Similar presentations:
Challenges and gaps in gene & cell therapy assessment and reimbursement pathway
1.
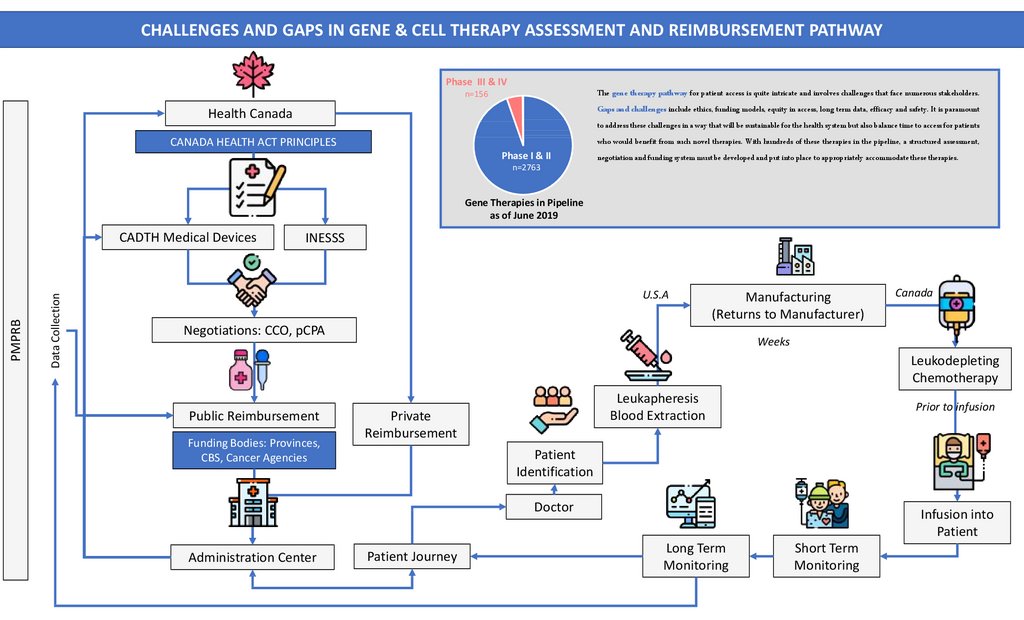
CHALLENGES AND GAPS IN GENE & CELL THERAPY ASSESSMENT AND REIMBURSEMENT PATHWAYPhase III & IV
n=156
Health Canada
CANADA HEALTH ACT PRINCIPLES
Phase I & II
n=2763
The gene therapy pathway for patient access is quite intricate and involves challenges that face numerous stakeholders.
Gaps and challenges include ethics, funding models, equity in access, long term data, efficacy and safety. It is paramount
to address these challenges in a way that will be sustainable for the health system but also balance time to access for patients
who would benefit from such novel therapies. With hundreds of these therapies in the pipeline, a structured assessment,
negotiation and funding system must be developed and put into place to appropriately accommodate these therapies.
Gene Therapies in Pipeline
as of June 2019
Data Collection
PMPRB
CADTH Medical Devices
INESSS
U.S.A
Manufacturing
(Returns to Manufacturer)
Negotiations: CCO, pCPA
Canada
Weeks
Leukodepleting
Chemotherapy
Public Reimbursement
Funding Bodies: Provinces,
CBS, Cancer Agencies
Leukapheresis
Blood Extraction
Private
Reimbursement
Prior to infusion
Patient
Identification
Doctor
Administration Center
Patient Journey
Infusion into
Patient
Long Term
Monitoring
Short Term
Monitoring
 medicine
medicine








