition rate constant.
[I] : molar concentration of initiator.
[M ·] : molar concentration of radical.
POLYMER CHEMISTRY
25.
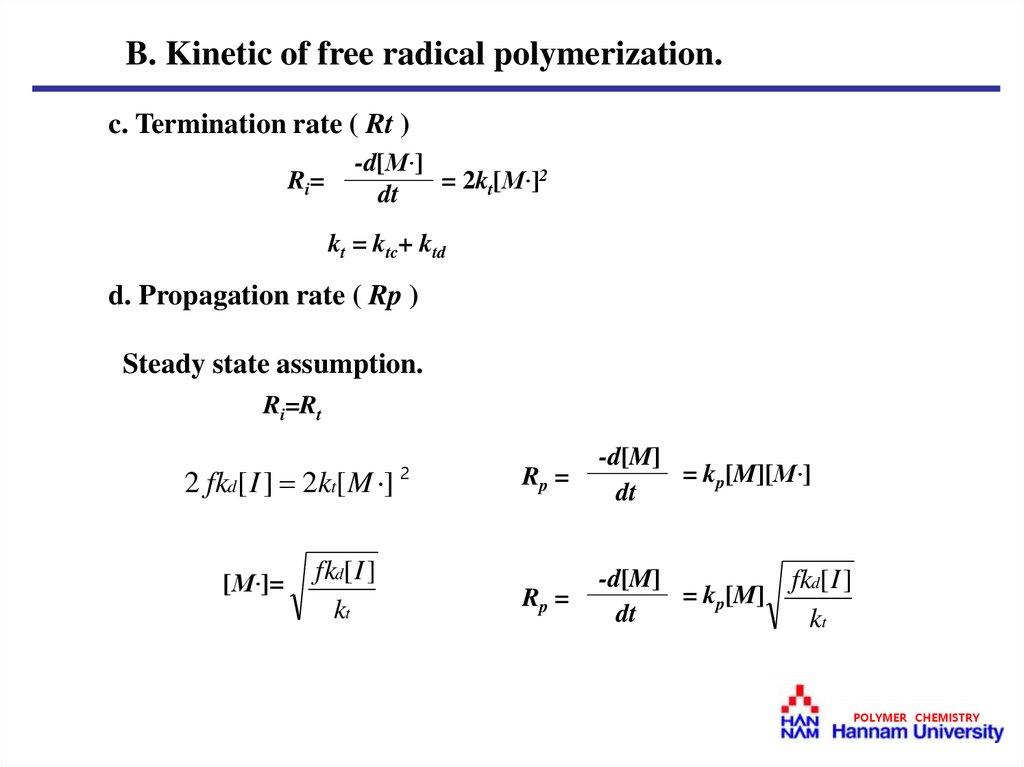
B. Kinetic of free radical polymerization.c. Termination rate ( Rt )
Ri =
-d[M·]
= 2kt[M·]2
dt
kt = ktc+ ktd
d. Propagation rate ( Rp )
Steady state assumption.
Ri=Rt
2 fkd[ I ] 2kt[ M ]
[M·]=
fkd[ I ]
kt
2
Rp =
-d[M]
= kp[M][M·]
dt
Rp =
-d[M]
= kp[M]
dt
fkd[ I ]
kt
POLYMER CHEMISTRY
26.
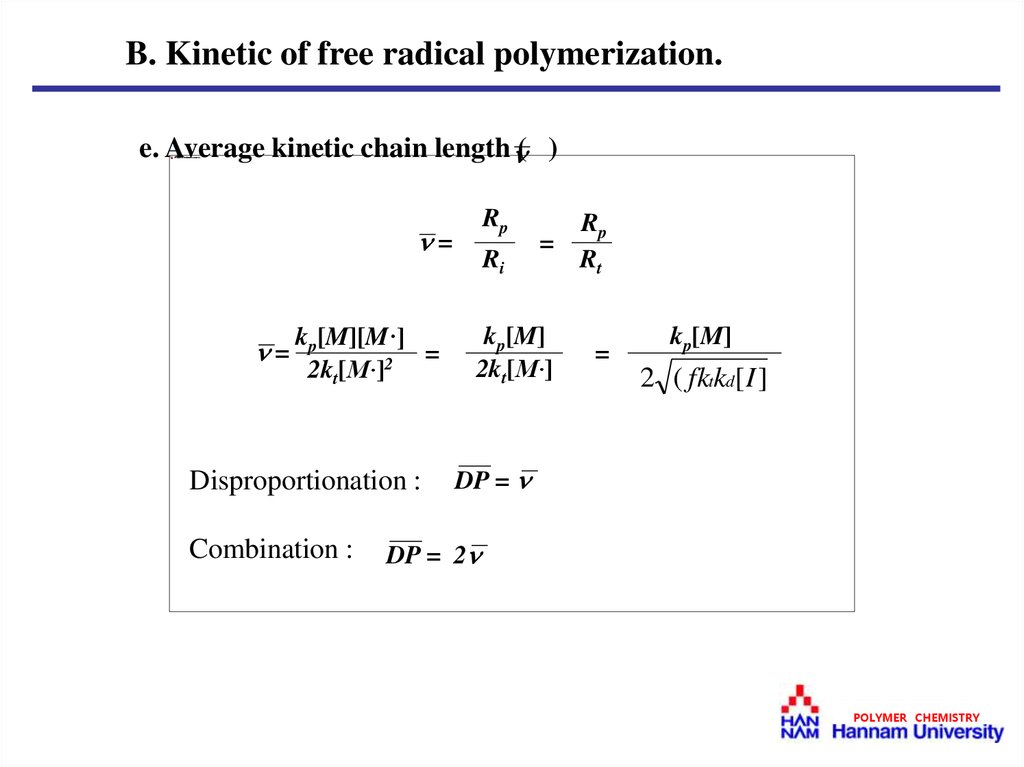
B. Kinetic of free radical polymerization.e. Average kinetic chain length ( )
Rp
=
Rp
Rt
kp[M]
2kt[M·]
=
=
=
kp[M][M·]
=
2kt[M·]2
Disproportionation :
Combination :
Ri
kp[M]
2 ( fktkd[ I ]
DP =
DP = 2
POLYMER CHEMISTRY
27.
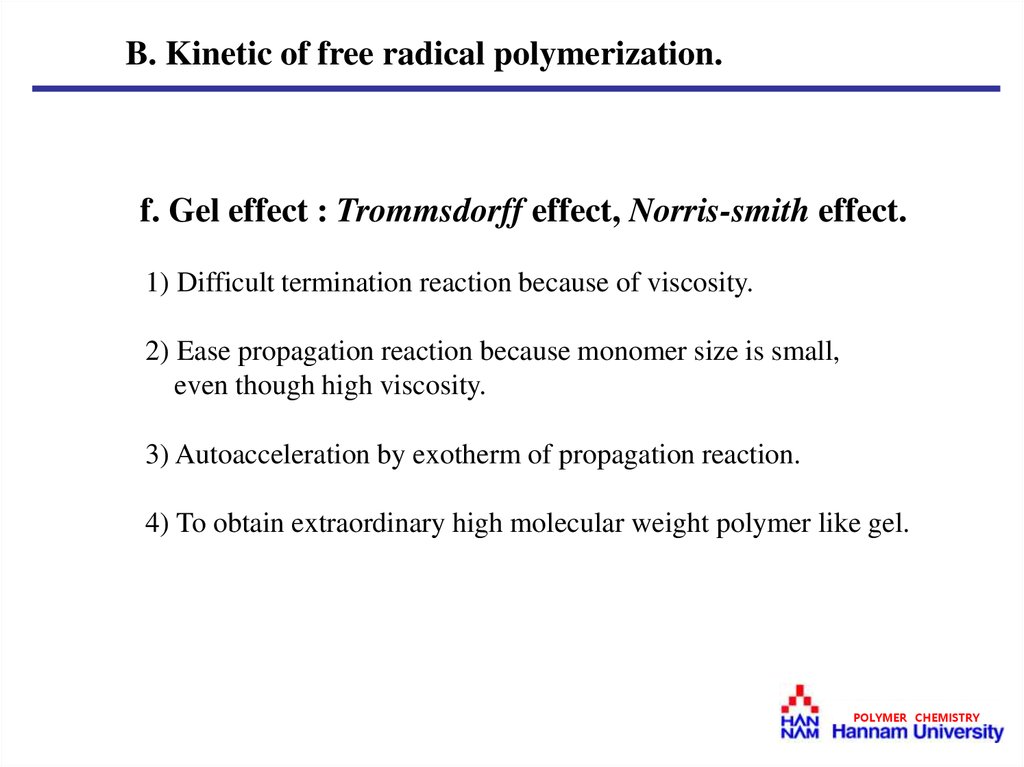
B. Kinetic of free radical polymerization.f. Gel effect : Trommsdorff effect, Norris-smith effect.
1) Difficult termination reaction because of viscosity.
2) Ease propagation reaction because monomer size is small,
even though high viscosity.
3) Autoacceleration by exotherm of propagation reaction.
4) To obtain extraordinary high molecular weight polymer like gel.
POLYMER CHEMISTRY
28.
C. Chain transfer reactions : Growing radicals move to other partsby hydrogen abstracting.
Lowering average kinetic chain length.
a. Growing radicals move to other polymer chain.
CH
Y
+
CH2CH
CH2
Y
+
CH2C
Y
Y
(6.32)
CH2CHY
CH2C
CH2
CHY
Y
b. Backbiting self polymer chain.
(6.33)
POLYMER CHEMISTRY
LDPE : branching polymer.
29.
C. Chain transfer reactionsc. Moving to initiators or monomers.
(6.34)
(6.35)
d. Moving to solvent.
(6.36)
(6.37)
POLYMER CHEMISTRY
30.
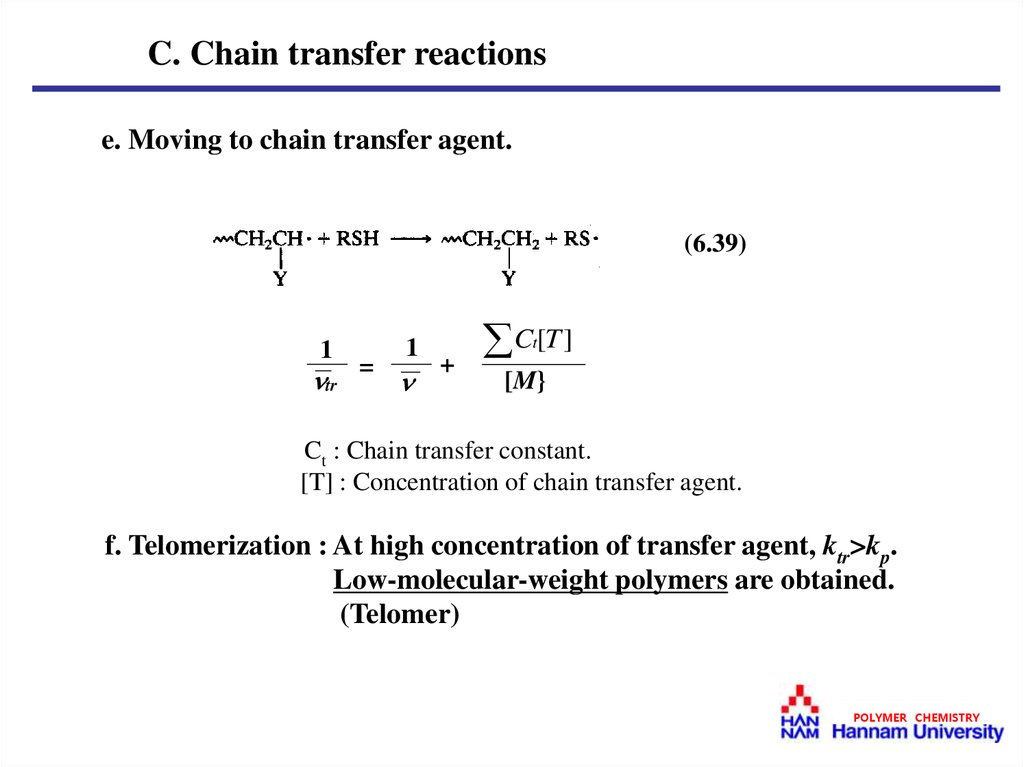
C. Chain transfer reactionse. Moving to chain transfer agent.
(6.39)
1
tr
=
1
C [T ]
t
+
[M}
Ct : Chain transfer constant.
[T] : Concentration of chain transfer agent.
f. Telomerization : At high concentration of transfer agent, ktr>kp.
Low-molecular-weight polymers are obtained.
(Telomer)
POLYMER CHEMISTRY
31.
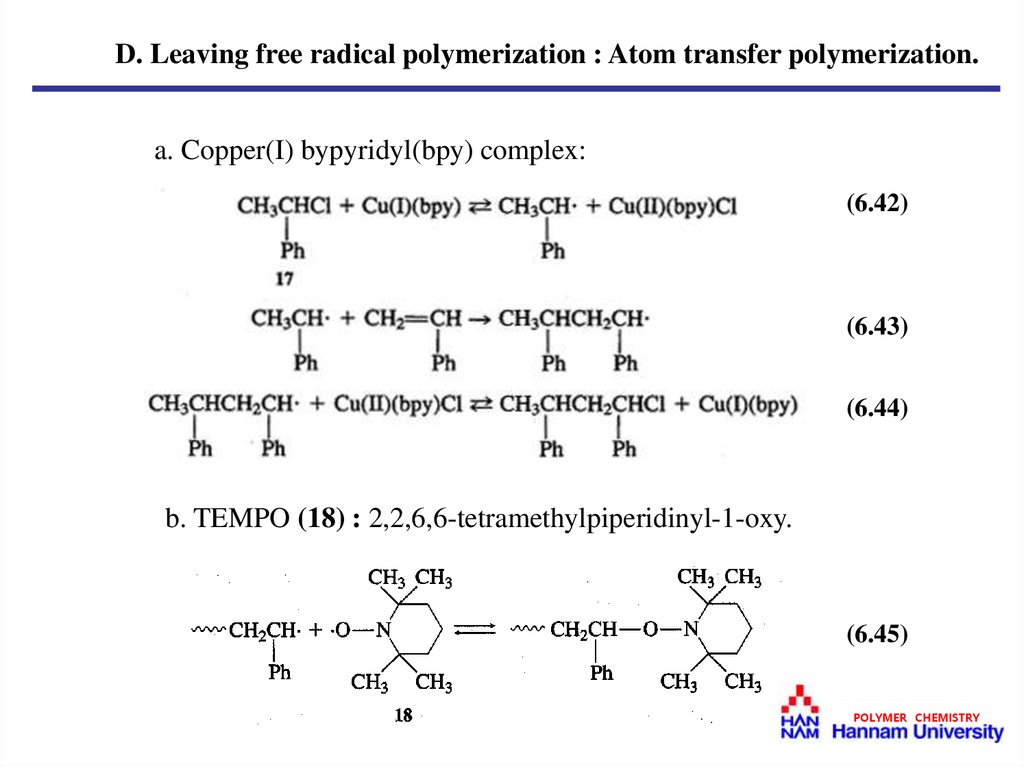
D. Leaving free radical polymerization : Atom transfer polymerization.a. Copper(I) bypyridyl(bpy) complex:
(6.42)
(6.43)
(6.44)
b. TEMPO (18) : 2,2,6,6-tetramethylpiperidinyl-1-oxy.
(6.45)
POLYMER CHEMISTRY
32.
6.4 Kinetic and Mechanism of polymerization.c. Synthesis of block copolymers like anionic polymerization.
d. Monodisperse polymerization (PI=1.05).
E. Kinetics of Emulsion polymerization.
a.
N
Rp kp[ M ]( )
2
N : the number of particles.
b.
kp[ M ] kp[ M ]N
Ri
2 fkd[ I ]
N
POLYMER CHEMISTRY
33.
6.5 Stereochemistry of polymerization.A. General consideration.
a. Stereoregular polymerization : Ionic and complex coordination
polymerization.
1) Terminal ion pair : counter ion.
2) Terminal complex active site.
3) Low temperature.
b. Stereo-irregular polymerization : Free-radical polymerization.
1) No stereoregulating radical terminal group.
2) Somewhat higher temperature.
POLYMER CHEMISTRY
34.
6.5 Stereochemistry of polymerization.B. Factors influencing stereochemistry in free-radical polymerization.
a. Interaction between the terminal chain carbon and the
approaching monomer molecule.
C. Stereoregular free-radical polymerization of PMMA.
(syndiotatic PMMA)
a. Polymerization temperature : below 0℃.
b.
(6.48)
POLYMER CHEMISTRY
35.
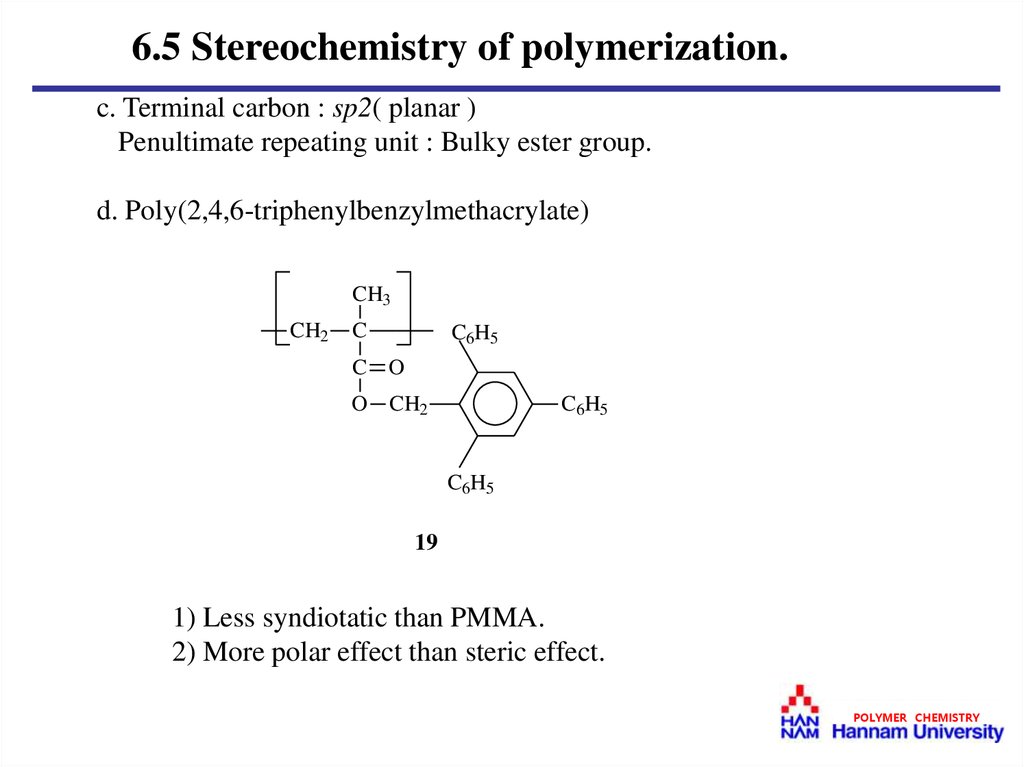
6.5 Stereochemistry of polymerization.c. Terminal carbon : sp2( planar )
Penultimate repeating unit : Bulky ester group.
d. Poly(2,4,6-triphenylbenzylmethacrylate)
CH3
CH2
C
C6H5
C O
O CH2
C6H5
C6H5
19
1) Less syndiotatic than PMMA.
2) More polar effect than steric effect.
POLYMER CHEMISTRY
36.
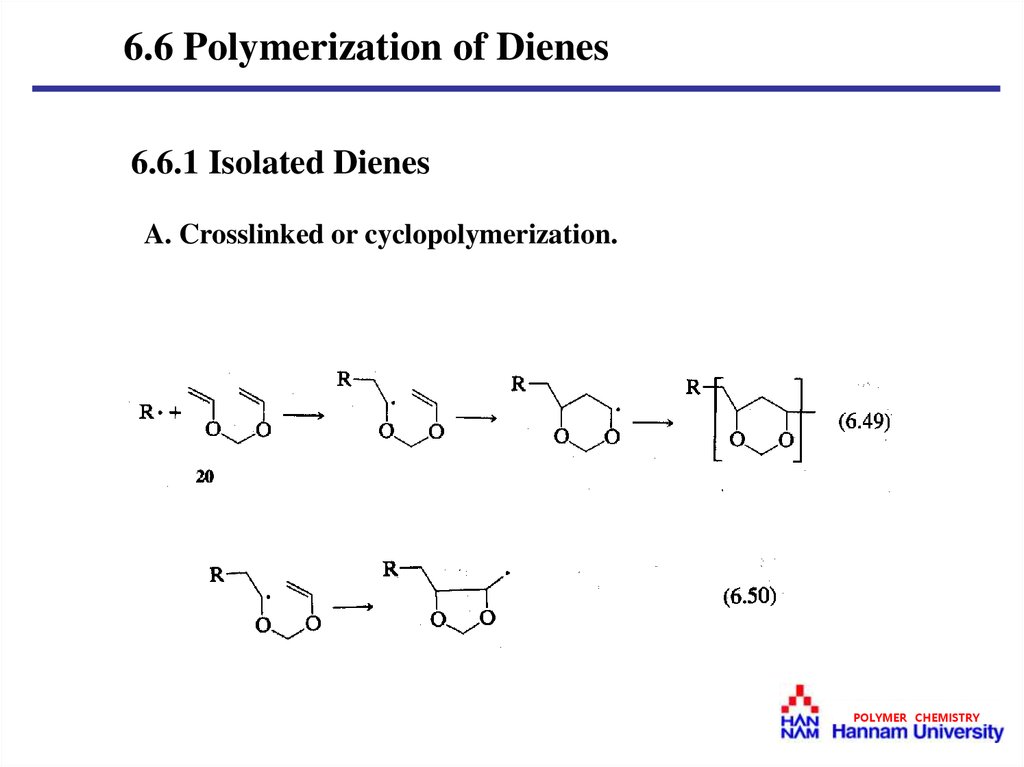
6.6 Polymerization of Dienes6.6.1 Isolated Dienes
A. Crosslinked or cyclopolymerization.
POLYMER CHEMISTRY
37.
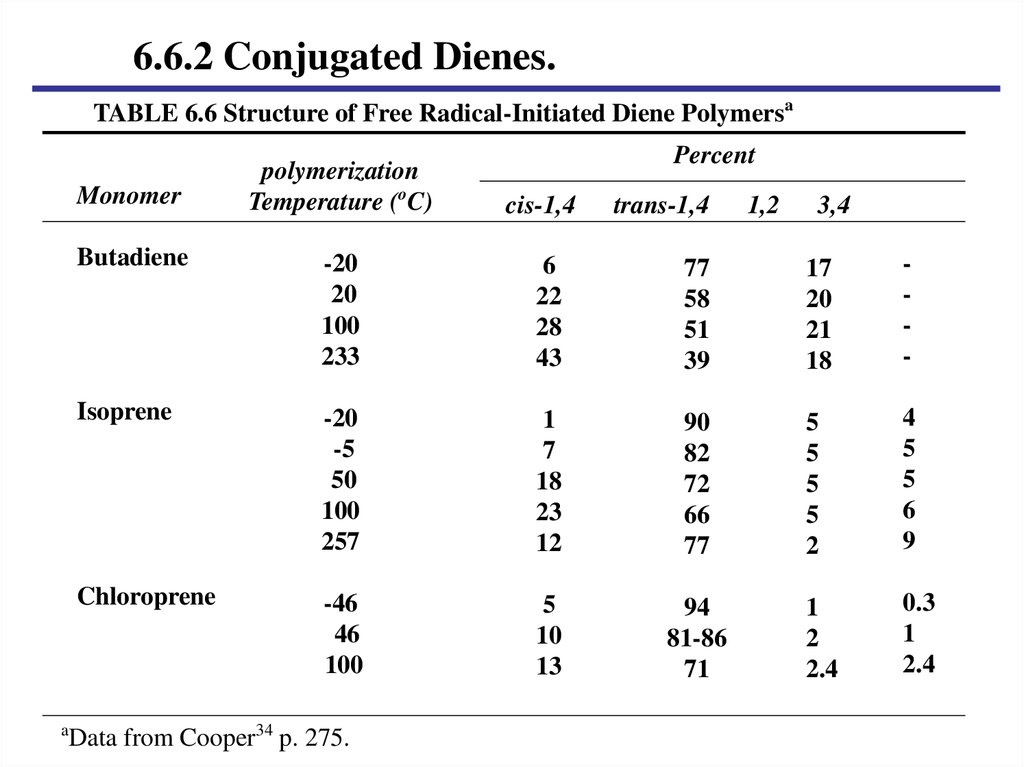
6.6.2 Conjugated Dienes.A. Structure of conjugated Diene monoer.
CH3
CH2
CH CH CH2
CH2
CH CH
CH2
Isoprene
23
B. a. 1,2-Addition : Pendent vinyl group.
CH2CH
CH
CH2
25
b. Stereochemistry : isotactic, syndiotactic, atactic.
POLYMER CHEMISTRY
38.
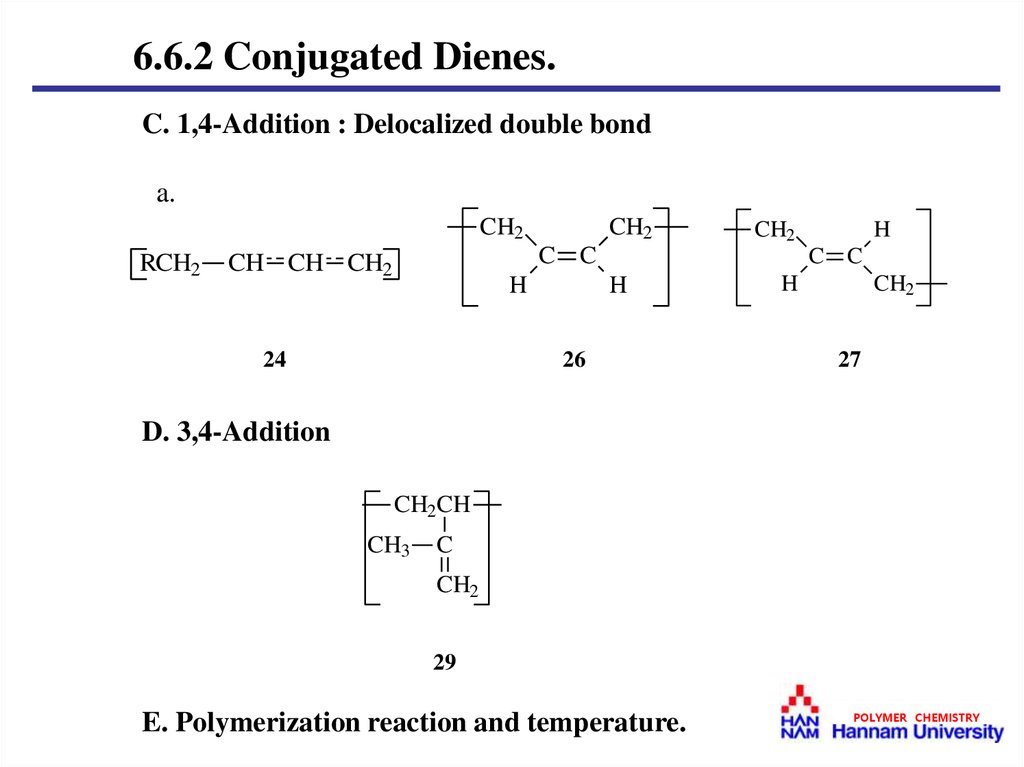
6.6.2 Conjugated Dienes.C. 1,4-Addition : Delocalized double bond
a.
CH2
RCH2
CH2
CH2
C C
CH CH CH2
H
24
H
C C
H
26
H
CH2
27
D. 3,4-Addition
CH2CH
CH3
C
CH2
29
E. Polymerization reaction and temperature.
POLYMER CHEMISTRY
39.
6.6.2 Conjugated Dienes.TABLE 6.6 Structure of Free Radical-Initiated Diene Polymersa
Monomer
a
polymerization
Temperature (oC)
Percent
cis-1,4
trans-1,4
1,2
3,4
Butadiene
-20
20
100
233
6
22
28
43
77
58
51
39
17
20
21
18
-
Isoprene
-20
-5
50
100
257
1
7
18
23
12
90
82
72
66
77
5
5
5
5
2
4
5
5
6
9
Chloroprene
-46
46
100
5
10
13
94
81-86
71
1
2
2.4
0.3
1
2.4
Data from Cooper34 p. 275.
40.
6.6.2 Conjugated Dienes.F. s-cis and s-trans
POLYMER CHEMISTRY
41.
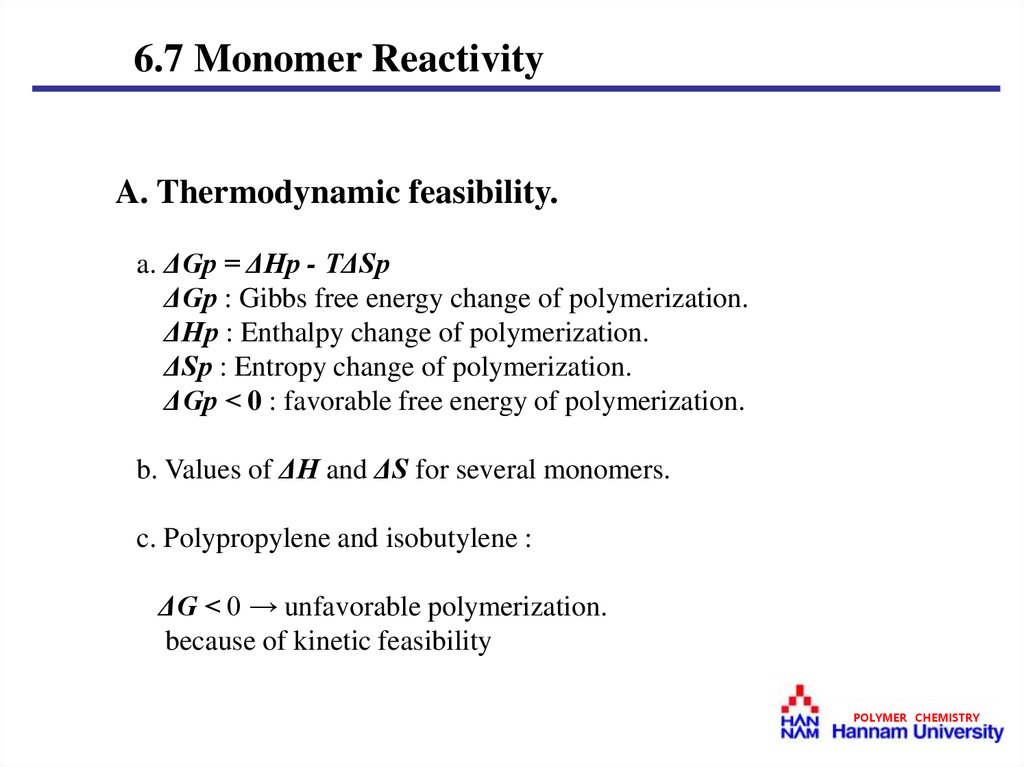
6.7 Monomer ReactivityA. Thermodynamic feasibility.
a. ΔGp = ΔHp - TΔSp
ΔGp : Gibbs free energy change of polymerization.
ΔHp : Enthalpy change of polymerization.
ΔSp : Entropy change of polymerization.
ΔGp < 0 : favorable free energy of polymerization.
b. Values of ΔH and ΔS for several monomers.
c. Polypropylene and isobutylene :
ΔG < 0 → unfavorable polymerization.
because of kinetic feasibility
POLYMER CHEMISTRY
42.
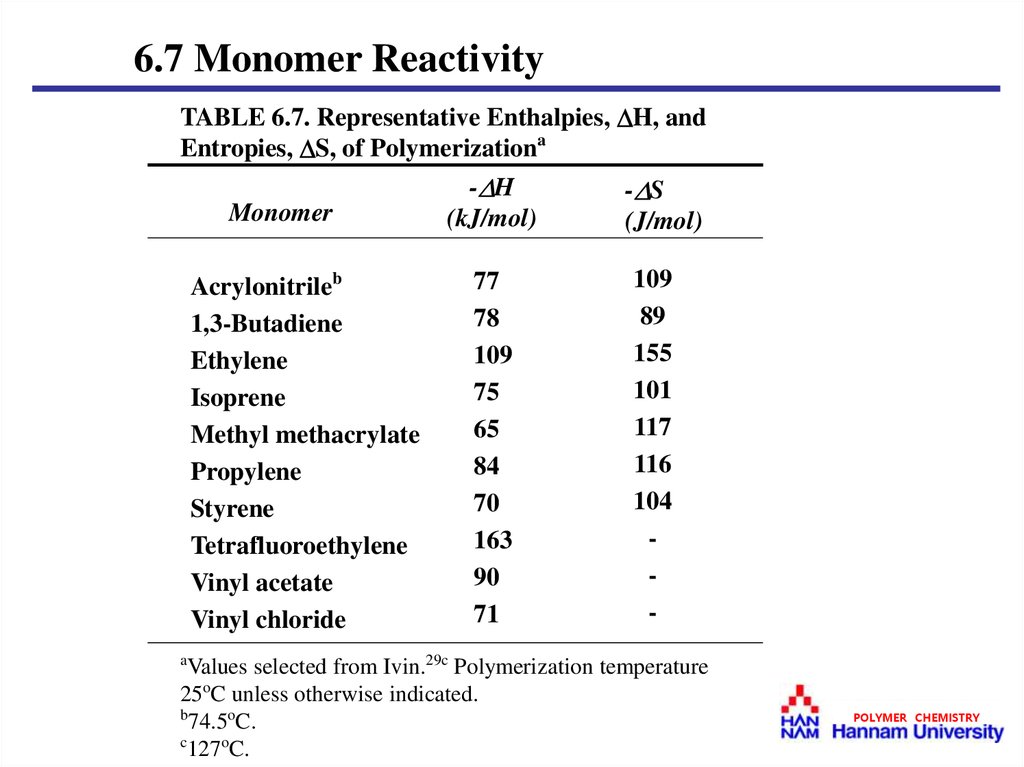
6.7 Monomer ReactivityTABLE 6.7. Representative Enthalpies, H, and
Entropies, S, of Polymerizationa
Monomer
Acrylonitrileb
1,3-Butadiene
Ethylene
Isoprene
Methyl methacrylate
Propylene
Styrene
Tetrafluoroethylene
Vinyl acetate
Vinyl chloride
- H
(kJ/mol)
77
78
109
75
65
84
70
163
90
71
- S
(J/mol)
109
89
155
101
117
116
104
-
a
Values selected from Ivin.29c Polymerization temperature
25oC unless otherwise indicated.
b
74.5oC.
c
127oC.
POLYMER CHEMISTRY
43.
6.7 Monomer ReactivityB. Factors of monomer reactivity in free radical polymerization.
a. The stability of the monomer toward addition of a free radical.
b. The stability of the monomer radicals.
c. Order of monomer reactivity.
Acrylonitrile > Styrene > Vinyl acetate.
d. Order of benzoyloxy radical initiation.
Syrene > Vinyl acetate > Acrylonitrile
Benzoyloxy radical : Ph14CO2․
POLYMER CHEMISTRY
44.
6.7 Monomer ReactivityC. The inverse relationship between monomer stability and
polymerization rate.
a. Vinyl acetate: not Stable monomer but high rate constant.
b. Steric and polar effects: Not clear-cut generalization.
Lower rate constant of MMA than MA.
c. 1,2 disubstituted monomer difficult to polymerize in free radical.
Exception: Tetrafluoroethylene.
POLYMER CHEMISTRY
45.
6.7 Monomer ReactivityD. Ceiling temperature (Tc)
a.
b. Definition of ceiling temperature.
ΔGp = 0 : equal forward and backword reactions.
Hp
Tc
Sr
c. High Tc : favorable polymerization.
Low Tc : unfavorable polymerization.
Exception : α-methylstyrene (Tc=66℃).
POLYMER CHEMISTRY
46.
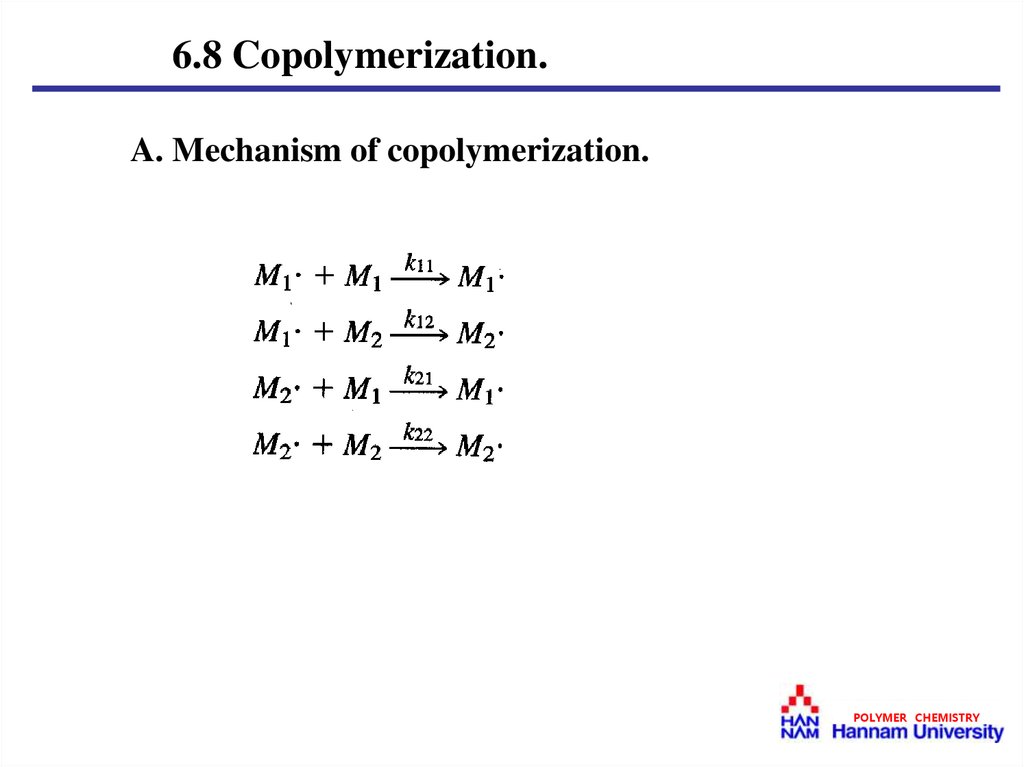
6.8 Copolymerization.A. Mechanism of copolymerization.
POLYMER CHEMISTRY
47.
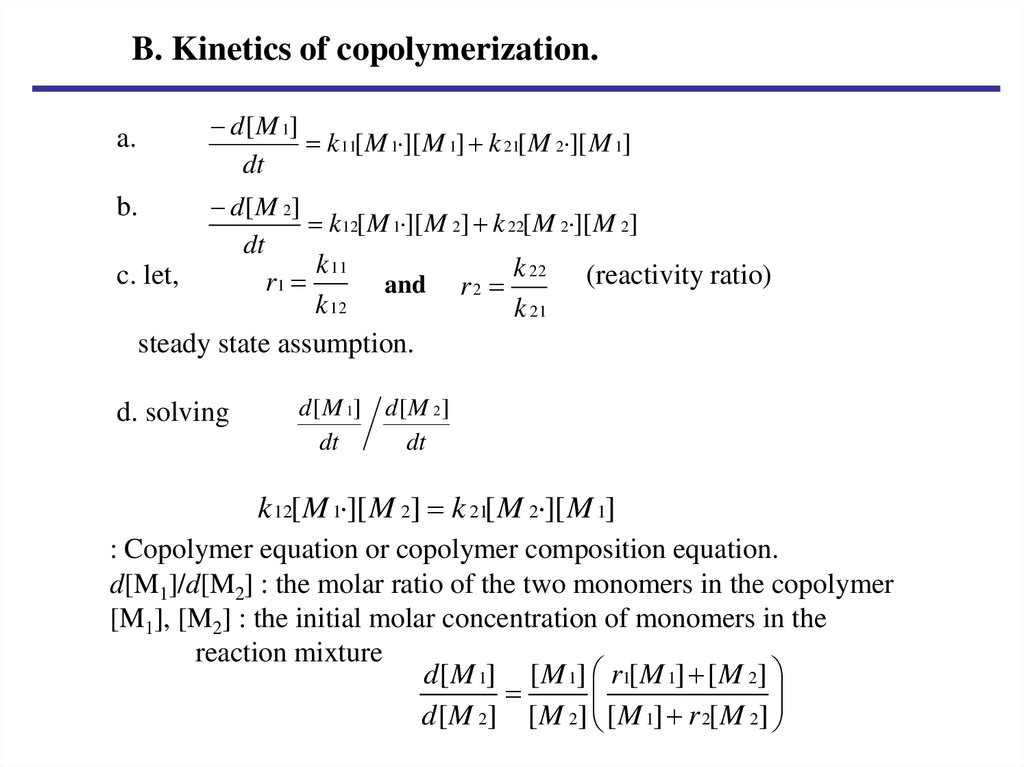
B. Kinetics of copolymerization.d [ M 1]
k 11[ M 1 ][ M 1] k 21[ M 2 ][ M 1]
dt
a.
b.
c. let,
d [ M 2]
k 12[ M 1 ][ M 2] k 22[ M 2 ][ M 2]
dt
k 11
k 22 (reactivity ratio)
r1
and r 2
k 12
steady state assumption.
d. solving
k 21
d [ M 1] d [ M 2]
dt
dt
k 12[ M 1 ][ M 2] k 21[ M 2 ][ M 1]
: Copolymer equation or copolymer composition equation.
d[M1]/d[M2] : the molar ratio of the two monomers in the copolymer
[M1], [M2] : the initial molar concentration of monomers in the
reaction mixture
d [ M 1] [ M 1] r1[ M 1] [ M 2]
d [ M 2] [ M 2] [ M 1] r 2[ M 2]
48.
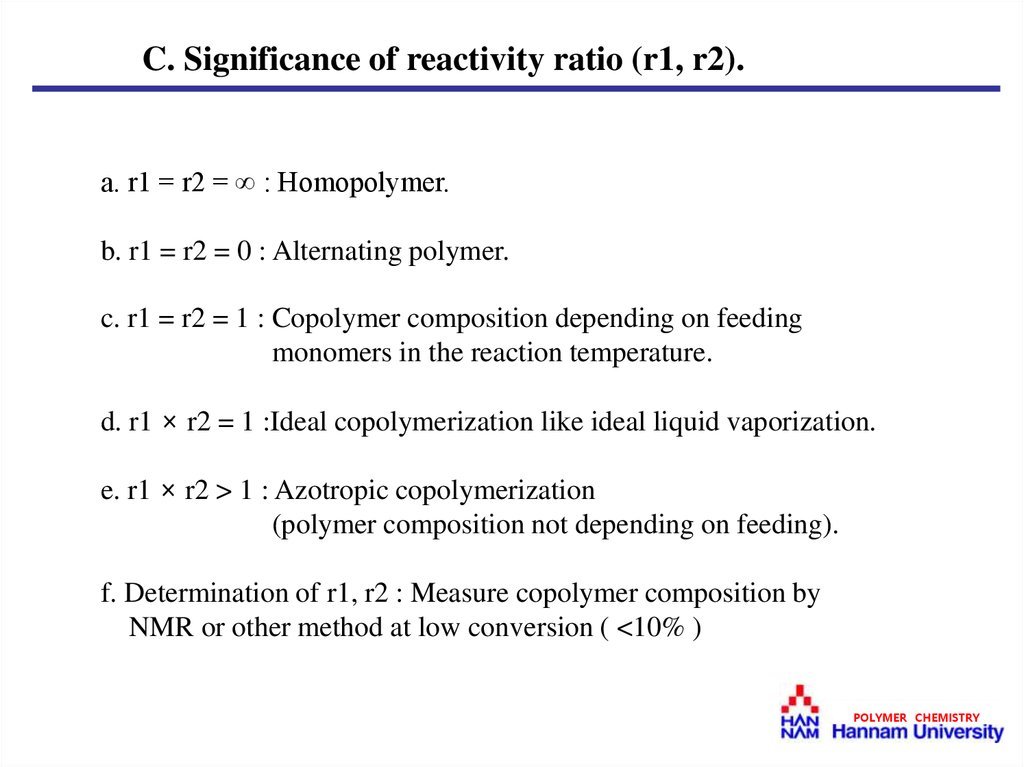
C. Significance of reactivity ratio (r1, r2).a. r1 = r2 = ∞ : Homopolymer.
b. r1 = r2 = 0 : Alternating polymer.
c. r1 = r2 = 1 : Copolymer composition depending on feeding
monomers in the reaction temperature.
d. r1 × r2 = 1 :Ideal copolymerization like ideal liquid vaporization.
e. r1 × r2 > 1 : Azotropic copolymerization
(polymer composition not depending on feeding).
f. Determination of r1, r2 : Measure copolymer composition by
NMR or other method at low conversion ( <10% )
POLYMER CHEMISTRY
49.
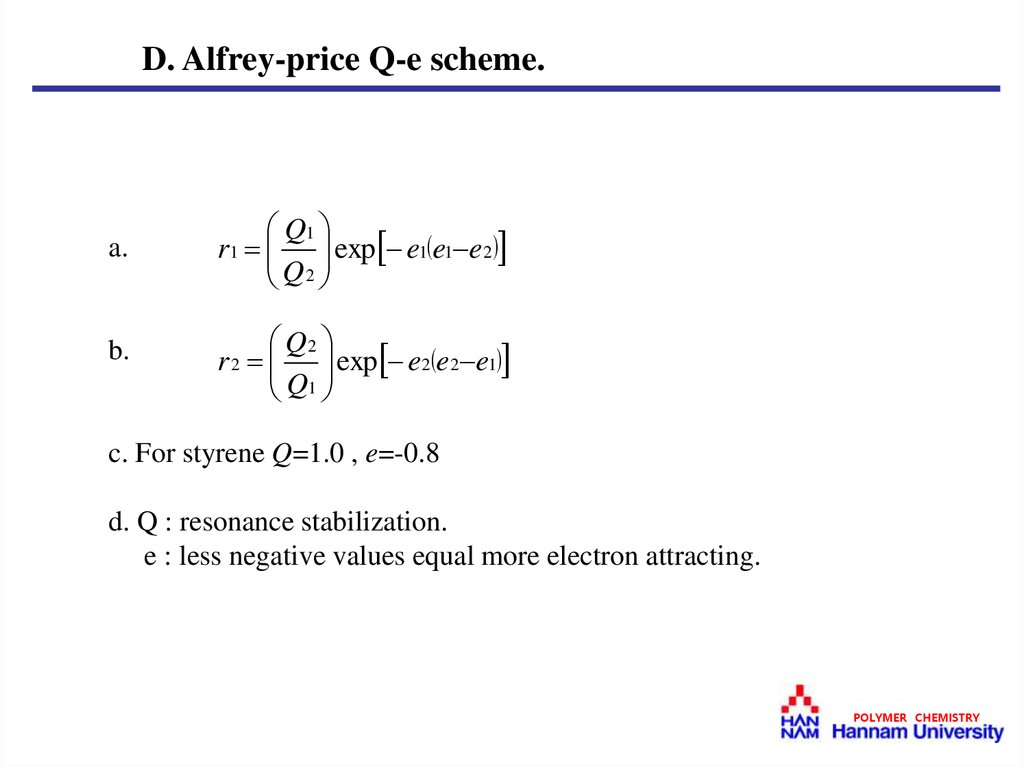
D. Alfrey-price Q-e scheme.a.
Q1
r1 exp e1 e1 e2
Q2
b.
Q2
r 2 exp e2 e2 e1
Q1
c. For styrene Q=1.0 , e=-0.8
d. Q : resonance stabilization.
e : less negative values equal more electron attracting.
POLYMER CHEMISTRY
50.
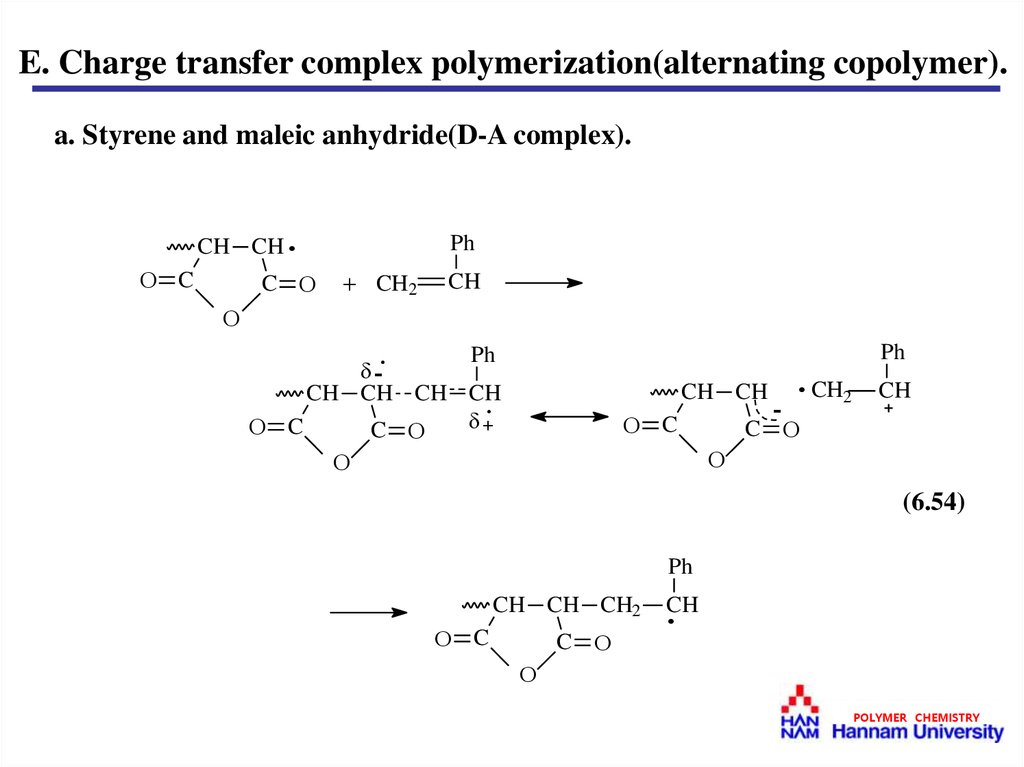
E. Charge transfer complex polymerization(alternating copolymer).a. Styrene and maleic anhydride(D-A complex).
C
CH CH ·
C
Ph
CH2
CH
Ph
-·
CH CH CH CH
+·
C
C
CH CH
C
-
· CH2
Ph
CH
C
(6.54)
Ph
CH CH CH2
C
C
·
CH
POLYMER CHEMISTRY
51.
E. Charge transfer complex polymerization (alternating copolymer).b.
R
H2C
CH +
CO
CH2
R
O
CH
C
(6.57)
c.
R
R
H2C
CH
+
SO2
CH2
CH
SO2
(6.58)
POLYMER CHEMISTRY
























 chemistry
chemistry