Similar presentations:
CHE1226 Physical Chemistry School of Chemical Engineering Lecture 3 – Internal Energy. First Law of Thermodynamics
1. CHE1226 Physical Chemistry
School of Chemical EngineeringLecture 3 – Internal Energy. First Law of Thermodynamics
2. Table of contents
• Internal Energy• Work
• Heat
• ΔU Cases
Learning Objective: Quantify the internal energy of a gas and discuss how energy is transferred to/from
the gas to its surroundings through work and heat.
References:
P. Atkins and J. de Paula, Elements of Physical Chemistry, Chapter 2
Chang, Physical Chemistry, Chapter 2
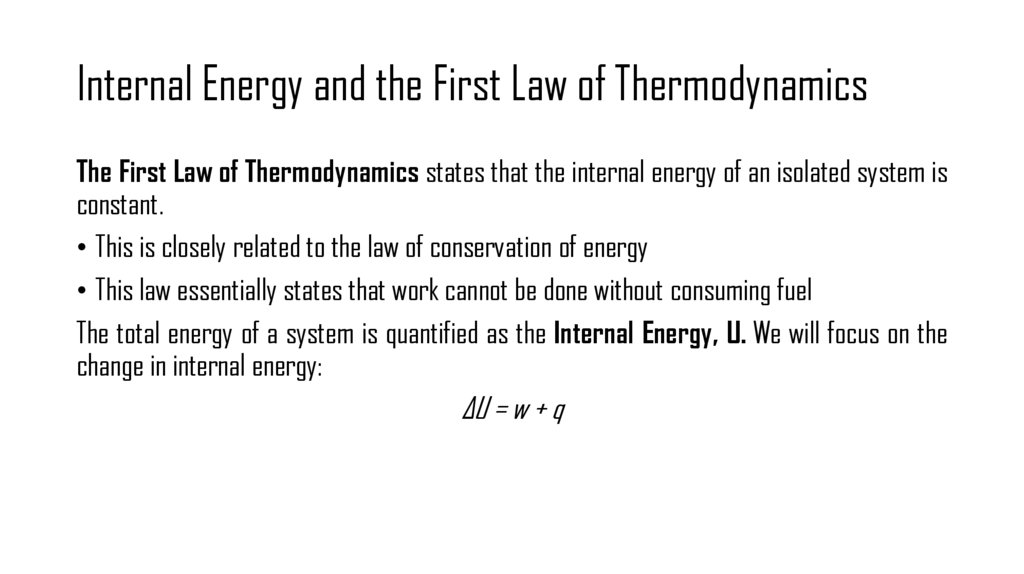
3. Internal Energy and the First Law of Thermodynamics
The First Law of Thermodynamics states that the internal energy of an isolated system isconstant.
• This is closely related to the law of conservation of energy
• This law essentially states that work cannot be done without consuming fuel
The total energy of a system is quantified as the Internal Energy, U. We will focus on the
change in internal energy:
ΔU = w + q
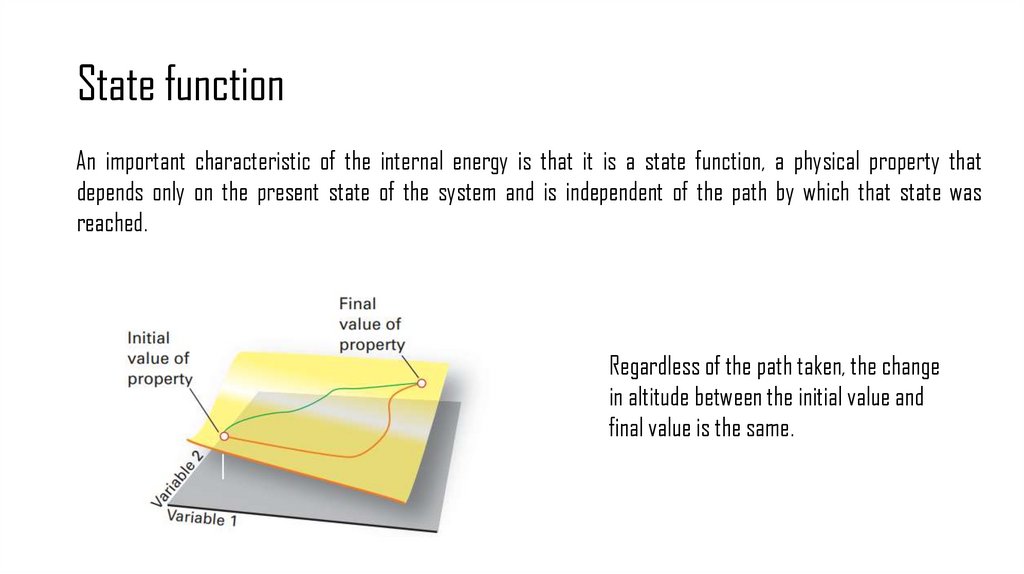
4. State function
An important characteristic of the internal energy is that it is a state function, a physical property thatdepends only on the present state of the system and is independent of the path by which that state was
reached.
Regardless of the path taken, the change
in altitude between the initial value and
final value is the same.
5. Systems and Surroundings
We need to define where reactions take place:• The Surroundings: From where we make observations. It also acts as a very large reservoir
adsorbing/delivering pressure, volume and heat while remaining unchanged.
• The System: The part of the world where we have interest. The types of systems exists:
1. An Open System can exchange both energy and matter with the surroundings.
2. A Closed System can only exchange energy with the surroundings.
3. An Isolated System can not exchange energy or matter with the surroundings.
The transfer of energy between the system and surroundings is
quantified as work and heat
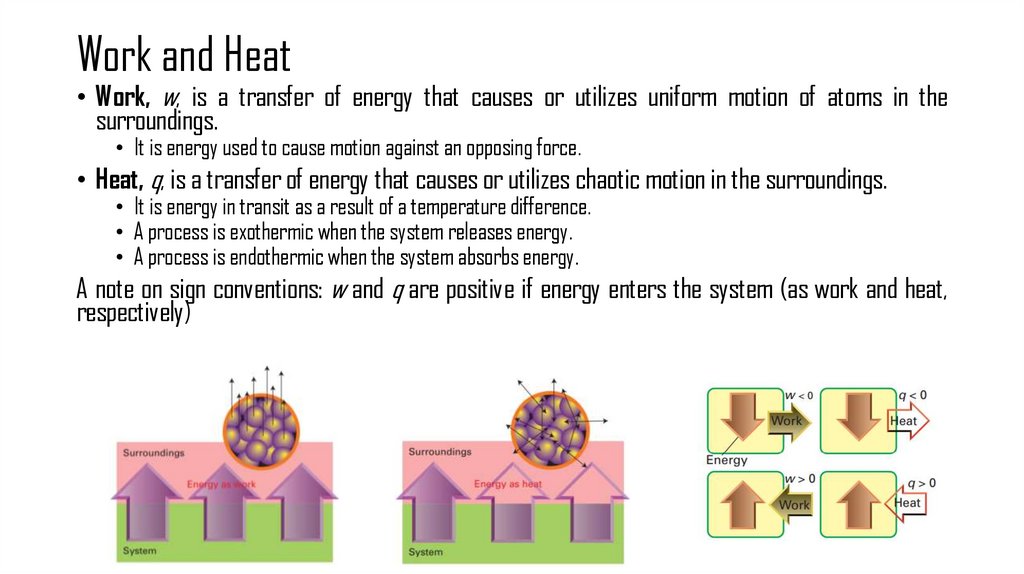
6. Work and Heat
• Work, w, is a transfer of energy that causes or utilizes uniform motion of atoms in thesurroundings.
• It is energy used to cause motion against an opposing force.
• Heat, q, is a transfer of energy that causes or utilizes chaotic motion in the surroundings.
• It is energy in transit as a result of a temperature difference.
• A process is exothermic when the system releases energy.
• A process is endothermic when the system absorbs energy.
A note on sign conventions: w and q are positive if energy enters the system (as work and heat,
respectively)
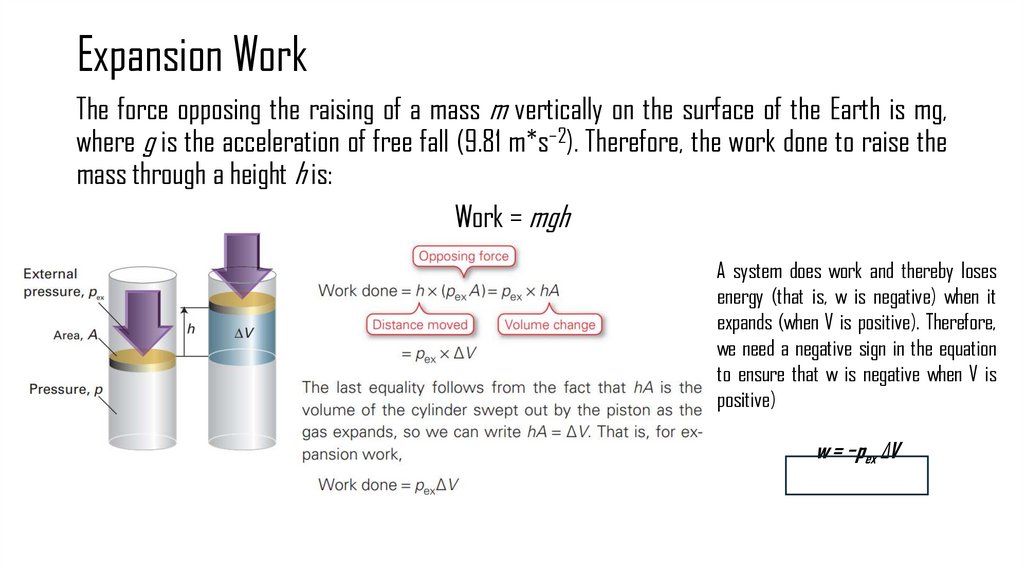
7. Expansion Work
The force opposing the raising of a mass m vertically on the surface of the Earth is mg,where g is the acceleration of free fall (9.81 m*s−2). Therefore, the work done to raise the
mass through a height h is:
Work = mgh
A system does work and thereby loses
energy (that is, w is negative) when it
expands (when V is positive). Therefore,
we need a negative sign in the equation
to ensure that w is negative when V is
positive)
w = −pex ΔV
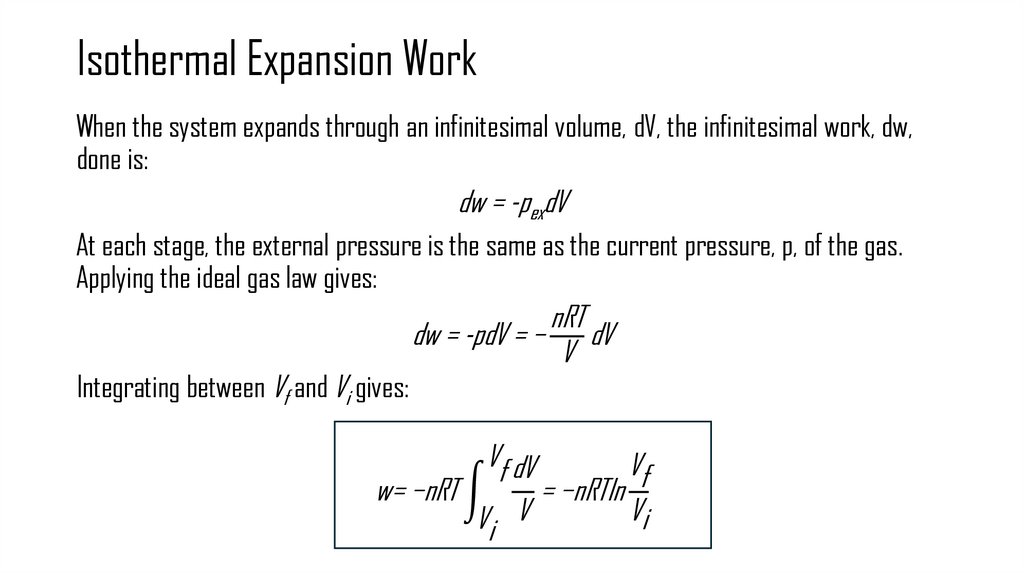
8. Isothermal Expansion Work
When the system expands through an infinitesimal volume, dV, the infinitesimal work, dw,done is:
dw = -pexdV
At each stage, the external pressure is the same as the current pressure, p, of the gas.
Applying the ideal gas law gives:
Integrating between Vf and Vi gives:
nRT
dw = -pdV = − dV
V
Vf dV
Vf
w= −nRT න
= −nRTln
Vi
Vi V
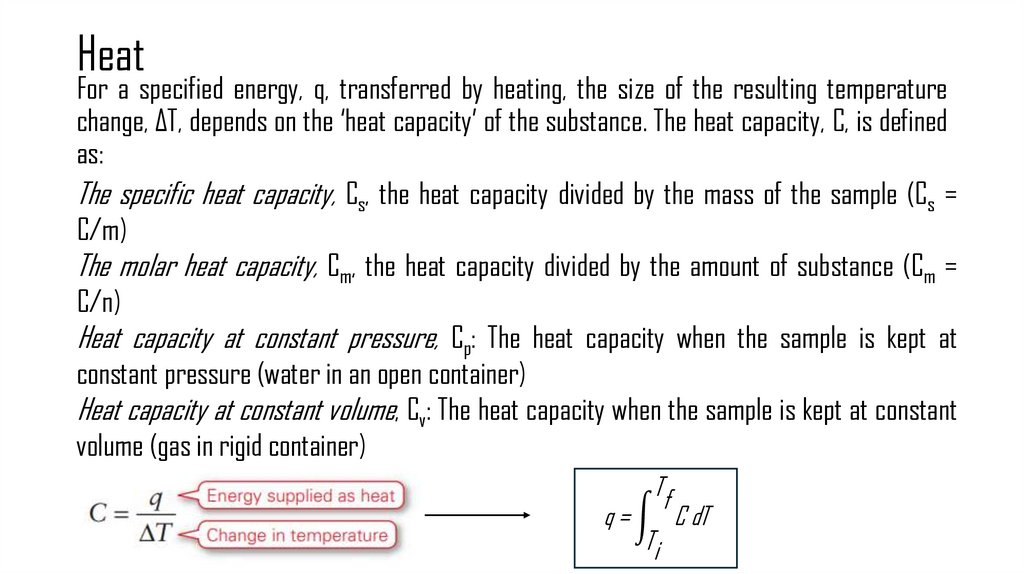
9. Heat
For a specified energy, q, transferred by heating, the size of the resulting temperaturechange, ΔT, depends on the ‘heat capacity’ of the substance. The heat capacity, C, is defined
as:
The specific heat capacity, Cs, the heat capacity divided by the mass of the sample (Cs =
C/m)
The molar heat capacity, Cm, the heat capacity divided by the amount of substance (Cm =
C/n)
Heat capacity at constant pressure, Cp: The heat capacity when the sample is kept at
constant pressure (water in an open container)
Heat capacity at constant volume, Cv: The heat capacity when the sample is kept at constant
volume (gas in rigid container)
Tf
q = න C dT
Ti
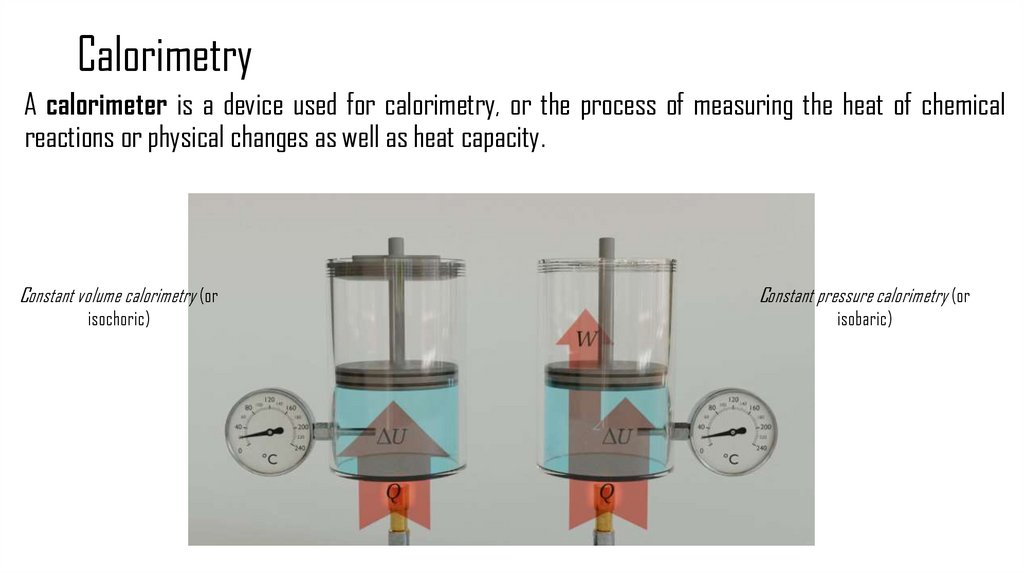
10. Calorimetry
A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemicalreactions or physical changes as well as heat capacity.
Constant volume calorimetry (or
Constant pressure calorimetry (or
isochoric)
isobaric)
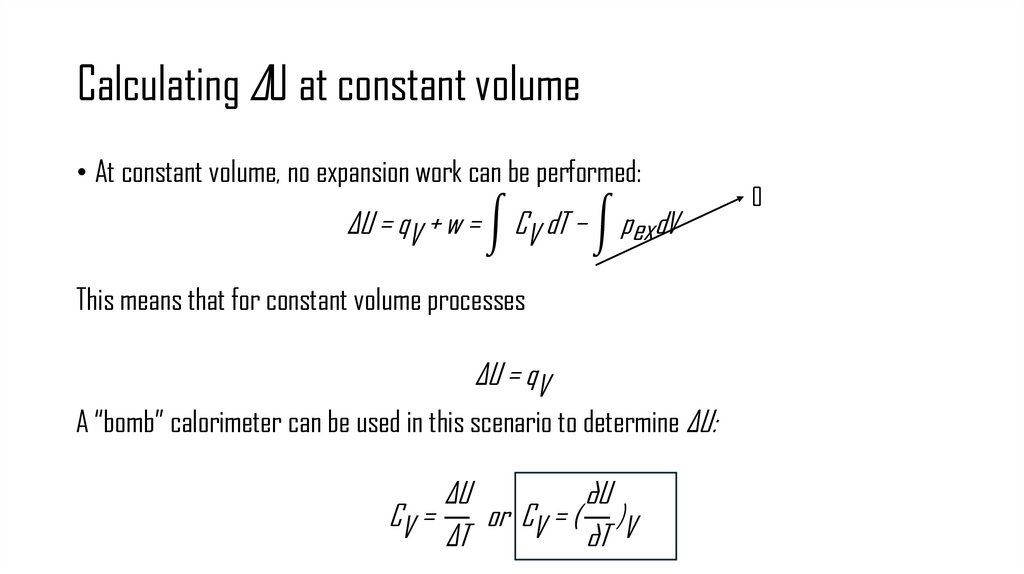
11. Calculating ΔU at constant volume
• At constant volume, no expansion work can be performed:ΔU = qV + w = න CV dT − න pex dV
This means that for constant volume processes
ΔU = qV
A “bomb” calorimeter can be used in this scenario to determine ΔU:
ΔU
∂U
CV =
or CV = ( )V
ΔT
∂T
0
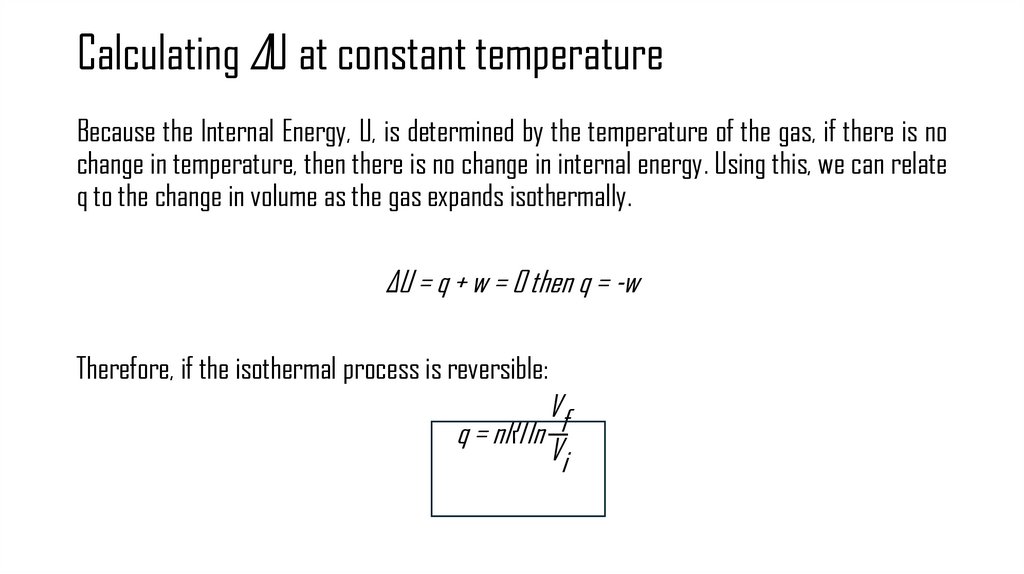
12. Calculating ΔU at constant temperature
Because the Internal Energy, U, is determined by the temperature of the gas, if there is nochange in temperature, then there is no change in internal energy. Using this, we can relate
q to the change in volume as the gas expands isothermally.
ΔU = q + w = 0 then q = -w
Therefore, if the isothermal process is reversible:
Vf
q = nRTln
Vi



 physics
physics








