Similar presentations:
Ionic polymerization
1.
Chapter 7. Ionic polymerization7.1 Introduction
7.2 Cationic polymerization
7.3 Anionic polymerization
7.4 Group transfer polymerization
2.
7.1 IntroductionPresence of counterions (= gegenions)
Influence of counterions
• Solvation effect
more complex than free radical polymerizations
but more versatile
ex)
counterion
Cl
CH3
CH3
C
C Cl
CH3
CH3
+ BCl3
Cl
CH3
CH3
C
C
CH3
CH3
+
BCl-4
3.
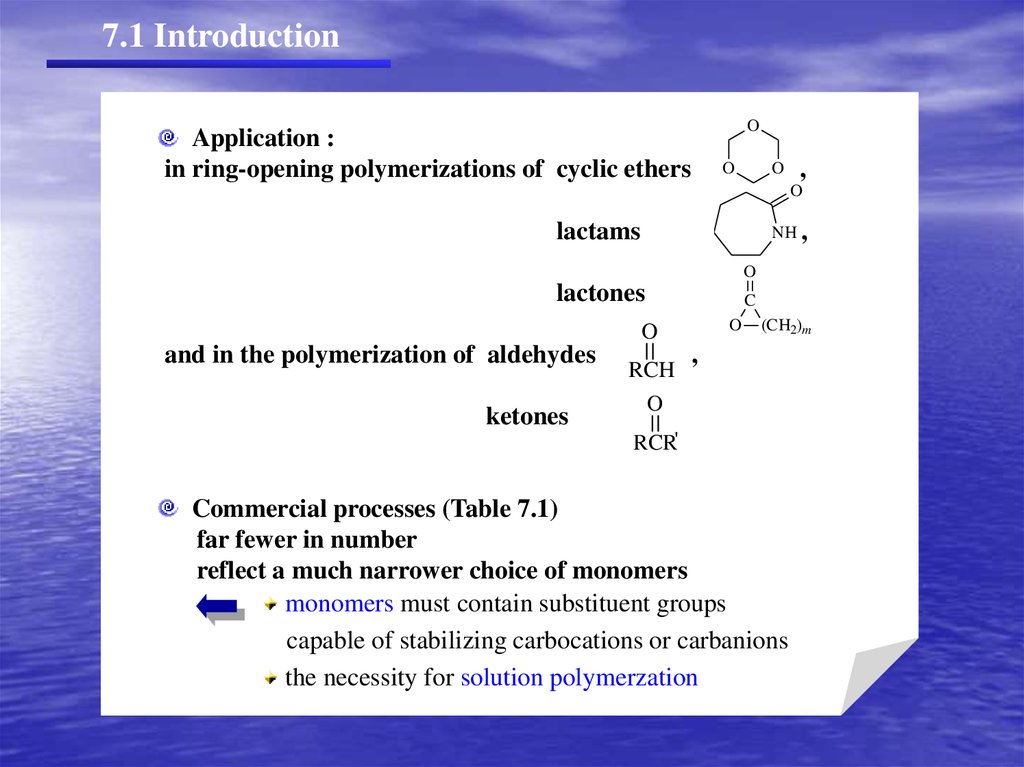
7.1 IntroductionO
Application :
in ring-opening polymerizations of cyclic ethers
O
O
,
O
lactams
NH ,
O
lactones
C
O
O
and in the polymerization of aldehydes
ketones
RCH
(CH2)m
,
O
RCR'
Commercial processes (Table 7.1)
far fewer in number
reflect a much narrower choice of monomers
monomers must contain substituent groups
capable of stabilizing carbocations or carbanions
the necessity for solution polymerzation
4.
TABLE 7.1. Commercially Important Polymers Prepared by Ionic PolymerizationPolymer or Copolymer
Cationica
Polyisobutylene and polybuteneb
(low and high molecular weight)
Isobutylene-isoprene copolymerc
(“butyl rubber”)
Isobutylene-cyclopentadiene
copolymer
Hydrocarbond and polyterpene resins
Coumarone-indene resinse
Poly(vinyl ether)s
Anionicf
cis-1,4-Polybutadiene
cis-1,4-Polisoprene
Styrene-butadiene rubber (SBR)g
Styrene-butadiene block and star
copolymers
ABA block copolymers (A= styrene,
B=butadiene or isoprene)
polycyanoacrylateh
aAlCl
3 and BF3
b”Polybutenes”
Major Uses
Adhesives, sealants, insulating oils, lubricating oil and
grease additives, moisture barriers
Inner tubes, engine mounts and springs, chemical tank
linings, protective clothing, hoses, gaskets, electrical
insulation
Ozone-resistant rubber
Inks, varnishes, paints, adhesives, sealants
Flooring, coatings, adhesives
Polymer modifiers, tackifiers, adhesives
Tires
Tires, footware, adhesives, coated fabrics
Tire treads, belting, hose, shoe soles, flooring, coated
fabrics
Flooring, shoe soles, artificial leather, wire and cable
insulation
Thermoplastic elastomers
Adhesives
most frequently used coinitiators.
are copolymers based on C4 alkenes and lesser amounts of propylene and C5 and higher alkenes from
refinery streams.
cTerpolymers of isobutylene, isoprene, and divinylbenzene are also used in sealant and adhesive formulations.
dAliphatic and aromatic refinery products.
eCoumarone (benzofuran) and indene (benzocyclopentadiene) are products of coal tar.
fn-Butyllithium most common initiator.
gContains higher cis content than SBR prepared by free radical polymerization.
hMonomer polymerized by adventitious water.
5.
7.2 Cationic polymerization7.2.1 Cationic initiators
7.2.2 Mechanism, kinetics, and reactivity in cationic polymerization
7.2.3 Stereochemistry of cationic polymerization
7.2.4.Cationic copolymerization
7.2.5 Isomerization in cationic polymerization
6.
7.2.1 Cationic InitiatorsThe propagating species : carbocation
Initiation
E+ + CH2
Initiator
+
(7.1)
ECH2CR2
CR2
mineral acid : H2SO4, H3PO4
lewis acid : AlCl3, BF3, TiCl4, SnCl4
Coinitiator
- +
BF3 + H2O
HOBF3 H
AlCl3 + RCl
AlCl4 R
- +
7.
7.2.1 Cationic InitiatorsVery active Lewis acid
autoionization
-
+
AlBr4 AlBr2
2AlBr3
Other initiators
(C6H5)3CCl
(C6H5)3C
(7.5)
+ Cl
+
Cl
(7.6)
Cl
CR2 + HI
ICH
I2 + CH2
CR2
ICH2CIR2
+
ICH2CR2I
-
(7.7)
(7.8)
8.
7.2.1 Cationic InitiatorsOther initiators
M+A
M
+
+ A
-
(7.9)
CH CH2
+
CH CH2
N
N
+ RNO2
·
+
· -2
RNO
(7.10)
9.
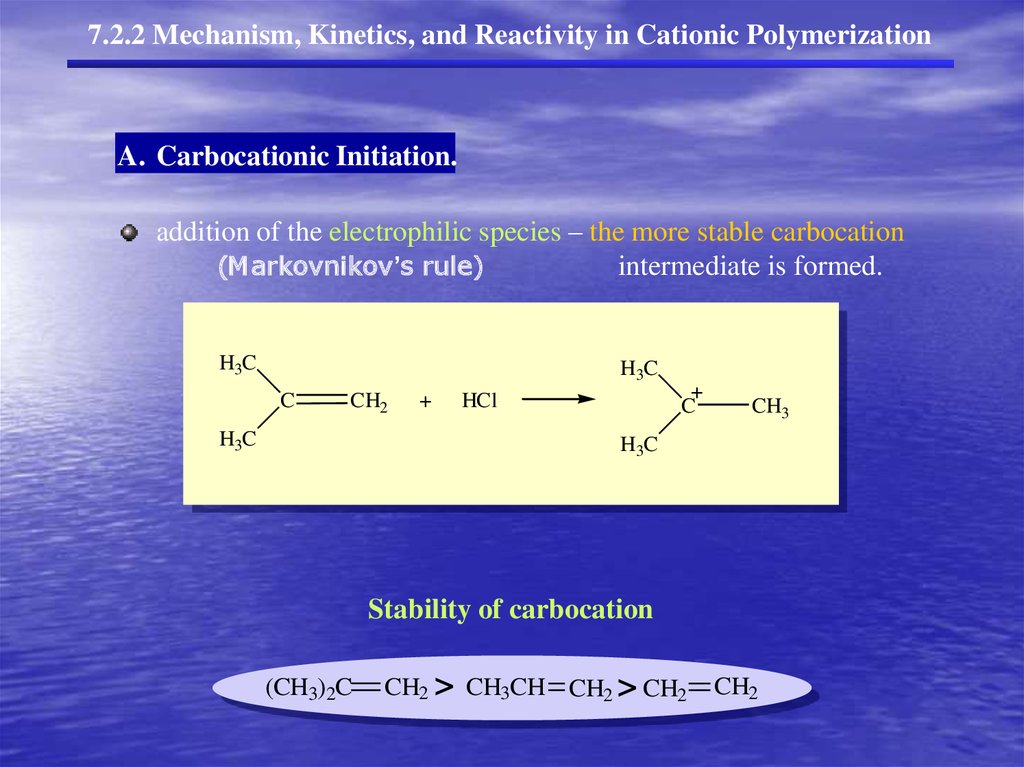
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationA. Carbocationic Initiation.
addition of the electrophilic species – the more stable carbocation
(Markovnikov’s rule)
intermediate is formed.
H3C
H3C
C
CH2
H3C
+
+
C
HCl
CH3
H3C
Stability of carbocation
(CH3)2C
CH2 > CH3CH CH2 > CH2
CH2
10.
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationA. Carbocationic Initiation.
For a series of para-substituted styrenes, the reactivity for substituent group
CH2
CH
X
OCH3
> CH3 > H > Cl
(Because of steric hindrance)
X
Vinyl ethers
CH2
CHOR
R'+
R'CH2
+
CH OR
R'CH2
+
CH OR (7.11)
11.
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationB. Propagation Step
Two Step
① -complex of chain end and approaching monomer
② formation of covalent bond
①
+
+ CH2
CR2
②
slow
CR2
+
CH2
fast
+
CH2CR2
(7.12)
12.
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationC. Influences polymerization rate
① Solvent polarity
(polarity
favors the initiation step)
② Degree of association between the cationic chain end and counterion (A-)
A
Covalent
+ A
Intimate
ion pair
+
A
-
Solvent-separated
ion pair
+ + A
Solvated ions
(7.13)
13.
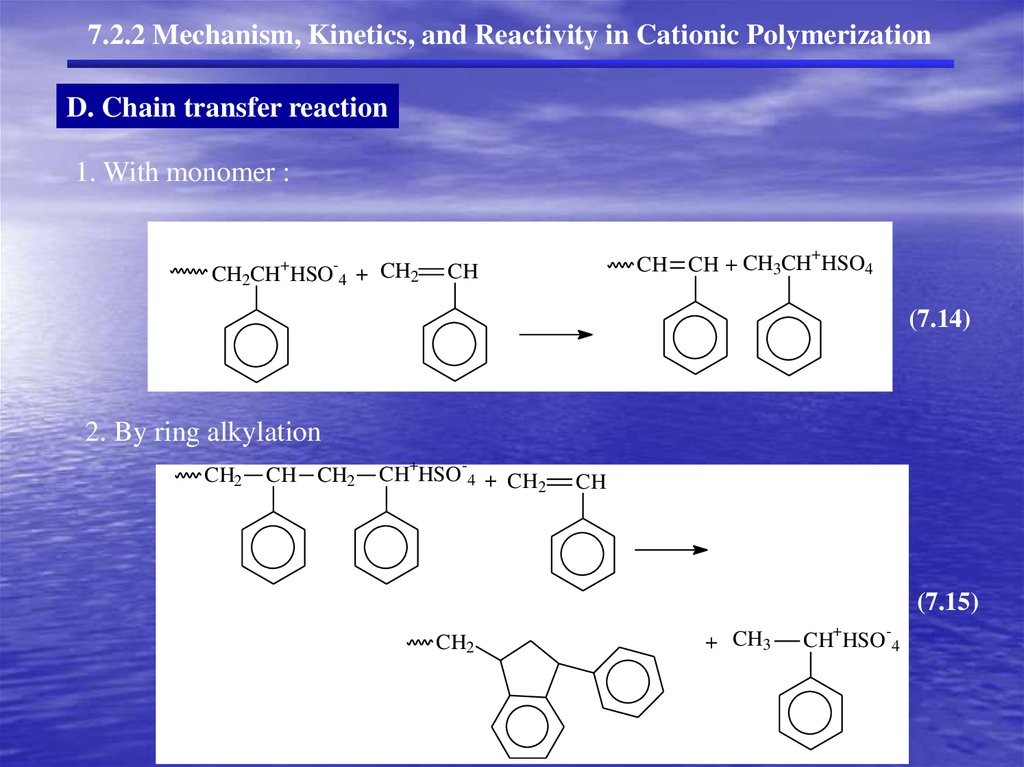
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationD. Chain transfer reaction
1. With monomer :
+
CH2CH
HSO 4
+ CH2
+
CH CH + CH3CH HSO4
CH
(7.14)
2. By ring alkylation
CH2
CH CH2
+
-
CH HSO 4 + CH
2
CH
(7.15)
CH2
+ CH3
+
-
CH HSO 4
14.
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationD. Chain transfer reaction
3. By hydride abstraction from the chain to form a more stable ion :
-
CH2CH+HSO-4 +
CH2CH2 +
CH2CHCH2
+ HSO 4
CH2CCH2
(7.16)
4. With solvent-for example, benzene-by electrophilic substitution :
+
CH2CH HSO 4 +
+ CH2
CH
(7.17)
CH2CH
+
-
+ CH3CH HSO 4
15.
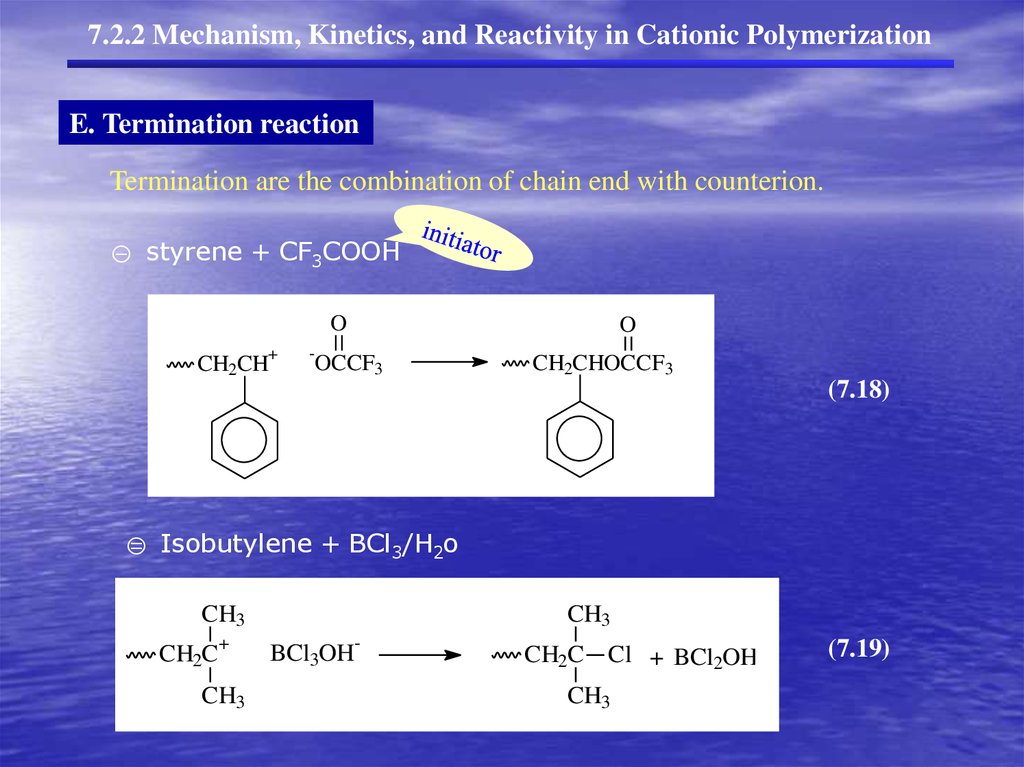
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationE. Termination reaction
Termination are the combination of chain end with counterion.
① styrene + CF3COOH
O
+
CH2CH
O
-
OCCF3
CH2CHOCCF3
(7.18)
② Isobutylene + BCl3/H2o
CH3
CH2C
+
CH3
CH3
-
BCl3OH
CH2C Cl + BCl2OH
CH3
(7.19)
16.
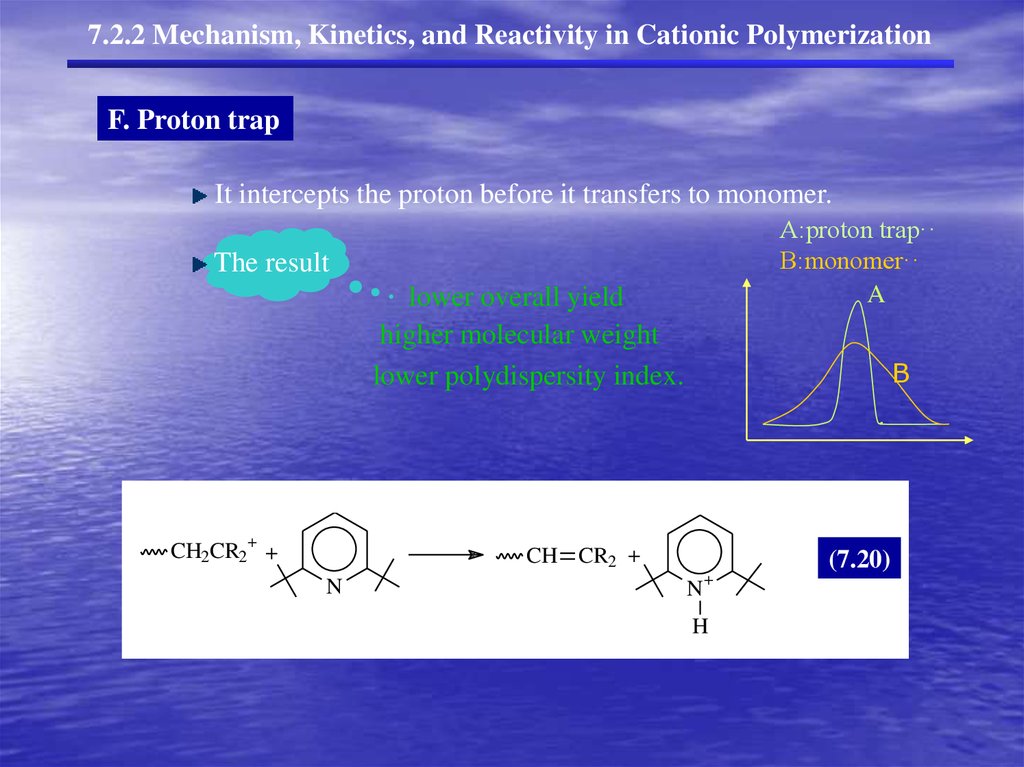
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationF. Proton trap
It intercepts the proton before it transfers to monomer.
A:proton trap··
B:monomer··
A
The result
lower overall yield
higher molecular weight
lower polydispersity index.
+
CH2CR2 +
B
CH CR2 +
N
(7.20)
N
+
H
17.
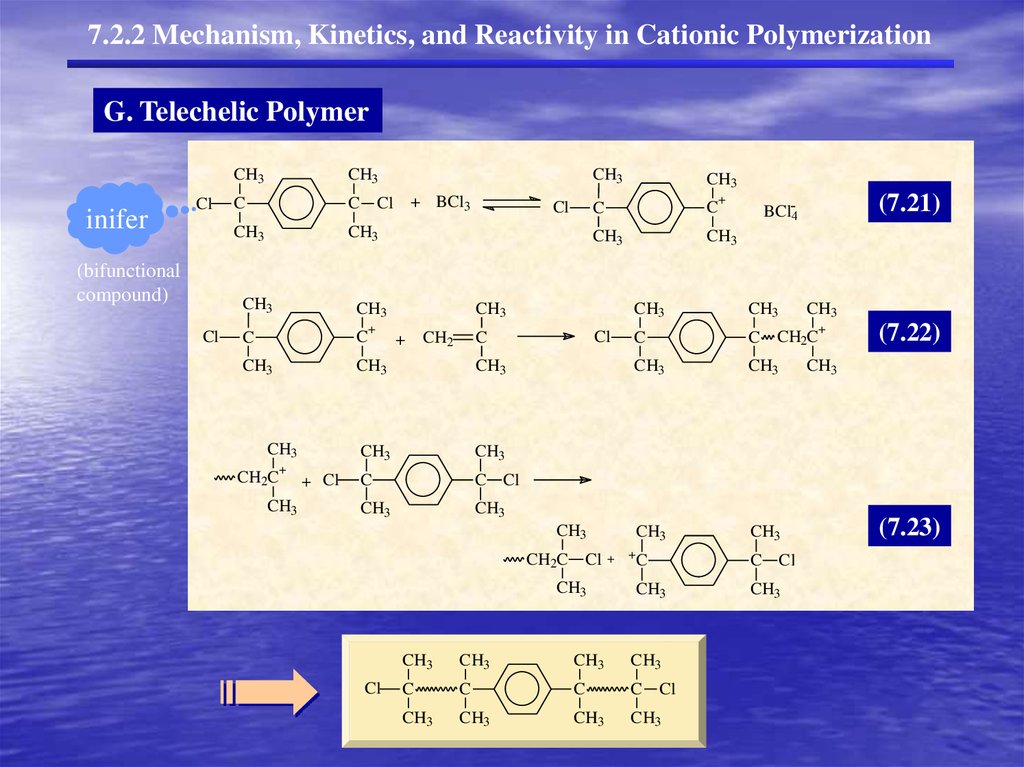
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationG. Telechelic Polymer
inifer
Cl
(bifunctional
compound)
CH3
CH3
C
C Cl
CH3
CH3
CH3
Cl
+ BCl3
CH3
+
Cl
C
CH3
CH3
CH3
CH3
CH3
C
C Cl
CH3
CH3
CH2C
+
CH3
+ Cl
CH3
C
C
CH3
CH3
CH3
C
CH3
CH3
+
Cl
C
CH2
CH3
Cl
+
(7.21)
BCl-4
CH3
CH3
C
C CH2C
CH3
CH3
CH3
CH3
C
C Cl
CH3
CH3
CH3
CH2C Cl
+
+
CH3
CH3
CH3
CH3
C
C
C
C Cl
CH3
CH3
CH3
CH3
CH3
+
(7.22)
CH3
(7.23)
18.
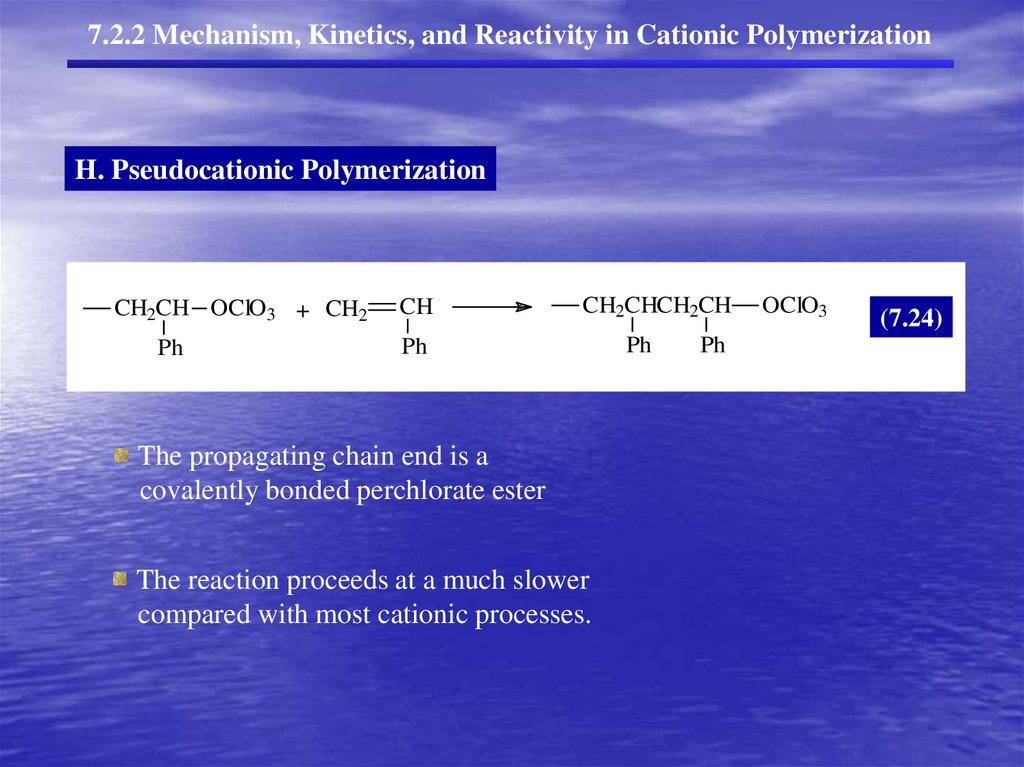
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationH. Pseudocationic Polymerization
CH2CH OClO3 + CH2
Ph
CH
CH2CHCH2CH
Ph
The propagating chain end is a
covalently bonded perchlorate ester
The reaction proceeds at a much slower
compared with most cationic processes.
Ph
Ph
OClO3
(7.24)
19.
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationI. To prepare living polymers under cationic conditions.
(Termination or chain transfer reaction 없이 중합반응이 종결되는 예)
ex1) Tertiary ester + BCl3 / Isobutylene polymerization
① formation of tertiary carbocation-initiating species.
O
O
R3C+
R3COCCH3 + BCl3
BCl3
(7.27)
-
OCCH3
② Polymerization to yield polyisobutylene terminated
: appearance of a very tightly bound – but still active – ion pair
CH2
C(CH3)2
CH3
R3C
CH2C
CH3
CH3
CH2C
–
CH3
O
…
OCCH3
BCl3
(7.26)
20.
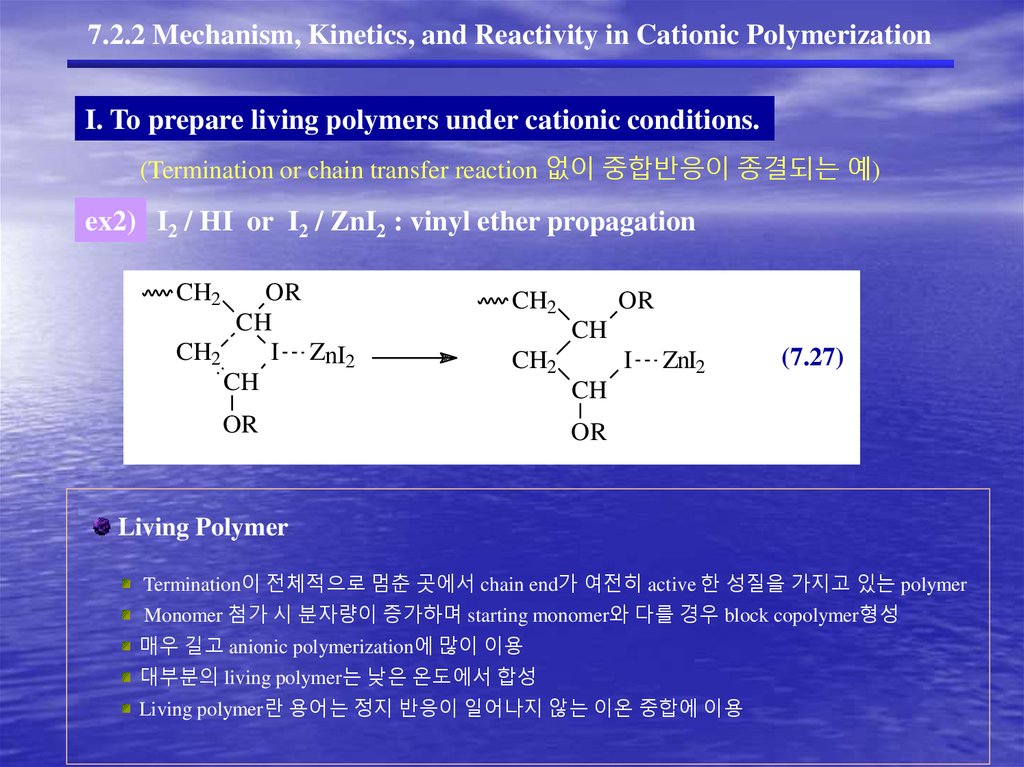
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationI. To prepare living polymers under cationic conditions.
(Termination or chain transfer reaction 없이 중합반응이 종결되는 예)
ex2) I2 / HI or I2 / ZnI2 : vinyl ether propagation
CH2
OR
CH
CH2
I ZnI2
CH
CH2
OR
CH
CH2
OR
I
ZnI2
(7.27)
CH
OR
Living Polymer
Termination이 전체적으로 멈춘 곳에서 chain end가 여전히 active 한 성질을 가지고 있는 polymer
Monomer 첨가 시 분자량이 증가하며 starting monomer와 다를 경우 block copolymer형성
매우 길고 anionic polymerization에 많이 이용
대부분의 living polymer는 낮은 온도에서 합성
Living polymer란 용어는 정지 반응이 일어나지 않는 이온 중합에 이용
21.
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationJ. Kinetics
Expression of general initiation, propagation, termination, and transfer rates
Ri ki [ I ][ M ]
[I ]
R p k p [ M ][ M ]
: molar concentration of initiation
[ M ] : molar concentration of monomer
[ M ] : molar concentration of
Rt kt [ M ]
Rtr ktr [ M ][ M ]
cationic chain end
As with free radical polymerization approximation to a steady state
for the growing chain end.
thus Ri Rt
ki [ I ][ M ] kt [ M ]
or
[M ]
ki [ I ][ M ]
kt
22.
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationTABLE 7.2. Representative Cationic propagation Rate Constants,
Monomer
Styrene
-Methylstyrene
i-Butyl vinyl ether
i-Butyl vinyl ether
i-Butyl vinyl ether
Methyl vinyl ether
2-Chloroethyl vinyl ether
aData
RP
Solvent
Temperature (oC)
Initiator
None
None
None
CH2Cl2
CH2Cl2
CH2Cl2
CH2Cl2
15
0
30
0
0
0
0
Radiation
Radiation
Radiation
C7H7+SbCl6C7H7+SbCl6C7H7+SbCl6C7H7+SbCl6-
from Ledwith and Sherrington.19
a
kp (L/mol s)
3.5 106
4 106
3 105
5 103
3.5 103
1.4 102
2 102
23.
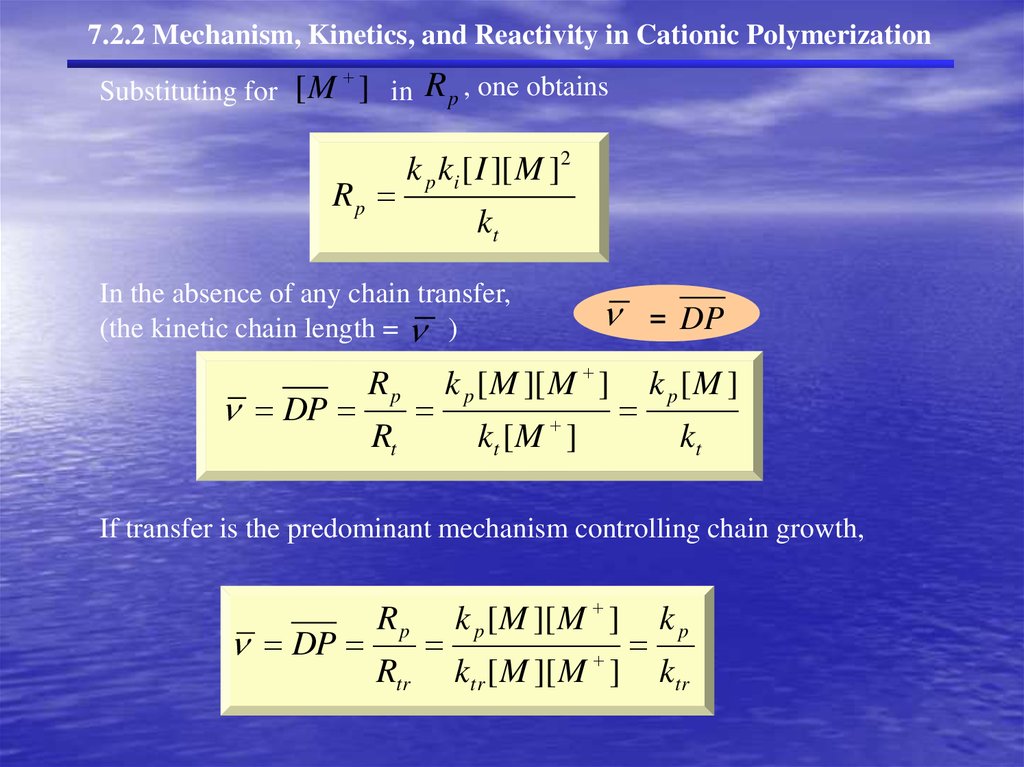
[ M ] ki [ I ][ M ] kt [ M ][ I ] Kinetics, and Reactivity in Cationic Polymerization
7.2.2 Mechanism,
Ri Rt
ki [ I ][ M ]
[
M
]
] R p , one obtains
Substituting for [ M[ M] in
k[iM
[ I ][
M ] kt [ M ] kt
]
2
k
[
I
][
M
]
k
k
[
I
][
M
]
[R
Mi ] R t Rip p i
kt
k
ki [ I ][ M ] kt [ M2 ] t
k p ki [ I ][ M ] R p k p [ M ][ M ] k p [ M ]
In the absence
of any
kchain
[ IDP
][transfer,
M ]
R[ M
i
p
]
=
DP
(the kinetic chain lengthk=t ) Rt
kt [ M ]
kt
k
t
R
k
[
M
][
M
]
k
[
M] ] k p
2
R
k
[
M
][
M
p
p
p
p
p
k
k
[
I
][
M
]
R DP p i DP
p
Rkt
Rktrt [ M k]tr [ M ][ Mkt ] ktr
t
RRp kkp [[M
][
M
kkp [ M ]
]
M
][
M
]
p
p
the
DP
p chain growth,
If transfer is
predominant
controlling
DP R mechanism
][ M
] ktr k
Rtrt ktr [kM
[
M
]
t
t
DP
Rp
Rtr
k p [ M ][ M ]
ktr [ M ][ M ]
kp
ktr
24.
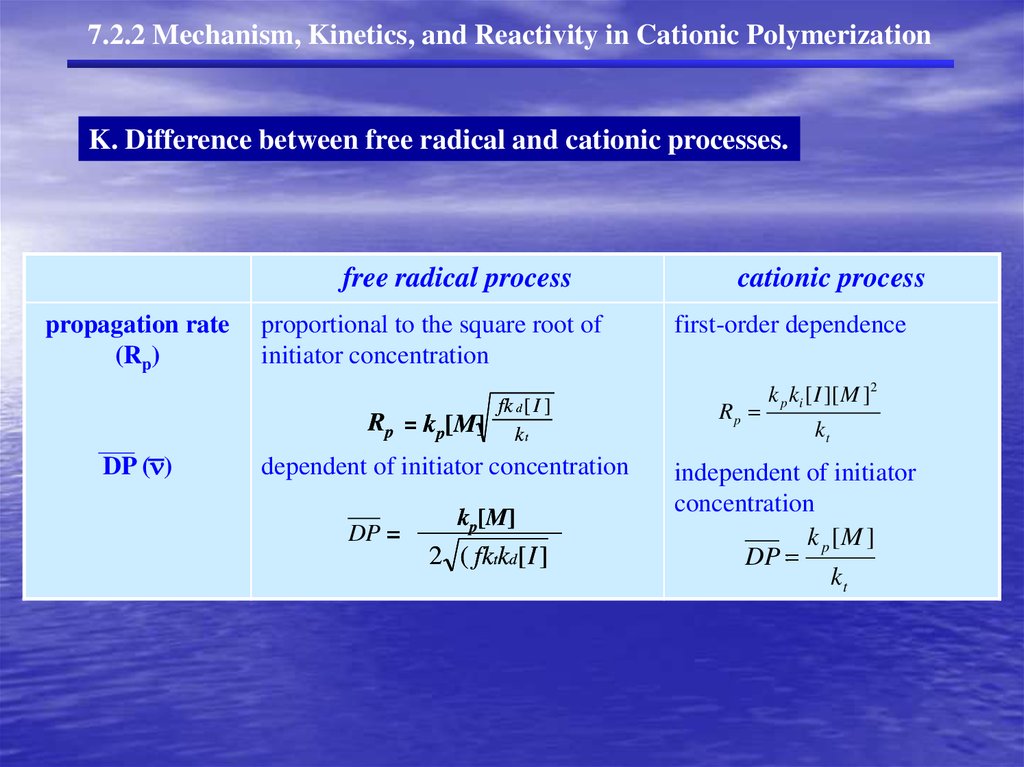
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationK. Difference between free radical and cationic processes.
[I ]
[I ]
[I ]
[ I[]M ]
[M ]
[M ]
[M
[ M] ]
[M ]
Ri ] Rt
[M
[M ]
cationic
Ri free
Rt radical process
][tM ] kt [process
M ]
Riki [ I R
Ri Rt
propagation rate proportional
root
ki [ I ][ M ]to
kthe
]k [ I ][
t [ Msquare
ki[[M
I ][ ]M dependence
]k i [ Ik][t [MM] ]
M ]of
kt [ M ]first-order
i
(Rp)
initiator concentration
kt
k [ I ][ M ]
k
[
I
][ M ]2
k
[
I
][
M
]
[M ] i
i
i
[
M
]
[
M
]
k
k
[
I
][ M ]
kt -d[M]fk d [ I ]
p i
fk d [ I ]
R
-d[M]
k
k
p
t
=k t kp[M] tk t
kp[M]
Rp = dtkRkp [=I ][
2
k
dt
t
M]
p i
2
2
k
k
[
I
][
M
]
k
k
[
I
][
M
]
R
p
i
p
i
p
R pinitiator
k p [ M ][ M ] k p [
DP ( )
dependent of initiator
concentration
independent
of
Rp
R p
kt
DP k
kt
Rtt
kt [ M ]
k
concentration
R p kkpp[M]
[ M ][ M ] k p [ M ]
kp[M]
kp[M][M·]
=
=
=
DP
R p k p [ M ][ M ] kR
[
M
]
k
[
M
][
M
R
k
[
M
][
M
] ] k pk
2
p
pp
pp
2kt[M·]
2kt[M·]
Rt2 ( fk ktkt [d
M
]
k
[ IDP
]
DP
t
DP
k
[
M
][
M
]
ktr
R
k
[
M
]
k
R
k
[
M
]
tr
tr t
t
t t
R p k p [ M ][ M ] kt p
DP
R
k
[
M
][
M
]
k
R
k
[
M
][
M
] k
p
p
p
p
p
Rtr ktr [ M ][DP
M
] ktr
DP
Rtr ktr [ M ][ M ] kRtrtr ktr [ M ][ M ] k
25.
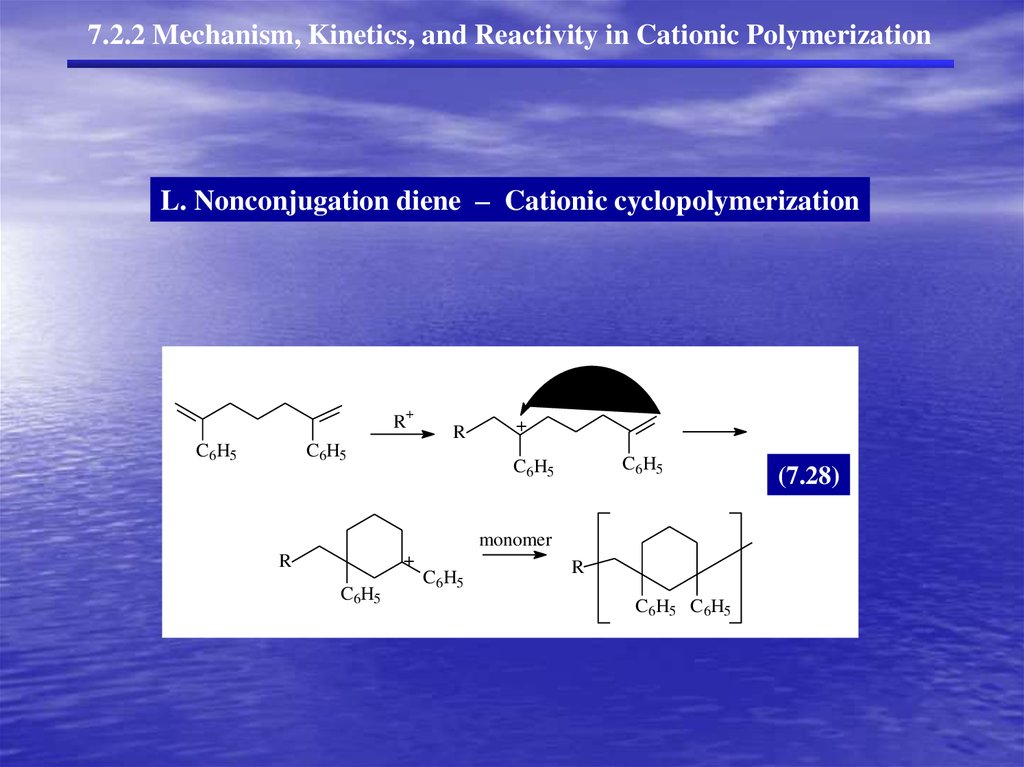
7.2.2 Mechanism, Kinetics, and Reactivity in Cationic PolymerizationL. Nonconjugation diene – Cationic cyclopolymerization
+
R
C6H5
R
C6H5
+
C6H5
C6H5
monomer
+
R
C6H5
C6H5
R
C6H5 C6H5
(7.28)
26.
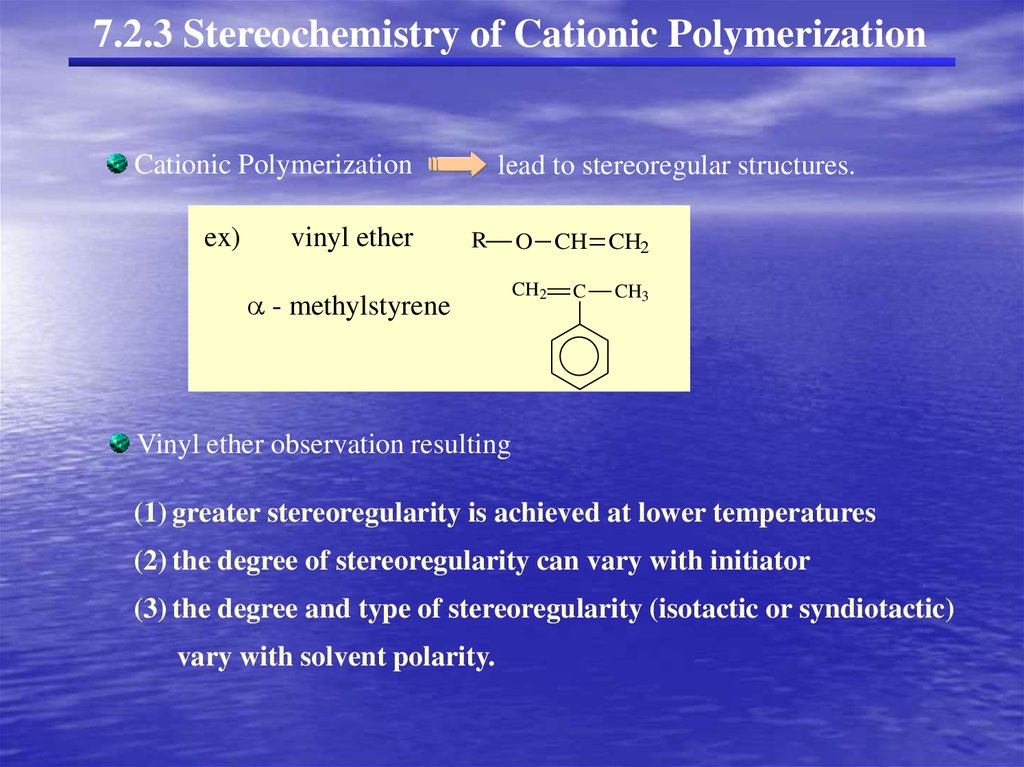
7.2.3 Stereochemistry of Cationic PolymerizationCationic Polymerization
ex)
vinyl ether
lead to stereoregular structures.
R
- methylstyrene
O CH CH2
CH2
C
CH3
Vinyl ether observation resulting
(1) greater stereoregularity is achieved at lower temperatures
(2) the degree of stereoregularity can vary with initiator
(3) the degree and type of stereoregularity (isotactic or syndiotactic)
vary with solvent polarity.
27.
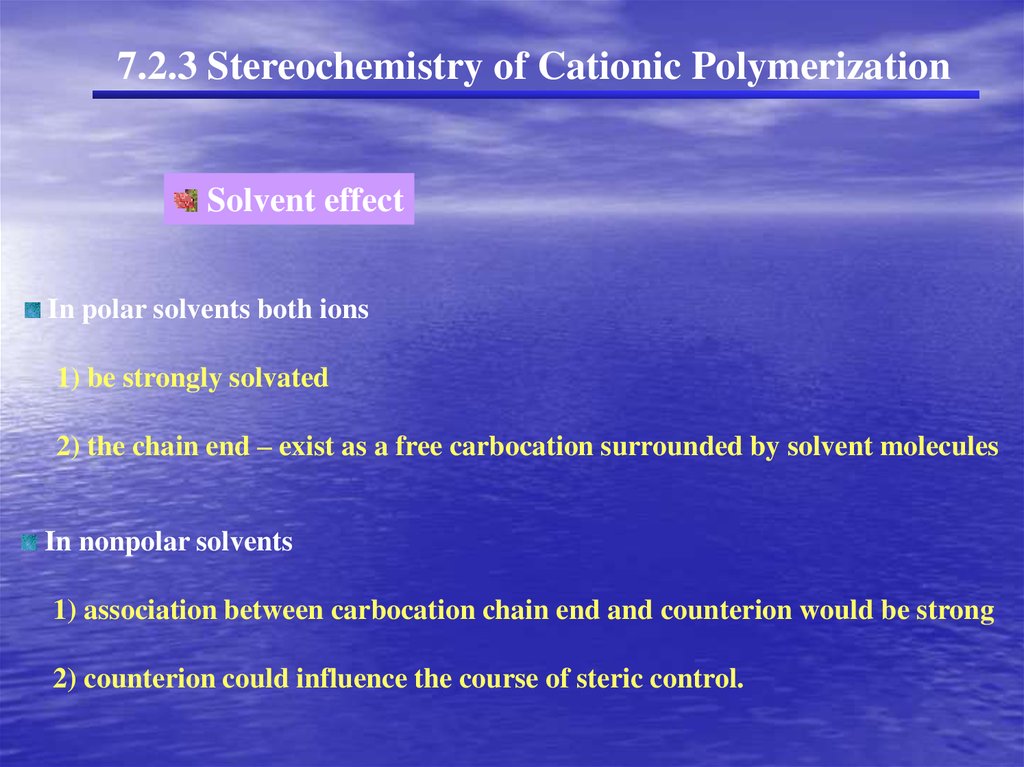
7.2.3 Stereochemistry of Cationic PolymerizationSolvent effect
EX) t-butyl vinyl ether
forms isotactic polymer in nonpolar solvents.
forms mainly syndiotactic polymer in polar solvents.
( cationic chain end and the counterion are associated )
28.
7.2.3 Stereochemistry of Cationic PolymerizationSolvent effect
In polar solvents both ions
1) be strongly solvated
2) the chain end – exist as a free carbocation surrounded by solvent molecules
In nonpolar solvents
1) association between carbocation chain end and counterion would be strong
2) counterion could influence the course of steric control.
29.
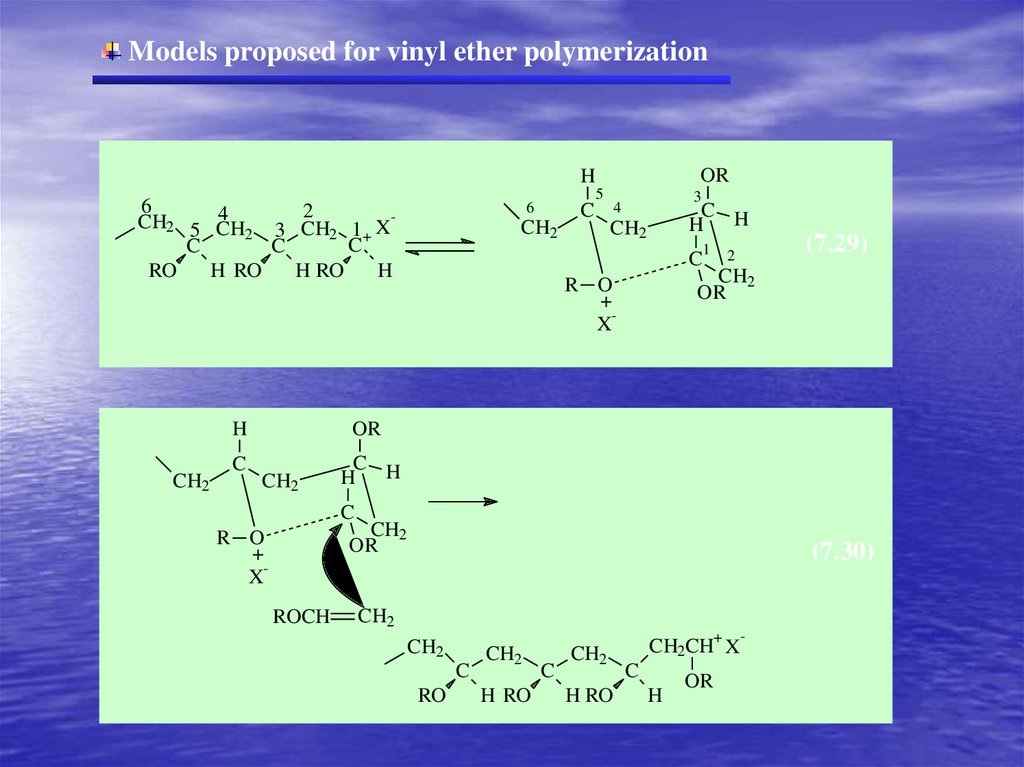
Models proposed for vinyl ether polymerizationOR
H
5
6
2
4
CH2 5 CH
CH
3
2
2 1+ X
C
C
C
RO
H RO
H RO
H
C
CH2
C
C
H H
4
CH2
1
2
C
(7.29)
CH2
OR
R O
+
X
OR
H
CH2
6
3
CH2
C
H H
C
CH2
OR
R O
+
X
ROCH
(7.30)
CH2
+
CH2
C
RO
CH2
H RO
C
CH2
H RO
CH2CH X
C
H
OR
-
30.
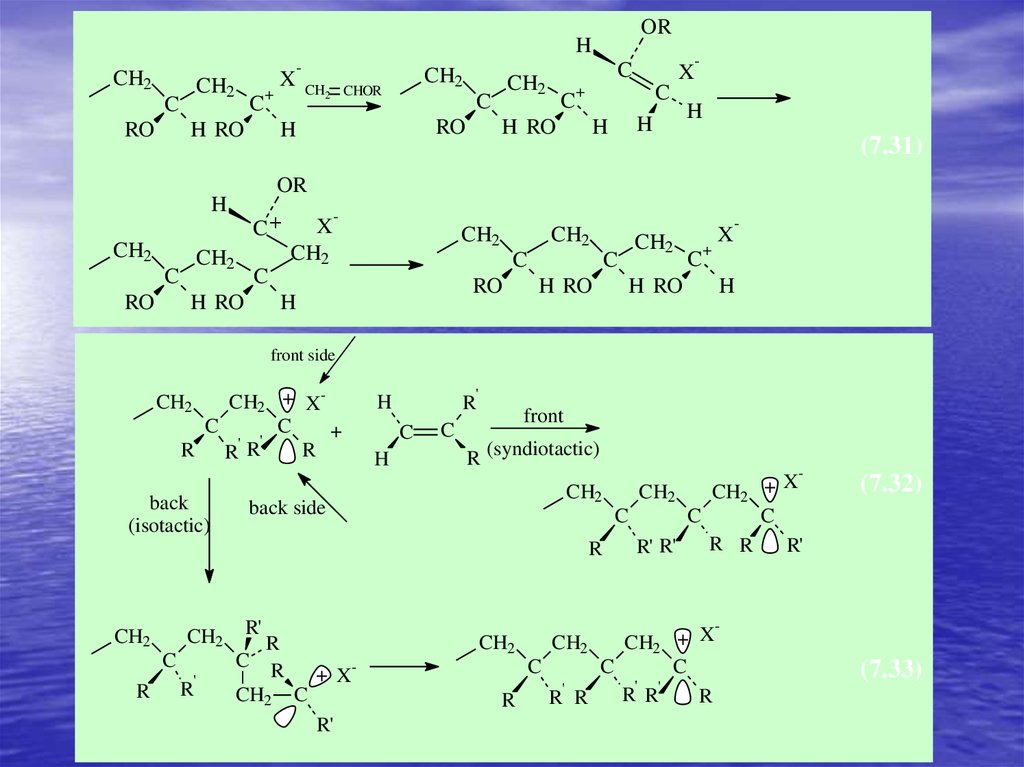
ORH
CH2
CH2
C
RO
C
+
H RO
RO
CH2 CHOR
CH2
C
RO
C
C
X
C
+
H RO
H
H
-
H
(7.31)
OR
C+
CH2
C
CH2
H
H
CH2
X
-
X
CH2
-
CH2
C
C
H RO
CH2
RO
H
C
H RO
CH2
C
+
H RO
X
-
H
front side
CH2 + XC
C
+
' R'
R R
R
CH2
back
(isotactic)
CH2
CH2
C
R
back side
R'
C
'
R
R
R
CH2
+ XC
R'
'
H
R
C
H
C
front
R (syndiotactic)
-
CH2 + X
C
C
C
R R
R'
R' R'
R
CH2
CH2
CH2
CH2
CH2 + X
C
C
C
' '
'
R R
R
R R
R
(7.32)
-
(7.33)
31.
7.2.4 Cationic CopolymerizationA. Copolymerization equation
- the situation is complication by counterion effects.
B. Reactivity ratios vary with initiator type and solvent polarity.
C. Temperature – unpredictable effect
D. Steric effects (Table 7.3)
E. commercial cationic copolymers – butyl rubber
(prepared from isobutylene and isoprene.)
protective clothing
tire inner tubes
32.
TABLE 7.3. Representative Cationic Reactivity Rations (r)aMonomer 1
Isobutylene
Styrene
p-Chlorostyrene
Ethyl vinyl ether
2-Chloroethyl
vinyl ether
aData
r1
r2
-100
-103
-103
-78
0
-92
-78
0
-78
0
43
115
2.5
0.60
1.60
9.02
1.2
0.05
0.33
1.80
0
0
0.4
4.5
1.17
1.99
5.5
2.90
1.74
1.10
Monomer 2
1,3-Butadiene
1,3-Butadiene
Isoprene
Cyclopentadiene
Styrene
Styrene
-Methylstyrene
-Methylstyrene
p-Methylstyrene
trans- -Methylstyrene
cis- -Methylstyrene
trans- -Methylstyrene
i-Butyl vinyl
ether
-Methylstyrene
AlEtCl2
AlCl3
AlCl3
BF3·OEt2
SnCl4
AlCl3
TiCl4
SnCl4
SnCl4
SnCl4
CH3Cl
CH3Cl
CH3Cl
PhCH3
EtCl
CH3Cl
PhCH3
EtCl
CCl4
CH2Cl2
SnCl4
CCl4/PhNO2(1:1)
0
1.0
0.32
SnCl4
CCl4/PhNO2(1:1)
0
0.74
0.32
BF3
CH2Cl2
-78
1.30
0.92
BF3
CH2Cl2
-23
6.02
0.42
from Kennedy and Marechal.5
bEt = C H , Ph = phenyl.
2 5
Solventb
Temperature
(oC)
Coinitiatorb
33.
7.2.5 Isomerization in Cationic PolymerizationCH2
CH
CH3
CH
CH3
CH3
H:shift
CH CH
CH3
+
X
XCH2
CH3
XCH2
CH2
C
+
CH3
+
CH3
monomer
CH2
CH2
C
CH3
R
R
R
(7.34)
+
(7.35)
+
34.
7.3 Anionic Polymerization7.3.1 Anionic initiators
7.3.2 Mechanism, kinetics, and reactivity in anionic
polymerization
7.3.3 Stereochemistry of anionic polymerization
7.3.4 Anionic copolymerization
35.
7.3.1 Anionic InitiatorsPropagating chain - carbanion
Nu + CH2
CHR
NuCH2
-
CH
(7.36)
R
Monomers having substituent group – stabilizing a carbanion
resonance or induction
Examples – nitro, cyano, carboxyl, vinyl, and phenyl.
36.
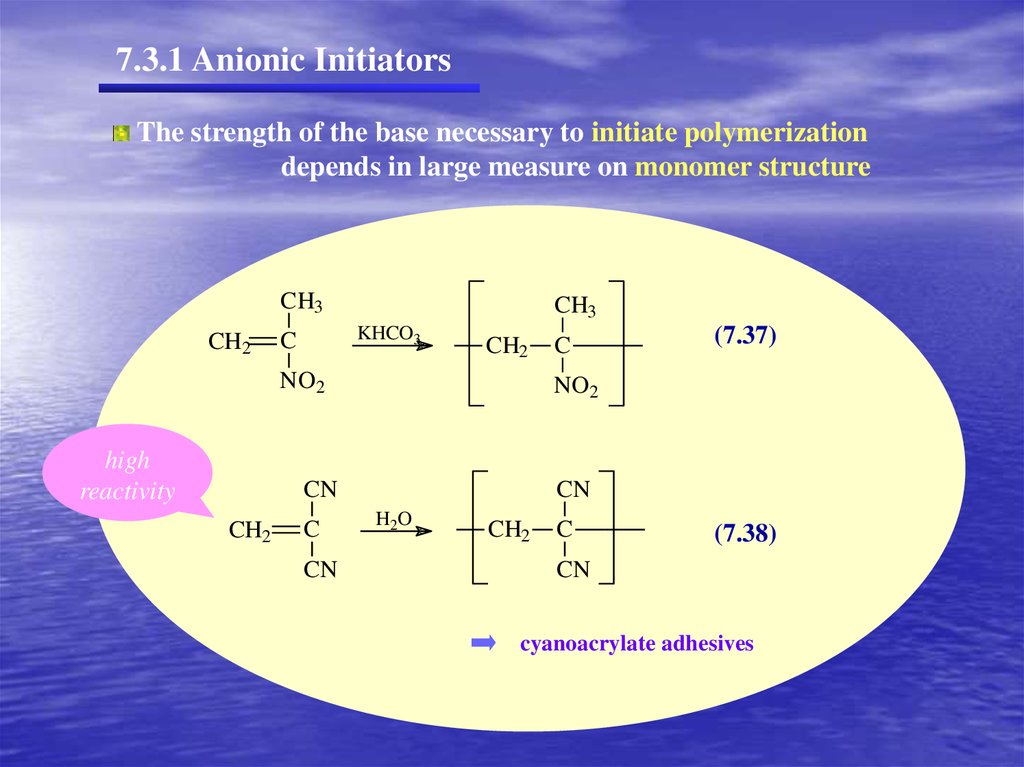
7.3.1 Anionic InitiatorsThe strength of the base necessary to initiate polymerization
depends in large measure on monomer structure
CH3
CH2
CH3
KHCO3
C
CH2
NO2
high
reactivity
C
CN
(7.37)
NO2
CN
CH2
C
CN
H2O
CH2
C
(7.38)
CN
cyanoacrylate adhesives
37.
7.3.1 Anionic InitiatorsTwo basic types
that react by addition of a negative ion
that undergo electron transfer.
① The most common initiators that react by addition of a negative ion
simple organometallic compounds of the alkali metals
For example : butyllithium
Character of organolithium compounds
- low melting
- soluble in inert organic solvents.
Organometallic compounds of the higher alkali metals
- more ionic character
- generally insoluble
38.
7.3.1 Anionic Initiators② Electron transfer (charge transfer)
by free alkali metal : solutions in liquid ammonia or ether solvents
suspensions in inert solvents
by addition complex of alkali metal and unsaturated or aromatic compounds.
Electron transfer processes (involving metal donor D· , monomer M)
D +M
_
+
D + M
O
Na+
Na +
Na + Ph CH
CH Ph
CH Ph] Na +
[Ph CH
O
_
D
_
+ M
Na + PhCPh
D + M
2 PhCPh
Na+
O
PhCPh
Na+
Na O
ONa
Ph2C
CPh2
39.
7.3.2 Mechanism, kinetics, and reactivity in anionic polymerizationA. Mechanism을 변화시킬 수 있는 요인
a. solvent polarity
R
+
R- Me
R-Me+
metal
R- + Me+
solvent separated solvated ion
ion pair
ion pair
Degree of association of ion
counterion의 역할
polar solvent : solvated ion 우세
-
X + CH2
CH
XCH2
R
non polar solvent : 이온들간의 association우세
CH
R
- complex형성
CHR'
R
Li + CH2
CH
R
RCH2CHLi
Li
CH2
R'
R'
40.
7.3.2 Mechanism, kinetics, and reactivity in anionic polymerizationb. Type of cation (counterion)
c. Temperature
B. The rate of initiation
- initiator 와 monomer의 structure에 의존
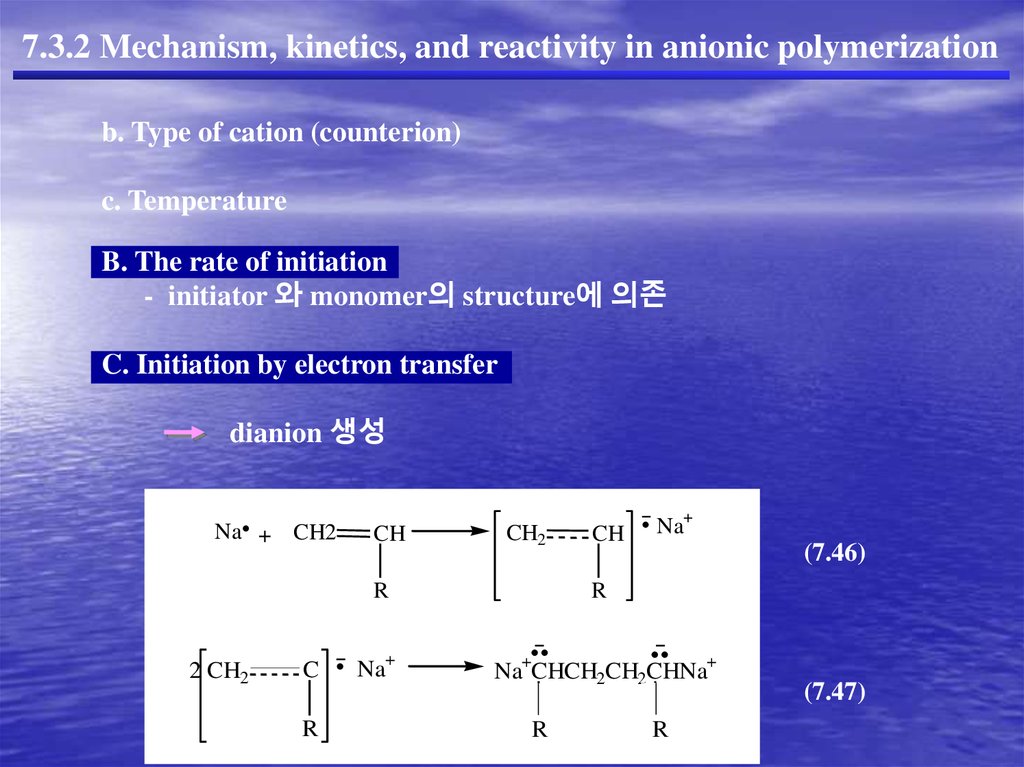
C. Initiation by electron transfer
dianion 생성
Na + CH2
2 CH2
C
R
CH
CH2
CH
Na+
(7.46)
R
R
Na+
Na+CHCH2CH2CHNa+
R
R
(7.47)
41.
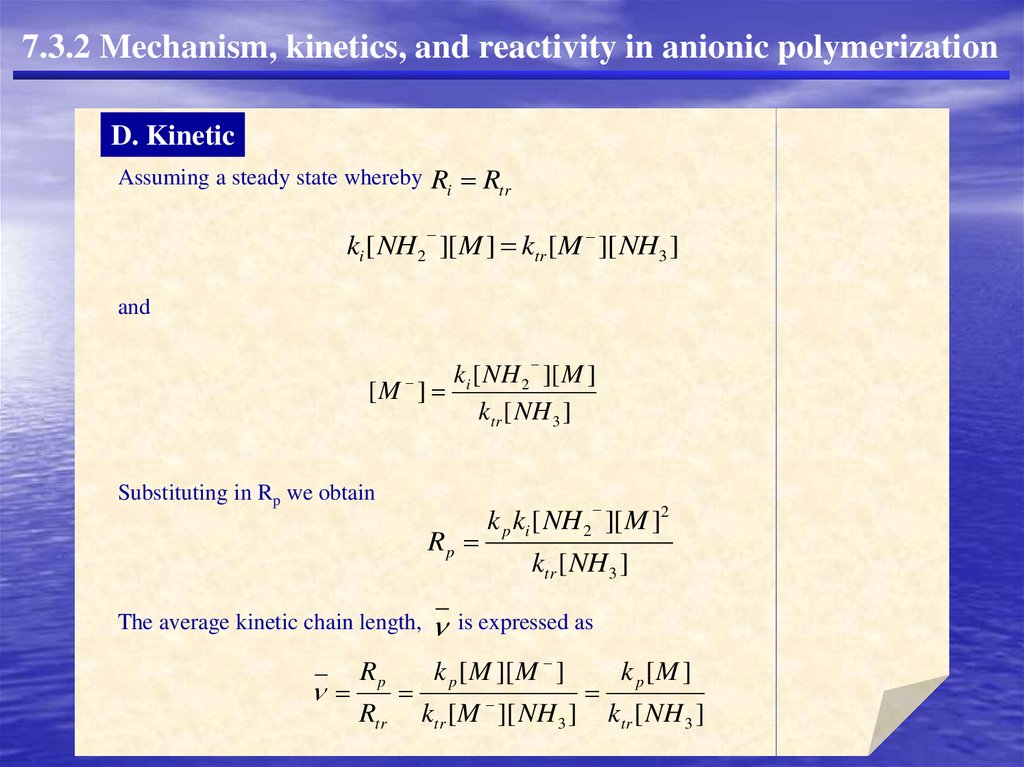
7.3.2 Mechanism, kinetics, and reactivity in anionic polymerizationD. Kinetic
KNH2
NH2- + M
K+ +
H2N
NH2M-
DP
Because the second step is slow relative to the first,
Ri ki [ NH2 ][ M ]
Chain termination is known to result primarily by transfer to solvent:
H2N(M)n-
+ NH3
H2N(M)nMH + NH2-
Rate expressions for propagation and transfer may be written in the conventional way:
Rp k p [M ][ M ]
Rtr ktr [ M ][ NH 3 ]
42.
7.3.2 Mechanism, kinetics, and reactivity in anionic polymerizationD. Kinetic
Assuming a steady state whereby
Ri Rtr
ki [ NH2 ][ M ] ktr [M ][ NH3 ]
and
k [ NH 2 ][ M ]
[M ] i
ktr [ NH 3 ]
Substituting in Rp we obtain
Rp
The average kinetic chain length,
Rp
Rtr
k p ki [ NH 2 ][ M ]2
ktr [ NH3 ]
is expressed as
k p [ M ][ M ]
ktr [ M ][ NH 3 ]
k p [M ]
ktr [ NH 3 ]
43.
7.3.2 Mechanism, kinetics, and reactivity in anionic polymerizationE. Other types of transfer reactions
CH2CH
+ CH2
CN
CH2
+
CH2
CH
CN
C
O
C
CH2
CN
C
CN
CH3
C
CN
CN
C
CH2
CH2CH2 + CH2
CH
CH2CH
CN
CN
CH3
CH2
C
H2
C
CO2CH3
C
CH2
H3C
CH3
CH2
_
+ OCH3
O
OCH3
C
O
CO2CH3
OCH3
H3C
CH2
CO2CH3
CH3
44.
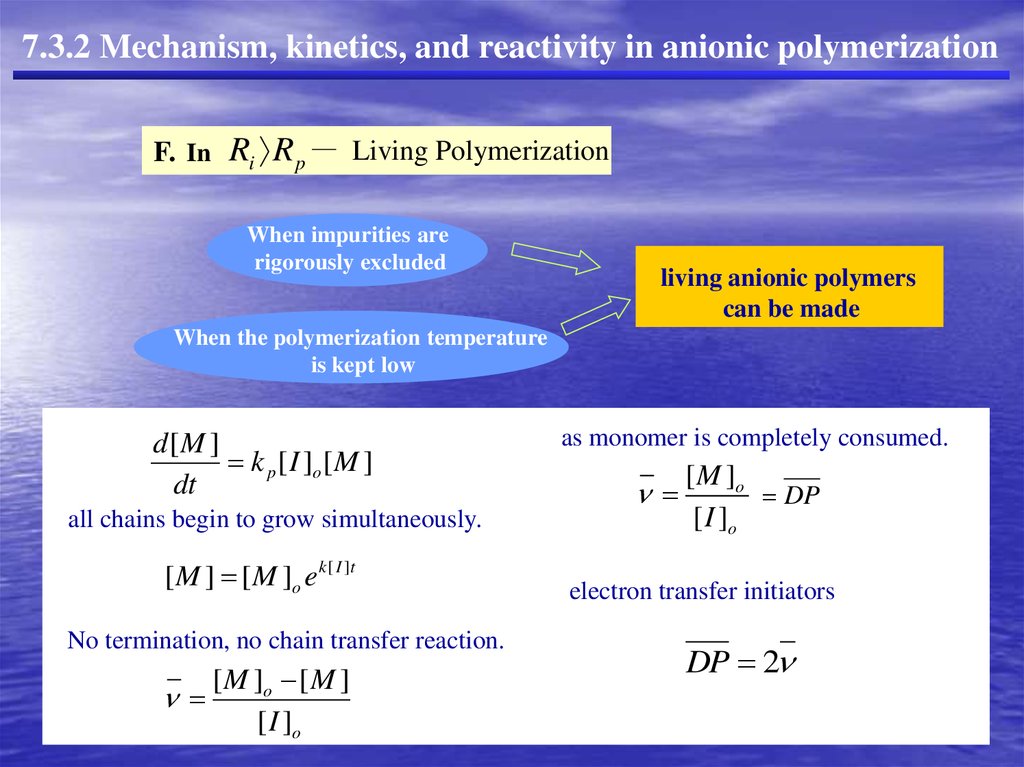
7.3.2 Mechanism, kinetics, and reactivity in anionic polymerizationF. In Ri R p
Living Polymerization
When impurities are
rigorously excluded
living anionic polymers
can be made
When the polymerization temperature
is kept low
d[M ]
k p [ I ]o [ M ]
dt
all chains begin to grow simultaneously.
[ M ] [ M ]o e k [ I ]t
No termination, no chain transfer reaction.
[ M ]o [ M ]
[ I ]o
as monomer is completely consumed.
[ M ]o
DP
[ I ]o
electron transfer initiators
DP 2
45.
7.3.2 Mechanism, kinetics, and reactivity in anionic polymerizationG. Important factor in propagation rate.
a. Association between counterion and terminal carbanion
Li
2
CH2CHLi
R
CH2CH
R
CHCH2
Li
(7.54)
R
TABLE 7.4. Representative Anionic Propagation
Rate Constants, kp, for Polystyrenea
Counterion
Na+
Na+
Li+
Li+
Li+
Solvent
kp (L/mol s)b
Tetrahydrofuran
80
aData from Morton.30
1,2-Dimethoxyethane
3600
Bat 25oC unless otherwise noted.
Tetrahydrofuran
160
cVariable temperature.
Benzene
10-3-10-1c
Cyclohexane
(5-100) 10-5c
46.
7.3.2 Mechanism, kinetics, and reactivity in anionic polymerizationG. Important factor in propagation rate.
b. Monomer structure
inductive destabilization of the carbanion
CH3
CH2
CHCN
> CH2
CCO2CH3
> CH2
CHC6H5
> CH2
CH
steric effect
CH3
CH3
CH2
CHCN
>
CH2
CCN
CH
> CH2
CHCO2CH3
> CH2
CCO2CH3
CH2
47.
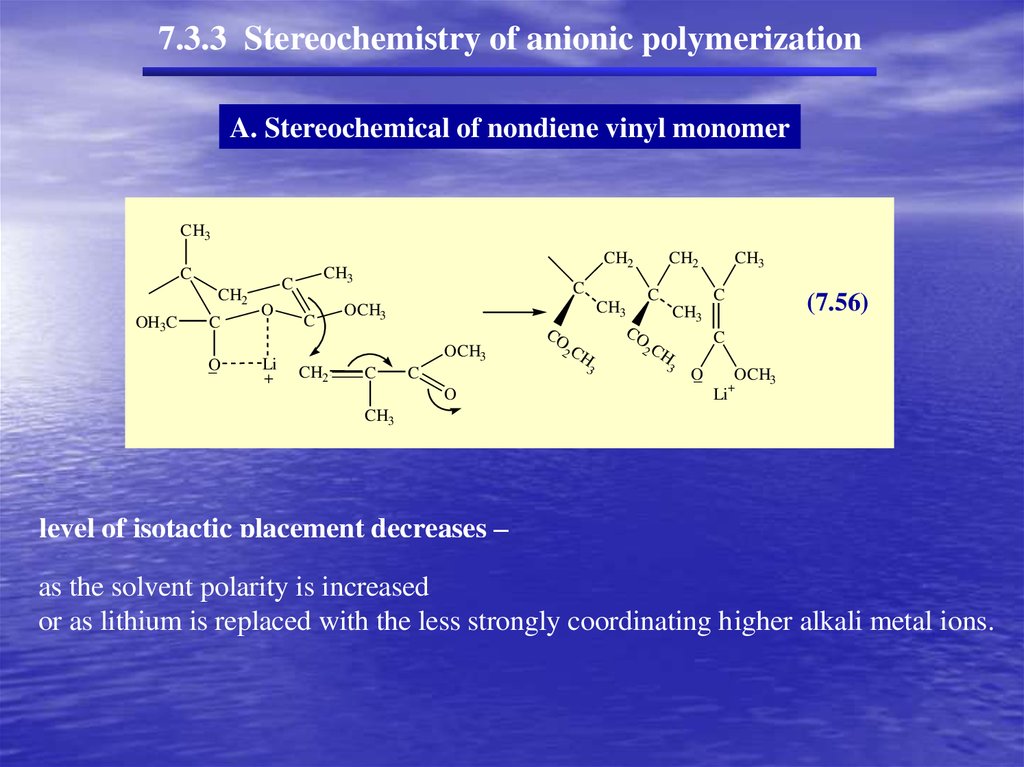
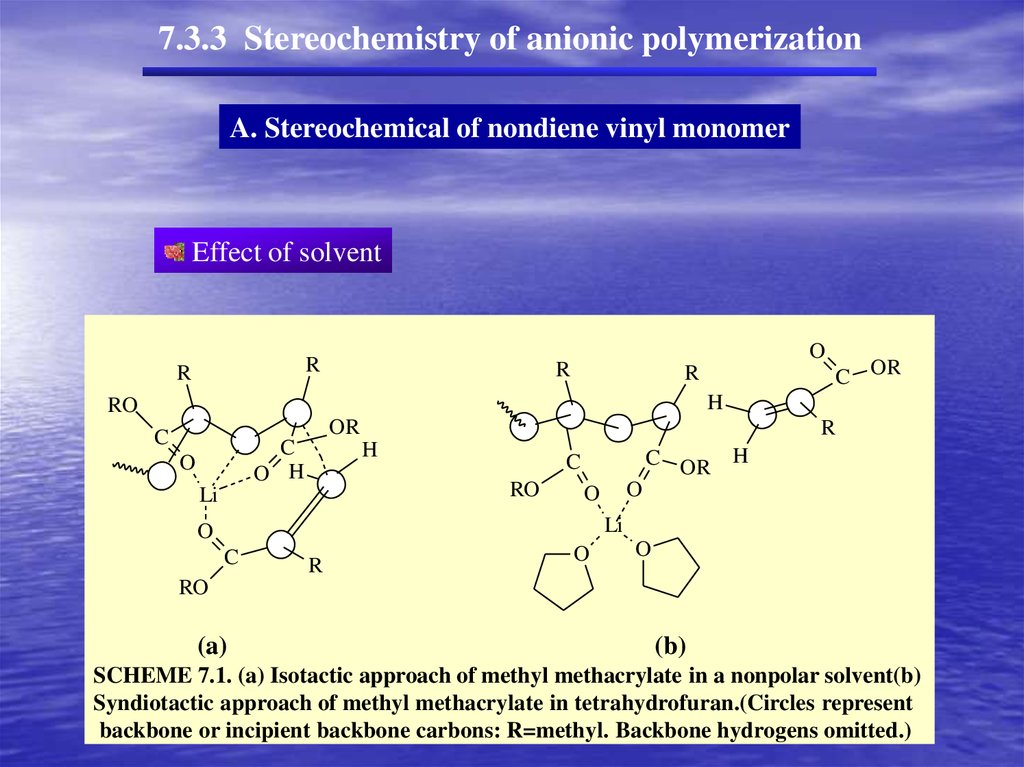
7.3.3 Stereochemistry of anionic polymerizationA. Stereochemical of nondiene vinyl monomer
With soluble anionic initiators (homogeneous conditions)
at low temperatures,
polar solvents favor syndiotactic placement
nonpolar solvents favor isotactic placement.
(stereochemistry depends in large measure on the degree of association with counterion,
as it does in cationic polymerization)
C
CH3O
C
C
CH3
-
O
CH3
CH2
CH3
C
O
Li
C
OCH3
+
CH2
CH
O
_
CH3
CH3
C
O
Li
+
C
OCH3
(7.55)
48.
7.3.3 Stereochemistry of anionic polymerizationA. Stereochemical of nondiene vinyl monomer
CH3
C
CH2
OH3C
C
O
_
C
O
Li
+
CH2
CH3
C
C
OCH3
OCH3
CH2
CH2
C
C
O
CO
2 CH
3
CH3
CO
C
2 CH
3
CH3
C
(7.56)
CH3
C
O
_
OCH3
Li
+
CH3
level of isotactic placement decreases –
level
of solvent
isotactic
placement
decreases –
as the
polarity
is increased
as
solvent is
polarity
is increased
orthe
as lithium
replaced
with the less strongly coordinating higher alkali metal ions.
or as lithium is replaced with the less strongly coordinating higher alkali metal ions.
49.
7.3.3 Stereochemistry of anionic polymerizationA. Stereochemical of nondiene vinyl monomer
Effect of solvent
R
R
O
R
R
C OR
H
RO
OR
C
C
O H
O
R
H
RO
Li
C OR H
C
O
O
Li
O
C
R
O
O
RO
(a)
(b)
SCHEME 7.1. (a) Isotactic approach of methyl methacrylate in a nonpolar solvent(b)
Syndiotactic approach of methyl methacrylate in tetrahydrofuran.(Circles represent
backbone or incipient backbone carbons: R=methyl. Backbone hydrogens omitted.)
50.
7.3.3 Stereochemistry of anionic polymerizationB. Stereochemical of Dienes
CH3
H2C
C
H
C
CH2
isoprene
H2C
CH
H
C
1,3-butadiene
catalyst, solvent의 영향
Li-based initiator/nonpolar solvents
cis-1,4 polymer의 생성이 증가
ex) Isoprene/BuLi/pentane or hexane
cis-1,4 polyisoprene
CH2
51.
7.3.3 Stereochemistry of anionic polymerizationformation of cis-polyisoprene – lithium’s ability
s-cis comformation by pi complexation – hold isoprene
CH2
CH3
CH2Li + CH2
C
CH
CH2Li
CH2
CH2
C
H
(7.57)
C
CH3
forming a six-membered ring transition state
– “lock” the isoprene into a cis-configuration
+
Li
- CH2
C C
H
CH3
CH2
+ CH2
CH
C
CH2
CH3
(7.58)
CH2
CH2
steric effect
CH2
H
CH3
CH2
H
C
C
C
CH3
Li
C
C
CH3
C
CH2
CH2
C
H
CH2
CH
CH3
(7.59)
CH2
52.
7.3.4 Anionic CopolymerizationComplicating factors of counterion.
① solvating polar of the solvent
Table 7.5
② temperature effect
③ electron transfer initiator 사용
free radical polymerization
Anionic polymerization
++
Li CHCH2CH2CHLi + Cl R Cl
X
X
competition
CHCH2CH2CH R
X
X
(7.60)
④ contrasts between homogeneous and heterogeneous
polymerization systems.
relatively few reactivity ratios
53.
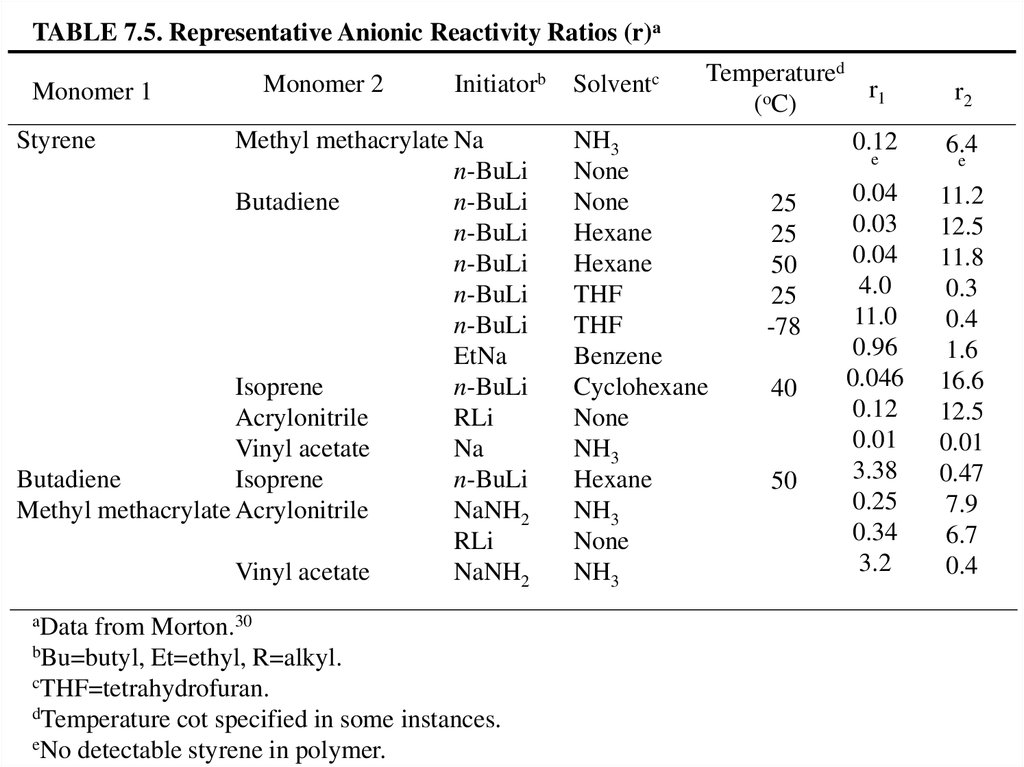
TABLE 7.5. Representative Anionic Reactivity Ratios (r)aMonomer 1
Monomer 2
Initiatorb
Styrene
Methyl methacrylate Na
n-BuLi
Butadiene
n-BuLi
n-BuLi
n-BuLi
n-BuLi
n-BuLi
EtNa
Isoprene
n-BuLi
Acrylonitrile
RLi
Vinyl acetate
Na
Butadiene
Isoprene
n-BuLi
Methyl methacrylate Acrylonitrile
NaNH2
RLi
Vinyl acetate
NaNH2
aData
from Morton.30
bBu=butyl, Et=ethyl, R=alkyl.
cTHF=tetrahydrofuran.
dTemperature cot specified in some instances.
eNo detectable styrene in polymer.
Solventc
Temperatured
r1
(oC)
0.12
6.4
0.04
0.03
0.04
4.0
11.0
0.96
0.046
0.12
0.01
3.38
0.25
0.34
3.2
11.2
12.5
11.8
0.3
0.4
1.6
16.6
12.5
0.01
0.47
7.9
6.7
0.4
NH3
None
None
Hexane
Hexane
THF
THF
Benzene
Cyclohexane
None
NH3
Hexane
NH3
None
NH3
e
25
25
50
25
-78
40
50
r2
e
54.
7.3.4 Anionic Copolymerizationformation of block copolymers by the living polymer method.
CH3
-
n CH2
CH
C6H5
R:
-
CH2CH:
CH2CH
C6H5
C6H5
n-1
(7.61)
CH3
CH2CH
C6H5 n
CCO2CH3
m CH2
CH2C
CH3
-
CH2C:
CO2CH3 m-1
CO2C
55.
7.3.4 Anionic CopolymerizationCommercial block copolymers
ABA triblock polymers – Greatest commercial success
ex) styrene-butadiene-styrene
B
initiator
- combination -
B:
-
:BB:
B
-
- S
:BBBBBBBBBB:
(7.62)
-
-
:SSSSSBBBBBBBBBBSSSSS:
star-block (radial)
– much lower melt viscosities, even at very high molecular weights
ex) silicon tetrachloride
4
-
: + SiCl4
Si
(7.63)
56.
7.4 Group Transfer Polymerization (GTP)(In the 1980s a new method for polymerizing acrylic-type monomers)
GTP의 특성
① Anionic polymerization에서 흔히 사용되는 monomer를 사용
Living polymer로 전환
② Propagating chain
Covalent character
③ Organosilicon이 개시제로 사용
R
C
C
R
OSiR3
(R
CH3
OR
+ CH2
C
CH2
RO2C
CO2CH3
CH3
C CH2C
C
C
OSiR3
OCH3
R
CH3)
(7.64)
CH3
n
R
-
HF2
R
CO2CH3
RO2C
CH3
C CH2C
CH3
CH2C C
OSiR3
living
polyme
r
Organosilicon에서 SiR3가 transfer되어
중합을 형성(GTP)
R
CO2CH3 n
OCH3
57.
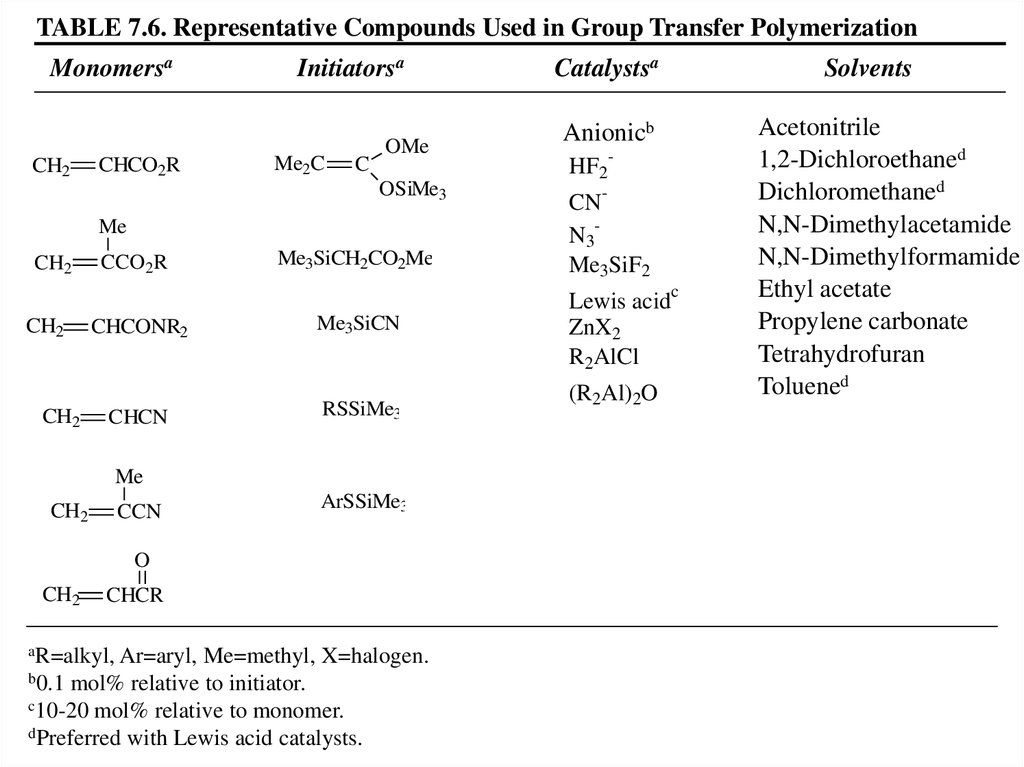
TABLE 7.6. Representative Compounds Used in Group Transfer PolymerizationMonomersa
CH2
CHCO2R
Initiatorsa
Me2C
C
OMe
OSiMe3
Me
CH2
CH2
CH2
CCO2R
Me3SiCH2CO2Me
CHCONR2
Me3SiCN
CHCN
RSSiMe3
Me
CH2
CCN
ArSSiMe3
O
CH2
CHCR
aR=alkyl,
Ar=aryl, Me=methyl, X=halogen.
b0.1 mol% relative to initiator.
c10-20 mol% relative to monomer.
dPreferred with Lewis acid catalysts.
Catalystsa
Solvents
Anionicb
HF2
-
CNN3
Me3SiF2
Lewis acid
ZnX2
R2AlCl
(R2Al)2O
c
Acetonitrile
1,2-Dichloroethaned
Dichloromethaned
N,N-Dimethylacetamide
N,N-Dimethylformamide
Ethyl acetate
Propylene carbonate
Tetrahydrofuran
Toluened
58.
7.4 Group Transfer Polymerization (GTP)* Synthesis of initiator
R2CHCO2R
R'2N-Li+
R2CCO2R
R2C
C
O
-
R3SiCl
CH2SSiMe3
+ CH2
CHCO2R
ZnI2
OSiR3
사슬의 양끝에서 성장
CO2R
CH2SSiMe3
C
OR
OR
두 개의 작용기를 갖는 개시제 사용
R2C
OSiMe3
CH2S
CH2CH CH2CH C OR
CH2S
CH2CH CH2CH C OR
CO2R
OSiMe3
(7.65)
59.
7.4 Group Transfer Polymerization (GTP)Speciality
① Once the monomer is consumed, a different monomer may be added
② chain can be terminated by removal of catalyst.
③ chain can be terminated by removal by protonation or alkylation.
CH3
CH3OH
CH3
CH2C C
CH2CH
OSiR3
CO2CH3
OCH3
CH3
C6H5CH2Br
CH2CCH2C6H5
CO2CH3
(7.66)
(7.67)
60.
7.4 Group Transfer Polymerization (GTP)GPT mecahanism
R
C
OR
C
R
Nu
OSiR3
-
-
R
C
C
R
O
CH2
C
CH3
R
R
C
C
CH2
OR
O
C
CH3
OSiR3
C
OCH3
Nu
OR
SiR3
O
C
OCH3
(7.68)
61.
7.4 Group Transfer Polymerization (GTP)Chain transfer of GPT
CH2
C C
CH3
-
O
OSiR3
OCH3
+
CH2
C
C
CH3
CH3O
CH2
CH2
O Si (R3) O
C C
CH3
(7.69)
C C
OCH3 CH3O
CH3





































 chemistry
chemistry








