Similar presentations:
Class water
1. Water
You will:- explain the properties of water and its importance for living organisms
2. Properties of water
• Universal solvent• Surface tension
• Cohesion
• Adhesion
• High heat capacity
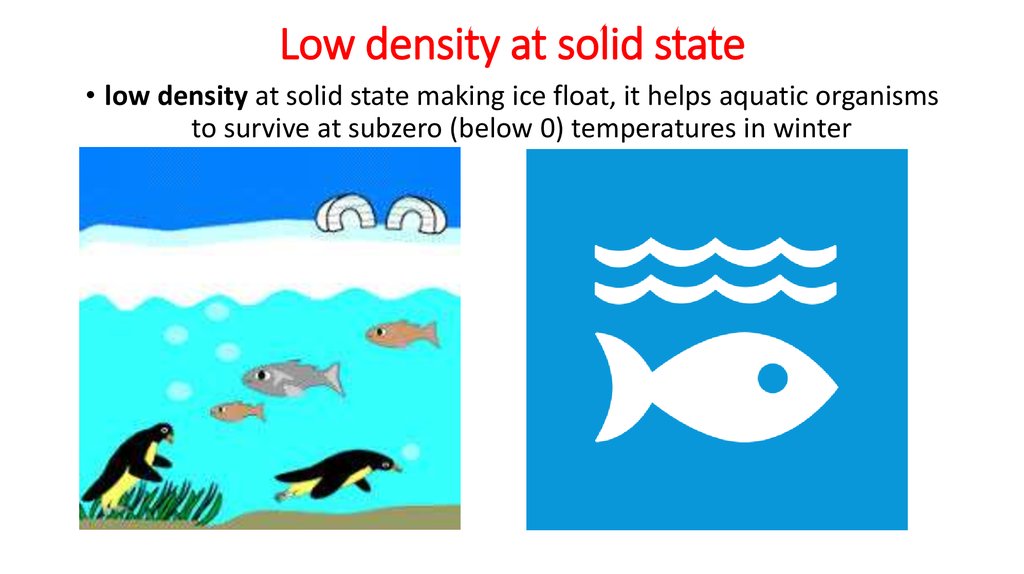
• Low density at solid state
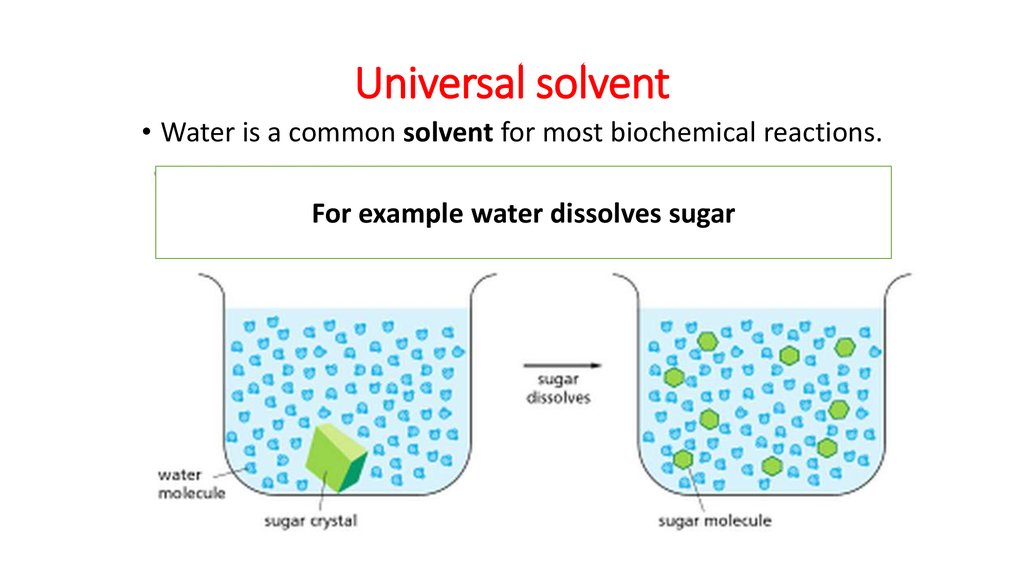
3. Universal solvent
• Water is a common solvent for most biochemical reactions.For example water dissolves sugar
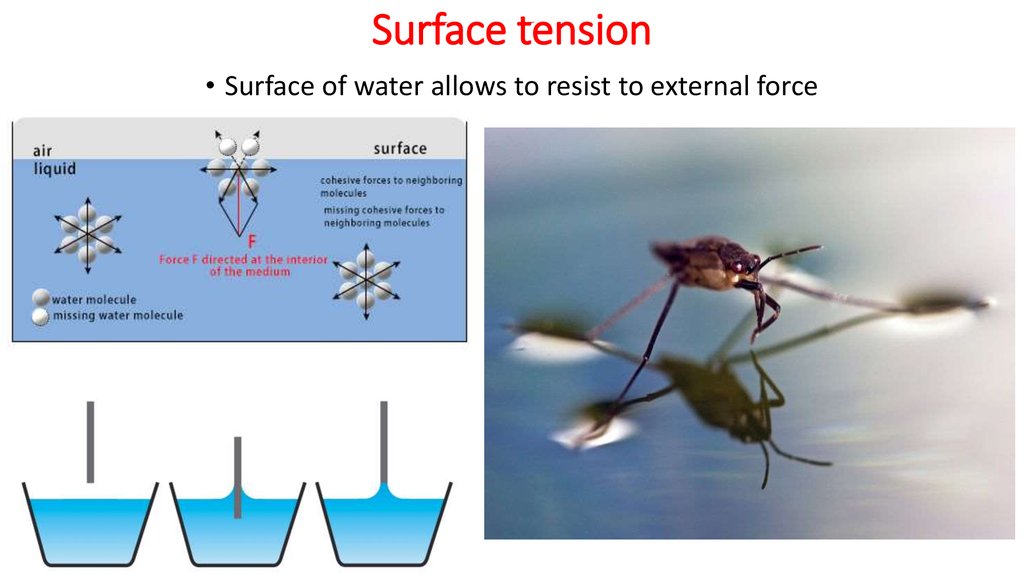
4. Surface tension
• Surface of water allows to resist to external force5. Cohesion
• sticking of water molecules to each other6. Adhesion
• sticking of water molecules to another (not water)molecules
7.
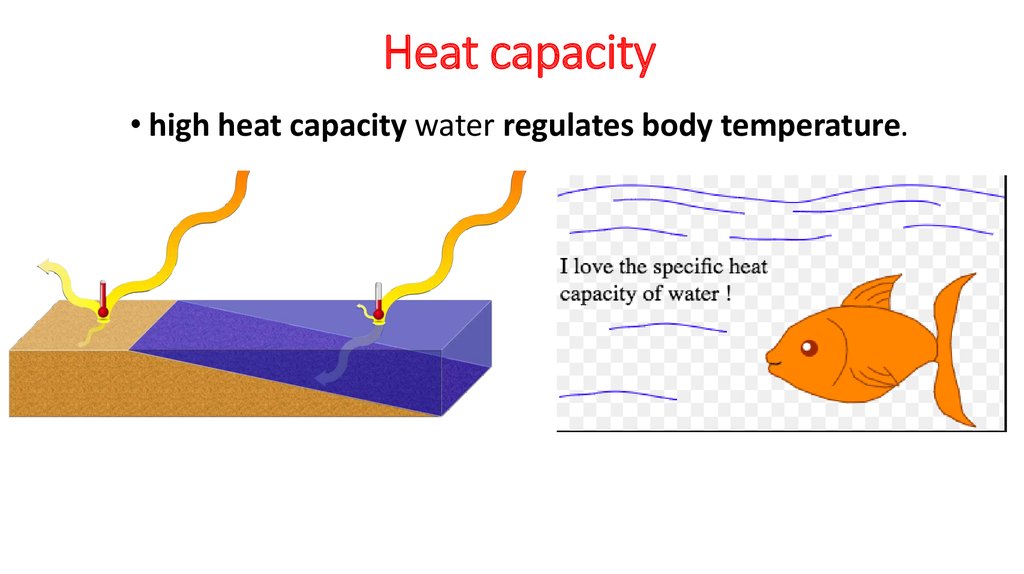
8. Heat capacity
• high heat capacity water regulates body temperature.9. Low density at solid state
• low density at solid state making ice float, it helps aquatic organismsto survive at subzero (below 0) temperatures in winter




 biology
biology








