Similar presentations:
Elrazi university
1.
ELRAZI UNIVERSITYFaculty of medicine
ENDOCRINE PATHOLOGY
ADRENAL GLAND
G. M. ELIMAIRI
2.
ADRENAL GLANDS3.
ADRENALSThe adrenals consist essentially of two separate
endocrine glands within a single anatomical organ.
The medulla, is part of the sympathetic nervous
system; it secretes catecholamines, which are
essential in the physiological responses to stress, e.g.
infection, shock or injury.
The cortex, derived from mesoderm, synthesize a
range of steroid hormones with generalised effects on
metabolism, the immune system, and water and
electrolyte balance.
4.
ADRENAL MEDULLAHistologically, the adrenal medulla consists of
chromaffin cells and sympathetic nerve endings.
The adrenal medulla is the main source of
adrenaline (epinephrine), as it is produced there
from noradrenaline (norepinephrine) by the
enzyme phenyl-ethanolamine-N-methyl
transferase.
Elsewhere in the body, sympathetic nerve endings
lack this enzyme and their secretory product is
thus noradrenaline.
5.
ADRENAL MEDULLACatecholamines are secreted in states of stress
and of hypovolaemic shock, when they are vital in
the maintenance of blood pressure by causing
vasoconstriction in the skin, gut and skeletal
muscles.
At tissue level, these hormones bind to cell surface
receptors, altering cellular levels of a second
messenger, cyclic AMP, which brings about rapid
functional changes in the cell.
6.
TUMOURS OF ADRENAL MEDULLAPhaeochromocytoma
Derived from adrenal medullary chromaffin cells
Symptoms due to excess catecholamine secretion (e.g.
hypertension, sweating)
May be familial and associated with other endocrine
tumours
Occasionally malignant
A curable cause of secondary hypertension
7.
. The tumour presents through the effects of itscatecholamine secretions:
hypertension (which is sometimes intermittent),
pallor,
headaches,
sweating and nervousness.
Its presence should be suspected especially in younger
hypertensive patients.
Although it is a rare cause of hypertension,
phaeochromocytoma must not be overlooked.
8.
The diagnosis of phaeochromocytoma is usuallybased on estimating the urinary excretion of
vanillylmandelic acid (VMA), a catecholamine
metabolite, which is generally at least doubled in
the presence of the tumour.
Localisation of the tumour is assisted by computed
tomography of the abdomen and by radioisotope
scanning .
9.
Phaeochromocytoma may be familial, associated withmedullary carcinoma of the thyroid or with
hyperparathyroidism as part of a multiple endocrine
neoplasia (MEN) syndrome.
The familial cases are frequently bilateral.
Other associations are with neurofibromatosis
Phaeochromocytomas are brown, and are highly
vascular .
Although most are benign, a few phaeochromocytomas
pursue a malignant course.
10.
NeuroblastomaNeuroblastoma is a rare and highly malignant tumour
found in infants and children.
Derived from sympathetic nerve cells it may, secrete
catecholamines, and there may be elevated levels of
their metabolites in the urine.
Neuroblastomas may also originate from parts of the
sympathetic chain outside the adrenal medulla.
Secondary spread to liver, skin and bones (especially
those of the skull) is common.
Surprisingly, neuroblastoma may occasionally mature
spontaneously to ganglioneuroma, a benign tumour.
11.
ADRENAL CORTEXHistologically, the adrenal cortex has three zones .
Beneath the capsule lies the zona glomerulosa.
This zone produces mineralocorticoid steroids such as
aldosterone.
Most of the adrenal cortex comprises the middle and
inner zones-zona fasciculata and zona reticularis,
respectively.
The middle zone is rich in lipid.
The inner zone cells convert lipid into corticosteroids,
principally glucocorticoids and sex steroids, for secretion.
12.
Steroid hormonesGlucocorticoids
The glucocorticoids have important effects on a wide
range of tissues and organs. At physiological levels they
Inhibit protein synthesis
Increase protein breakdown
Increase gluconeogenesis.
In excess, as a result of therapeutic administration or high
Levels of endogenous secretion, they cause:
Adiposity of face and trunk
Hypertension
Impaired wound healing
13.
GlucocorticoidsAnti-inflammatory effects
Immunosuppression
Growth inhibition
Osteoporosis
Peptic ulceration
A diabetic state.
The most important of the hormones is cortisol
(hydrocortisone), but other steroid metabolites have
similar effects. The synthesis and secretion of
glucocorticoids are controlled by ACTH from the
pituitary.
14.
MineralocorticoidsThe most important of the mineralocorticoids, aldosterone,
acts on the renal tubules to increase reabsorption of sodium
and chloride, reducing their loss in urine at the expense of
potassium exchange.
The synthesis and release of aldosterone is not under pituitary
control.
Low perfusion pressure in the kidney stimulates release of renin.
This converts angiotensinogen into angiotensin I.
Angiotensin I is then converted to angiotensin II by angiotensin
converting enzyme, mainly in the lung. Angiotensin II stimulates
secretion of aldosterone from the adrenal cortex.
15.
Sex steroidsThe production of sex steroids in the adrenal
cortex is low compared with that in the gonads.
However, virilising androgens may be produced
in conditions such as certain congenital enzyme
defects and adrenal cortical tumours, especially
if these are malignant.
Hyperfunction of the adrenal cortex produces
generalised effects, the nature of which
depends on whether glucocorticoids,
mineralocorticoids or sex steroids are produced
in excess.
16.
Cushing's syndromeDue to excess glucocorticoids
Main features include central obesity, hirsutism,
hypertension, diabetes and osteoporosis
Main causes are excess ACTH secretion from the
pituitary, adrenal cortical neoplasms, or the iatrogenic
effects of ACTH or steroid administration
Exogenous administration of glucocorticoids or ACTH is a
common iatrogenic cause of Cushing's syndrome.
The syndrome occurs most commonly in adult women,
and sometimes there is also excess androgen production
causing virilisation.
17.
Cushing Syndrome18.
CushingSyndrome –
Clinical
Features
19.
Diagnosis Of Cushing's syndromeDiagnosis is by demonstration of glucocorticoid
excess,
either as elevated plasma levels of cortisol
or as elevated urinary excretion of 17hydroxysteroids, degradation products of
glucocorticoids.
Further tests, such as measurement of plasma
ACTH levels, are essential to determine the
cause of the Cushing's syndrome .
20.
PathogenesisIatrogenic disease. The therapeutic administration of
glucocorticoids to the patient is by far the commonest
cause of the features of Cushing's syndrome.
An addition, three different types of natural disease can
cause the syndrome:
Excess ACTH secretion by the adenohypophysis
Adrenal cortical neoplasms
Ectopic ACTH secretion.
21.
HyperaldosteronismPrimary hyperaldosteronism (Conn's syndrome).
This is the autonomous secretion of excess
aldosterone.
The usual cause is an adenoma of the zona
glomerulosa, but generalised hyperplasia of the zona
is sometimes responsible.
The resulting renal retention of sodium and water
leads to hypertension, while potassium loss leads to
muscular weakness and cardiac arrhythmias.
The hypokalaemia is associated with metabolic
alkalosis, causing tetany and paraesthesiae.
22.
HyperaldosteronismSecondary hyperaldosteronism.
When renal glomerular perfusion is reduced, for
example through a fall in blood volume, the
renin-angiotensin system stimulates aldosterone
secretion from the zona glomerulosa in an
attempt to correct this.
This physiological response is known as
secondary hyperaldosteronism, which is by far
the commonest type of hyperaldosteronism.
23.
DiagnosisThe diagnosis of primary hyperaldosteronism
rests on two criteria: plasma aldosterone
must be raised while renin is low.
This is to distinguish it from secondary
hyperaldosteronism, in which aldosterone
levels are raised but are an appropriate
response to high renin levels.
24.
Hypersecretion of sex steroidsSome adrenal cortical adenomas secrete sex
steroids, most commonly androgens.
In Cushing's syndrome, quantities of androgens
are occasionally secreted along with the
glucocorticoids, causing virilisation of females,
especially those with adrenocortical carcinomas.
Failure of cortisol production leads to increased
ACTH secretion, resulting in hyperplasia of the
adrenal cortex.
25.
TumoursAdenoma. adrenal cortical adenomas Cushing's
or Conn's syndromes.
Carcinoma. Adrenal cortical carcinoma is rare;
these tumours are usually hormone-secreting, with
a tendency to produce androgens.
Examination of the adjacent adrenal cortex and
that of the opposite gland may give a clue as to
the function of the neoplasm; glucocorticoidsecreting tumours will suppress ACTH, resulting in
atrophy of the non-neoplastic adrenal cortex.
26.
Adrenal cortical insufficiencyClinical effects are due to lack of mineralocorticoids and
glucocorticoids
Main features include weight loss, lethargy, hypotension,
pigmentation and hyponatraemia
Causes include autoimmune adrenalitis, tuberculosis and
Waterhouse- Friderichsen syndrome
Adrenocortical hypofunction can be primary, due to
lesions within the adrenal gland, or secondary, due to
failure of ACTH secretion by the adenohypophysis.
27.
Adrenal cortical insufficiencyAcute primary insufficiency is called Waterhouse - Friderichsen
syndrome.
Causes of chronic primary insufficiency include:
Tuberculosis
Autoimmune adrenalitis
Amyloidosis
Haemochromatosis
Metastatic tumours
Atrophy due to prolonged steroid therapy.
Autoimmune adrenalitis selectively damages and destroy the adrenal
cortex, sparing the medulla; tuberculosis destroy the cortex and
medulla.
28.
Acute insufficiencyAcute insufficiency ('adrenal apoplexy') was first
noted in children by Waterhouse and Friderichsen
Other acute septicaemias, especially those due to
Gram-negative bacteria, may cause a similar effect.
The adrenal necrosis is probably due to disseminated
intravascular coagulation (DIC).
The symptoms are attributable to lack of
mineralocorticoids (salt and water loss with
hypovolaemic shock) and of glucocorticoids (failure
of gluconeogenesis resulting in hypoglycaemia).
29.
Chronic insufficiencyThomas Addison first described an association between destruction of the
adrenal cortex and the group of symptoms caused by the resulting chronic
insufficiency of adrenal cortical hormones (Addison's disease).
The effects are due to a combined lack of mineralocorticoids and
glucocorticoids:
Anorexia, weight loss, vomiting
Weakness
Lethargy
Hypotension
Skin pigmentation
Hyponatraemia with hyperkalaemia
Chronic dehydration
Sexual dysfunction.
30.
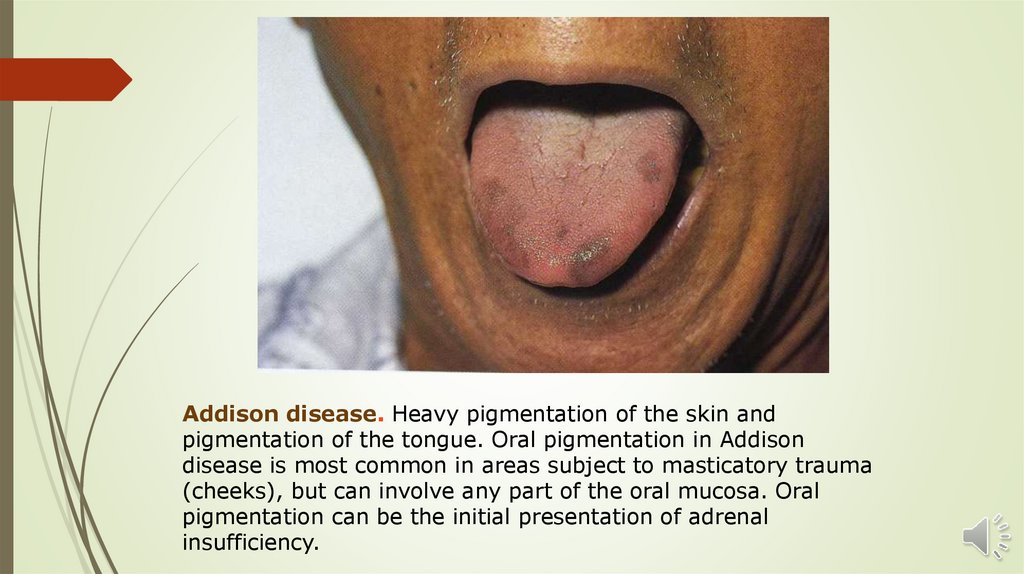
Addison disease. Heavy pigmentation of the skin andpigmentation of the tongue. Oral pigmentation in Addison
disease is most common in areas subject to masticatory trauma
(cheeks), but can involve any part of the oral mucosa. Oral
pigmentation can be the initial presentation of adrenal
insufficiency.
31.
Chronic insufficiencyPatients with chronic adrenocortical
insufficiency may develop an acute
Addisonian crisis, in which even minor
illnesses such as infections may cause
vomiting, fluid loss, electrolyte
disturbances and circulatory collapse.
The commonest cause of Addison's
disease was caseous necrosis of the
adrenal cortex due to tuberculosis.
32.
Chronic insufficiencyAutoimmune destruction of the cortex is now a
commoner cause; this is associated with other
'organ-specific' autoimmune diseases, such as
pernicious anaemia (also described by Addison),
thyroiditis, insulin-dependent diabetes mellitus and
parathyroid failure.
In all cases of Addison's disease, plasma cortisol
levels are low. Estimation of ACTH levels enables a
distinction to be made between primary
adrenocortical insufficiency (ACTH raised) and
secondary insufficiency (ACTH low).
33.
THANK YOUTHANK YOU



























 medicine
medicine








