Similar presentations:
Microbial Nutrition & Cultivation
1.
Microbial Nutrition &Cultivation
Copyright © McGraw-Hill Education. Permission required for reproduction or display.
1
2. Learning Objective
• List the essential nutrients of a bacterialcell.
• Differentiate between macronutrients and
micronutrients.
• List and define four different terms that
describe an organism’s sources of carbon
and energy.
• Compare and contrast the processes of
diffusion and osmosis, active transport.
3. Microbial Nutrition
• Essential nutrient: any substance that mustbe provided to an organism
• Macronutrients: required in relatively large
quantities and play principal roles in cell
structure and metabolism
- Carbon, hydrogen, and oxygen
• Micronutrients: also known as trace
elements
- Present in much smaller amounts and are
involved in enzyme function and maintenance of
protein structure
- Examples: manganese, zinc, nickel
4. Microbial Nutrition
• Inorganic nutrient- An atom or simple molecule that contains a
combination of atoms other than carbon and
hydrogen
- Found in the crust of the earth, bodies of water,
and the atmosphere
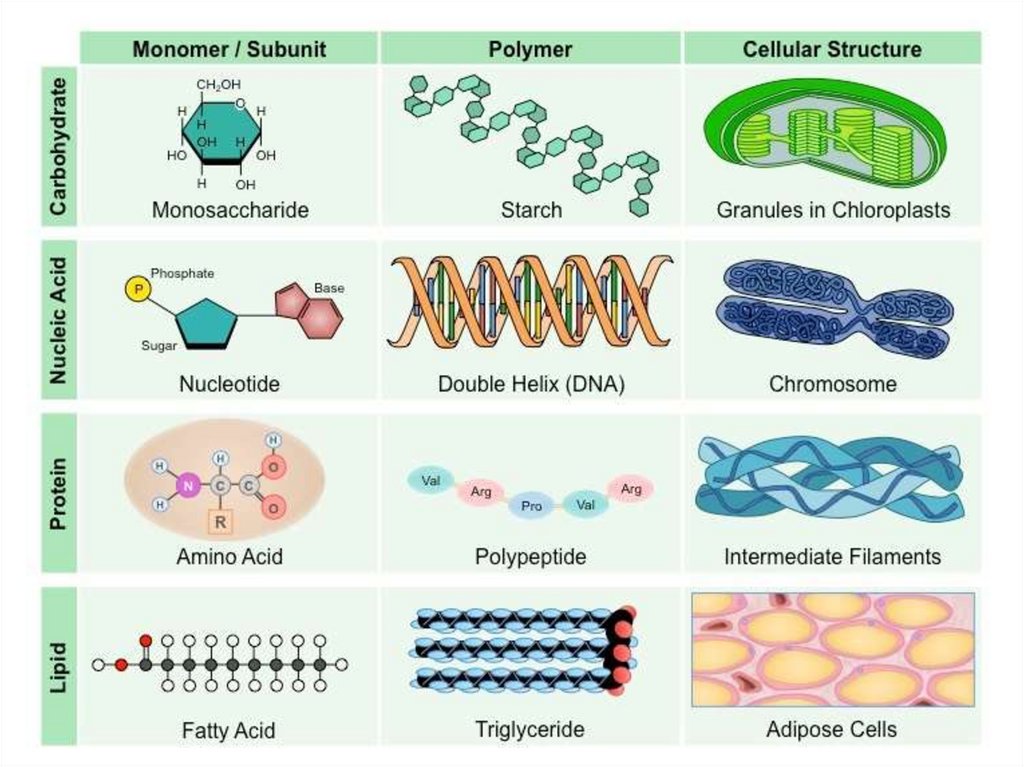
• Organic nutrients
- Contain carbon and hydrogen atoms and are the
products of living things
- Simple organic molecules such as methane
- Large polymers (carbohydrates, lipids, proteins,
nucleic acids)
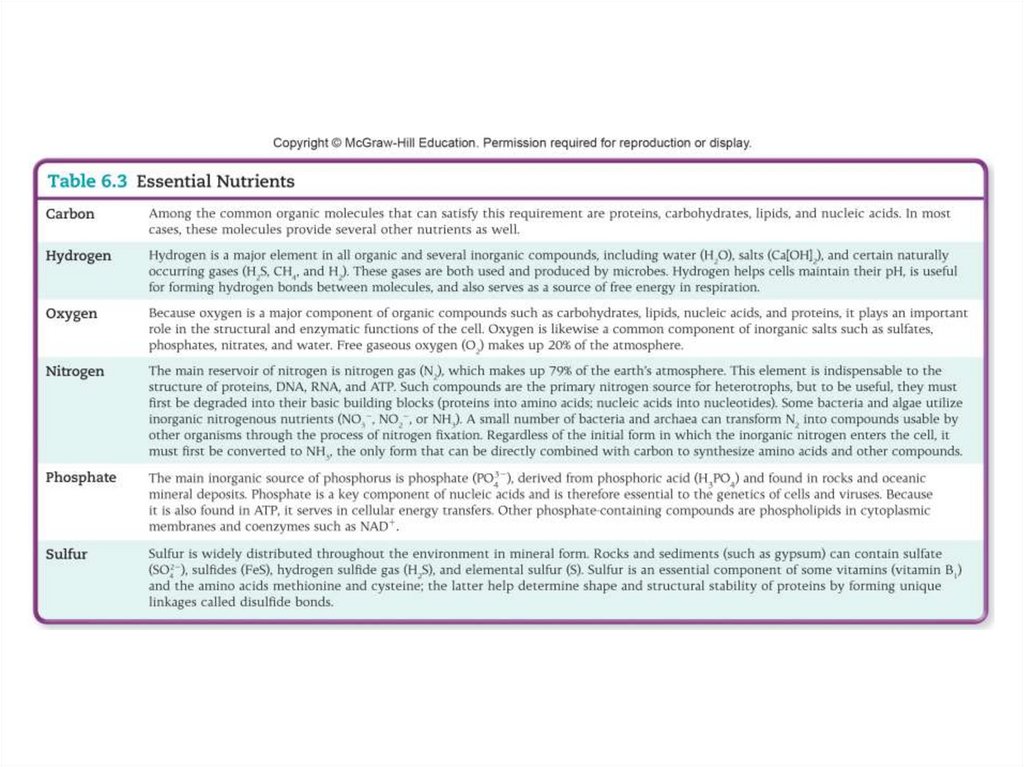
5. Chemical Analysis of the Microbial Cytoplasm
• Water – 70% of all components• Proteins
• Organic compounds – 97% of dry cell weight
• Elements CHONPS – 96% of dry cell weight
• Most chemical elements available to the cell
as compounds and not as pure elements
• Only a few types of nutrients needed to
synthesize over 5,000 different compounds
6.
7.
8.
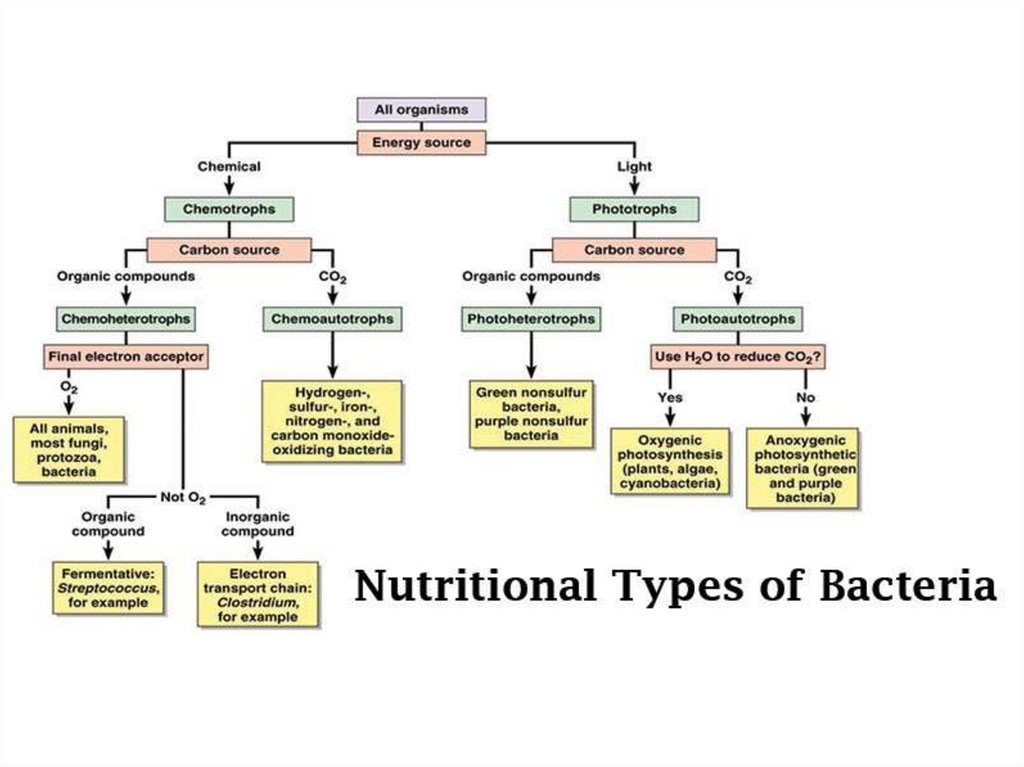
9. What Microbes Eat
• Heterotroph: an organism that must obtainits carbon in an organic form
• Autotroph: an organism that uses inorganic
CO2 as its carbon source
- Has the capacity to convert CO2 into organic
compounds
- Not nutritionally dependent on other living
things
• Phototroph: microbes that photosynthesize
• Chemotroph: microbes that gain energy from
chemical compounds
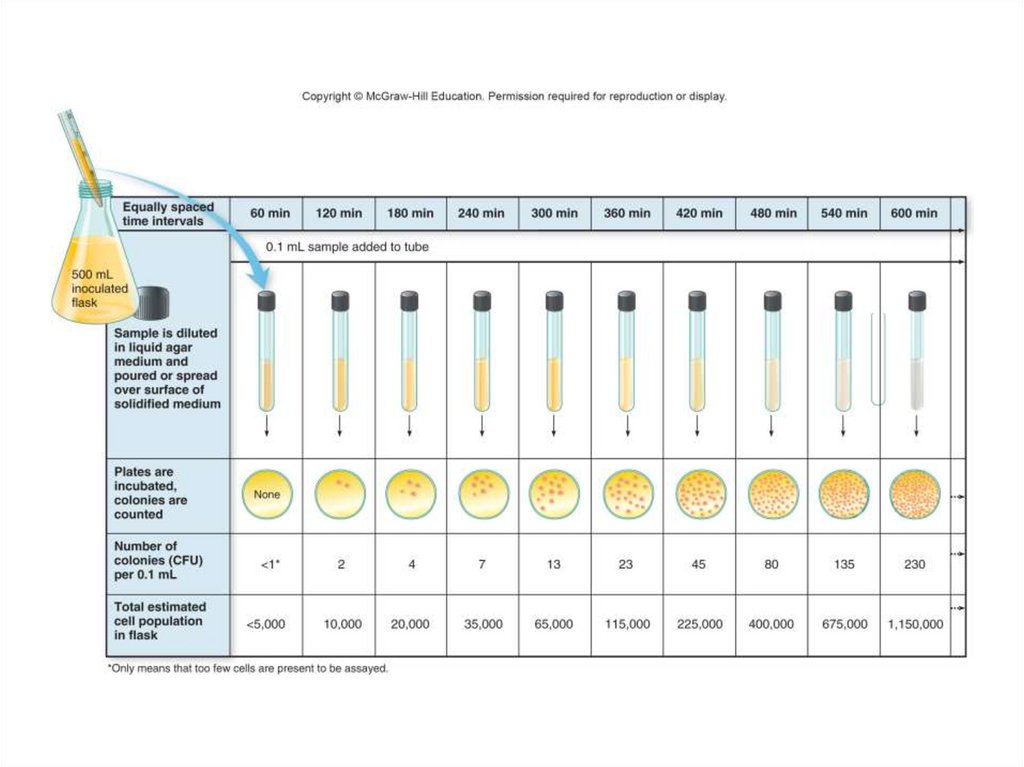
10.
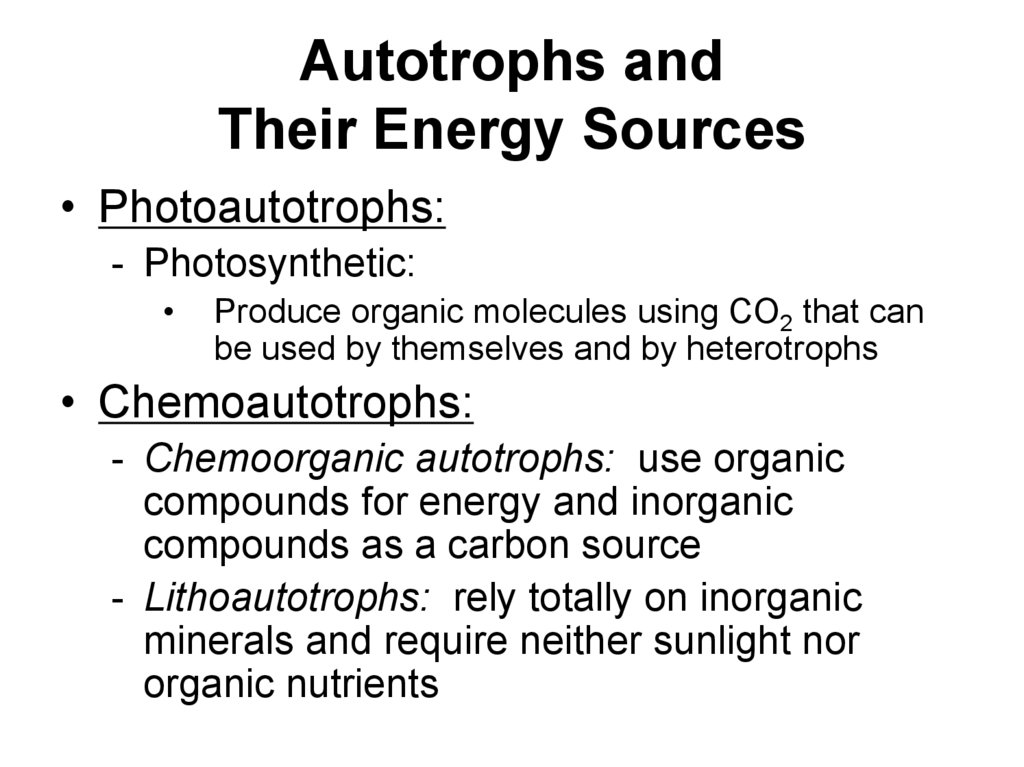
11. Autotrophs and Their Energy Sources
• Photoautotrophs:- Photosynthetic:
Produce organic molecules using CO2 that can
be used by themselves and by heterotrophs
• Chemoautotrophs:
- Chemoorganic autotrophs: use organic
compounds for energy and inorganic
compounds as a carbon source
- Lithoautotrophs: rely totally on inorganic
minerals and require neither sunlight nor
organic nutrients
12. Oxygenic Photosynthesis
Anoxygenic PhotosynthesisOccurs in green sulfur and nonsulfur
bacteria, purple bacteria,
heliobacteria, and acidobacteria.
13.
https://www.youtube.com/watch?v=eT1zF1-srag14. Heterotrophs and Their Energy Sources
• Chemoheterotrophs:- Derive both carbon and energy from organic
compounds
- Process these molecules through respiration or
fermentation
• Saprobes:
- Free-living organisms that feed on organic
detritus from dead organisms
- Decomposers of plant litter, animal matter, and
dead microbes
- Recycle organic nutrients
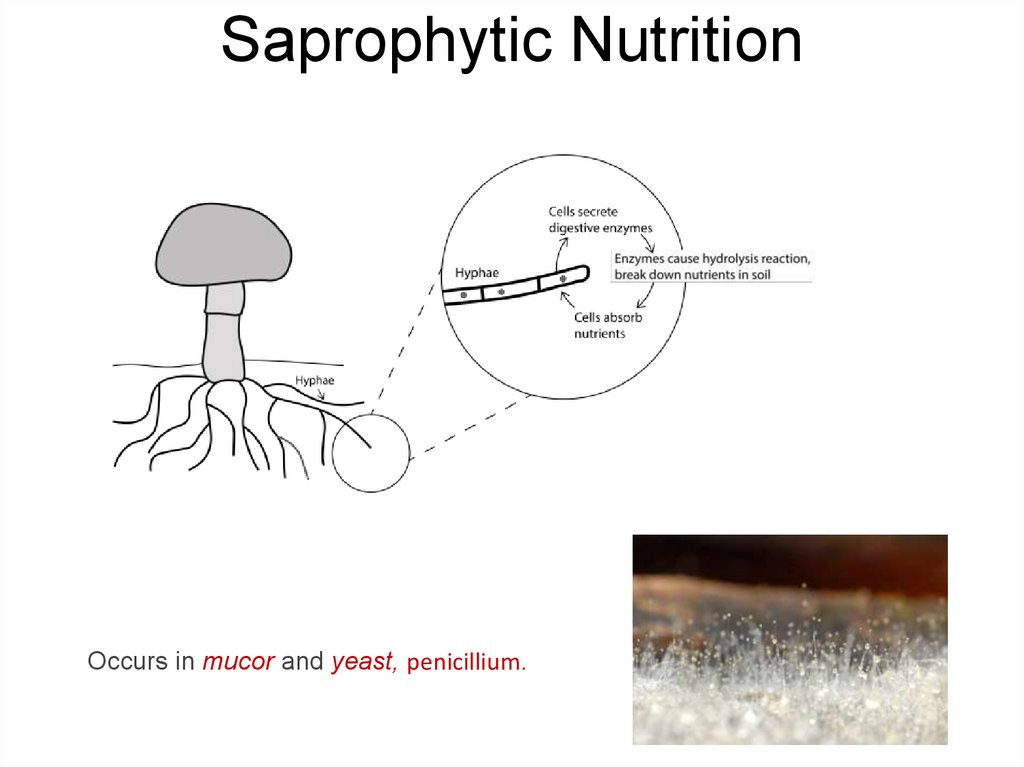
15. Saprophytic Nutrition
Occurs in mucor and yeast, penicillium.16. Heterotrophs and Their Energy Sources
• Parasites:- Derive nutrients from the cells or tissues of a
living host
- Pathogens: cause damage to tissues or even
death
- Range from viruses to helminths
- Ectoparasites: live on the body
- Endoparasites: live in the organs and tissues
- Intracellular parasites: live within cells such as
the leprosy bacillus and the syphilis spirochete
- Obligate parasites: unable to grow outside of a
living host
17.
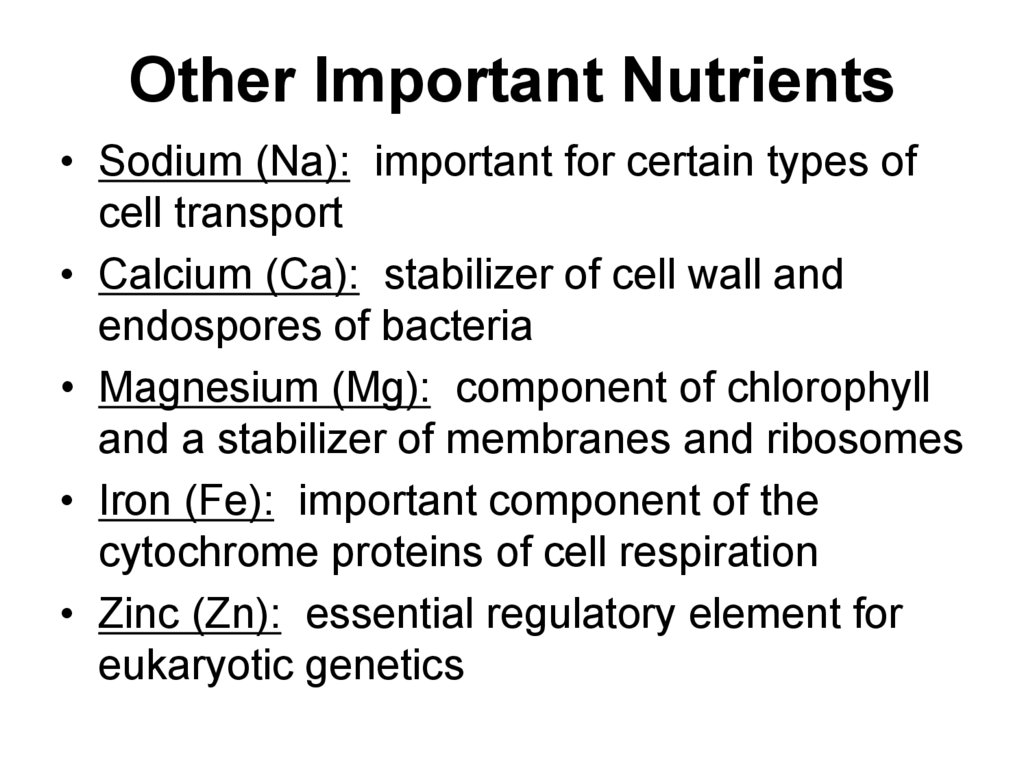
18. Other Important Nutrients
• Sodium (Na): important for certain types ofcell transport
• Calcium (Ca): stabilizer of cell wall and
endospores of bacteria
• Magnesium (Mg): component of chlorophyll
and a stabilizer of membranes and ribosomes
• Iron (Fe): important component of the
cytochrome proteins of cell respiration
• Zinc (Zn): essential regulatory element for
eukaryotic genetics
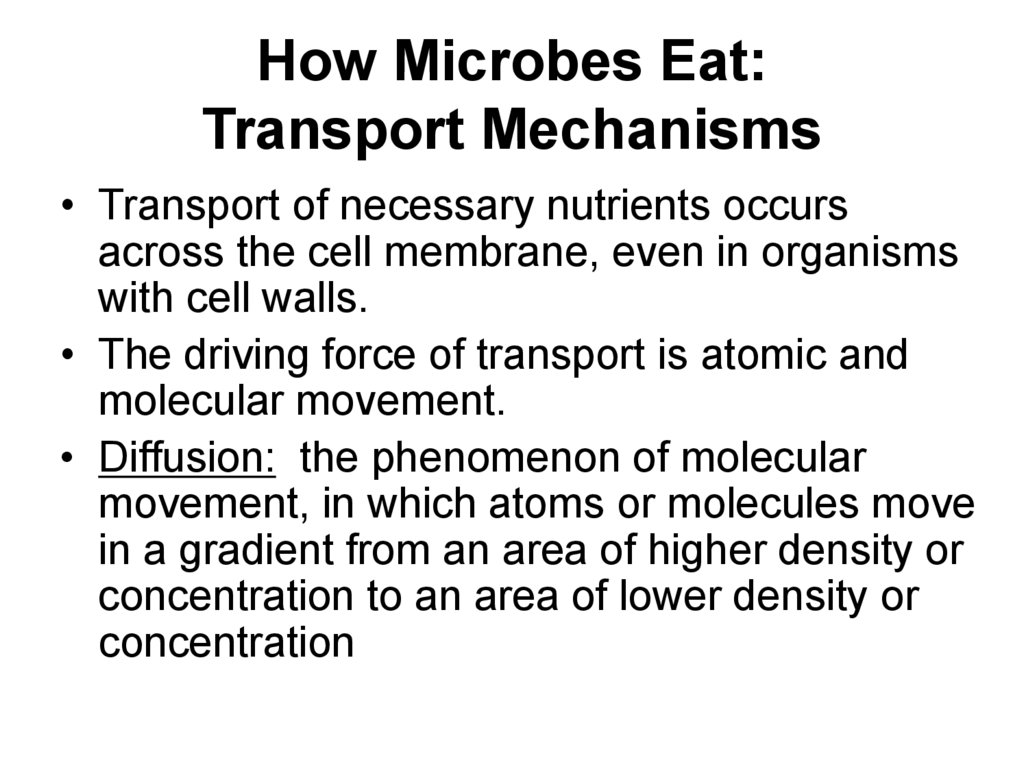
19. How Microbes Eat: Transport Mechanisms
• Transport of necessary nutrients occursacross the cell membrane, even in organisms
with cell walls.
• The driving force of transport is atomic and
molecular movement.
• Diffusion: the phenomenon of molecular
movement, in which atoms or molecules move
in a gradient from an area of higher density or
concentration to an area of lower density or
concentration
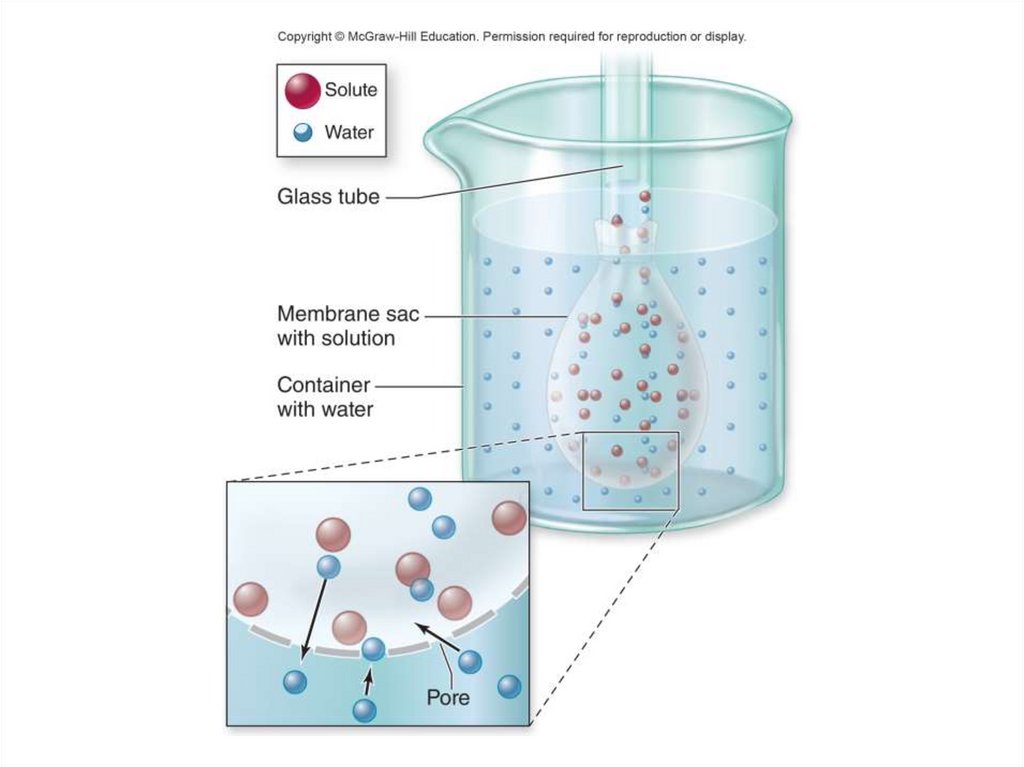
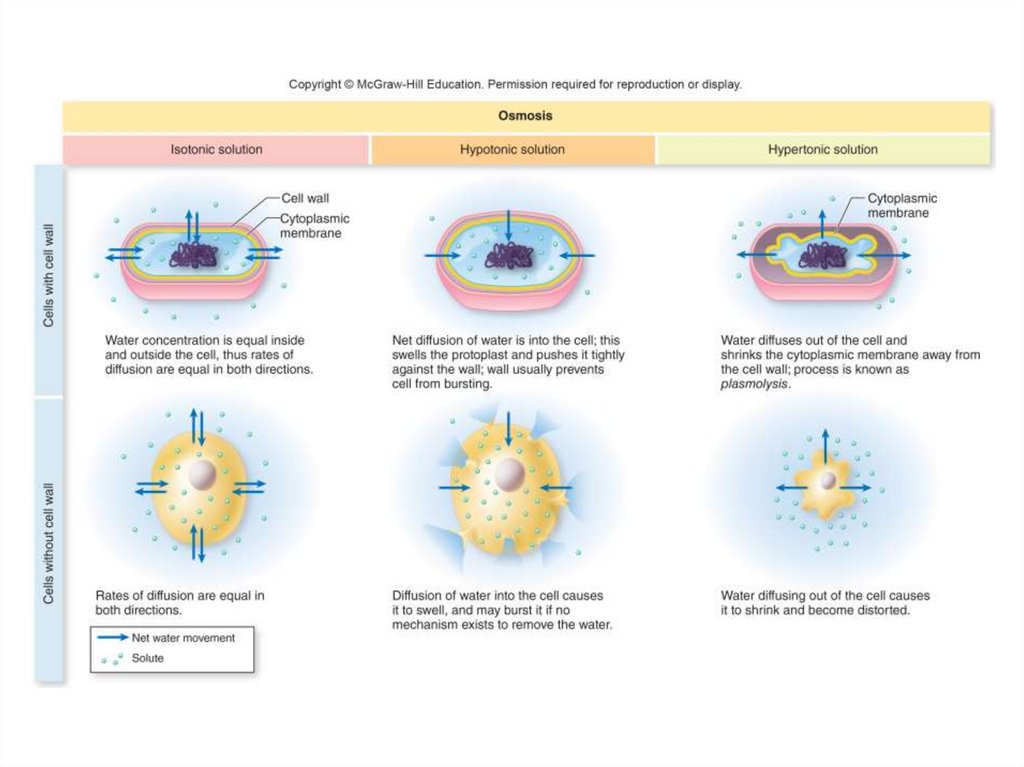
20. The Movement of Water: Osmosis
• Osmosis: the diffusion of water through aselectively, or differentially, permeable
membrane
- Has passageways that allow free diffusion of
water, but block certain other dissolved molecules
- When the membrane is placed between solutions
of differing concentrations of solute and the solute
cannot pass through the membrane, water will
diffuse at a faster rate from the side that has
more water to the side that has less water.
- This will continue until the concentration of water
is equalized on both sides of the membrane.
21.
22.
23.
24. Active Transport
• Active transport:- The transport of nutrients against the diffusion
gradient or in the same direction as the
natural gradient, but at a rate faster than by
diffusion alone
- The presence of specific membrane proteins
(permeases and pumps)
- The expenditure of energy
• Examples of substances transported
actively: monosaccharides, amino acids,
organic acids, phosphates, and metal ions
25. Endocytosis: Eating and Drinking By Cells
• Endocytosis:- Cell encloses the substance in its membrane
- Simultaneously forms a vacuole and engulfs the
substance
• Phagocytosis:
- Accomplished by amoebas and white blood cells
- Ingest whole cells or large solid matter
• Pinocytosis:
– Ingestion of liquids such as oils or molecules in
solution
26. Cellular transport mechanisms
KNOW WELLCellular transport mechanisms
No large, charged
or hydrophilic
Larger molecules,
charged ions and
polar molecules.
27.
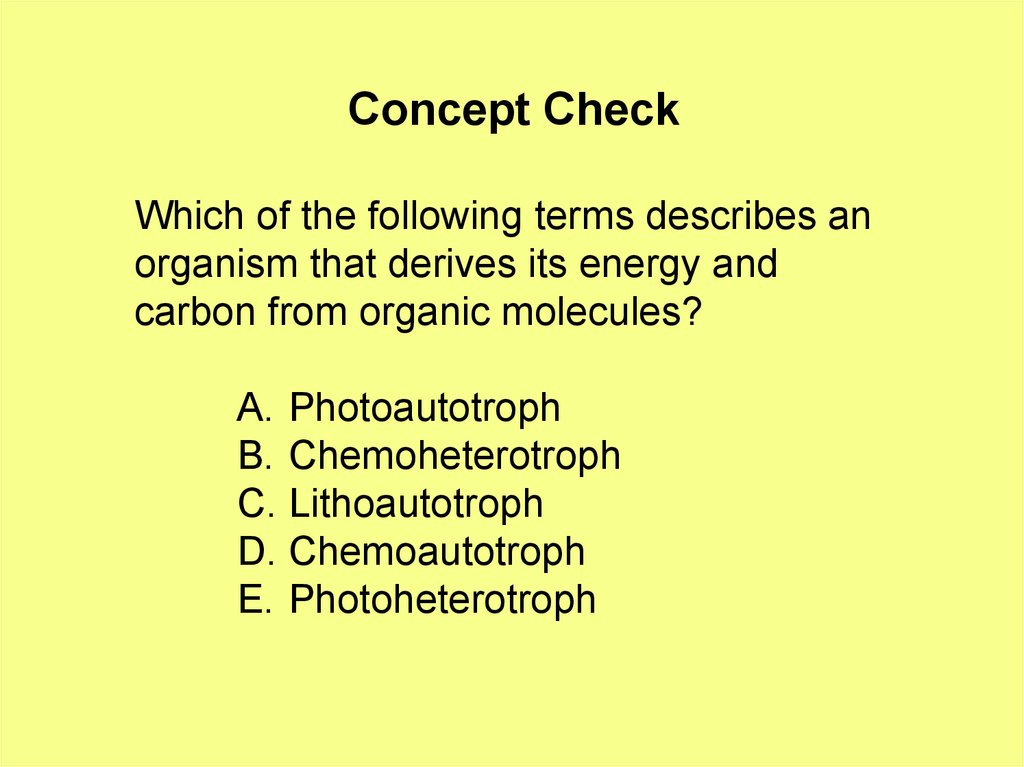
Concept CheckWhich of the following terms describes an
organism that derives its energy and
carbon from organic molecules?
A. Photoautotroph
B. Chemoheterotroph
C. Lithoautotroph
D. Chemoautotroph
E. Photoheterotroph
28. Learning Objectives
• List and define five terms used to expressa microbe’s optimal growth temperature.
• Summarize three ways in which
microorganisms function in the presence
of differing oxygen conditions.
• Identify three important environmental
factors (other than temperature and
oxygen) with which microorganisms must
cope.
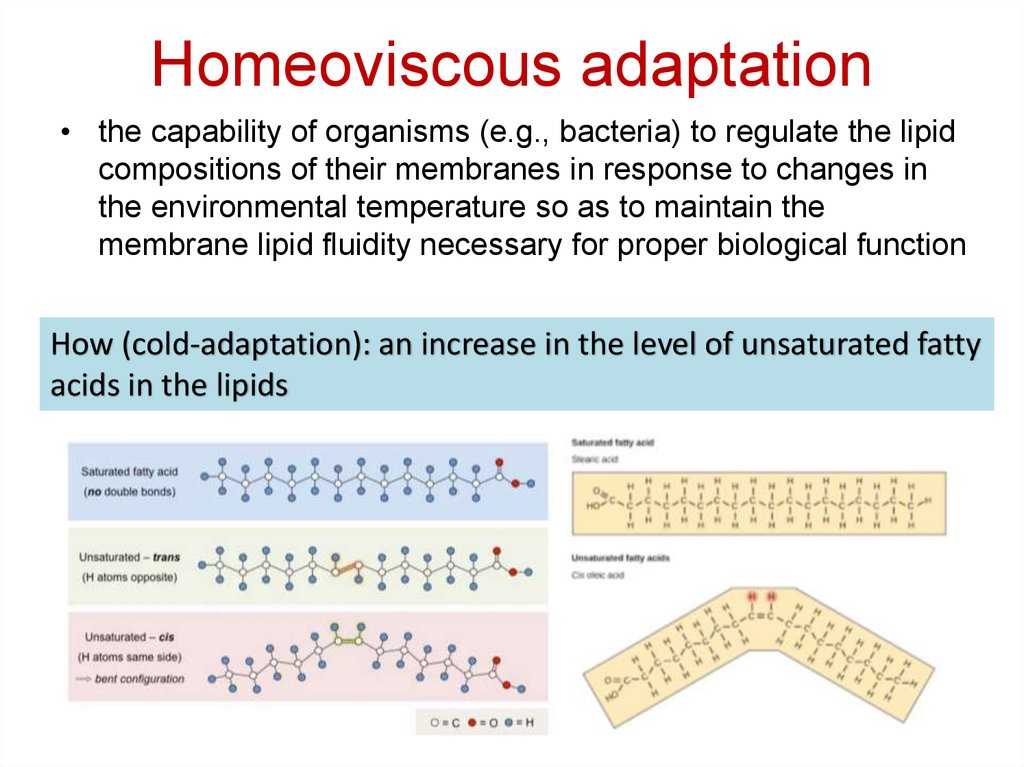
29. Homeoviscous adaptation
• the capability of organisms (e.g., bacteria) to regulate the lipidcompositions of their membranes in response to changes in
the environmental temperature so as to maintain the
membrane lipid fluidity necessary for proper biological function
How (cold-adaptation): an increase in the level of unsaturated fatty
acids in the lipids
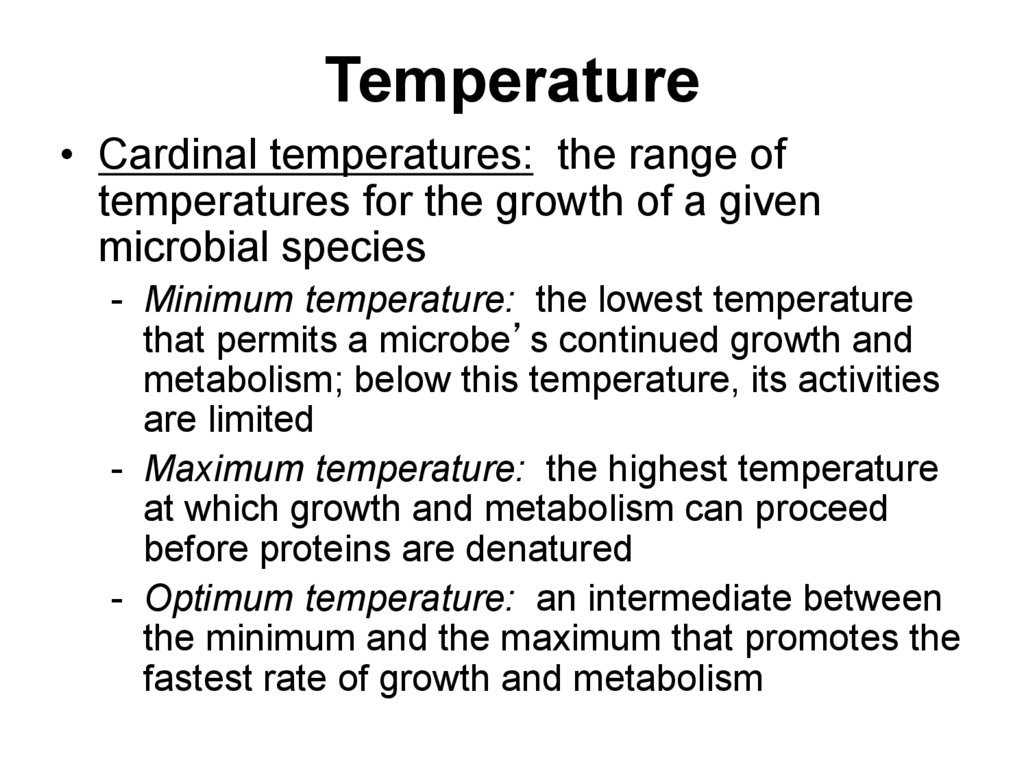
30. Temperature
• Cardinal temperatures: the range oftemperatures for the growth of a given
microbial species
- Minimum temperature: the lowest temperature
that permits a microbe’s continued growth and
metabolism; below this temperature, its activities
are limited
- Maximum temperature: the highest temperature
at which growth and metabolism can proceed
before proteins are denatured
- Optimum temperature: an intermediate between
the minimum and the maximum that promotes the
fastest rate of growth and metabolism
31. Temperature
• Psychrophiles:- Optimum temperature below 15°C
- Capable of growth at 0°C
- Obligate with respect to cold and cannot grow
above 20°C
- Storage at refrigerator temperature incubates
rather than inhibits them
- Natural habitats of psychrophilic bacteria,
fungi, and algae are lakes, rivers, snowfields,
polar ice, and the deep ocean.
- Rarely pathogenic
32. Temperature
• Psychrotrophs:- Grow slowly in the cold but have an
optimum temperature between 15°C
and 30°C
- Staphylococcus aureus and Listeria
monocytogenes are able to grow at
refrigerator temperatures and cause
food-borne disease.
33. Temperature
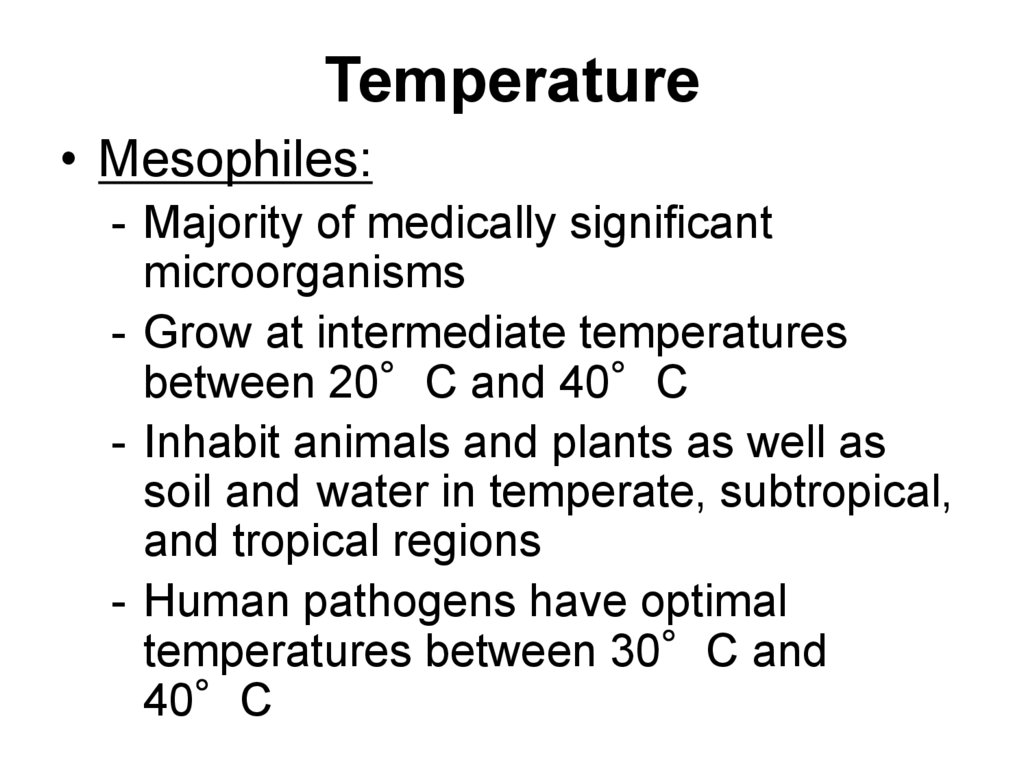
• Mesophiles:- Majority of medically significant
microorganisms
- Grow at intermediate temperatures
between 20°C and 40°C
- Inhabit animals and plants as well as
soil and water in temperate, subtropical,
and tropical regions
- Human pathogens have optimal
temperatures between 30°C and
40°C
34. Temperature
• Thermoduric:- Can survive short exposure to high
temperatures but are normally
mesophiles
- Common contaminants of heated or
pasteurized foods
- Examples are heat-resistant cysts such
as Giardia and sporeformers such as
Bacillus and Clostridium.
35. Temperature
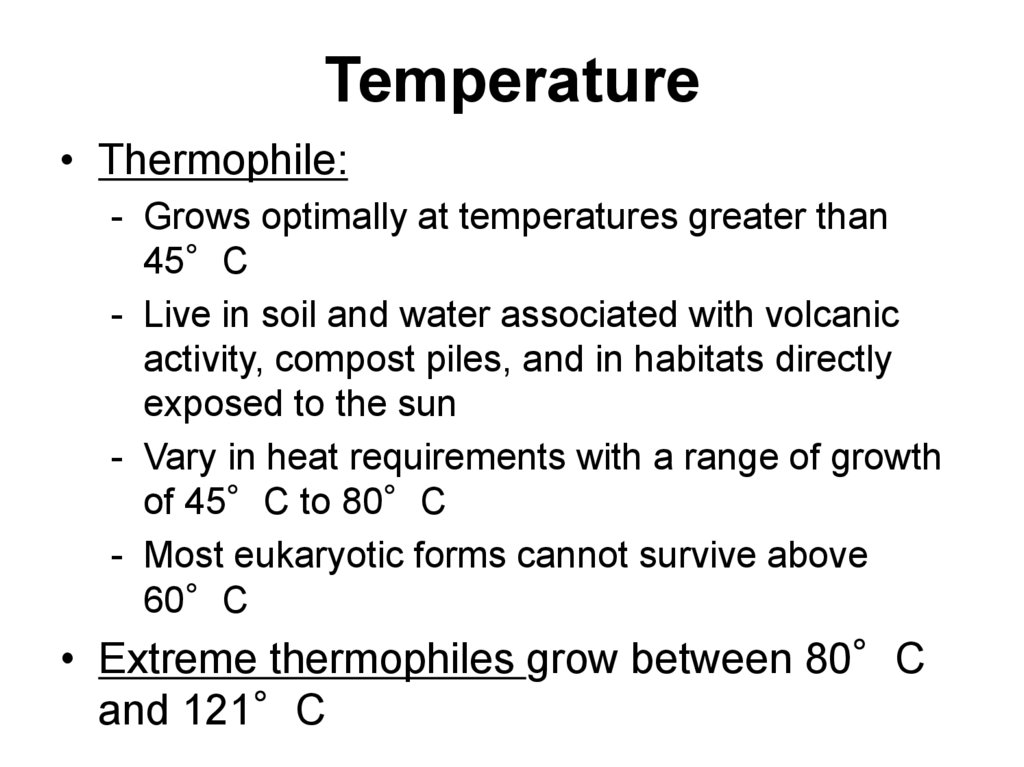
• Thermophile:- Grows optimally at temperatures greater than
45°C
- Live in soil and water associated with volcanic
activity, compost piles, and in habitats directly
exposed to the sun
- Vary in heat requirements with a range of growth
of 45°C to 80°C
- Most eukaryotic forms cannot survive above
60°C
• Extreme thermophiles grow between 80°C
and 121°C
36.
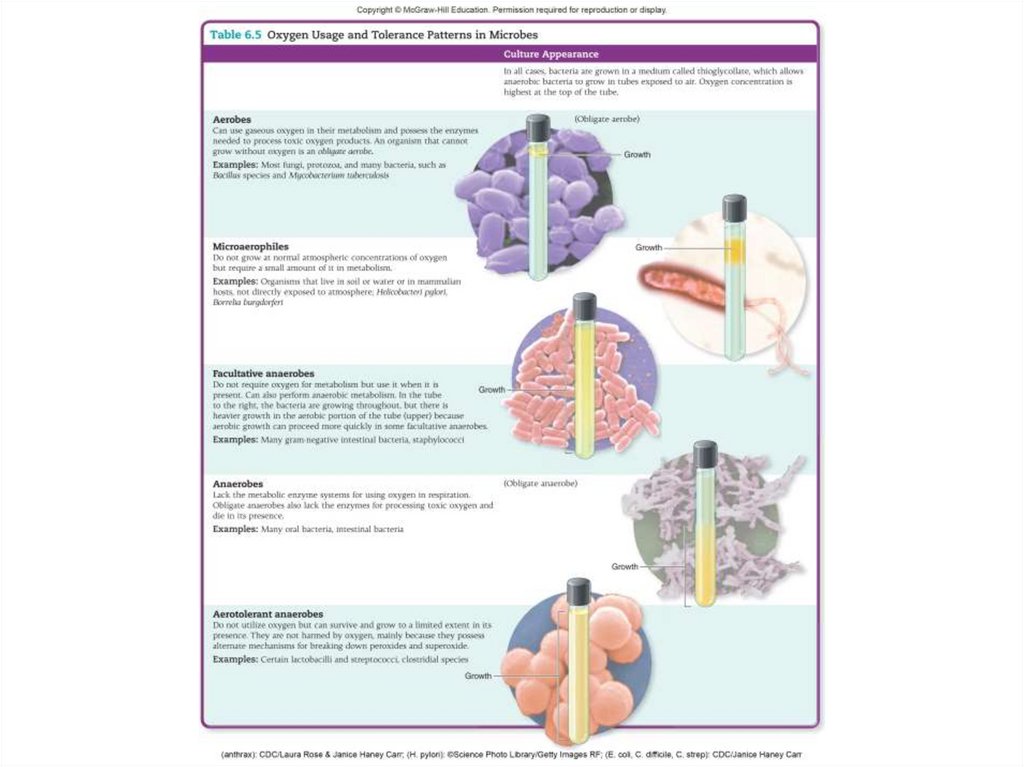
37. Gases
• The atmospheric gases that influencemicrobial growth are O2 and CO2
- O2 has the greatest impact on microbial growth.
- O2 is an important respiratory gas and a
powerful oxidizing agent.
• Microbes fall into one of three categories:
- Those that use oxygen and detoxify it.
- Those that can neither use oxygen nor detoxify
it.
- Those that do not use oxygen but can detoxify it.
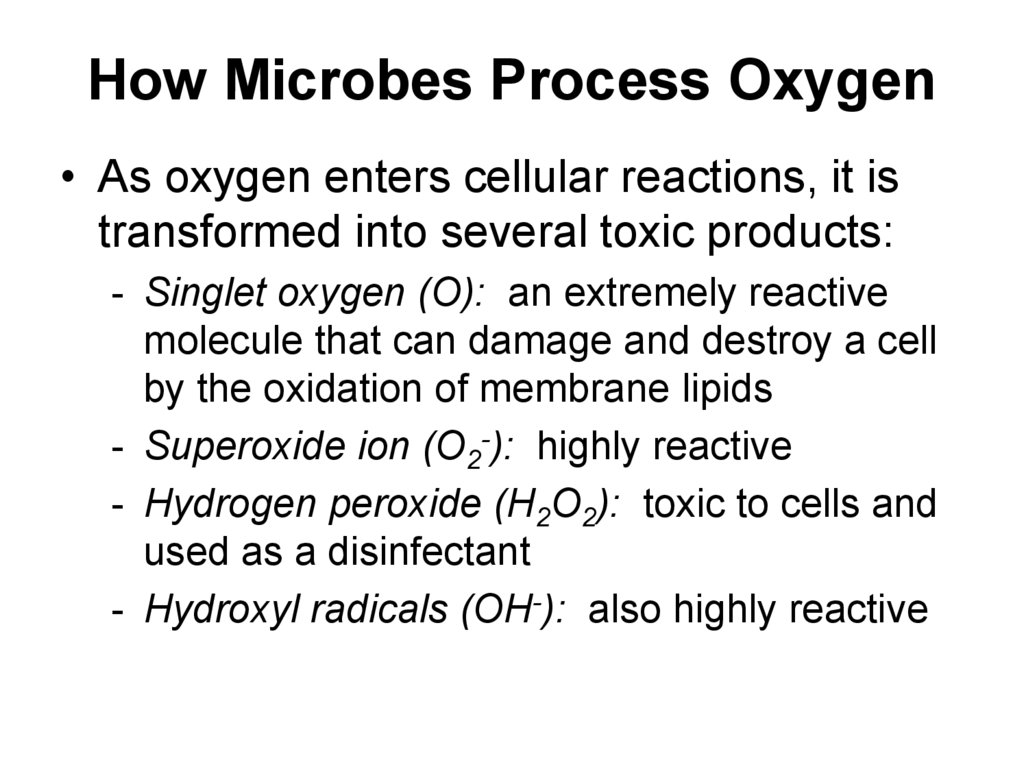
38. How Microbes Process Oxygen
• As oxygen enters cellular reactions, it istransformed into several toxic products:
- Singlet oxygen (O): an extremely reactive
molecule that can damage and destroy a cell
by the oxidation of membrane lipids
- Superoxide ion (O2-): highly reactive
- Hydrogen peroxide (H2O2): toxic to cells and
used as a disinfectant
- Hydroxyl radicals (OH-): also highly reactive
39. How Microbes Process Oxygen
• Most cells have developed enzymes that scavengeand neutralize reactive oxygen byproducts.
• Two-step process requires two enzymes:
• Superoxide ion is converted into hydrogen peroxide by
superoxide dismutase.
• Hydrogen peroxide is converted into harmless water and
oxygen by catalase.
40.
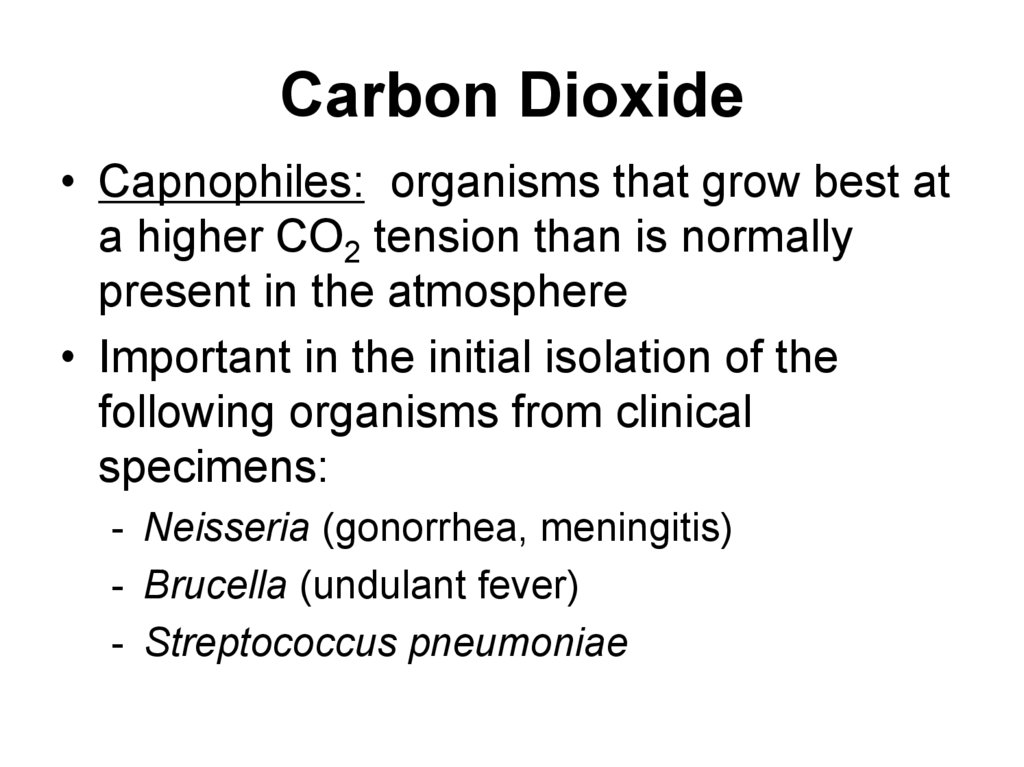
41. Carbon Dioxide
• Capnophiles: organisms that grow best ata higher CO2 tension than is normally
present in the atmosphere
• Important in the initial isolation of the
following organisms from clinical
specimens:
- Neisseria (gonorrhea, meningitis)
- Brucella (undulant fever)
- Streptococcus pneumoniae
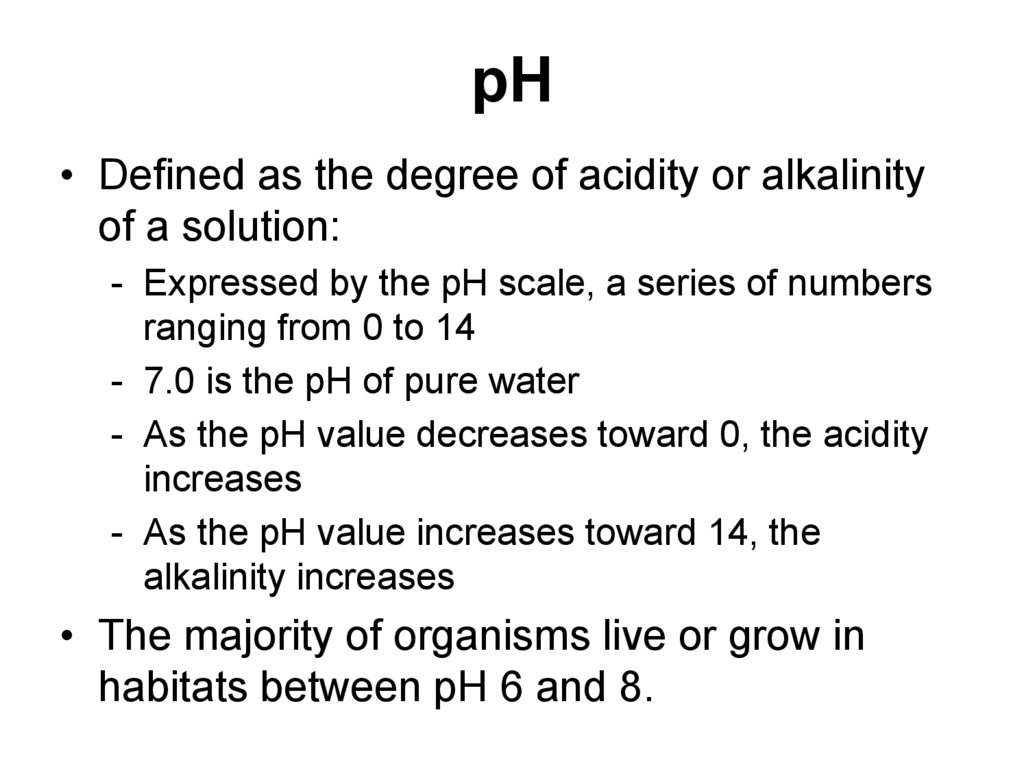
42. pH
• Defined as the degree of acidity or alkalinityof a solution:
- Expressed by the pH scale, a series of numbers
ranging from 0 to 14
- 7.0 is the pH of pure water
- As the pH value decreases toward 0, the acidity
increases
- As the pH value increases toward 14, the
alkalinity increases
• The majority of organisms live or grow in
habitats between pH 6 and 8.
43. pH
• Acidophiles: organisms that thrive in acidicenvironments
- Euglena mutabilis: grows in acid pools between pH 0 and 1
- Thermoplasma: lives in coal piles at a pH of 1 or 2
- Picrophilus: thrives at a pH of 7, but can live at a pH of 0
- Many molds and yeasts tolerate acid and are the primary
spoilage agents of pickled foods
• Alkalinophiles: organisms that thrive in alkaline
conditions
- Natromonas: live in hot pools and soils at pH 12
- Proteus: can create alkaline conditions to neutralize urine
and colonize and infect the urinary system
44. Osmotic Pressure
• Osmophiles: live in habitats with high soluteconcentration
• Halophiles: prefer high concentration of salt
- Obligate halophiles: Halobacterium and Halococcus
grow optimally at solutions of 25% NaCl but require at
least 9% NaCl.
- Facultative halophiles: remarkably resistant to salt,
even though they do not normally reside in high salt
environments
- Staphylococcus aureus can grow on NaCl media
ranging from 0.1% to 20%.
45. Radiation
• Phototrophs use visible light rays as an energysource.
• Nonphotosynthetic microbes tend to be
damaged by the toxic oxygen products produced
by contact with light.
• Some microbial species produce yellow
carotenoid pigments to absorb and dismantle
toxic oxygen.
• Ultraviolet and ionizing radiation can be used in
microbial control.
46. Pressure
• Barophiles:– Exist under pressures that range from a
few times to over 1,000 times the
pressure of the atmosphere
- These bacteria are so strictly adapted to
high pressures that they will rupture
when exposed to normal atmospheric
pressure.
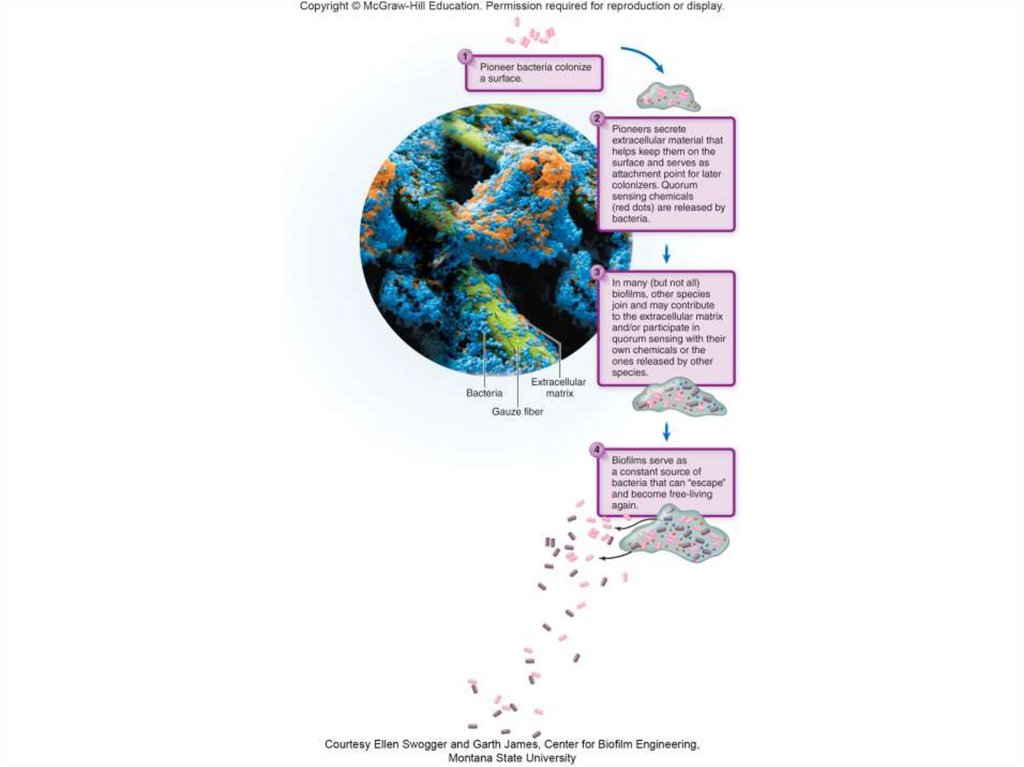
47. Biofilms: The Epitome of Synergy
• Mixed communities of bacteria and othermicrobes that are attached to a surface and
each other.
• Formation of a biofilm:
– A “pioneer” colonizer initially attaches to a surface.
– Other microbes then attach to those bacteria or a
polymeric sugar or protein substance secreted by
the microbial colonizers.
– Attached cells are stimulated to release chemicals
as the cell population grows.
48. Biofilms: The Epitome of Synergy
• Quorum sensing: used by bacteria to interactwith members of the same species as well as
members of other species that are close by
• Structure of the biofilm:
- Large, complex communities form with different
physical and biological characteristics.
- The bottom may have very different pH and oxygen
conditions than the surface.
- Partnership among multiple microbial inhabitants
- Cannot be eradicated by traditional methods
49. Biofilms: The Epitome of Synergy (cont’d)
• Bacteria in biofilms behave and respondvery differently than planktonic (free-living)
bacteria:
- Different genes are activated
- Behave and respond very differently to their
environments
50.
51.
Concept CheckWhich of the following describes an
association between microbes in which one
organism is benefitted and one is harmed in
some way?
A. Mutualism
B. Synergism
C. Commensalism
D. Parasitism
E. Antagonism
52. Learning Outcomes
• Summarize the steps of cell division usedby most bacteria.
• Define doubling time, and describe how it
leads to exponential growth.
• Compare and contrast the four phases of
growth in a bacterial growth curve.
• Identify one quantitative and one
qualitative method used for analyzing
bacterial growth.
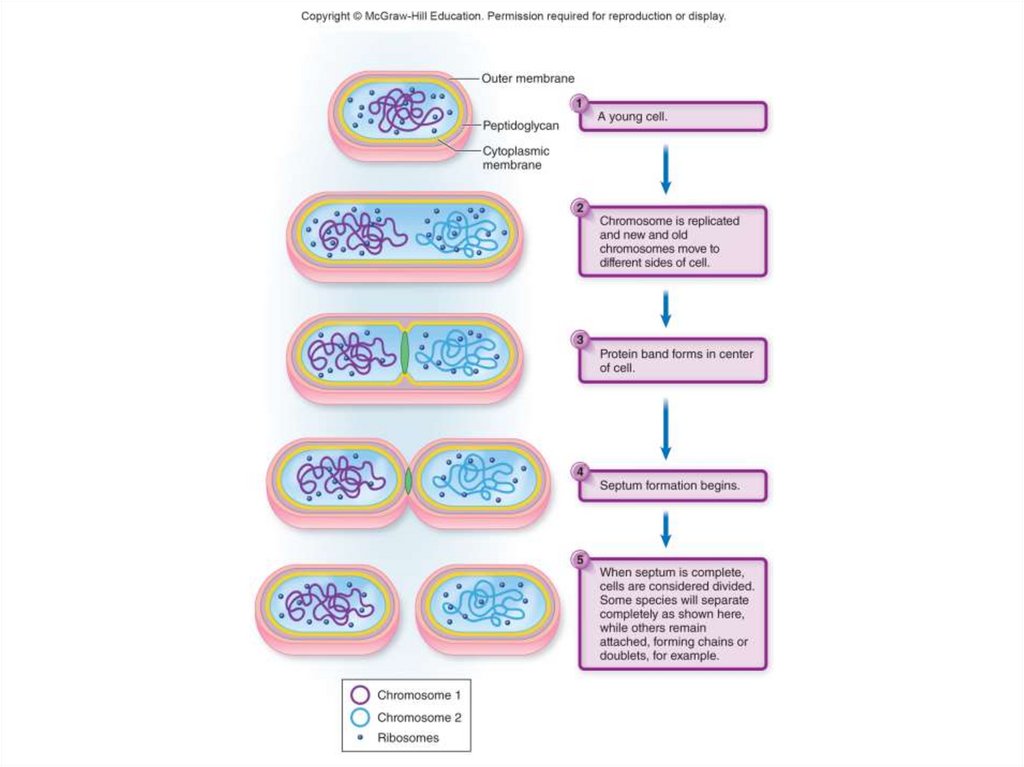
53. The Study of Bacterial Growth
• Binary fission- One cell becomes two
- Parent cell enlarges
- Duplicates its chromosome
- Starts to pull its cell envelope together
to the center of the cell
- Cell wall eventually forms a complete
central septum
54.
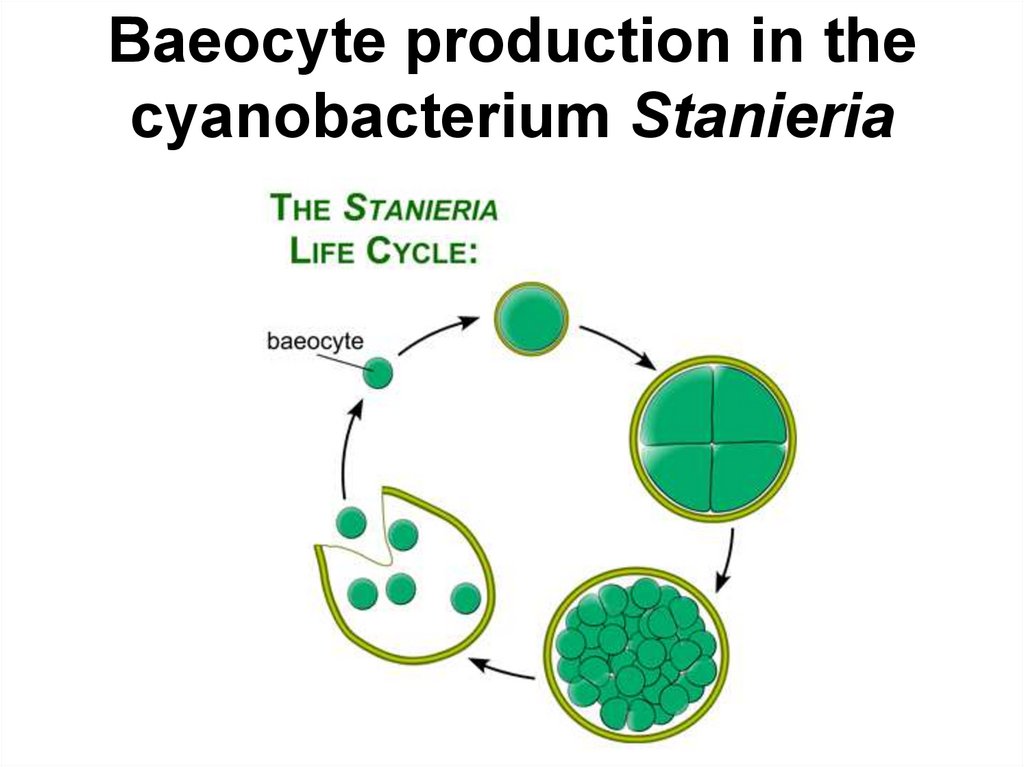
55. Baeocyte production in the cyanobacterium Stanieria
Baeocyte production in thecyanobacterium Stanieria
56.
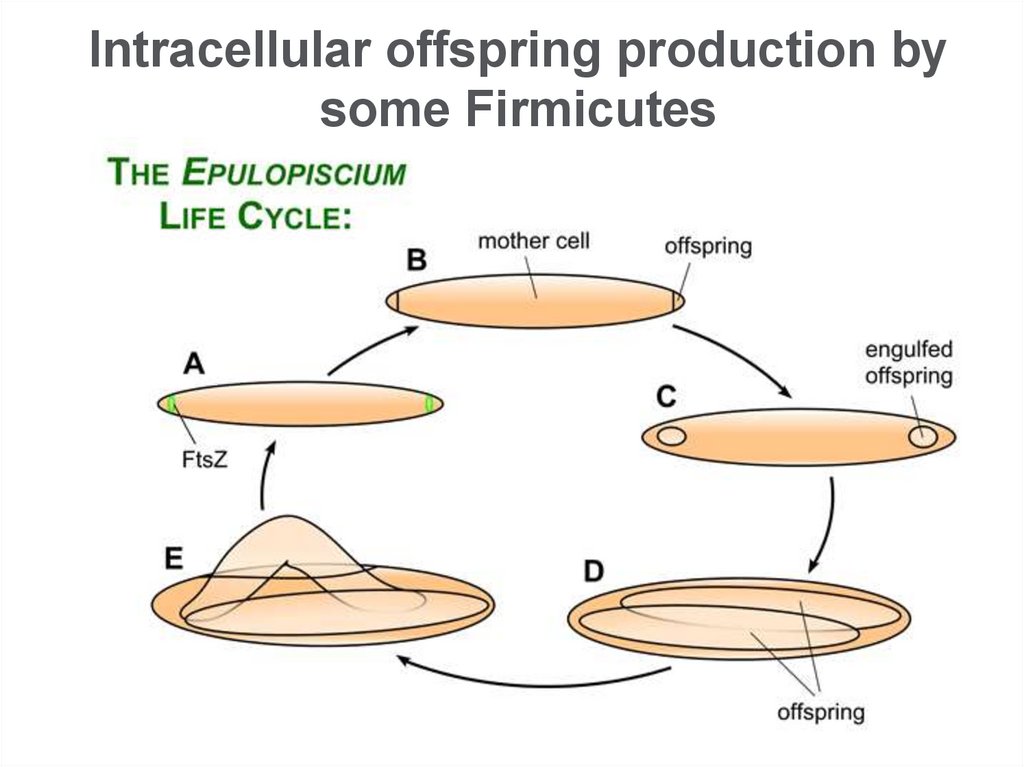
Intracellular offspring production bysome Firmicutes
57. Rate of Population Growth
• Generation time or doubling time:– The time required for a complete fission
cycle, from parent cell to two daughter
cells
- Generation: increases the population by
a factor of two
- As long as the environment remains
favorable, the doubling effect can
continue at a constant rate.
58. Rate of Population Growth
• The length of the generation time is ameasure of the growth rate of an organism:
- Average generation time is 30 – 60 minutes
- Shortest generation times can be 10 – 12 minutes
- Mycobacterium leprae has a generation time of
10 – 30 days.
- Environmental bacteria have generation times
measured in months.
- Most pathogens have relatively short generation
times.
59.
60. The Mathematics of Population Growth
• The size of a population can be calculatedby the following equation:
Nt = (Ni)2n
- Nt is the total number of cells in the
population, t denotes “at some point in time”
- Ni represents the starting number of cells
- The exponent n denotes the generation
number.
- 2n represents the number of cells in that
generation.
61. The Population Growth Curve
• Growth Curve: a predicable pattern of abacterial population growth in a closed
system can be measured by:
- Placing a tiny number of cells into a sterile
liquid medium
- Incubating the culture over a period of several
hours
- Sampling the broth at regular intervals
- Plating each sample onto solid media
- Counting the number of colonies present
62.
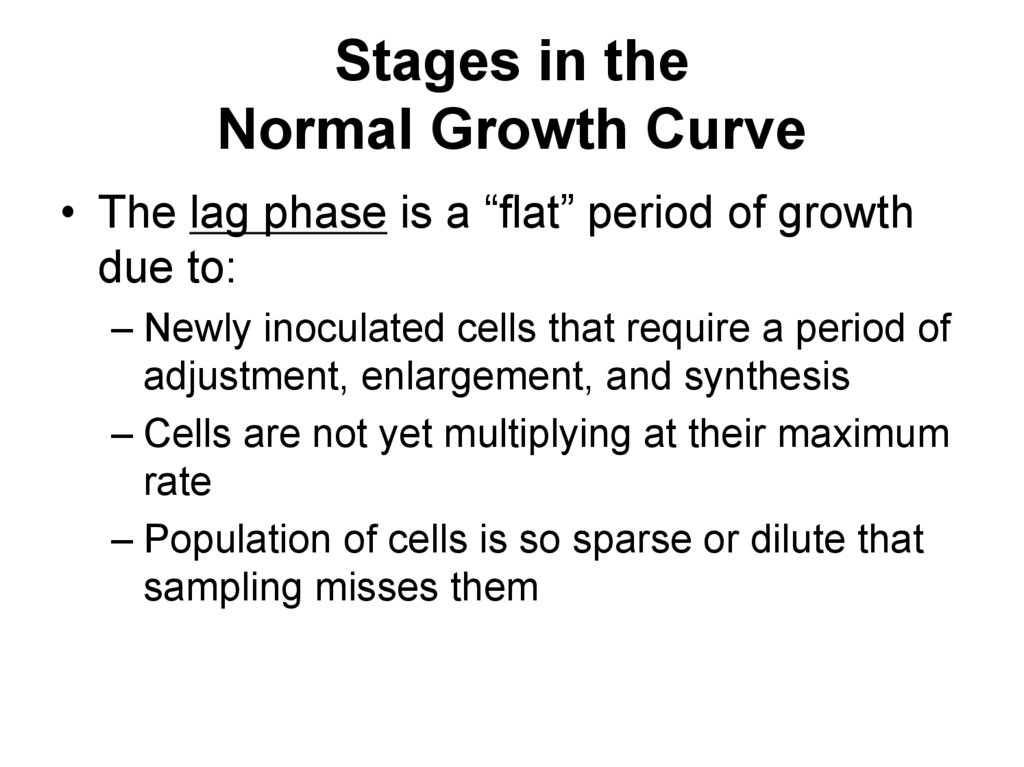
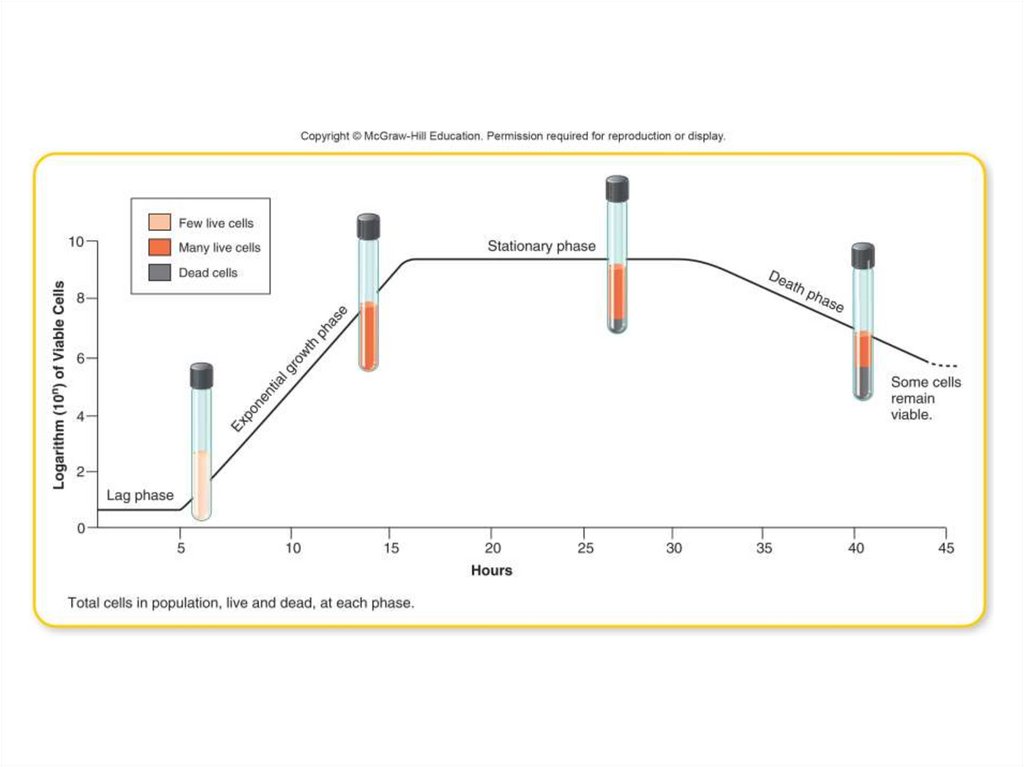
63. Stages in the Normal Growth Curve
• The lag phase is a “flat” period of growthdue to:
– Newly inoculated cells that require a period of
adjustment, enlargement, and synthesis
– Cells are not yet multiplying at their maximum
rate
– Population of cells is so sparse or dilute that
sampling misses them
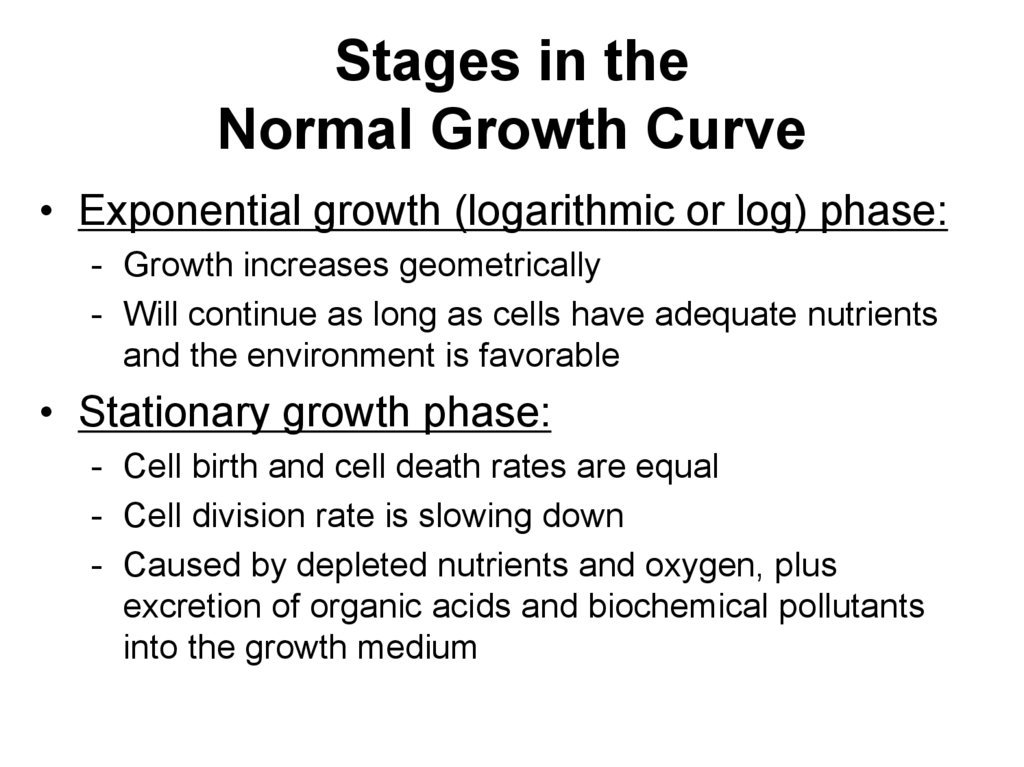
64. Stages in the Normal Growth Curve
• Exponential growth (logarithmic or log) phase:- Growth increases geometrically
- Will continue as long as cells have adequate nutrients
and the environment is favorable
• Stationary growth phase:
- Cell birth and cell death rates are equal
- Cell division rate is slowing down
- Caused by depleted nutrients and oxygen, plus
excretion of organic acids and biochemical pollutants
into the growth medium
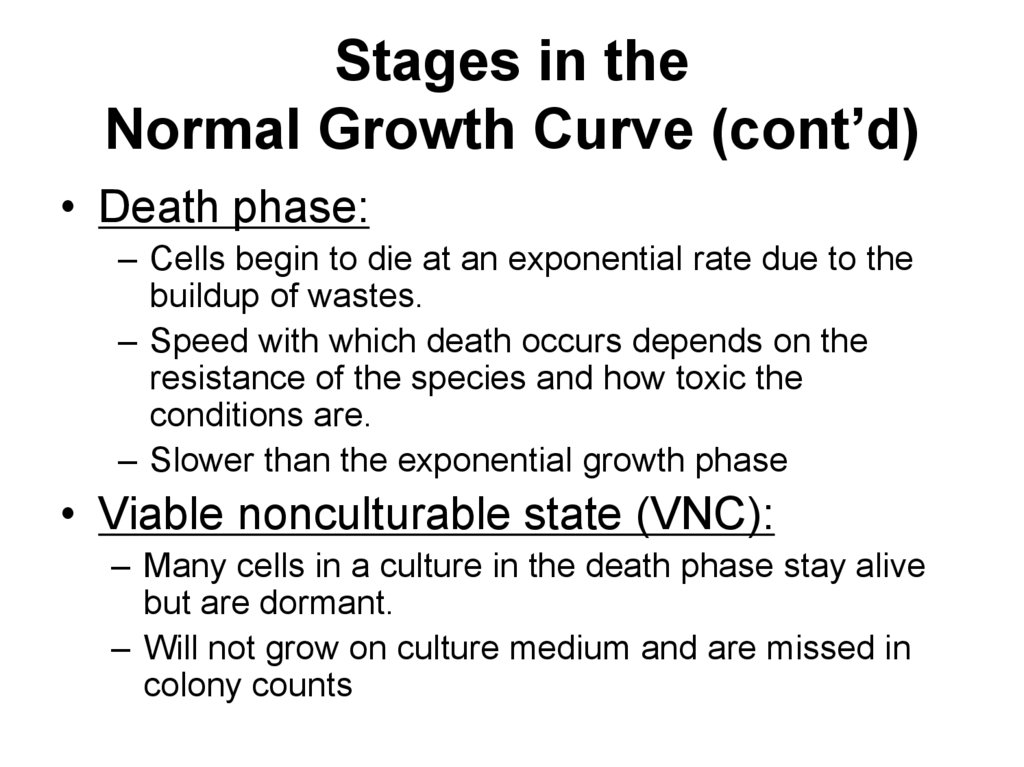
65. Stages in the Normal Growth Curve (cont’d)
• Death phase:– Cells begin to die at an exponential rate due to the
buildup of wastes.
– Speed with which death occurs depends on the
resistance of the species and how toxic the
conditions are.
– Slower than the exponential growth phase
• Viable nonculturable state (VNC):
– Many cells in a culture in the death phase stay alive
but are dormant.
– Will not grow on culture medium and are missed in
colony counts
66.
67. The Practical Importance of the Growth Curve
• The tendency for populations to exhibitphases of rapid growth, slow growth, and
death has implications in microbial control,
food microbiology, and culture technology:
– Microbes in the exponential growth phase are
more vulnerable to antimicrobial agents and heat.
– Actively growing cells are more vulnerable to
conditions that disrupt cell metabolism and binary
fission.
– A person actively shedding bacteria in the early
and middle stages of infection is more likely to
spread it than a person in the later stages.
68. The Practical Importance of the Growth Curve
• Chemostat- Automatic growth chamber
- Admits a steady stream of new nutrients
- Siphons off used media and old bacterial cells
- Stabilizes growth rate and cell number
- Used in research and industrial applications
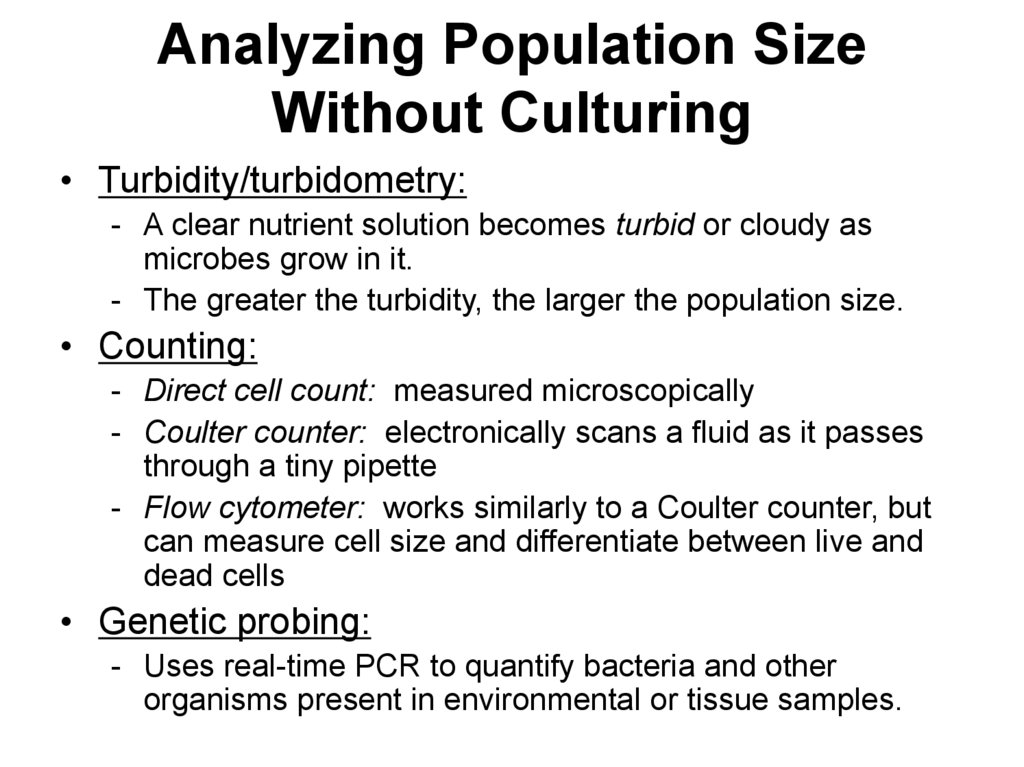
69. Analyzing Population Size Without Culturing
• Turbidity/turbidometry:- A clear nutrient solution becomes turbid or cloudy as
microbes grow in it.
- The greater the turbidity, the larger the population size.
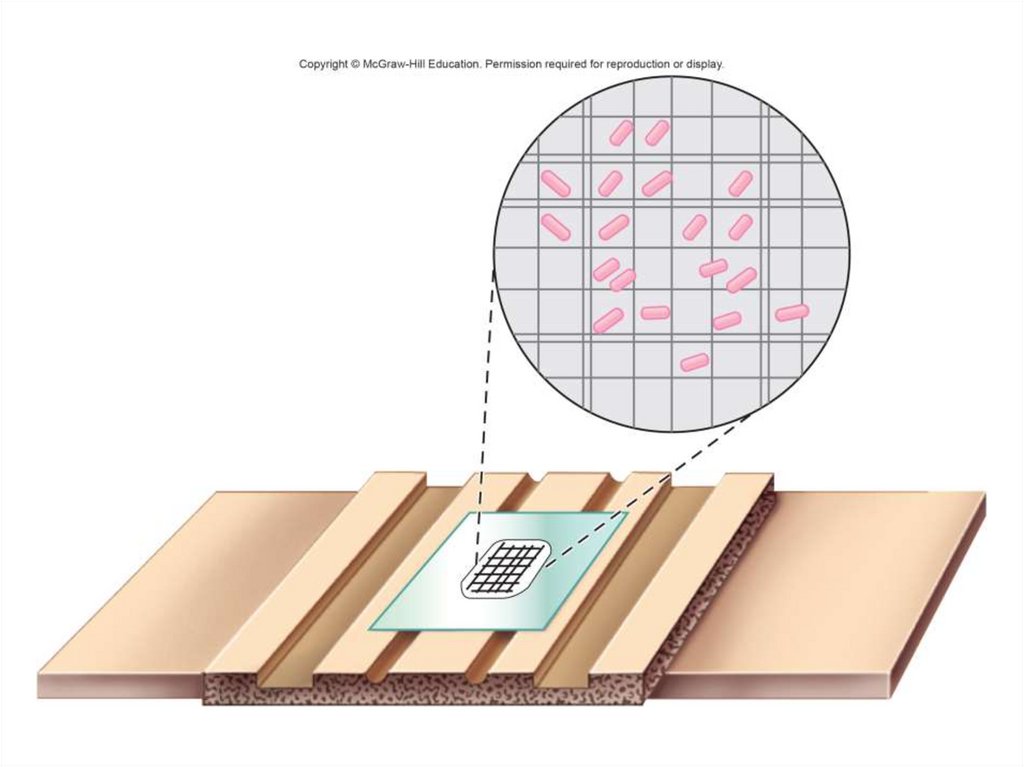
• Counting:
- Direct cell count: measured microscopically
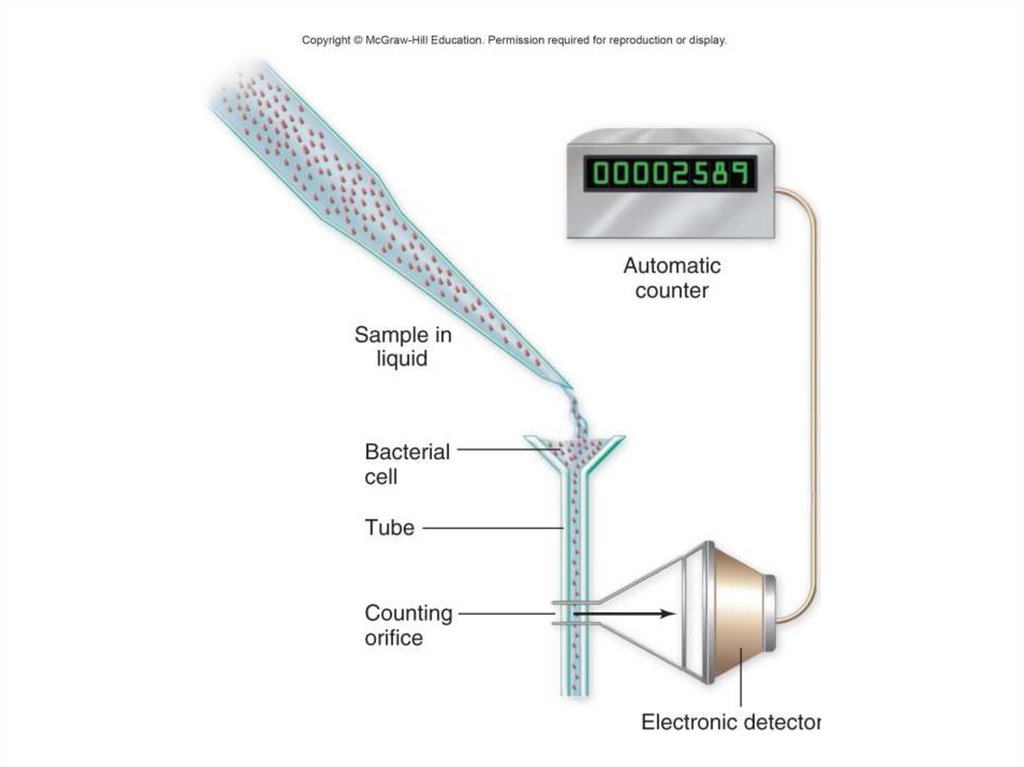
- Coulter counter: electronically scans a fluid as it passes
through a tiny pipette
- Flow cytometer: works similarly to a Coulter counter, but
can measure cell size and differentiate between live and
dead cells
• Genetic probing:
- Uses real-time PCR to quantify bacteria and other
organisms present in environmental or tissue samples.
70.
71.
72.
Concept CheckPut the steps of the bacterial growth curve
in the correct order:
A. Death phase
B. Lag phase
C. Exponential phase
D. Stationary phase




































 biology
biology