Similar presentations:
Carbohydrate Metabolism I: Aerobic oxidation of glucose. Anaerobic Glycolysis. Gluconeogenesis
1.
THE MINISTRY OF PUBLIC HEALTH OF UKRAINEZAPOROZHYE STATE MEDICAL UNIVERSITY
Department of biochemistry and laboratory diagnostics
Carbohydrate Metabolism I:
Aerobic oxidation of glucose
Anaerobic Glycolysis
Gluconeogenesis
by Rudko N.P., 2011
2. OBJECTIVES in Carbohydrate Metabolism
Consider the main metabolic pathways(the intermediates, enzymes, cofactors and regulation)
for carbohydrate metabolism:
1) Aerobic oxidation of glucose (complete
degradation to CO2&H2O)
2) Glycolysis
3) Gluconeogenesis
4) Pentose Phosphate Pathway
5) Glycogenesis
6) Glycogenolysis
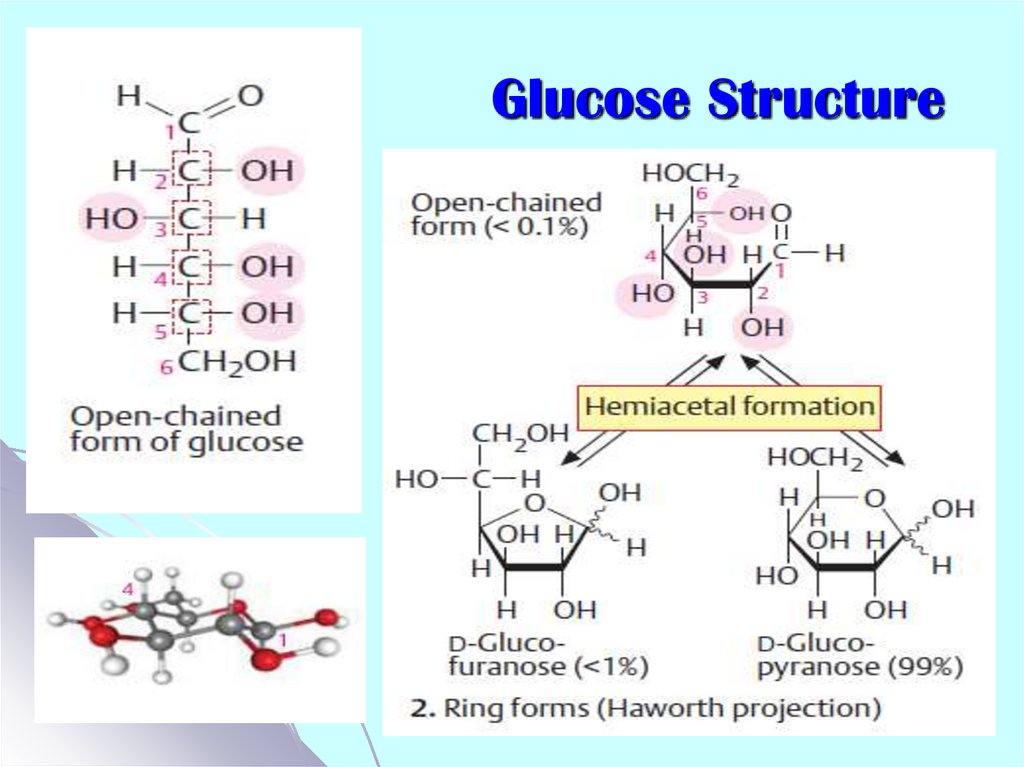
3. Glucose Structure
4.
Carbohydrate are Classified as:Monosaccharides: glucose, galactose,
fructose, ribose and deoxyribose
Oligosaccharides: sucrose (G-F),
lactose (G-Gal), maltose (G-G)
Polysaccharides:
homo: glycogen, starch, cellulose
hetero: mucopolysaccharides such as:
hyaluronic acid, heparine
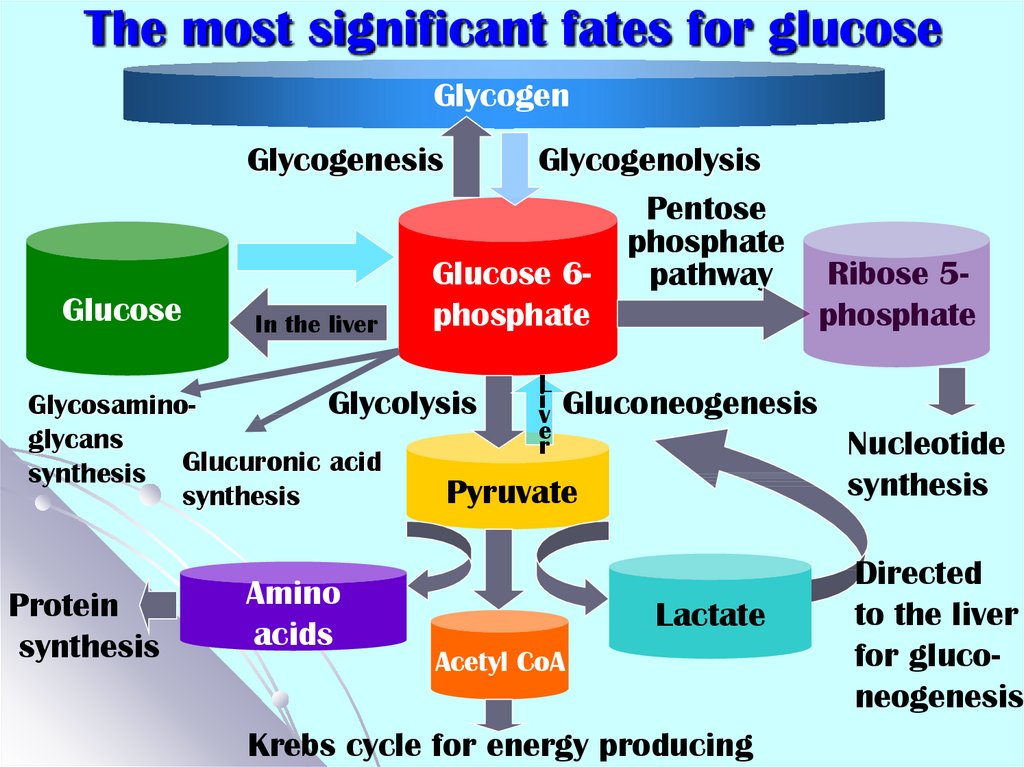
5. The most significant fates for glucose
GlycogenGlycogenesis
Glucose
In the liver
Glycogenolysis
Pentose
phosphate
Glucose 6Ribose 5pathway
phosphate
phosphate
L
i Gluconeogenesis
GlycosaminoGlycolysis
v
e
glycans
r
Glucuronic
acid
synthesis
Pyruvate
synthesis
Protein
synthesis
Amino
acids
Lactate
Acetyl CoA
Krebs cycle for energy producing
Nucleotide
synthesis
Directed
to the liver
for gluconeogenesis
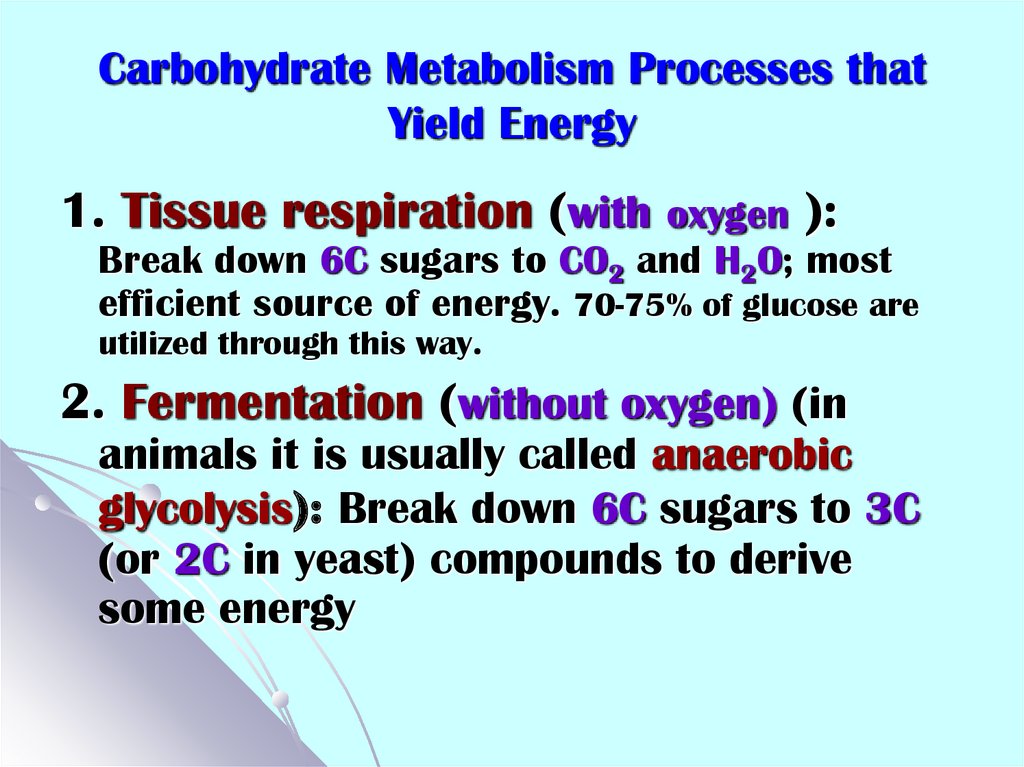
6. Carbohydrate Metabolism Processes that Yield Energy
1. Tissue respiration (with oxygen ):Break down 6C sugars to CO2 and H2O; most
efficient source of energy. 70-75% of glucose are
utilized through this way.
2. Fermentation (without oxygen) (in
animals it is usually called anaerobic
glycolysis): Break down 6C sugars to 3C
(or 2C in yeast) compounds to derive
some energy
7. Tissue Respiration (Aerobic Oxidation) for Glucose Consists of 3 Main Phases:
1Aerobic glycolysis &
oxidative decarboxylation of pyruvate
2
Krebs cycle
3
Electron transport in ETC
8. Aerobic Glycolysis
Definition:Aerobic Glycolysis is the metabolic pathway
in which monosaccharides (mainly glucose)
are split into two molecules of pyruvate
Location in the body : all type cells
Location within the cell : cytosol
Substrates: Glucose, galactose, fructose
Products: 2 pyruvates & 2 ATP & 2 NADH
Net reaction for aerobic Glycolysis:
C6H12O6 +2 NAD+ + 2ADP + 2Pi →
2 CH3-CO-COOH + 2 NADH + 2H+ + 2ATP
9.
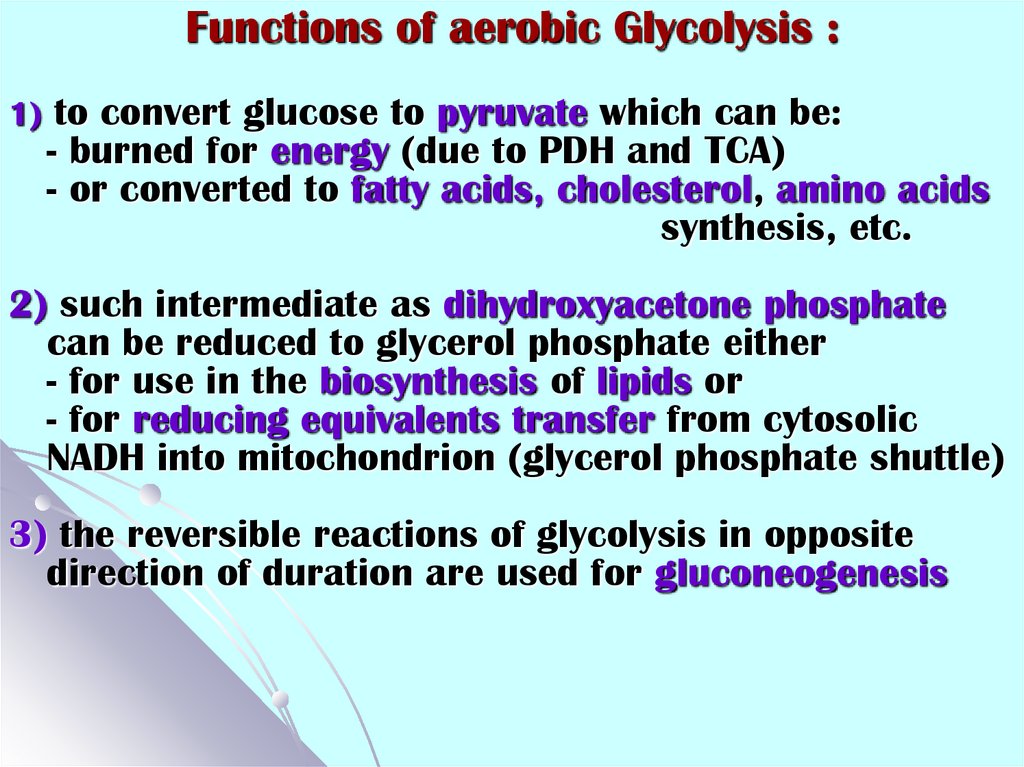
Functions of aerobic Glycolysis :1) to convert glucose to pyruvate which can be:
- burned for energy (due to PDH and TCA)
- or converted to fatty acids, cholesterol, amino acids
synthesis, etc.
2) such intermediate as dihydroxyacetone phosphate
can be reduced to glycerol phosphate either
- for use in the biosynthesis of lipids or
- for reducing equivalents transfer from cytosolic
NADH into mitochondrion (glycerol phosphate shuttle)
3) the reversible reactions of glycolysis in opposite
direction of duration are used for gluconeogenesis
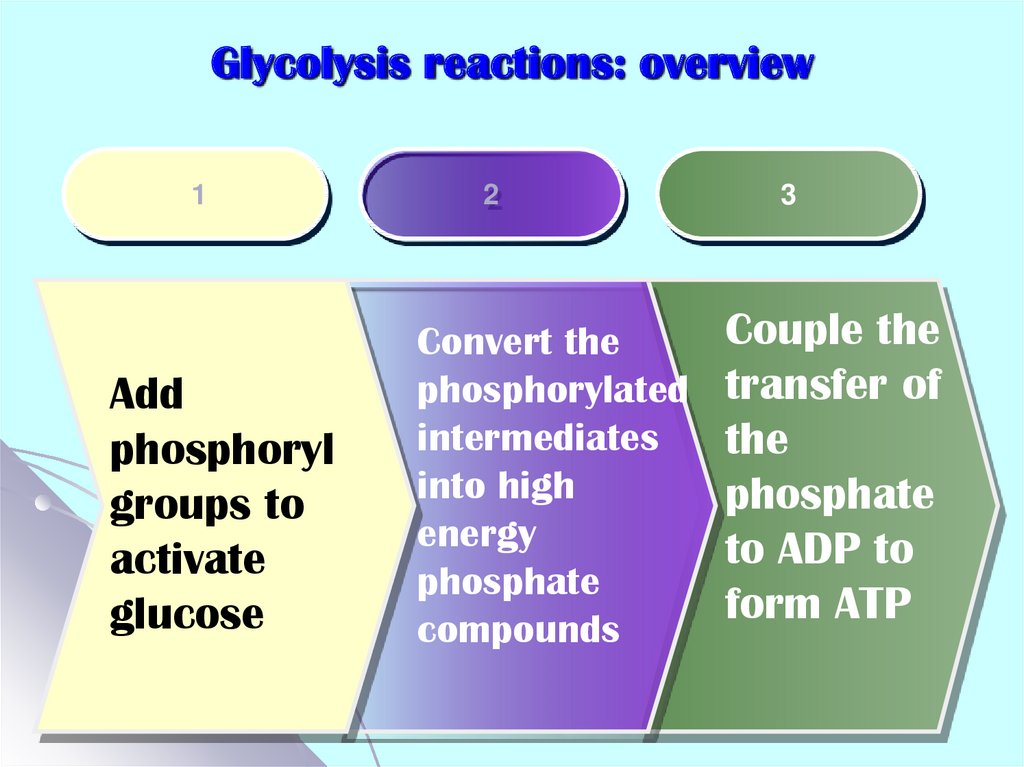
10. Glycolysis reactions: overview
1Add
phosphoryl
groups to
activate
glucose
2
Convert the
phosphorylated
intermediates
into high
energy
phosphate
compounds
3
Couple the
transfer of
the
phosphate
to ADP to
form ATP
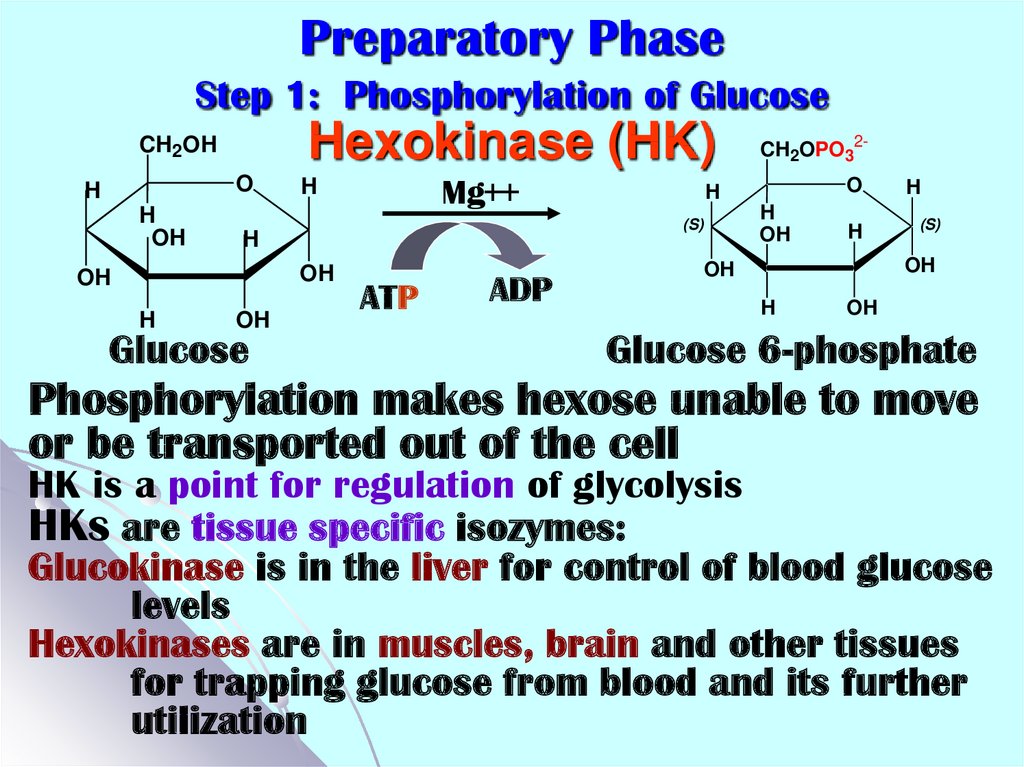
11. Preparatory Phase Step 1: Phosphorylation of Glucose Hexokinase (HK)
CH2OHO
H
H
OH
OH
OH
Glucose
ATP
ADP
O
H
H
OH
(S)
H
OH
H
Mg++
H
CH2OPO32-
H
H
(S)
OH
OH
H
OH
Glucose 6-phosphate
Phosphorylation makes hexose unable to move
or be transported out of the cell
HK is a point for regulation of glycolysis
HKs are tissue specific isozymes:
Glucokinase is in the liver for control of blood glucose
levels
Hexokinases are in muscles, brain and other tissues
for trapping glucose from blood and its further
utilization
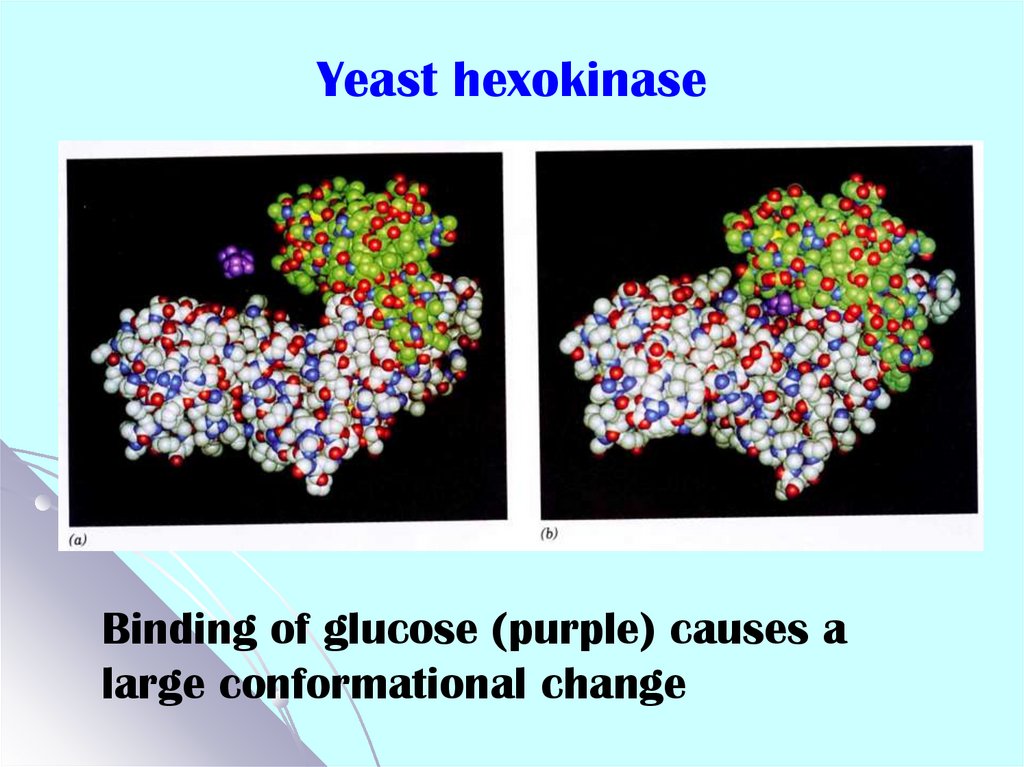
12. Yeast hexokinase
Binding of glucose (purple) causes alarge conformational change
13. Hexokinase characteristics
There are four important mammalianhexokinase isozymes. They are designated
hexokinases I, II, III, and IV
Hexokinases I, II, and III:
- are referred to as "low-Km" isozymes;
- also phosphorylate other hexose sugars;
- are inhibited by glucose 6-phosphate;
Hexokinase IV, also referred to as
glucokinase:
- its Km for glucose is 100 times higher
than that of hexokinases I, II, and III;
- phosphorylates only glucose;
- it is not allosterically inhibited by glucose6-phosphate
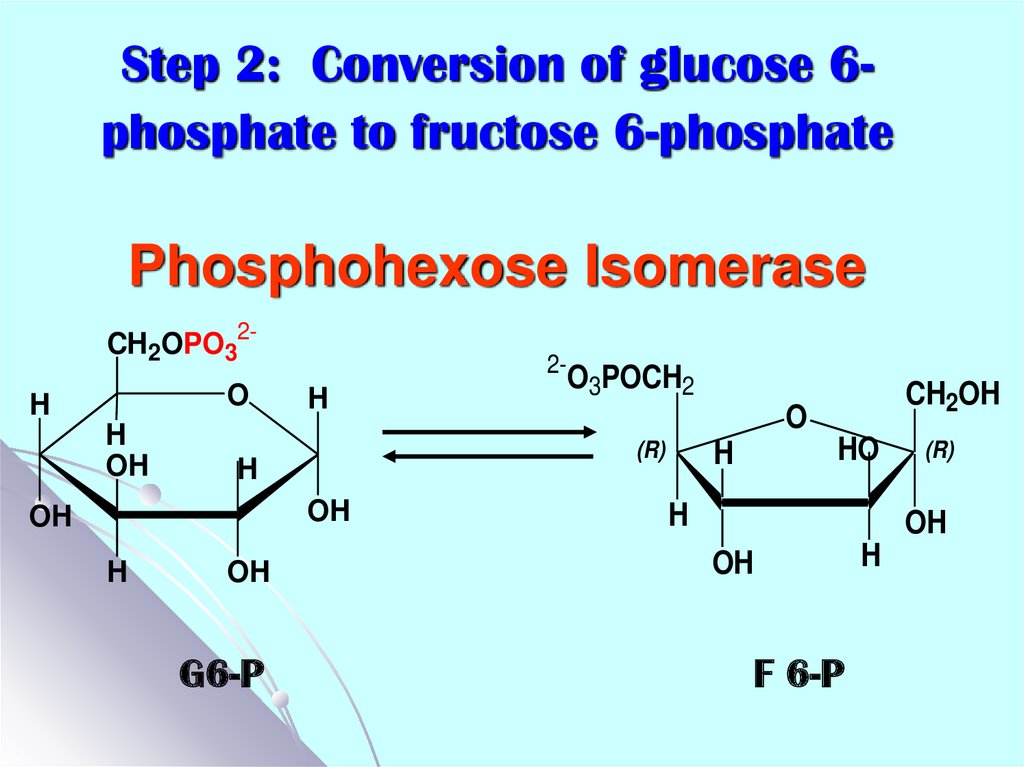
14. Step 2: Conversion of glucose 6-phosphate to fructose 6-phosphate Phosphohexose Isomerase
Step 2: Conversion of glucose 6phosphate to fructose 6-phosphatePhosphohexose Isomerase
CH2OPO32O
H
H
OH
2-
H
OH
H
OH
G6-P
CH2OH
O
(R)
H
OH
O3POCH2
HO
H
H
(R)
OH
OH
F 6-P
H
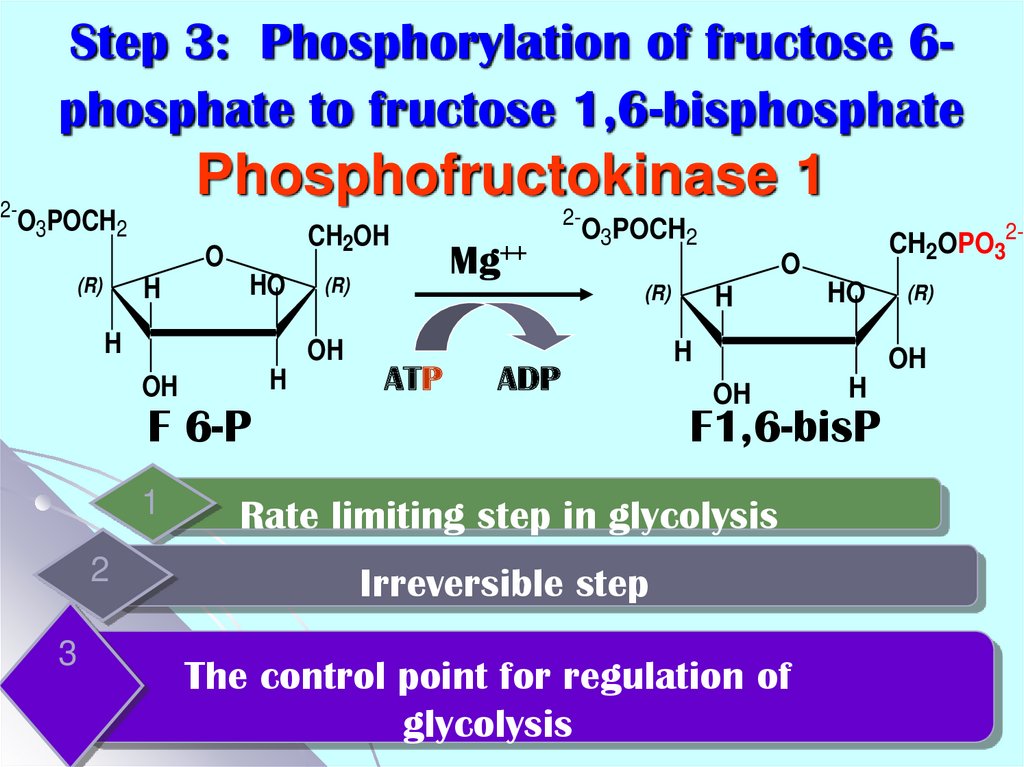
15. Step 3: Phosphorylation of fructose 6-phosphate to fructose 1,6-bisphosphate Phosphofructokinase 1
Step 3: Phosphorylation of fructose 6phosphate to fructose 1,6-bisphosphatePhosphofructokinase 1
2-
O3POCH2
CH2OH
O
(R)
H
HO
H
(R)
OH
H
OH
2-
Mg++
O3POCH2
O
(R)
ATP
ADP
F 6-P
1
2
3
CH2OPO32-
H
HO
H
(R)
OH
OH
H
F1,6-bisP
Rate limiting step in glycolysis
Irreversible step
The control point for regulation of
glycolysis
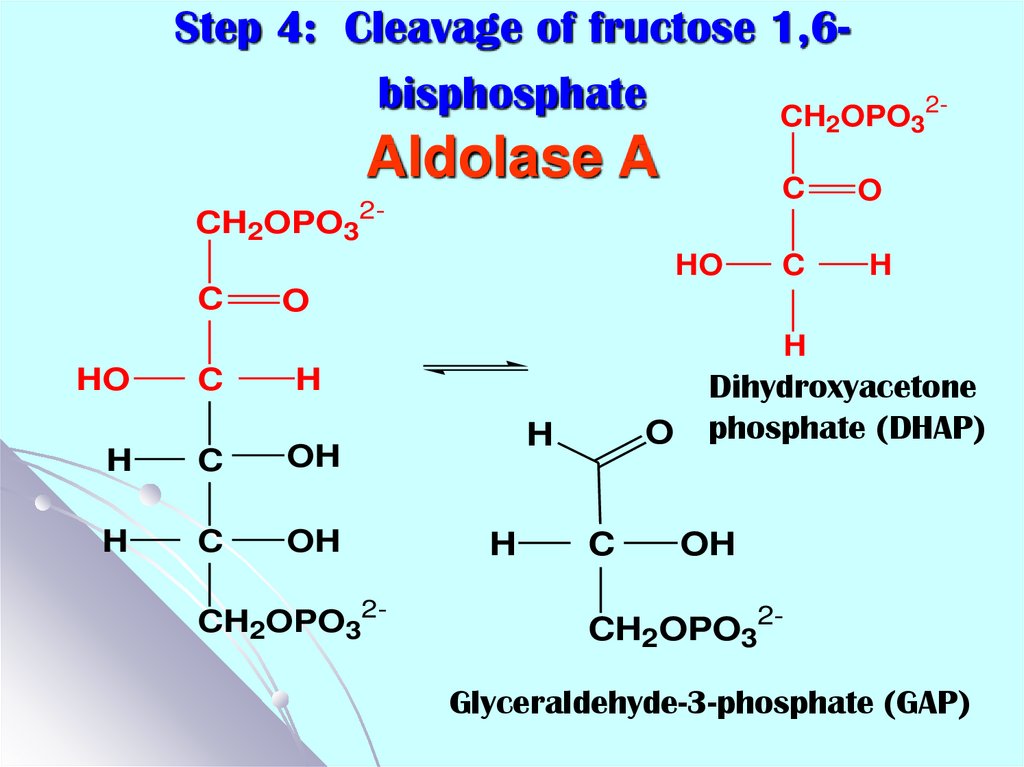
16. Step 4: Cleavage of fructose 1,6-bisphosphate Aldolase A
Step 4: Cleavage of fructose 1,6bisphosphateCH OPO
2
Aldolase A
C
CH2OPO32HO
C
C
3
2-
O
H
O
H
HO
C
H
H
C
OH
H
C
OH
CH2OPO32-
O
H
H
C
Dihydroxyacetone
phosphate (DHAP)
OH
CH2OPO32-
Glyceraldehyde-3-phosphate (GAP)
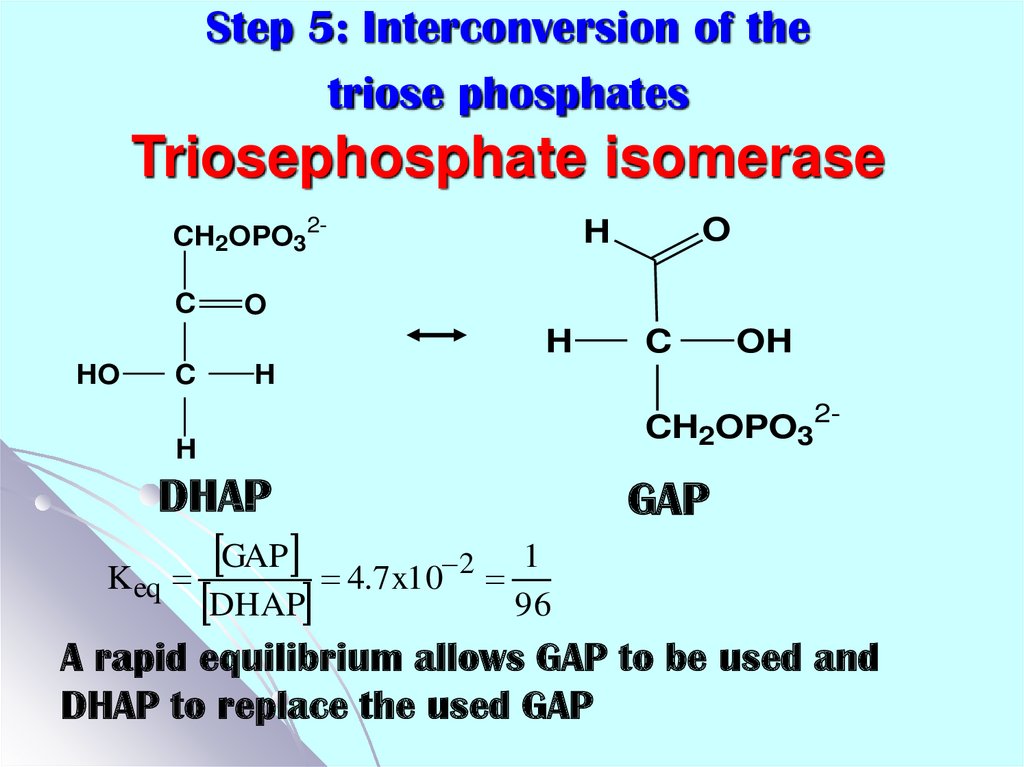
17. Step 5: Interconversion of the triose phosphates Triosephosphate isomerase
CH2OPO32CO
H
HO
C
C
OH
H
CH2OPO32-
H
DHAP
K eq
O
H
GAP
DHAP
GAP
2
4.7 x10
1
96
A rapid equilibrium allows GAP to be used and
DHAP to replace the used GAP
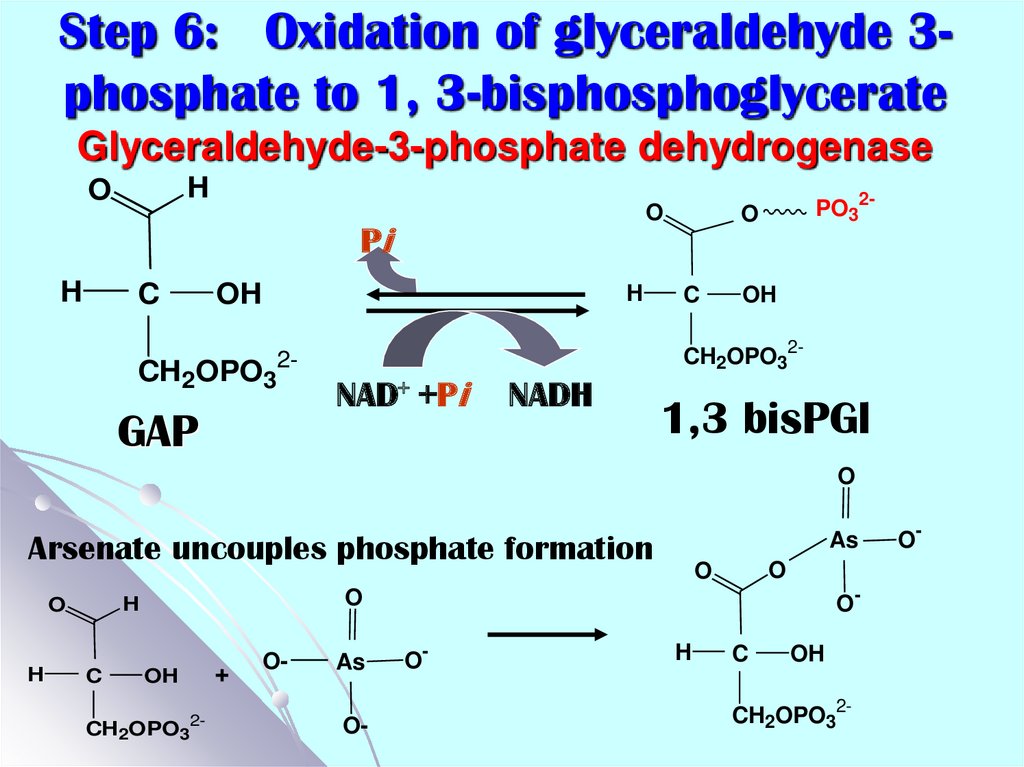
18. Step 6: Oxidation of glyceraldehyde 3-phosphate to 1, 3-bisphosphoglycerate Glyceraldehyde-3-phosphate dehydrogenase
Step 6: Oxidation of glyceraldehyde 3phosphate to 1, 3-bisphosphoglycerateGlyceraldehyde-3-phosphate dehydrogenase
H
O
O
Pi
H
C
OH
H
CH2OPO3
C
OH
CH2OPO32-
2-
GAP
PO32-
O
NAD+ +Pi
NADH
1,3 bisPGl
O
Arsenate uncouples phosphate formation
H
C
O
O
O-
O
H
O
As
+
OH
CH2OPO3
2-
O-
As
O-
O-
H
C
OH
CH2OPO32-
O-
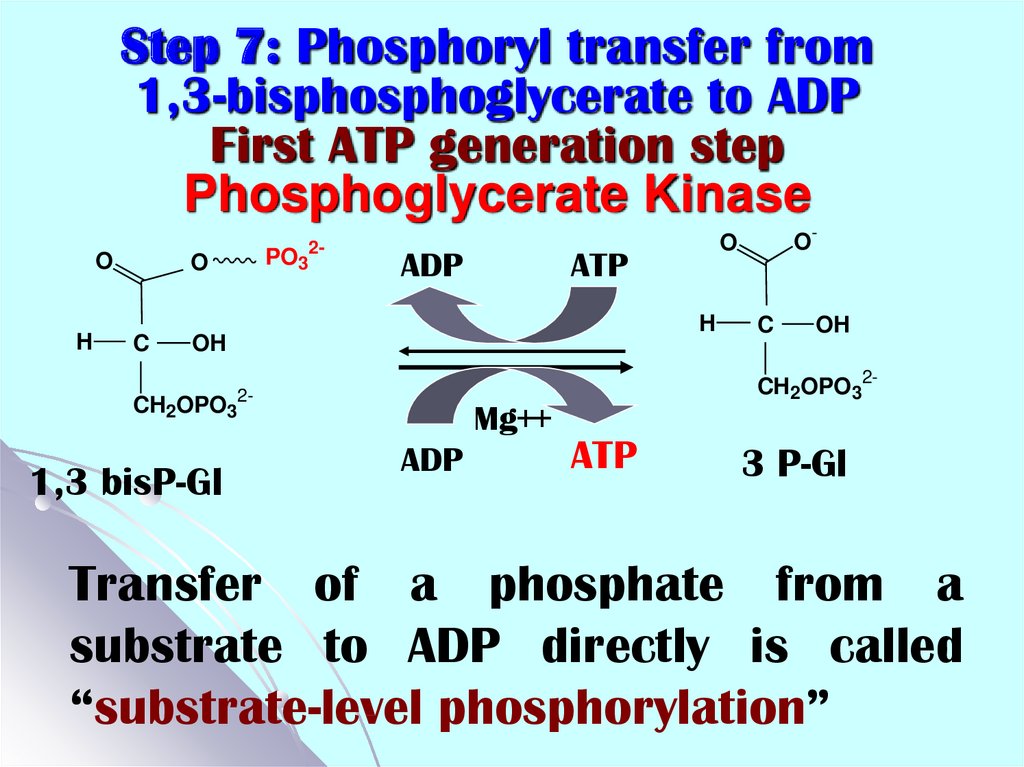
19. Step 7: Phosphoryl transfer from 1,3-bisphosphoglycerate to ADP First ATP generation step Phosphoglycerate Kinase
OH
O
C
PO32-
ADP
ATP
H
OH
CH2OPO32-
1,3 bisP-Gl
Mg++
ADP
O-
O
C
OH
CH2OPO32-
ATP
3 P-Gl
Transfer of a phosphate from a
substrate to ADP directly is called
“substrate-level phosphorylation”
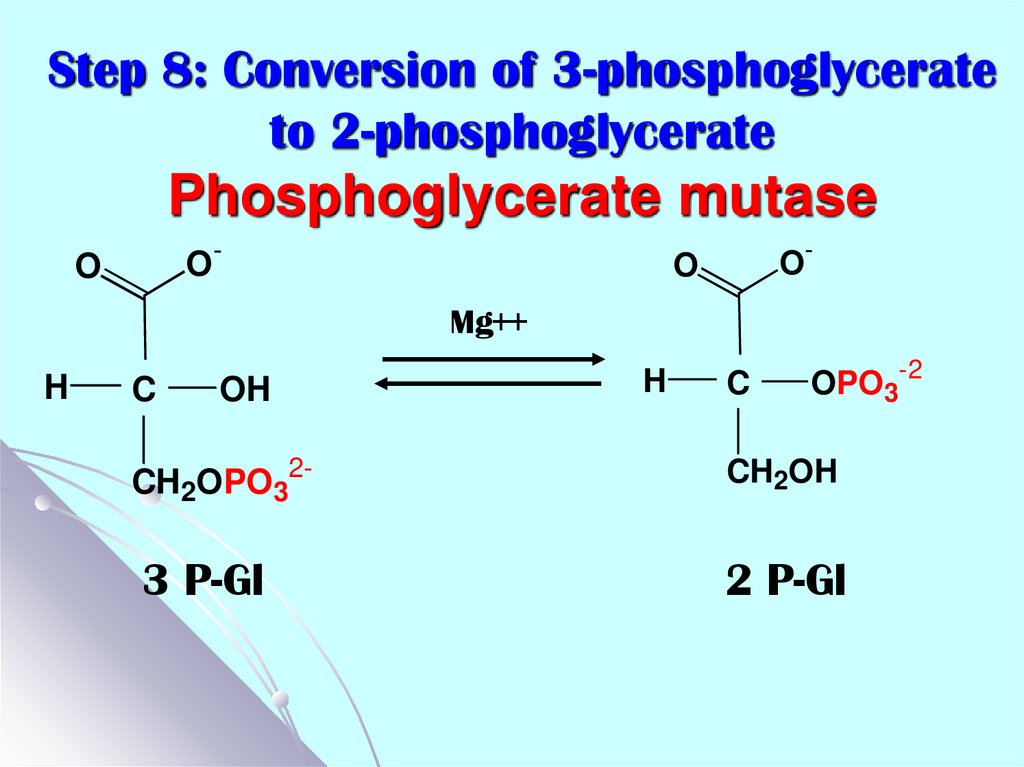
20. Step 8: Conversion of 3-phosphoglycerate to 2-phosphoglycerate Phosphoglycerate mutase
O-O
O-
O
Mg++
H
C
OH
CH2OPO32-
3 P-Gl
H
C
OPO3-2
CH2OH
2 P-Gl
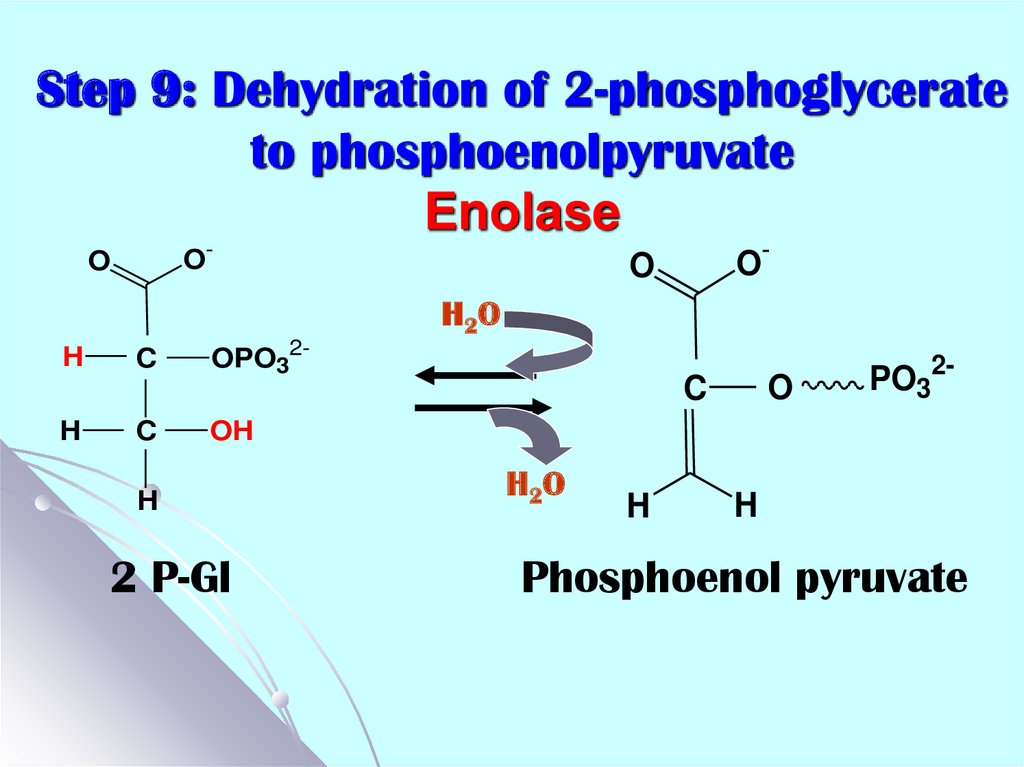
21. Step 9: Dehydration of 2-phosphoglycerate to phosphoenolpyruvate Enolase
O-O
O-
O
H 2O
H
H
C
C
OPO32-
O
C
PO32-
OH
H
2 P-Gl
H 2O
H
H
Phosphoenol pyruvate
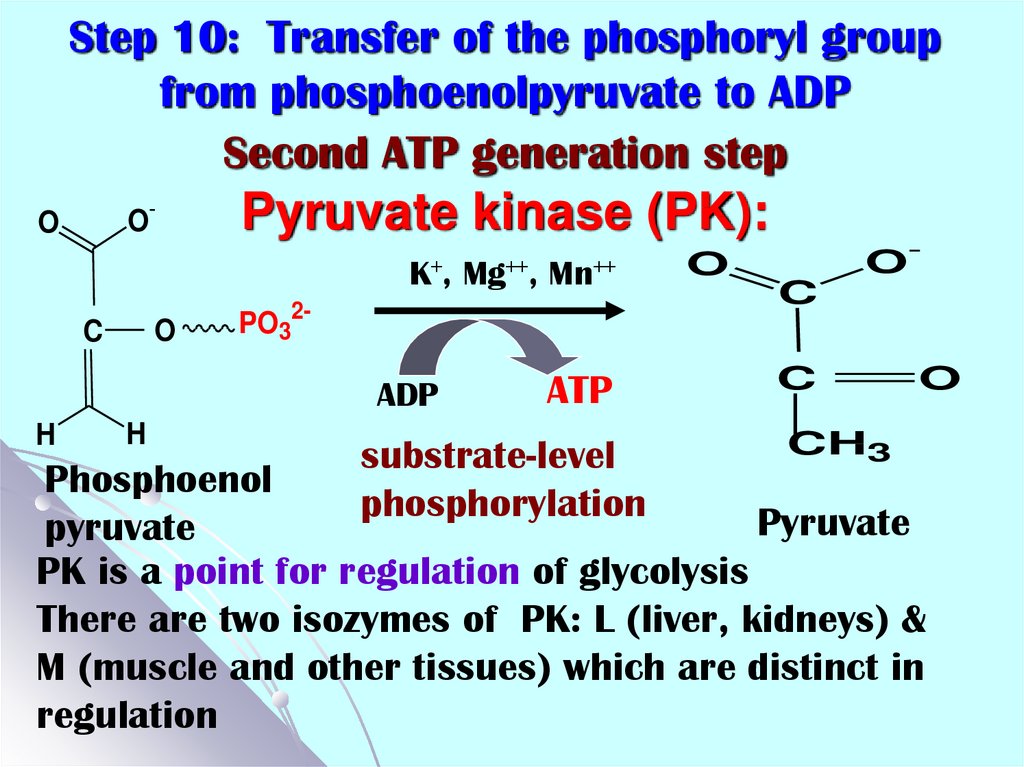
22. Step 10: Transfer of the phosphoryl group from phosphoenolpyruvate to ADP Second ATP generation step Pyruvate kinase (PK):
Step 10: Transfer of the phosphoryl groupfrom phosphoenolpyruvate to ADP
Second ATP generation step
O-
O
Pyruvate kinase (PK):
K+,
O
C
H
Mn++
PO32-
ADP
H
Mg++,
ATP
substrate-level
phosphorylation
O
OC
C
CH3
O
Phosphoenol
Pyruvate
pyruvate
PK is a point for regulation of glycolysis
There are two isozymes of PK: L (liver, kidneys) &
M (muscle and other tissues) which are distinct in
regulation
23. Oxidizing power of NAD+ must be recycled
22
2
Aerobic condition
Aerobic conditions
Anaerobic conditions
8
2
2 AcetylCoA
2
anaerobic
glycolysis
8
2
2
6
8
In mammalian all type cells
2
2
2
In mammalian contracting Fermentation in yeast
muscle, erytrocytes etc
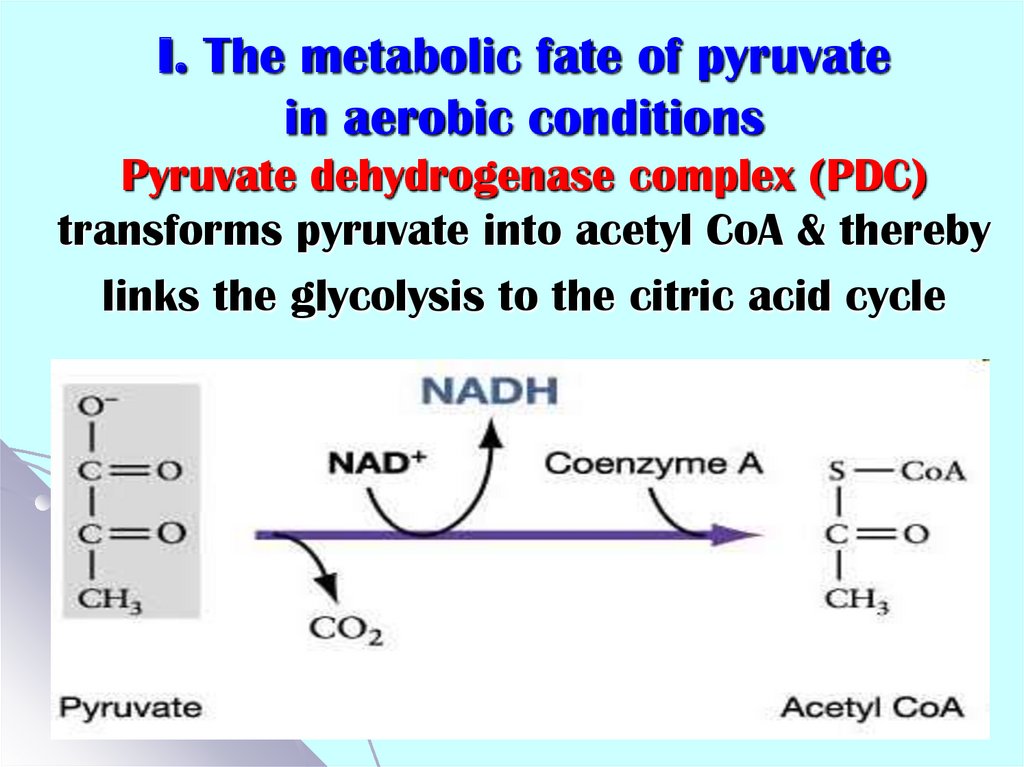
24. I. The metabolic fate of pyruvate in aerobic conditions Pyruvate dehydrogenase complex (PDC) transforms pyruvate into acetyl
CoA & therebylinks the glycolysis to the citric acid cycle
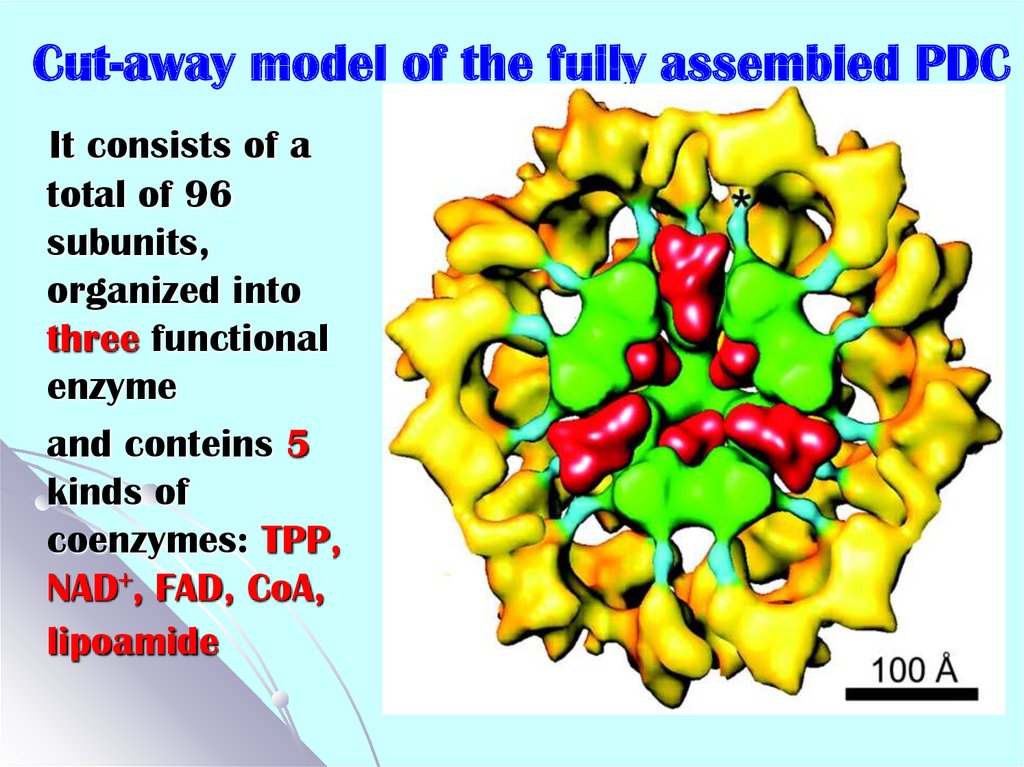
25. Cut-away model of the fully assembled PDC
It consists of atotal of 96
subunits,
organized into
three functional
enzyme
and conteins 5
kinds of
coenzymes: TPP,
NAD+, FAD, CoA,
lipoamide
26. Mechanism of PDC action (see in a text-book)
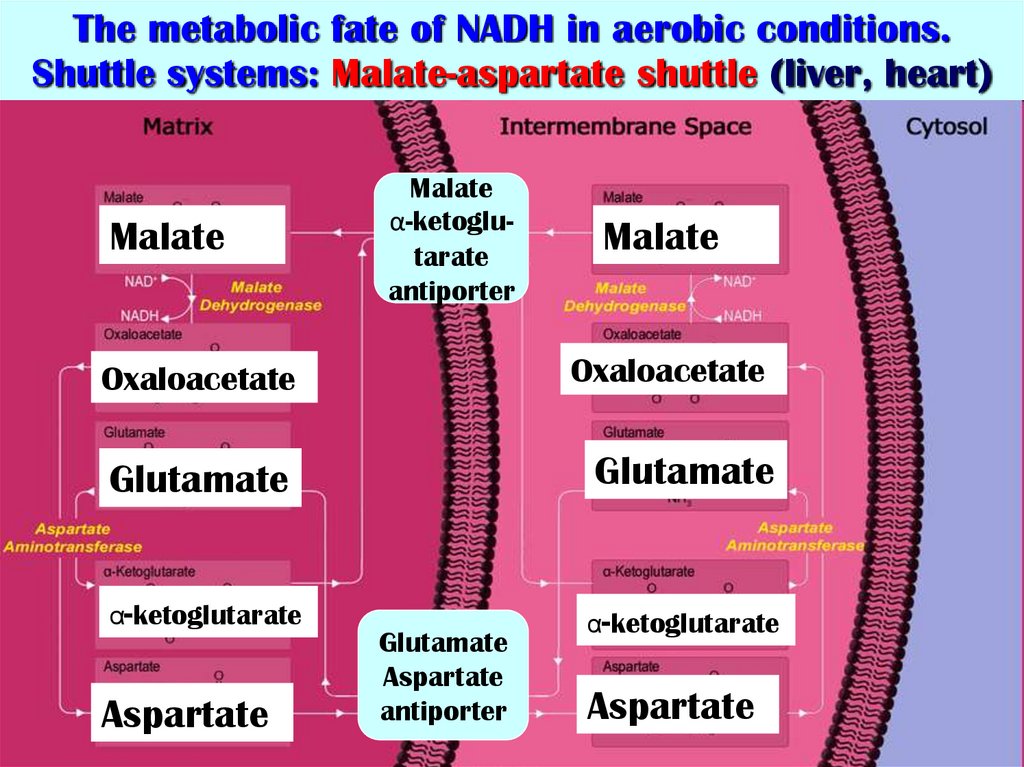
27. The metabolic fate of NADH in aerobic conditions. Shuttle systems: Malate-aspartate shuttle (liver, heart)
MalateMalate
α-ketoglutarate
antiporter
Malate
Oxaloacetate
Oxaloacetate
Glutamate
Glutamate
α-ketoglutarate
Aspartate
Glutamate
Aspartate
antiporter
α-ketoglutarate
Aspartate
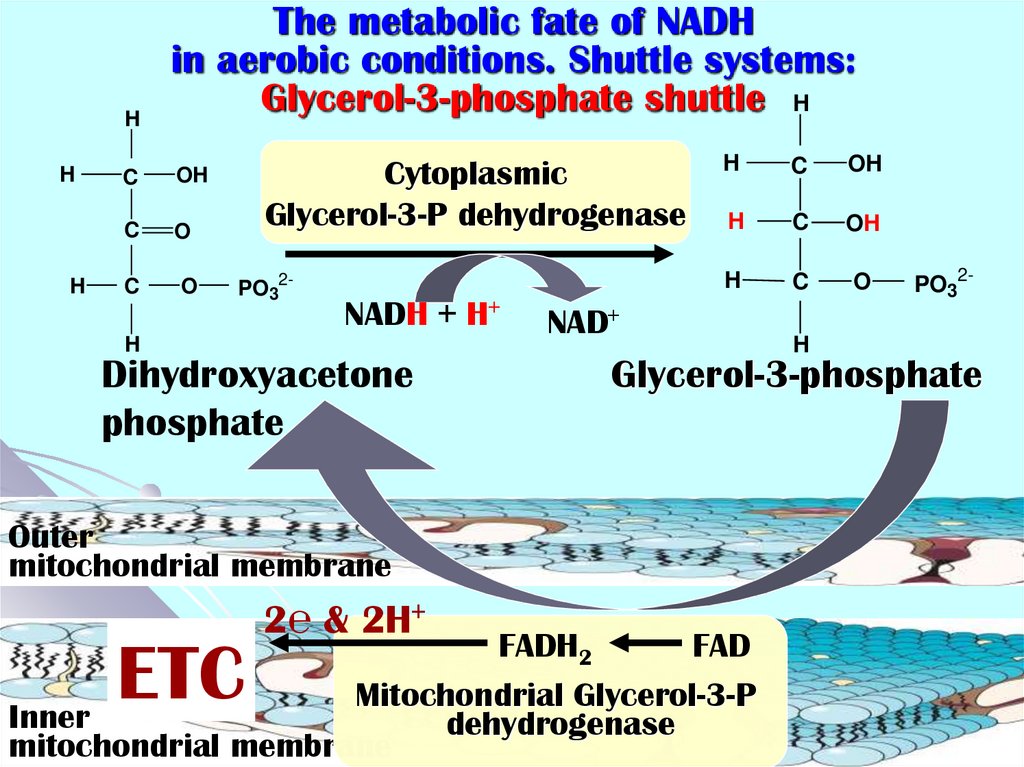
28. The metabolic fate of NADH in aerobic conditions. Shuttle systems: Glycerol-3-phosphate shuttle
HH
H
The metabolic fate of NADH
in aerobic conditions. Shuttle systems:
Glycerol-3-phosphate shuttle H
C
OH
C
O
C
O
Cytoplasmic
Glycerol-3-P dehydrogenase
PO32-
NADH + H+
H
Dihydroxyacetone
phosphate
H
C
OH
H
C
OH
H
C
O
NAD+
Glycerol-3-phosphate
Outer
mitochondrial membrane
2℮ & 2H+
FADH2
FAD
Mitochondrial Glycerol-3-P
Inner
dehydrogenase
mitochondrial membrane
ETC
H
PO32-
29. II. The metabolic fate of pyruvate in anaerobic conditions. Anaerobic glycolysis
Definition: Anaerobic Glycolysis is themetabolic pathway in which
monosaccharides (mainly glucose) are
split into two molecules of lactate
Location in the body : takes place in
erythrocytes, cornea, lens, skeletal muscle
tissue (significant at first 40-50 sec of
continuous muscle work)
Location within the cell : cytosol
Substrates:
Glucose
Products: 2 lactates & 2 ATP
30.
Functions of anaerobic Glycolysis :- ATP production
- 2,3 bisphosphoglycerate as powerful effector of O2
binding with haemoglobin in RBC is formed from
1,3 bisphosphoglycerate (glycolysis intermediate)
Anaerobic Glycolysis reactions:
All reactions of anaerobic glycolysis to pyruvate
are the same as they are in aerobic glycolysis but
one reaction is added else : Pyruvate is reduced by
NADH to lactate
Net reaction for anaerobic Glycolysis:
C6H12O6 + 2ADP + 2Pi →
2 CH3-CHOH-COOH + 2ATP
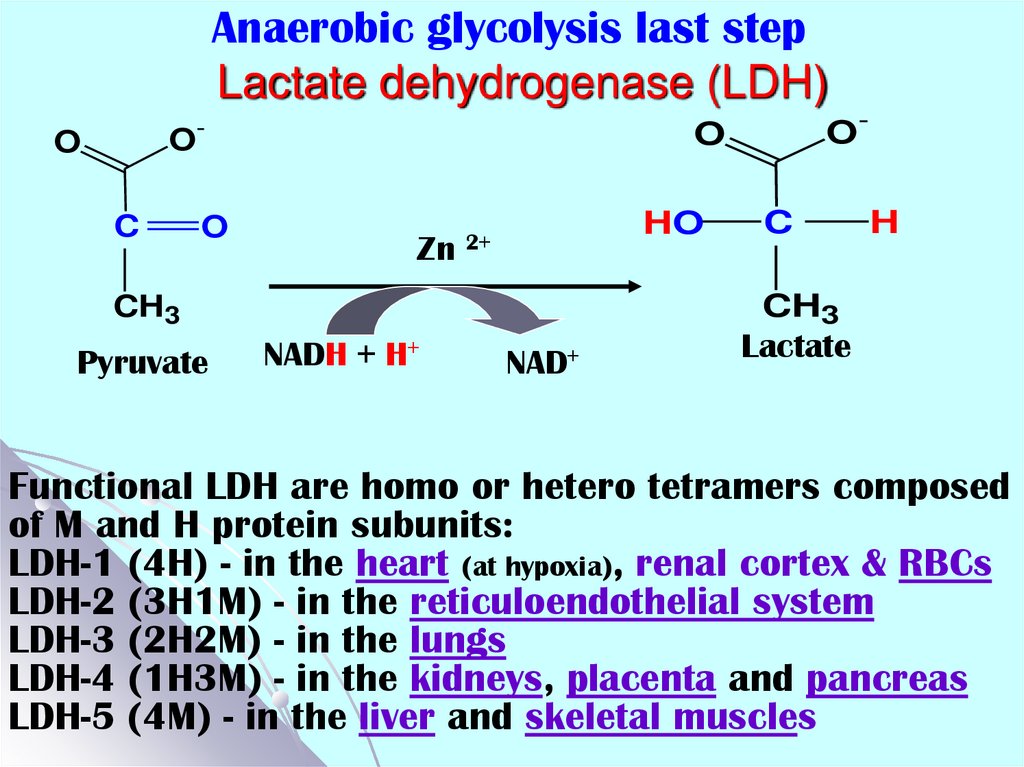
31. Anaerobic glycolysis last step Lactate dehydrogenase (LDH)
-O
O
C
O
Zn
HO
2+
C
H
CH3
CH3
Pyruvate
O-
O
NADH + H+
NAD+
Lactate
Functional LDH are homo or hetero tetramers composed
of M and H protein subunits:
LDH-1 (4H) - in the heart (at hypoxia), renal cortex & RBCs
LDH-2 (3H1M) - in the reticuloendothelial system
LDH-3 (2H2M) - in the lungs
LDH-4 (1H3M) - in the kidneys, placenta and pancreas
LDH-5 (4M) - in the liver and skeletal muscles
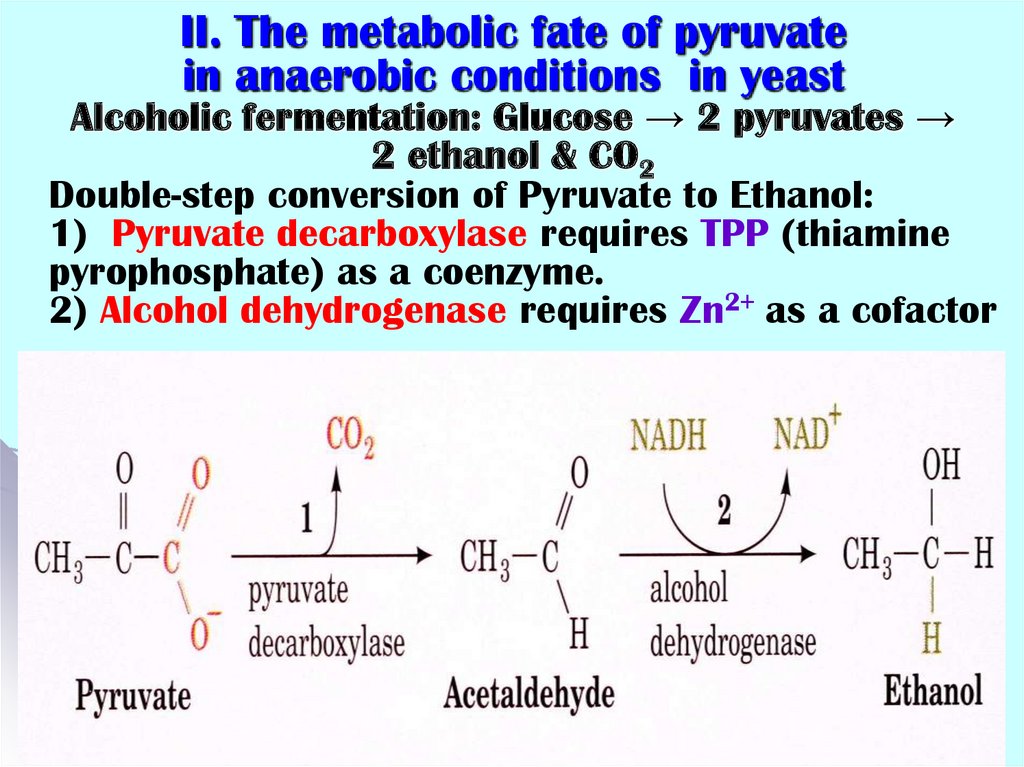
32. II. The metabolic fate of pyruvate in anaerobic conditions in yeast Alcoholic fermentation: Glucose → 2 pyruvates → 2 ethanol &
II. The metabolic fate of pyruvatein anaerobic conditions in yeast
Alcoholic fermentation: Glucose → 2 pyruvates →
2 ethanol & CO2
Double-step conversion of Pyruvate to Ethanol:
1) Pyruvate decarboxylase requires TPP (thiamine
pyrophosphate) as a coenzyme.
2) Alcohol dehydrogenase requires Zn2+ as a cofactor
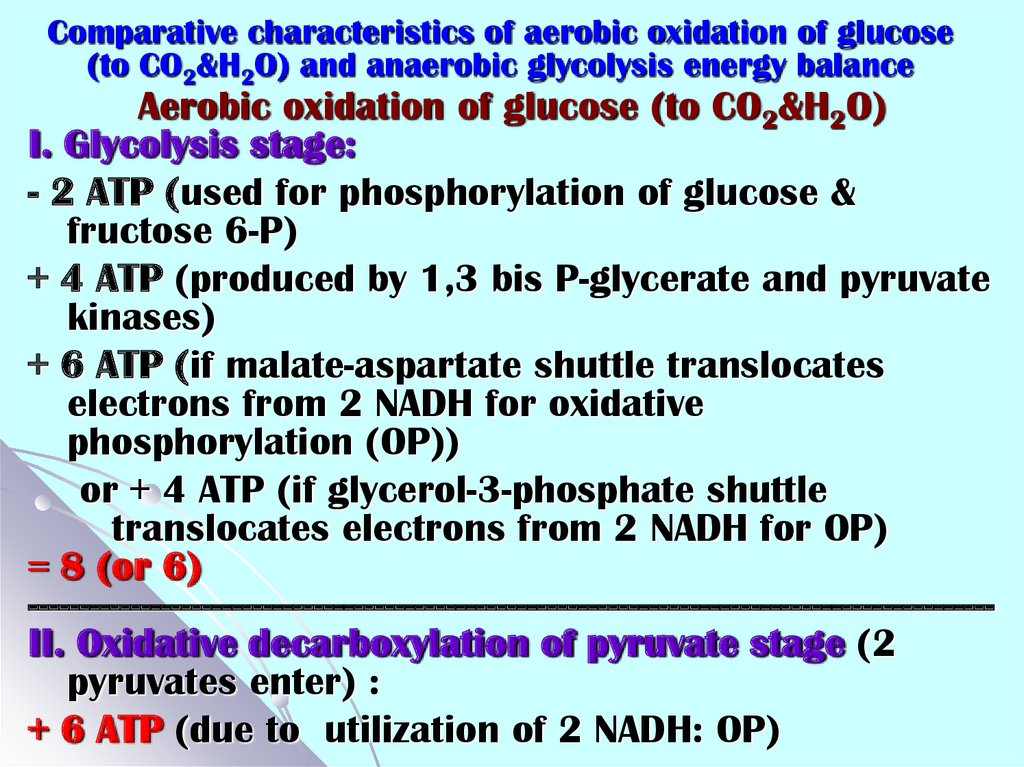
33. Comparative characteristics of aerobic oxidation of glucose (to CO2&H2O) and anaerobic glycolysis energy balance
Comparative characteristics of aerobic oxidation of glucose(to CO2&H2O) and anaerobic glycolysis energy balance
Aerobic oxidation of glucose (to CO2&H2O)
I. Glycolysis stage:
- 2 ATP (used for phosphorylation of glucose &
fructose 6-P)
+ 4 ATP (produced by 1,3 bis P-glycerate and pyruvate
kinases)
+ 6 ATP (if malate-aspartate shuttle translocates
electrons from 2 NADH for oxidative
phosphorylation (OP))
or + 4 ATP (if glycerol-3-phosphate shuttle
translocates electrons from 2 NADH for OP)
= 8 (or 6)
----------------------------------------------------------------------------------------------II. Oxidative decarboxylation of pyruvate stage (2
pyruvates enter) :
+ 6 ATP (due to utilization of 2 NADH: OP)
34.
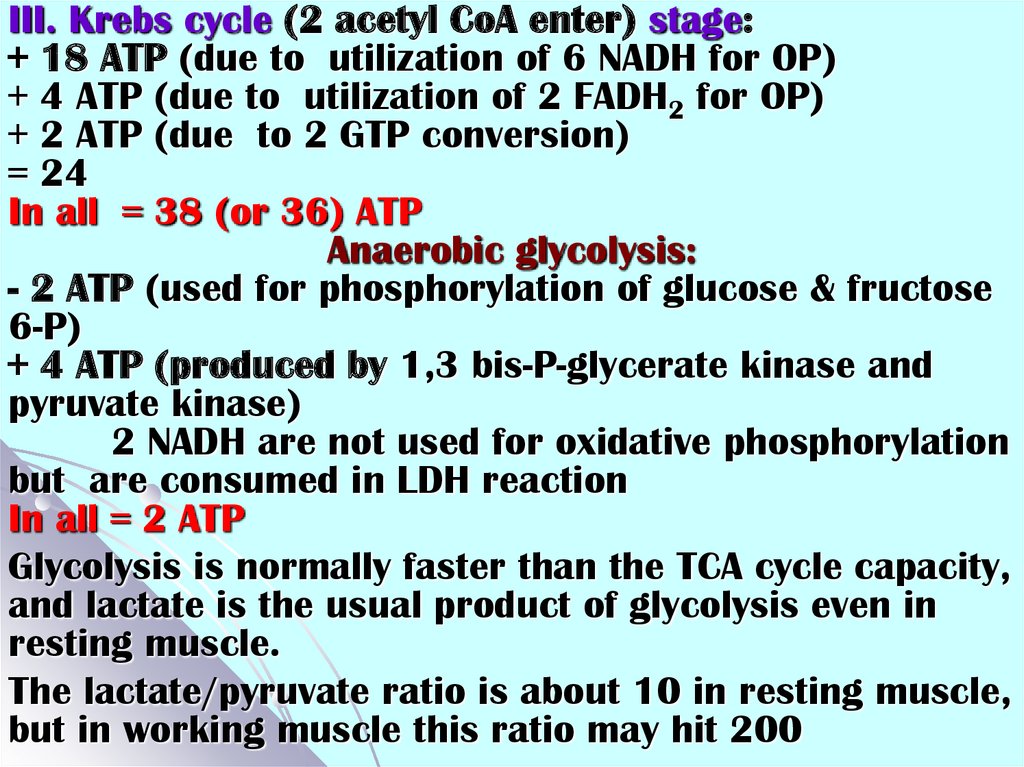
III. Krebs cycle (2 acetyl CoA enter) stage:+ 18 ATP (due to utilization of 6 NADH for OP)
+ 4 ATP (due to utilization of 2 FADH2 for OP)
+ 2 ATP (due to 2 GTP conversion)
= 24
In all = 38 (or 36) ATP
Anaerobic glycolysis:
- 2 ATP (used for phosphorylation of glucose & fructose
6-P)
+ 4 ATP (produced by 1,3 bis-P-glycerate kinase and
pyruvate kinase)
2 NADH are not used for oxidative phosphorylation
but are consumed in LDH reaction
In all = 2 ATP
Glycolysis is normally faster than the TCA cycle capacity,
and lactate is the usual product of glycolysis even in
resting muscle.
The lactate/pyruvate ratio is about 10 in resting muscle,
but in working muscle this ratio may hit 200
35.
36. Glycolysis is regulated at 3 steps involving non equilibrium reactions
Step1: Hexokinase
Step
3: Phosphofructokinase 1
Step
10: Pyruvate kinase
These three enzymes are key enzymes
for Glycolysis
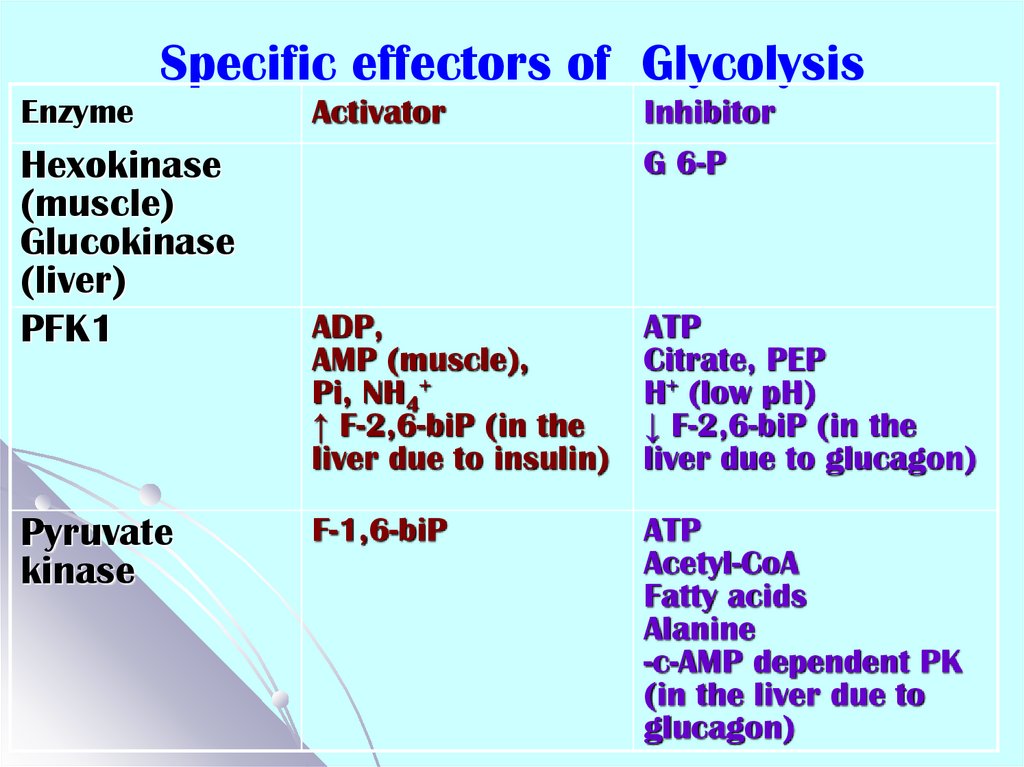
37. Specific effectors of Glycolysis
EnzymeHexokinase
(muscle)
Glucokinase
(liver)
PFK1
Pyruvate
kinase
Activator
Inhibitor
G 6-P
ADP,
AMP (muscle),
Pi, NH4+
↑ F-2,6-biP (in the
liver due to insulin)
ATP
Citrate, PEP
H+ (low pH)
↓ F-2,6-biP (in the
liver due to glucagon)
F-1,6-biP
ATP
Acetyl-CoA
Fatty acids
Alanine
-c-AMP dependent PK
(in the liver due to
glucagon)
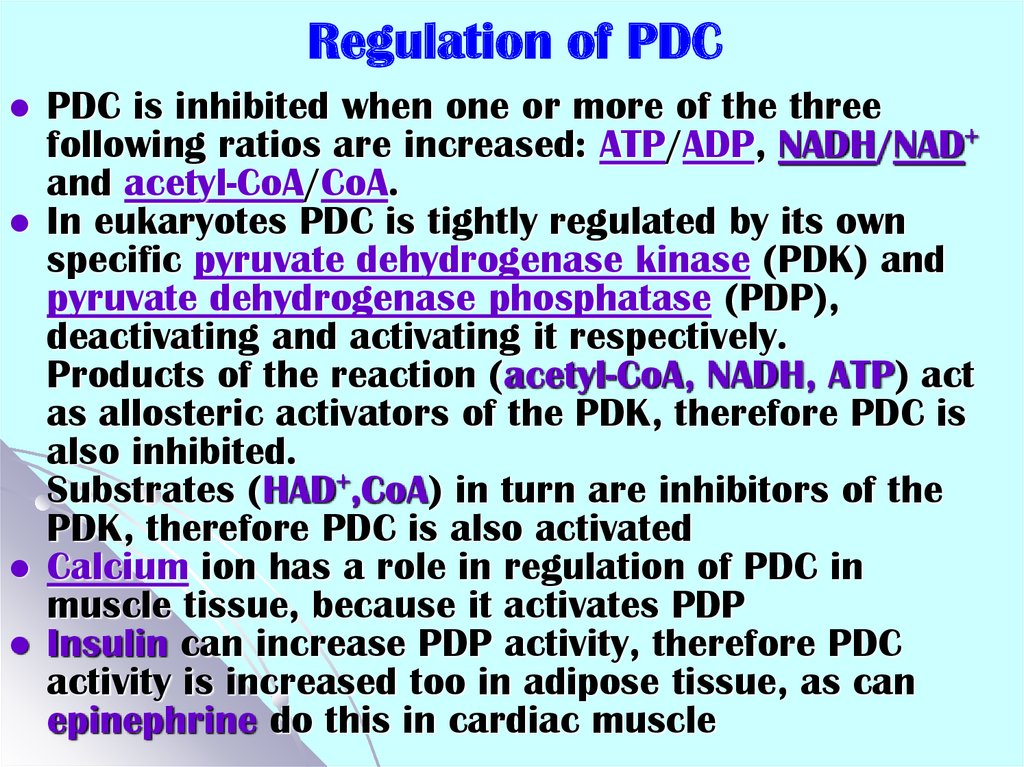
38. Regulation of PDC
PDC is inhibited when one or more of the threefollowing ratios are increased: ATP/ADP, NADH/NAD+
and acetyl-CoA/CoA.
In eukaryotes PDC is tightly regulated by its own
specific pyruvate dehydrogenase kinase (PDK) and
pyruvate dehydrogenase phosphatase (PDP),
deactivating and activating it respectively.
Products of the reaction (acetyl-CoA, NADH, ATP) act
as allosteric activators of the PDK, therefore PDC is
also inhibited.
Substrates (HAD+,CoA) in turn are inhibitors of the
PDK, therefore PDC is also activated
Calcium ion has a role in regulation of PDC in
muscle tissue, because it activates PDP
Insulin can increase PDP activity, therefore PDC
activity is increased too in adipose tissue, as can
epinephrine do this in cardiac muscle
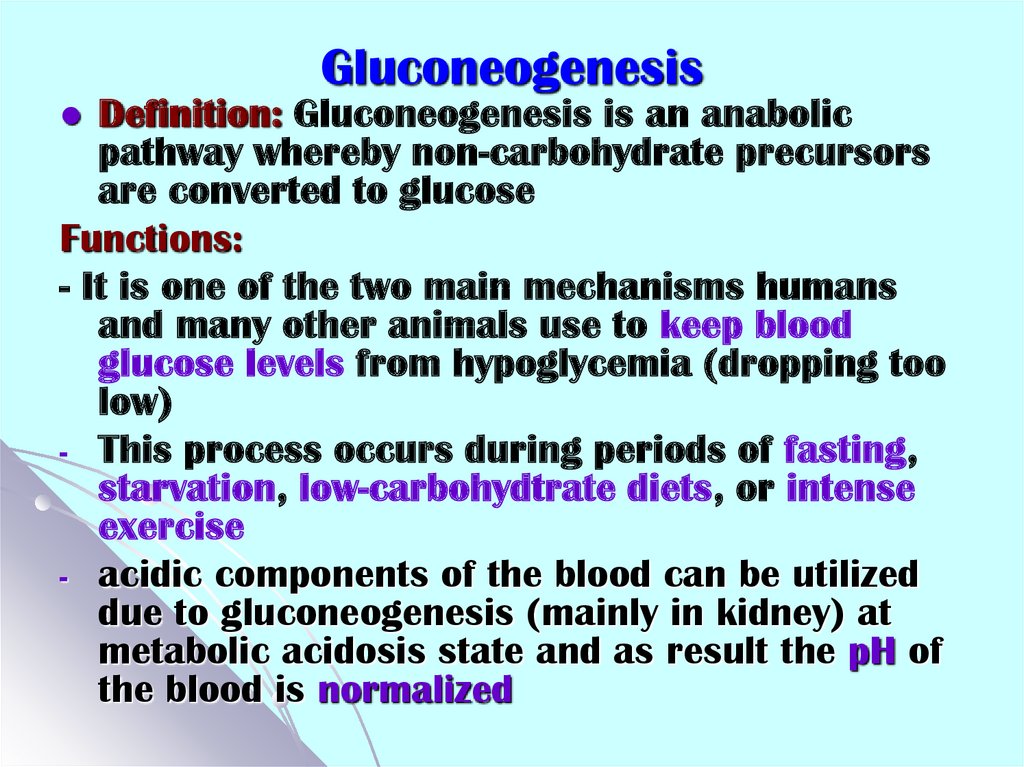
39. Gluconeogenesis
Definition: Gluconeogenesis is an anabolicpathway whereby non-carbohydrate precursors
are converted to glucose
Functions:
- It is one of the two main mechanisms humans
and many other animals use to keep blood
glucose levels from hypoglycemia (dropping too
low)
- This process occurs during periods of fasting,
starvation, low-carbohydtrate diets, or intense
exercise
- acidic components of the blood can be utilized
due to gluconeogenesis (mainly in kidney) at
metabolic acidosis state and as result the pH of
the blood is normalized
40. Gluconeogenesis
Location in the body :Glucose is synthesized between almost nil and
perhaps 200 g/day in adults
- Liver ( 90% )
- Kidney cortical layer (10%)
- Small intestine (0,1%)
Location within the cell (if pyruvate is the substrate):
- It is started in mitochondrion &
- is continued in cytoplasm &
- is finished in the lumen of the endoplasmic
reticulum
41. Gluconeogenesis
Substrates:Lactate ( produced in RBC, muscles)
Glycerol (produced in adipocytes due to lipolysis)
Glucogenic amino acids (all except Leu, Lys)
Propionyl CoA (due to oxidation of odd carbon chain
fatty acids from vegetable foodstuff mainly)
Most precursors must enter the Krebs cycle at
some point to be converted to oxaloacetate
Product:
- Glucose
Net reaction for Gluconeogenesis:
2 CH3-CO-COOH + 4ATP + 2GTP + 2NADH + 2H+ + 6H2O
C6H12O6 + 2 NAD+ + 4ADP + 2GDP + 6Pi
42.
Cori CycleLiver
Glucose
2 NAD+
2 NADH
6 ~P
2 Pyruvate
2 NADH
2 NAD+
2 Lactate
Blood
Muscle
Glucose
2 NAD+
2 NADH
2 ~P
2 Pyruvate
2 NADH
2 NAD+
2 Lactate
The major metabolic product produced under normal
circumstances by erythrocytes and by muscle cells
during intense exercise lactate is recycled to glucose
through the liver in the Cori cycle
43. Gluconeogenesis reactions
Synthesis of glucose from pyruvate utilizesmany of the same enzymes as Glycolysis.
Gluconeogenesis is not just the reverse of
glycolysis.
Three Glycolysis reactions are essentially
irreversible:
Hexokinase (or Glucokinase);
PFK1;
Pyruvate kinase
These steps must be bypassed in
gluconeogenesis
Two of the bypass reactions involve simple
hydrolysis reactions:
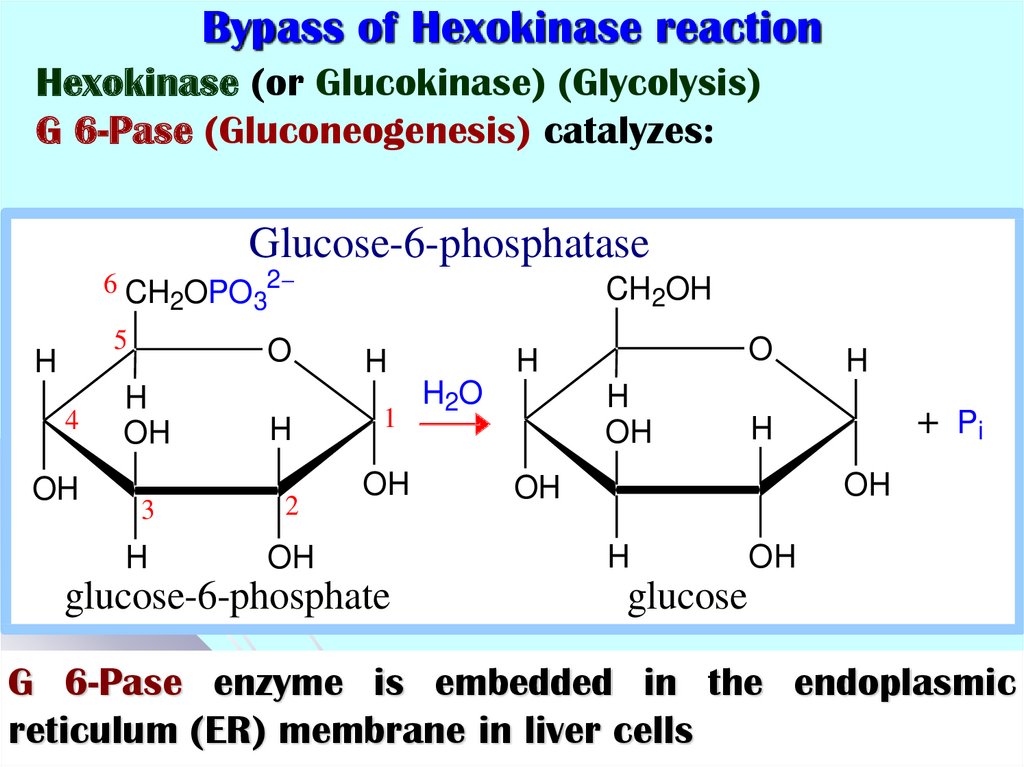
44. Bypass of Hexokinase reaction
Hexokinase (or Glucokinase) (Glycolysis)G 6-Pase (Gluconeogenesis) catalyzes:
Glucose-6-phosphatase
6 CH OPO 2
2
3
5
O
H
4
OH
H
OH
3
H
H
2
CH2OH
1
OH
OH
glucose-6-phosphate
O
H
H
H2O
H
OH
H
+ Pi
H
OH
OH
H
OH
glucose
G 6-Pase enzyme is embedded in the endoplasmic
reticulum (ER) membrane in liver cells
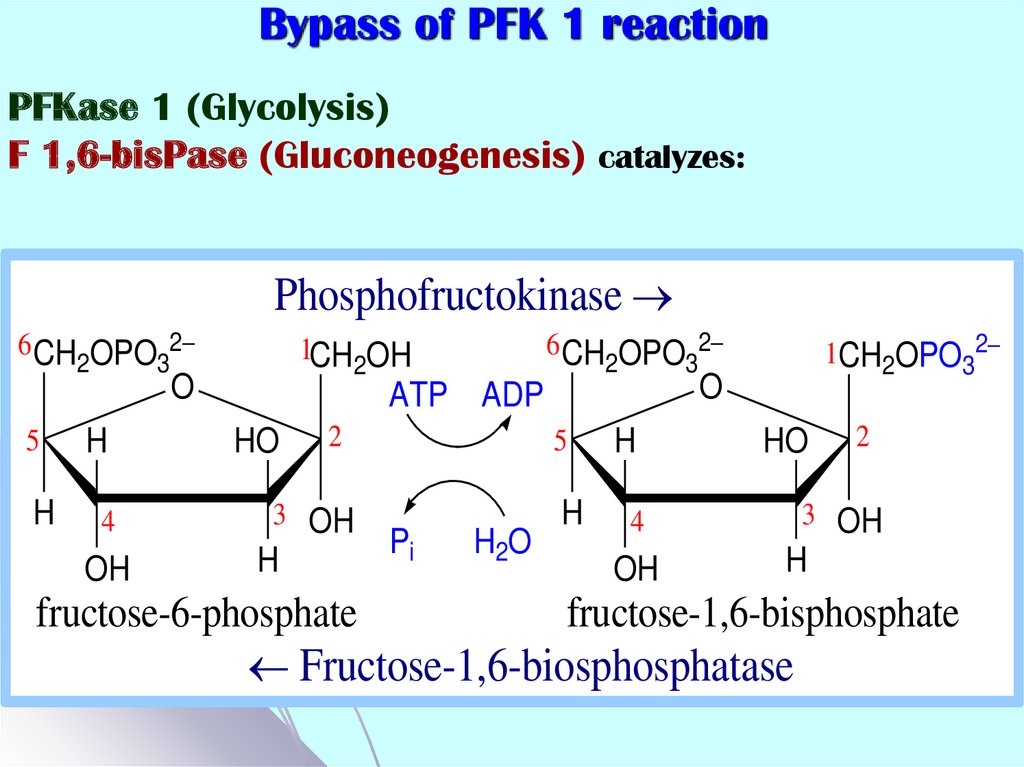
45. Bypass of PFK 1 reaction
PFKase 1 (Glycolysis)F 1,6-bisPase (Gluconeogenesis) catalyzes:
Phosphofructokinase
6 CH OPO 2
2
3
O
5
H
H
4
OH
6 CH OPO 2
2
3
1CH2OH
O
ATP ADP
HO
2
3 OH
H
fructose-6-phosphate
5
Pi
H2O
1CH2OPO32
H
H
HO
3 OH
4
OH
2
H
fructose-1,6-bisphosphate
Fructose-1,6-biosphosphatase
46. Bypass of Pyruvate Kinase reaction
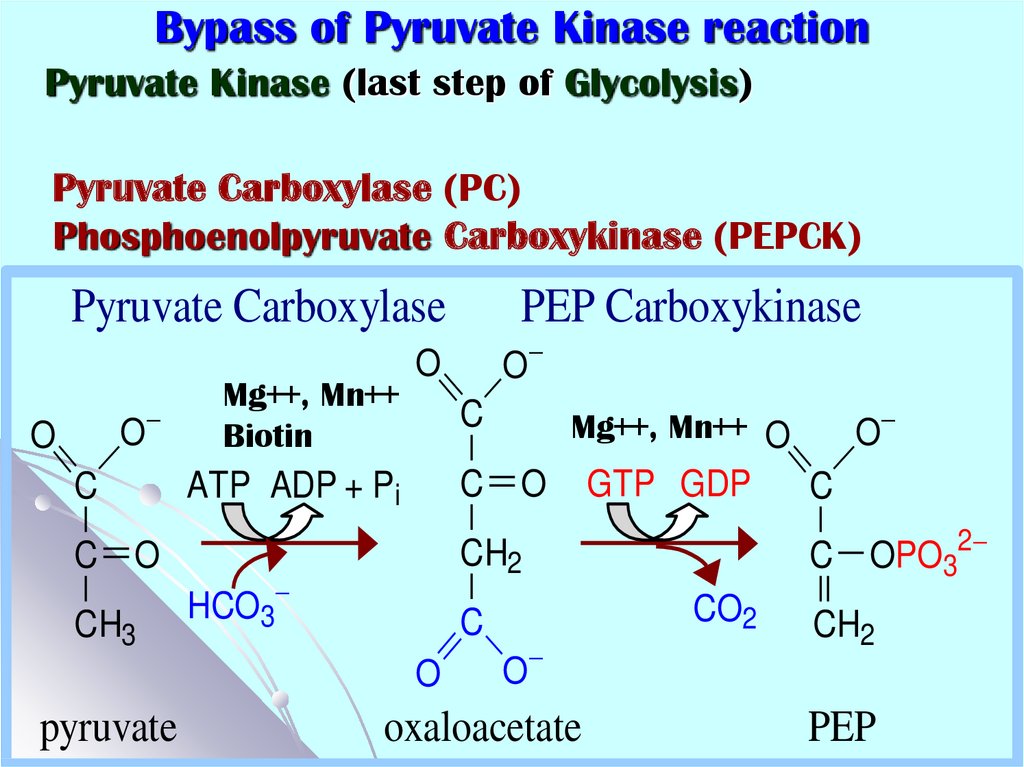
Pyruvate Kinase (last step of Glycolysis)Pyruvate Carboxylase (PC)
Phosphoenolpyruvate Carboxykinase (PEPCK)
Pyruvate Carboxylase
O
O
C
C O
CH3
Mg++, Mn++
Biotin
O
O
C
GTP GDP
C
CO2
O
oxaloacetate
C
C OPO32
CH2
HCO3
O
Mg++, Mn++ O
C O
ATP ADP + Pi
O
pyruvate
PEP Carboxykinase
CH2
PEP
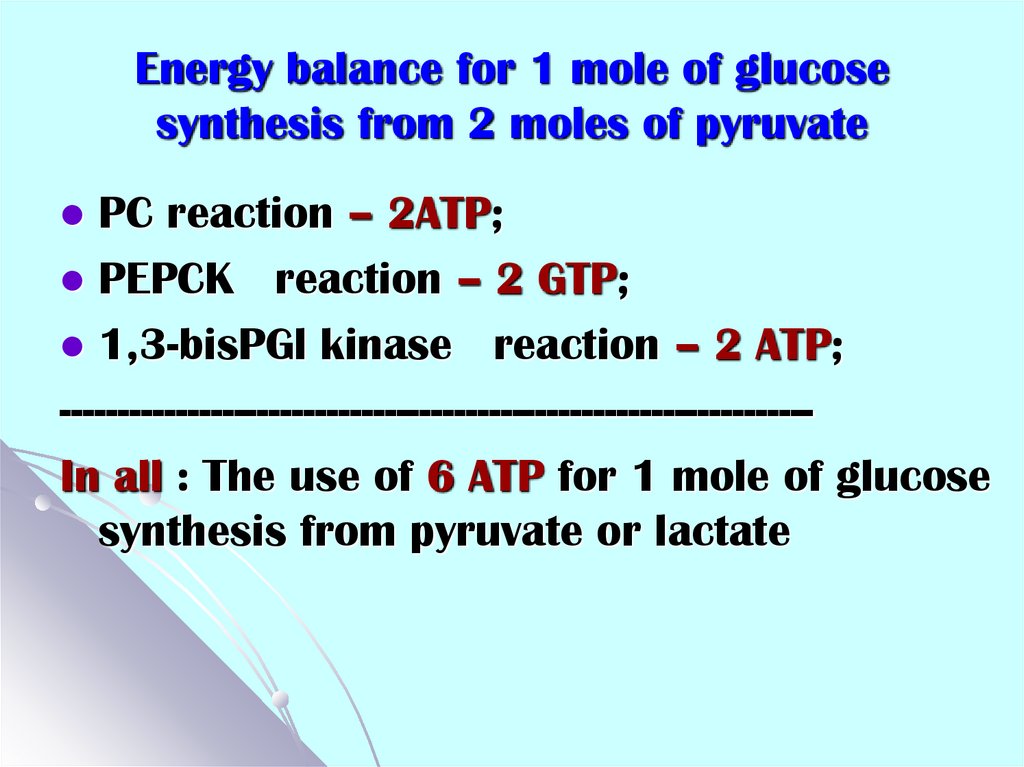
47. Energy balance for 1 mole of glucose synthesis from 2 moles of pyruvate
PC reaction – 2ATP;PEPCK reaction – 2 GTP;
1,3-bisPGl kinase reaction – 2 ATP;
----------------------------------------------------------------In all : The use of 6 ATP for 1 mole of glucose
synthesis from pyruvate or lactate
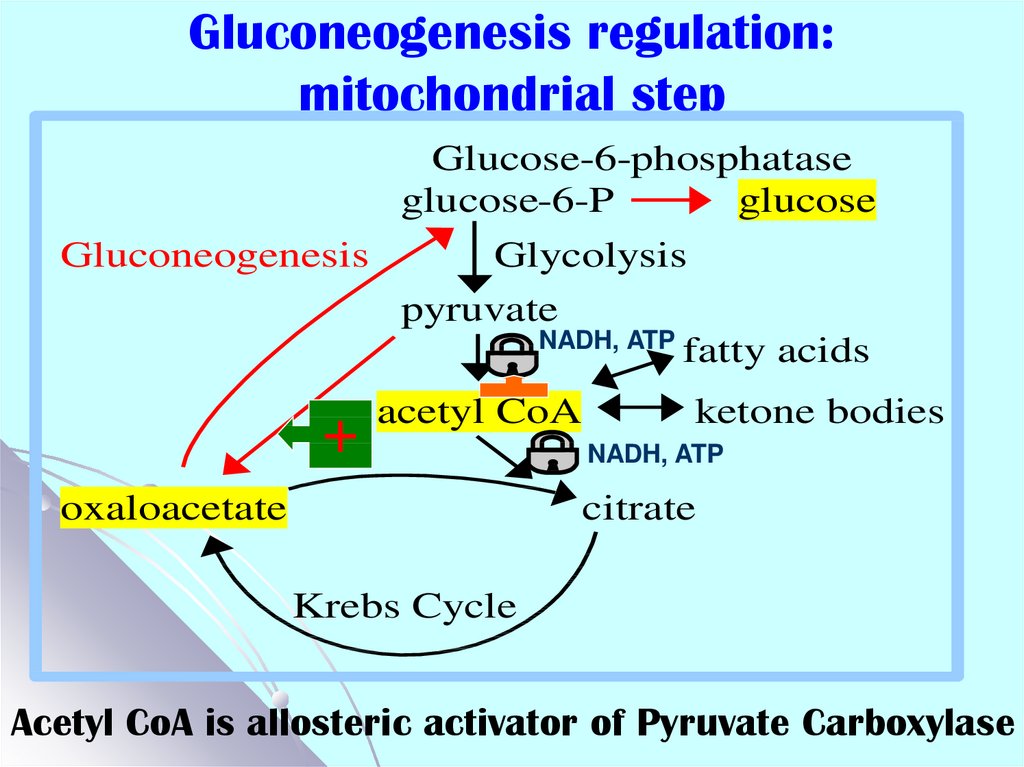
48. Gluconeogenesis regulation: mitochondrial step
Glucose-6-phosphataseglucose-6-P
glucose
Gluconeogenesis
+
Glycolysis
pyruvate
NADH, ATP
fatty acids
acetyl CoA
oxaloacetate
ketone bodies
NADH, ATP
citrate
Krebs Cycle
Acetyl CoA is allosteric activator of Pyruvate Carboxylase
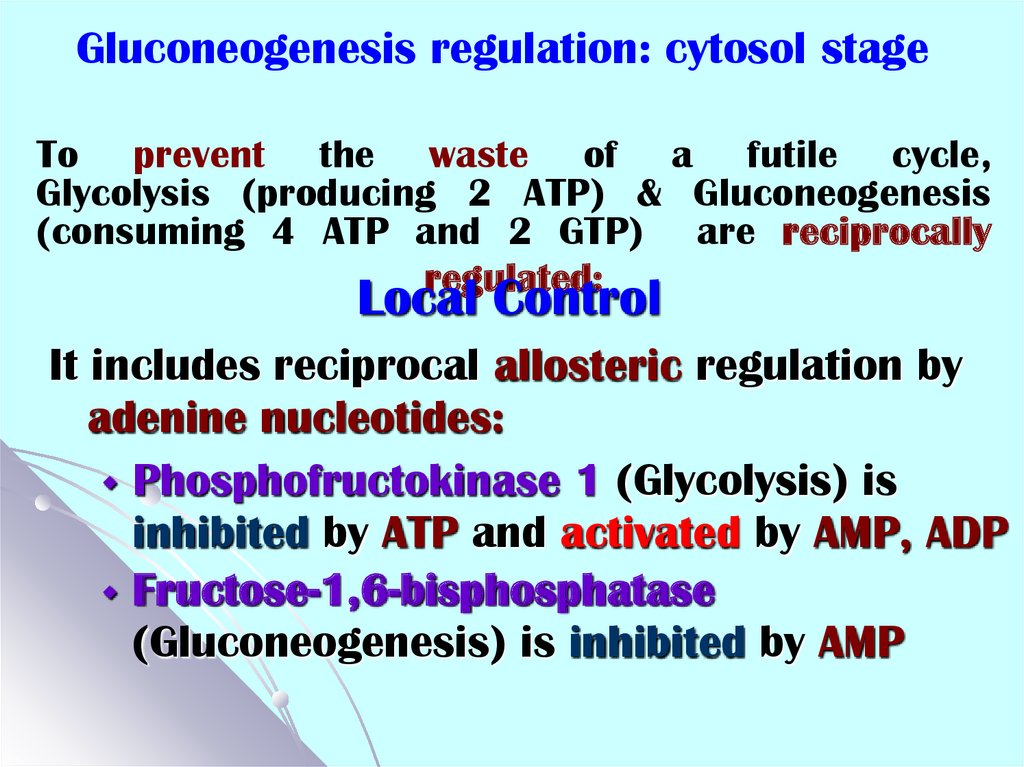
49. To prevent the waste of a futile cycle, Glycolysis (producing 2 ATP) & Gluconeogenesis (consuming 4 ATP and 2 GTP) are
Gluconeogenesis regulation: cytosol stageTo prevent the waste of a futile cycle,
Glycolysis (producing 2 ATP) & Gluconeogenesis
(consuming 4 ATP and 2 GTP) are reciprocally
regulated:
Local Control
It includes reciprocal allosteric regulation by
adenine nucleotides:
Phosphofructokinase 1 (Glycolysis) is
inhibited by ATP and activated by AMP, ADP
Fructose-1,6-bisphosphatase
(Gluconeogenesis) is inhibited by AMP
50. Global Control in liver cells
It includes reciprocal effects of a cyclic AMPcascade, triggered by the hormone glucagon
when blood glucose is low and epinephrine
during stress
Phosphorylation of enzymes & regulatory
proteins in liver by Protein Kinase A (cAMP
Dependent Protein Kinase) results in
inhibition of glycolysis
stimulation of gluconeogenesis,
making glucose available for release to the
blood
51. Global Control in liver cells
Enzymes relevant to these pathways that arephosphorylated by Protein Kinase A include:
Pyruvate
Kinase, a glycolysis enzyme that is
inhibited when phosphorylated.
CREB
(cAMP response element binding
protein) which activates, through other
factors, transcription of the gene for PEP
Carboxykinase, leading to increased
gluconeogenesis.
A
bi-functional enzyme that makes and
degrades an allosteric regulator, fructose2,6-bisphosphate
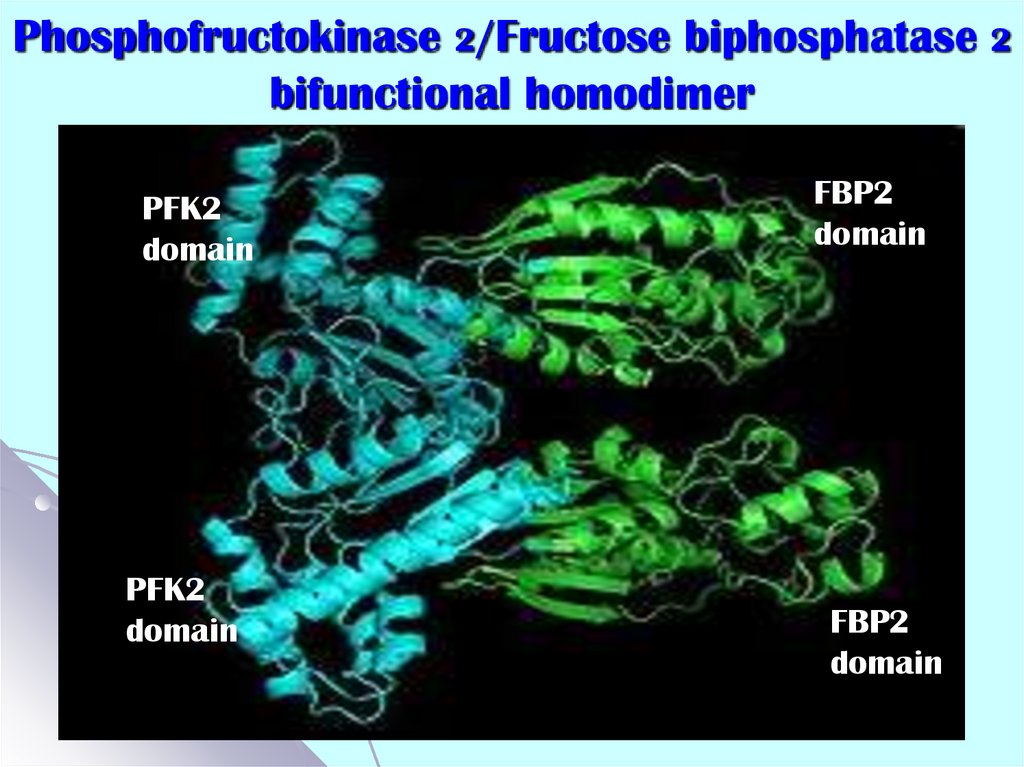
52. Phosphofructokinase 2/Fructose biphosphatase 2 bifunctional homodimer
PFK2domain
PFK2
domain
FBP2
domain
FBP2
domain
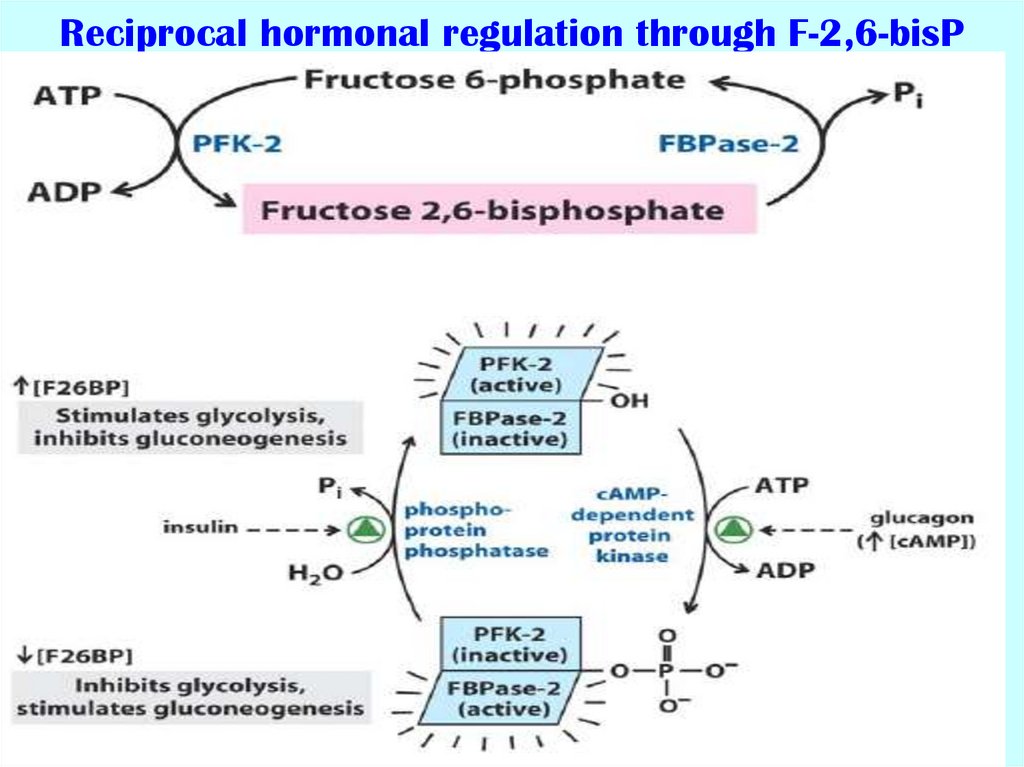
53. Reciprocal hormonal regulation through F-2,6-bisP
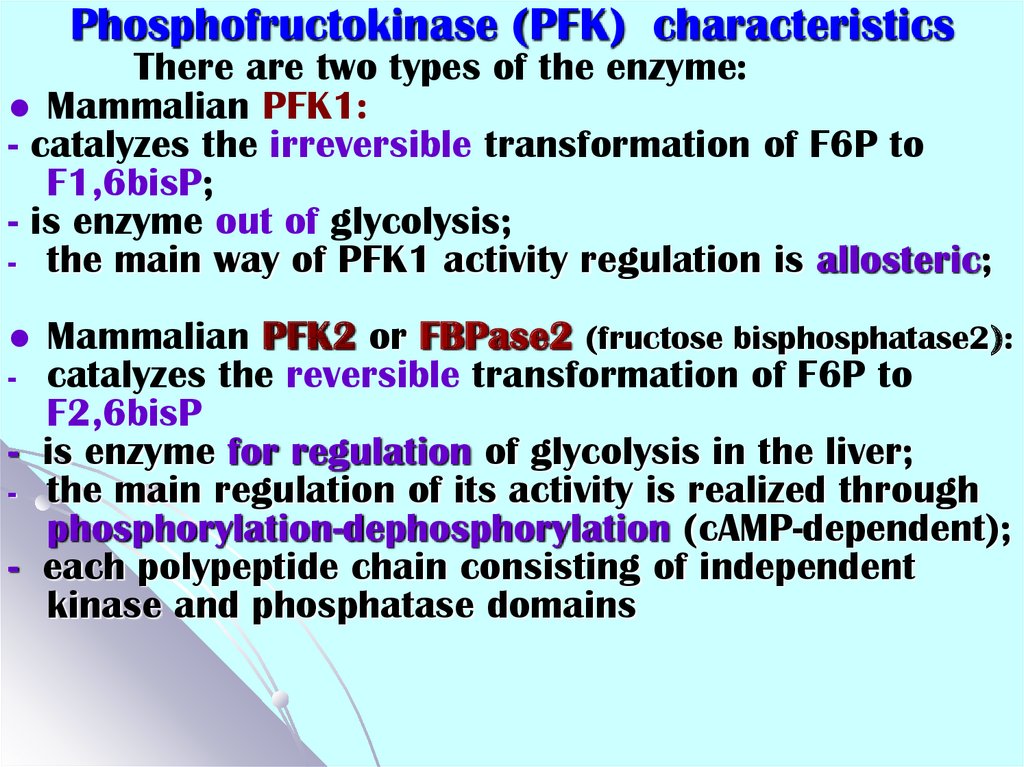
54. Phosphofructokinase (PFK) characteristics
There are two types of the enzyme:Mammalian PFK1:
- catalyzes the irreversible transformation of F6P to
F1,6bisP;
- is enzyme out of glycolysis;
- the main way of PFK1 activity regulation is allosteric;
Mammalian PFK2 or FBPase2 (fructose bisphosphatase2):
catalyzes the reversible transformation of F6P to
F2,6bisP
- is enzyme for regulation of glycolysis in the liver;
- the main regulation of its activity is realized through
phosphorylation-dephosphorylation (cAMP-dependent);
- each polypeptide chain consisting of independent
kinase and phosphatase domains
-
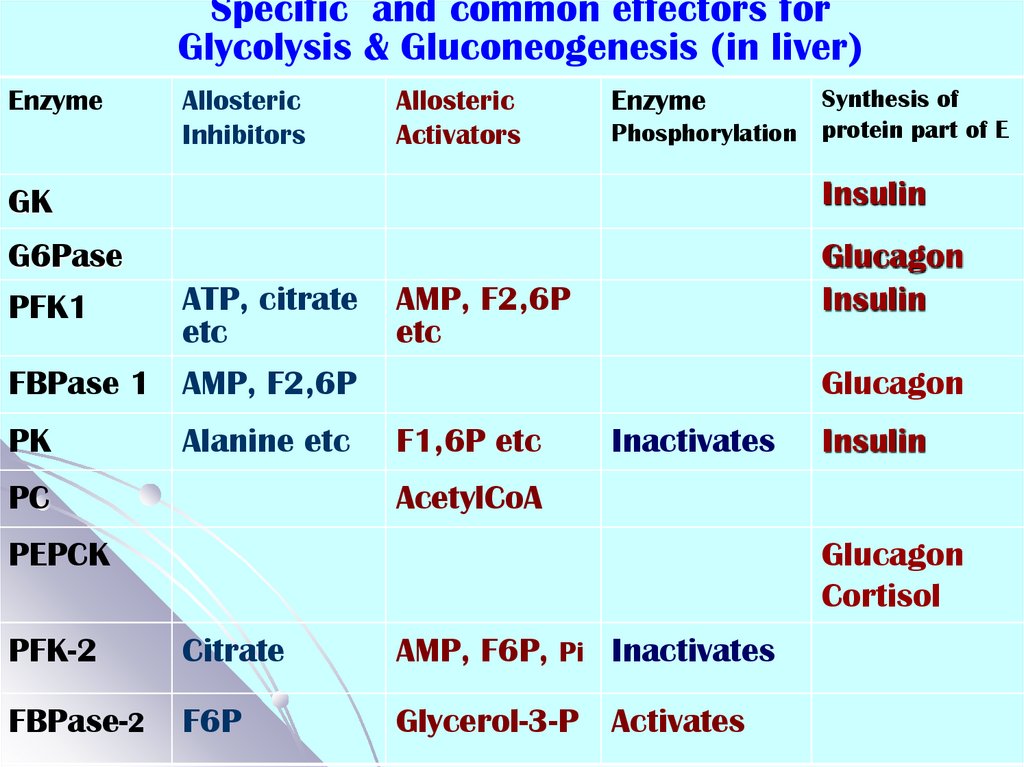
55. Specific and common effectors for Glycolysis & Gluconeogenesis (in liver)
Specific and common effectors forGlycolysis & Gluconeogenesis (in liver)
Enzyme
Allosteric
Inhibitors
Allosteric
Activators
Synthesis of
Phosphorylation protein part of E
Enzyme
GK
Insulin
G6Pase
PFK1
Glucagon
Insulin
ATP, citrate
etc
AMP, F2,6P
etc
FBPase 1 AMP, F2,6P
PK
Alanine etc
PC
Glucagon
F1,6P etc
Inactivates
Insulin
AcetylCoA
PEPCK
Glucagon
Cortisol
PFK-2
Citrate
AMP, F6P, Pi Inactivates
FBPase-2
F6P
Glycerol-3-P Activates















 biology
biology