Similar presentations:
Solid State Elementary Crystallography
1.
Solid StateElementary Crystallography
2.
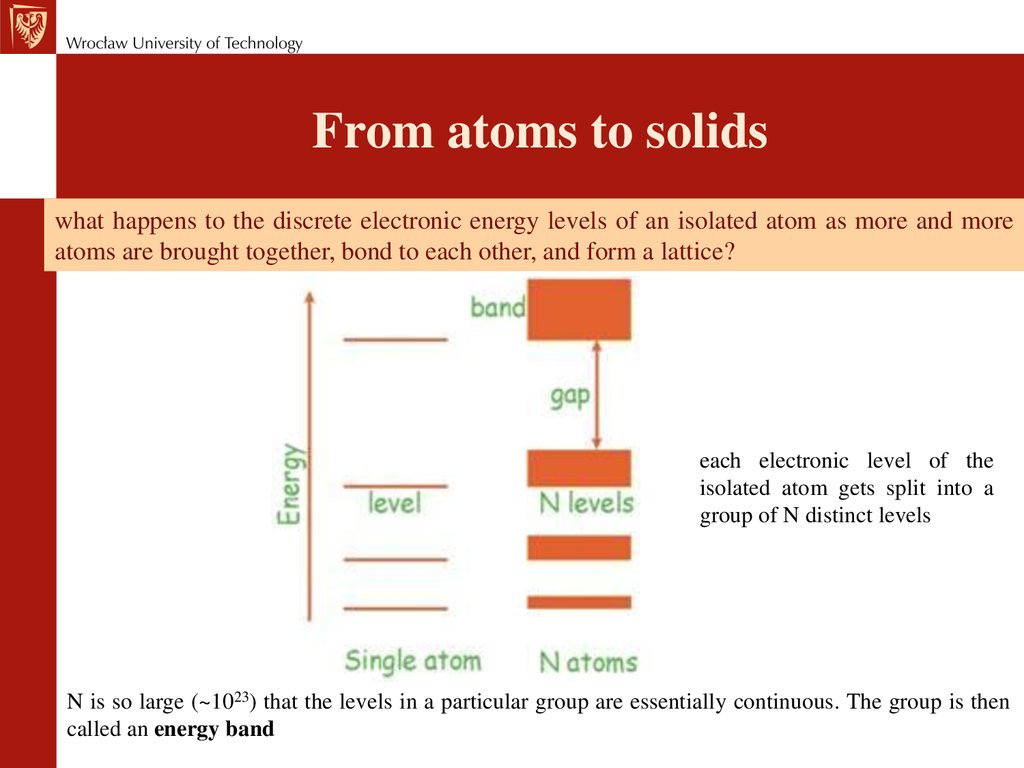
State of matter: attractive forces betweenparticles
retain a fixed volume and shape
GASES
rigid - particles locked into place
LIQUIDS
SOLIDS
Depending upon the type of component particles and
the nature of intermolecular forces between them:
Molecular Solids
Covalent Solids
Metallic Solids and Ionic solids
3.
SOLIDMATERIALS
TYPES
CRYSTALLINE
the atoms, ions or molecules are
arranged in a definite, repeating
pattern in three dimensions
long range order
Examples??????
AMORPHOUS
randomly orientated atoms, ions,
or molecules that do not form
defined patterns or lattice
structures.
no long-range order except for nearest
neighbors
4.
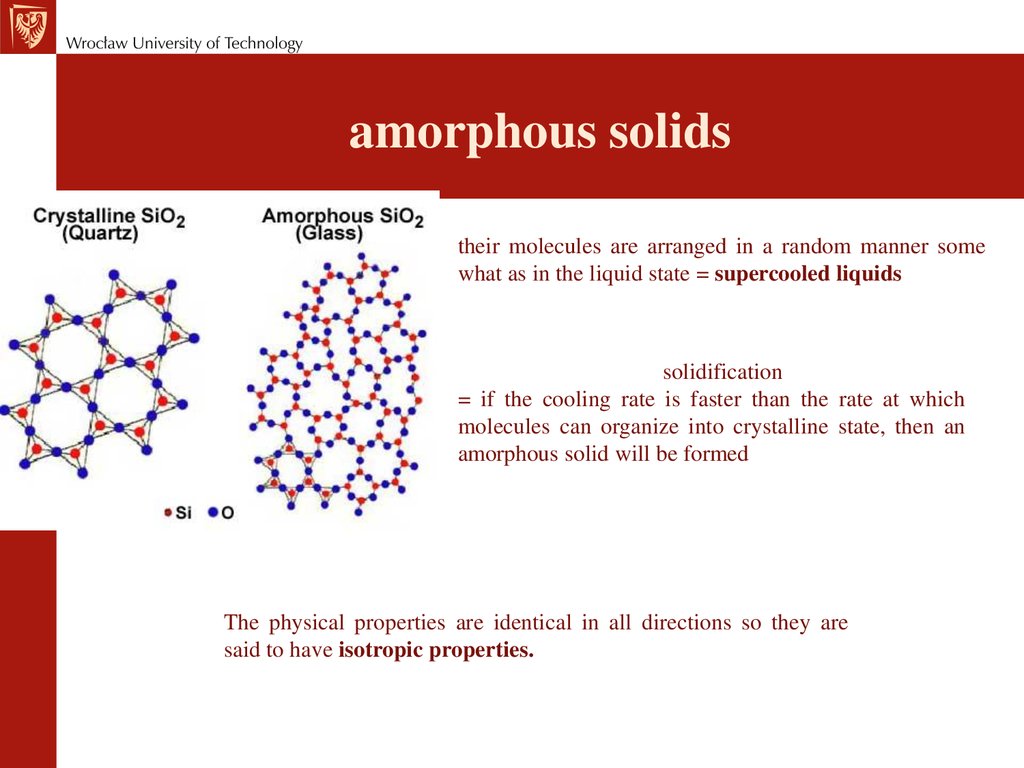
amorphous solidstheir molecules are arranged in a random manner some
what as in the liquid state = supercooled liquids
solidification
= if the cooling rate is faster than the rate at which
molecules can organize into crystalline state, then an
amorphous solid will be formed
The physical properties are identical in all directions so they are
said to have isotropic properties.
5.
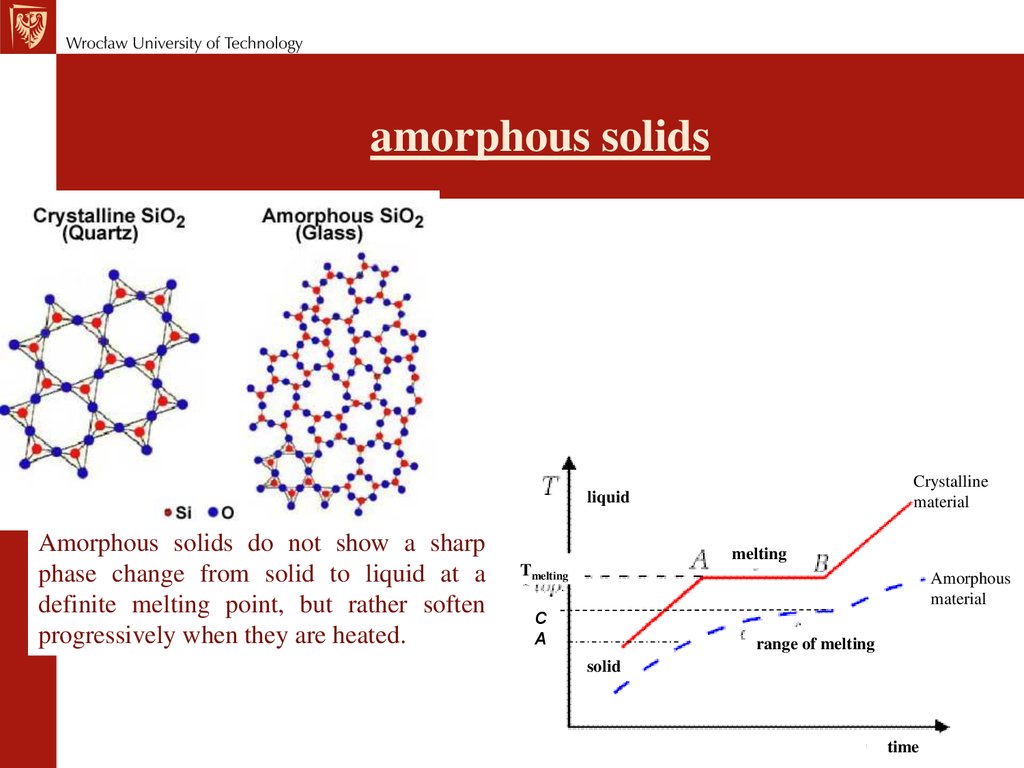
amorphous solidsCrystalline
material
liquid
Amorphous solids do not show a sharp
phase change from solid to liquid at a
definite melting point, but rather soften
progressively when they are heated.
melting
Tmelting
Amorphous
material
C
A
range of melting
solid
time
6.
AnisotropyDifferent directions in the crystal have
a different packing
- dependence of properties on a direction
The regular repeating pattern
of atoms = crystalline lattice
In crystalline solid, the properties like electrical conductance, refractive
index, thermal expansion, hardness etc., have different value in different
directions.
7.
naturally occurring crystalsQuartz (rock
crystal) (SiO2)
Pyrite FeS2
iron sulfide
Gypsum CaSO4×2H2O
Calcium sulphate
Corundum Al2O3
Ruby Al2O3
external shapes of crystals…
Amorphous solids
mineraloids
amber
Opal SiO2·nH2O
Agate SiO2 = Quartz variety
crystal glass??
Lead glass!!
8.
Single Crystal (monocrystal)and
Polycrystal
an aggregate of
many small single
crystals
with
different orientation
with respect to one
another
an atomic structure that repeats periodically
across its whole volume.
Even at infinite length scales, each atom is
related to every other equivalent atom in the
structure by translational symmetry.
Ordered in the same
manner regions, or single
crystal regions = grains
grain boundaries
9.
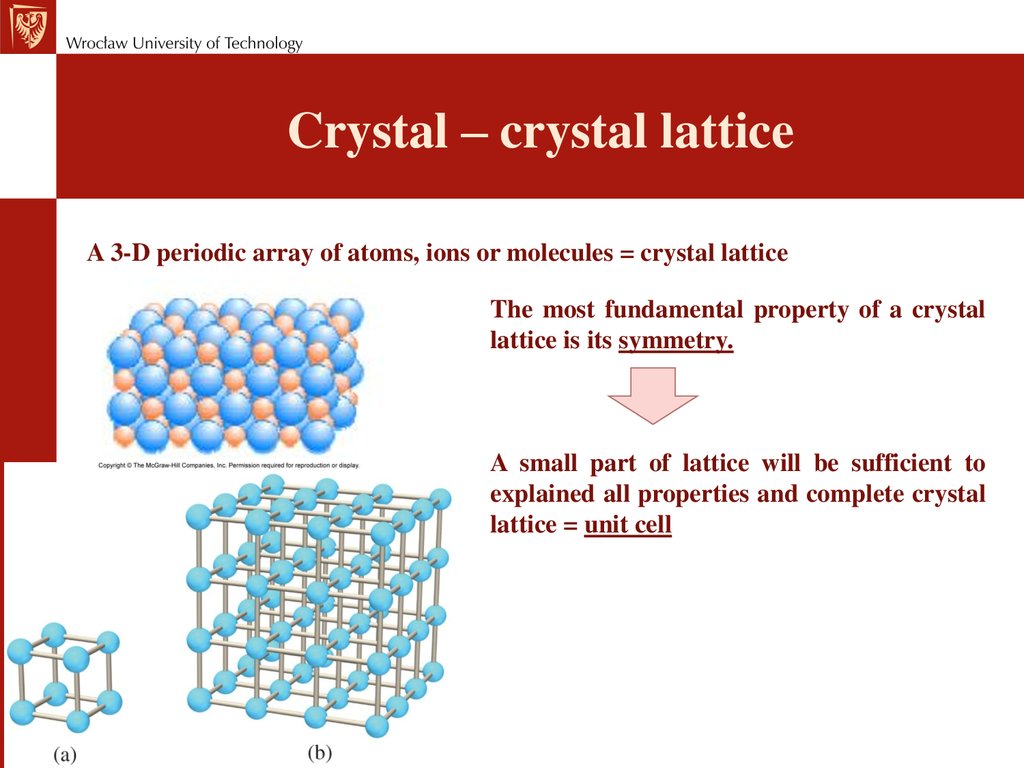
Crystal – crystal latticeA 3-D periodic array of atoms, ions or molecules = crystal lattice
The most fundamental property of a crystal
lattice is its symmetry.
A small part of lattice will be sufficient to
explained all properties and complete crystal
lattice = unit cell
10.
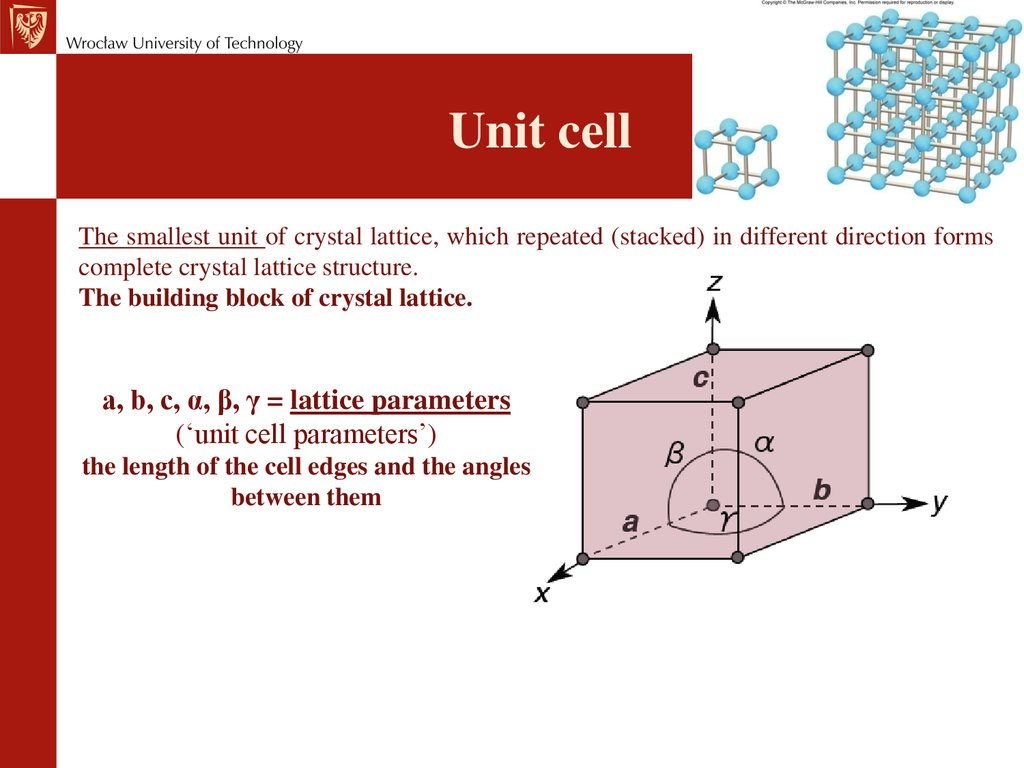
Unit cellThe smallest unit of crystal lattice, which repeated (stacked) in different direction forms
complete crystal lattice structure.
The building block of crystal lattice.
a, b, c, α, β, γ = lattice parameters
(‘unit cell parameters’)
the length of the cell edges and the angles
between them
11.
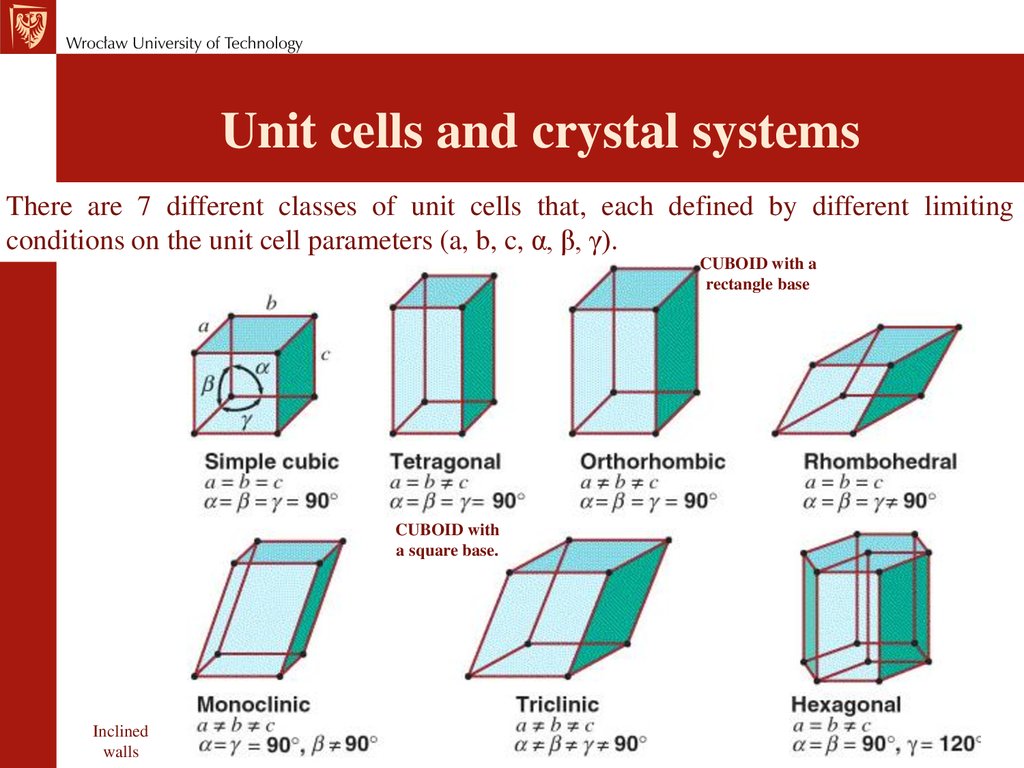
Unit cells and crystal systemsThere are 7 different classes of unit cells that, each defined by different limiting
conditions on the unit cell parameters (a, b, c, α, β, γ).
CUBOID with a
rectangle base
CUBOID with
a square base.
Inclined
walls
12.
Types of crystal lattice centeringPrimitive unit cell
Body centered unit cell
Face centered unit cell
Side centered unit cell
13.
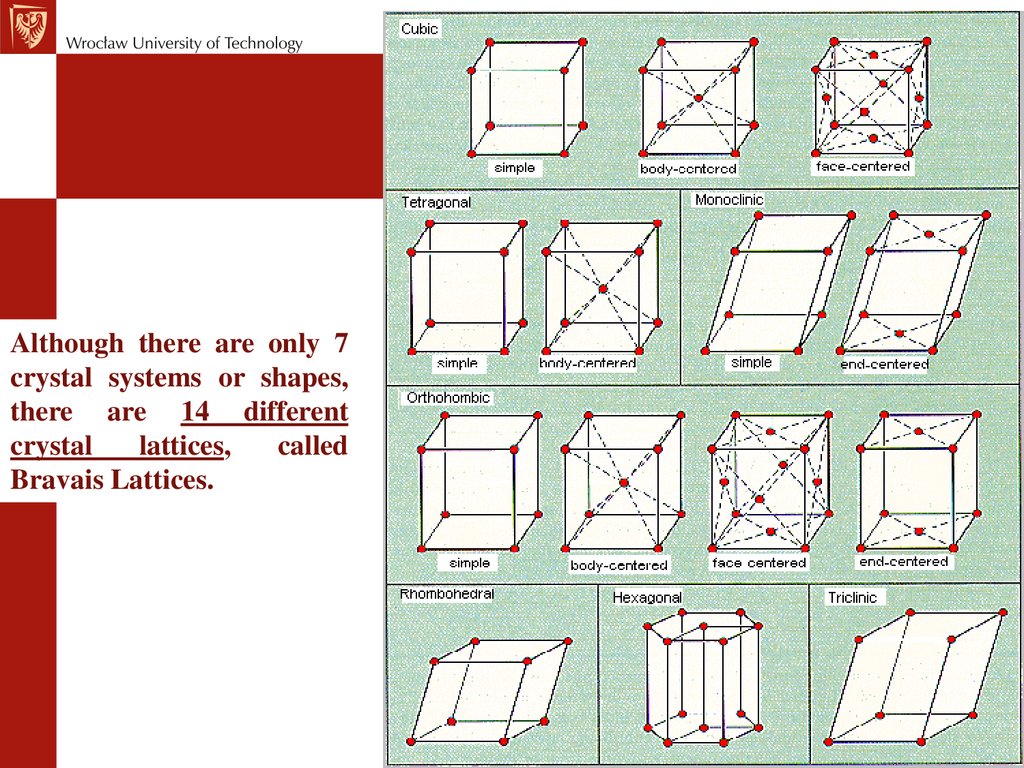
Although there are only 7crystal systems or shapes,
there are 14 different
crystal
lattices,
called
Bravais Lattices.
14.
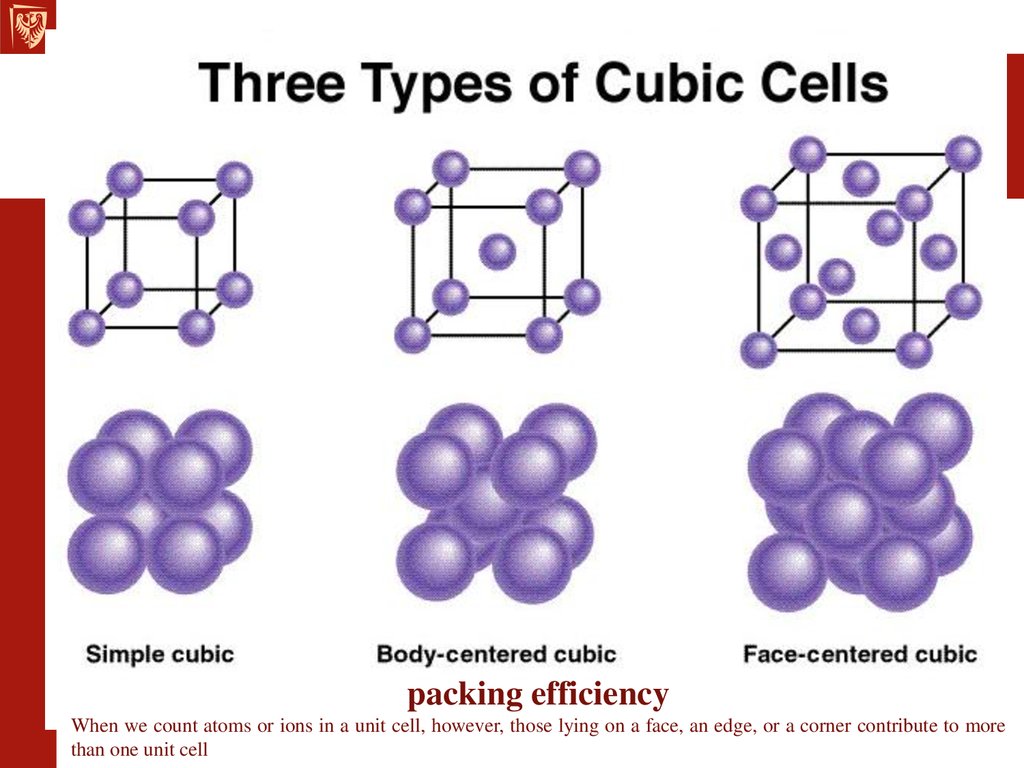
packing efficiencyWhen we count atoms or ions in a unit cell, however, those lying on a face, an edge, or a corner contribute to more
than one unit cell
15.
Atoms on the corners, edges, and faces of a unit cellare shared by more than one unit cell,
Shared by 8 unit cells
Shared by 2 unit cells
the number of atoms that lie in the unit cell???
16.
1 atom/unit cell(8 x 1/8 = 1)
2 atoms/unit cell
(8 x 1/8 + 1 = 2)
4 atoms/unit cell
(8 x 1/8 + 6 x 1/2 = 4)
17.
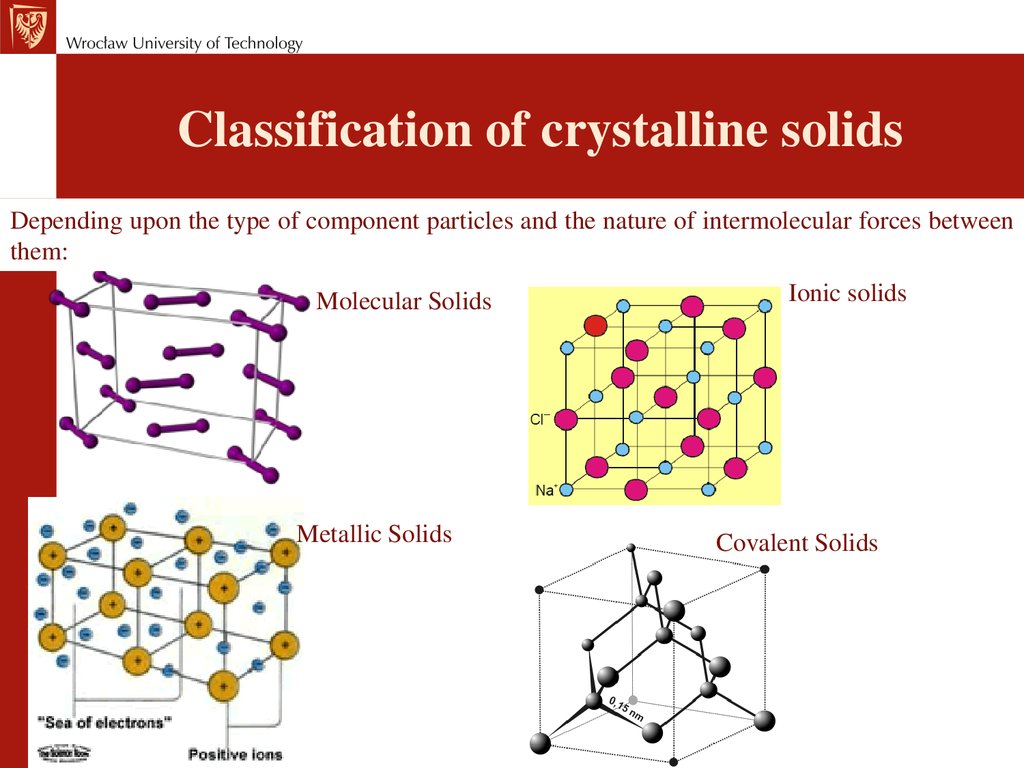
Classification of crystalline solidsDepending upon the type of component particles and the nature of intermolecular forces between
them:
Molecular Solids
Metallic Solids
Ionic solids
Covalent Solids
18.
Molecular Solids- solid composed of molecules held together by..??
- melting temperatures ?
-density and hardness ?
-electrical conduction?
I2
I2, is a bluish-black solid which evaporates (sublimes) to a purple vapour.
As the purple vapour is formed only the weak intermolecular bonds are being overcome. The covalent bonds between
the iodine atoms in I2 molecules are not broken.
19.
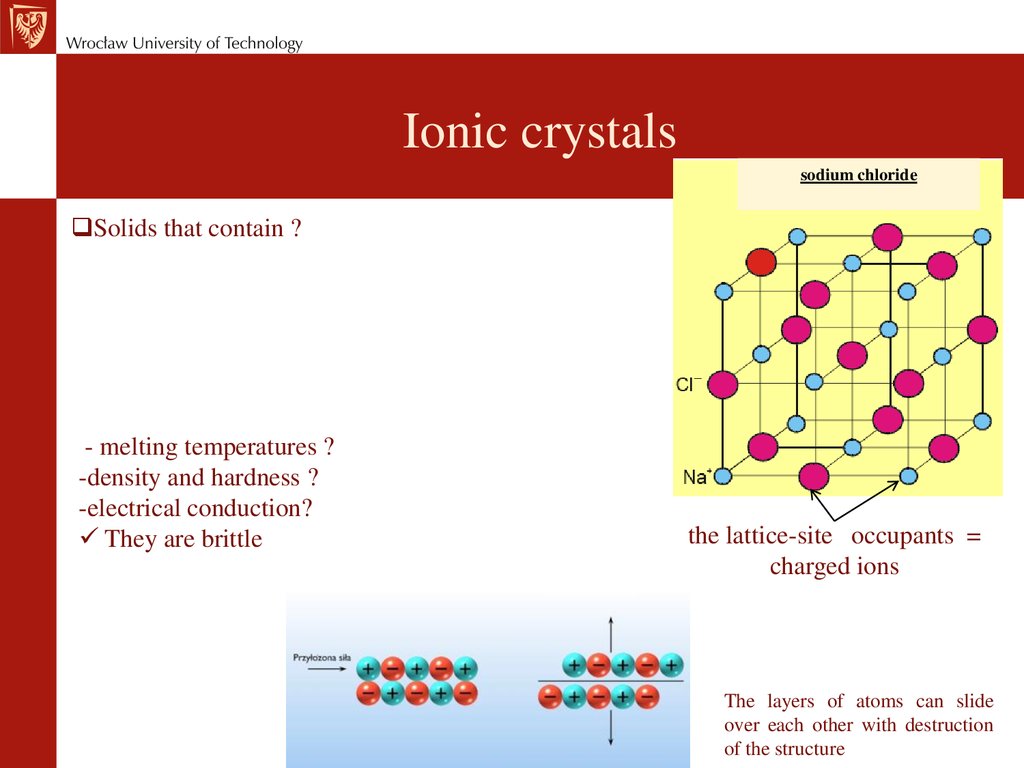
Ionic crystalssodium chloride
Solids that contain ?
- melting temperatures ?
-density and hardness ?
-electrical conduction?
They are brittle
the lattice-site occupants =
charged ions
The layers of atoms can slide
over each other with destruction
of the structure
20.
Metallic crystalsSolids that contain …?
- melting temperatures ?
-density and hardness ?
-electrical conduction?
-surface?
Metals are very malleable, they can be readily bent,
pressed or hammered into shape.
the lattice-site occupants = metal ions
21.
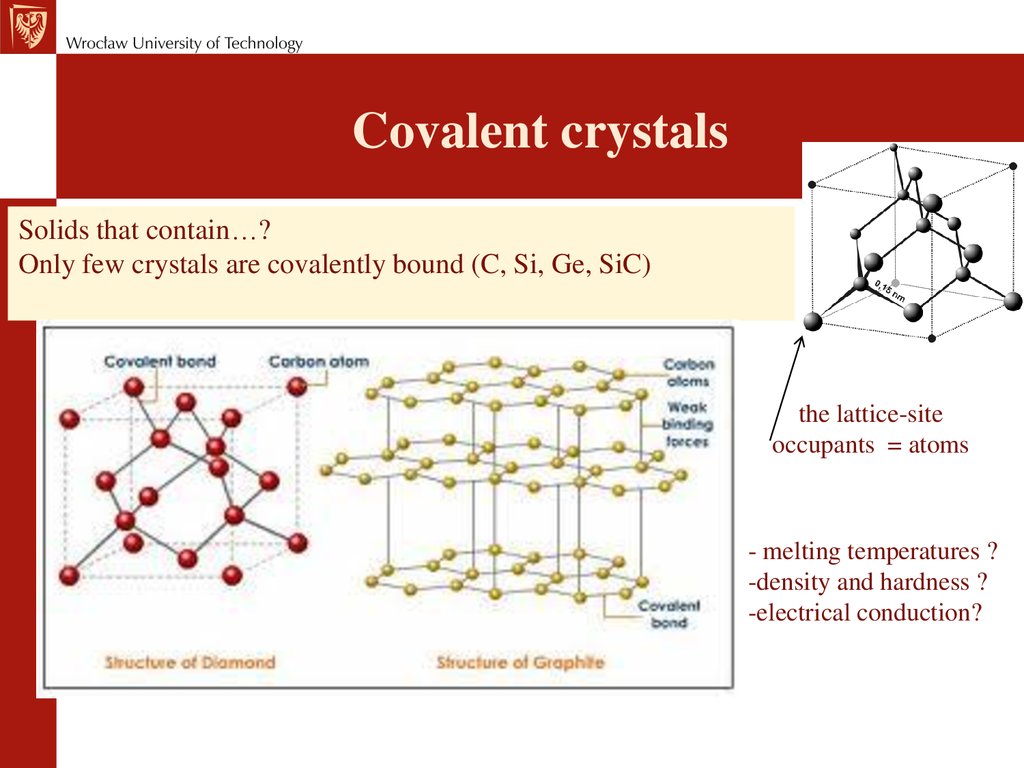
Covalent crystalsSolids that contain…?
Only few crystals are covalently bound (C, Si, Ge, SiC)
the lattice-site
occupants = atoms
- melting temperatures ?
-density and hardness ?
-electrical conduction?
22.
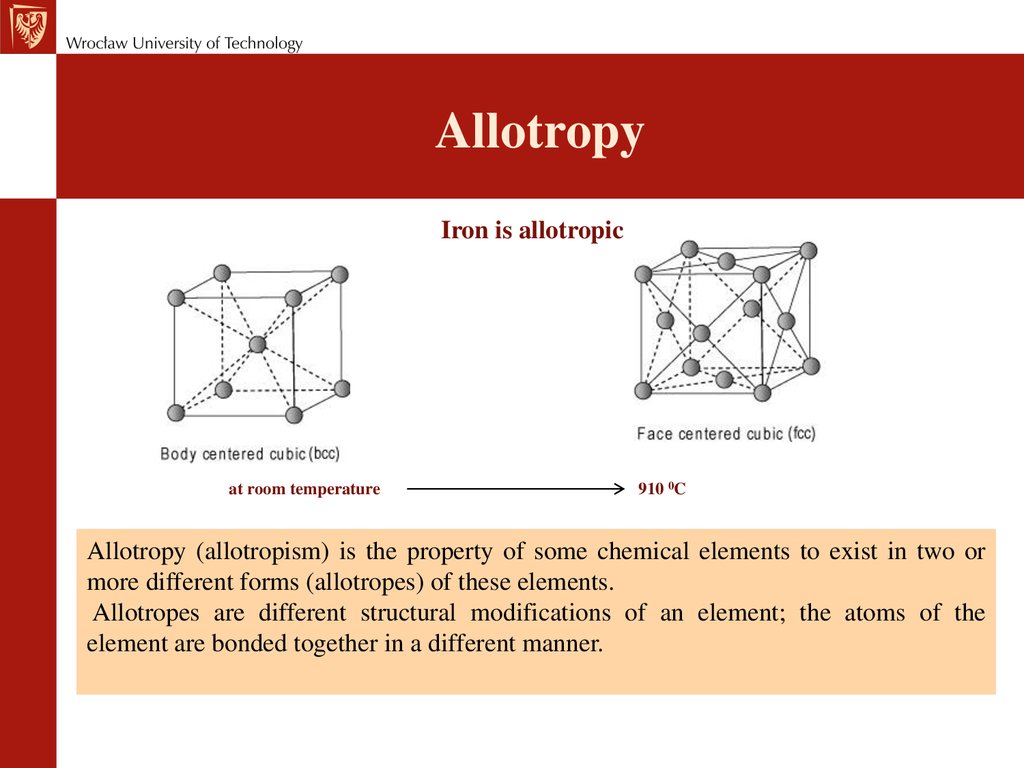
AllotropyIron is allotropic
at room temperature
910 0C
Allotropy (allotropism) is the property of some chemical elements to exist in two or
more different forms (allotropes) of these elements.
Allotropes are different structural modifications of an element; the atoms of the
element are bonded together in a different manner.
23.
Carbondiamond
graphite
fulleren
Graphite diamond and fulleren are made up of carbon atoms, but the arrangement of atoms
is different in each allotrope which results in different physical properties.
24.
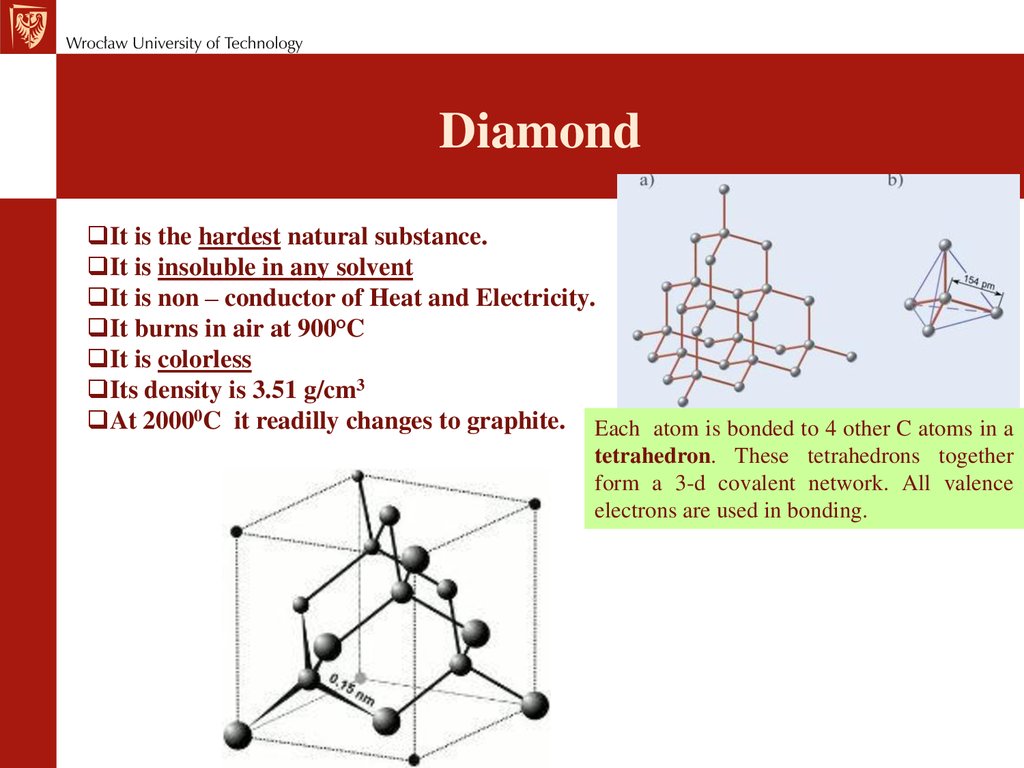
DiamondIt is the hardest natural substance.
It is insoluble in any solvent
It is non – conductor of Heat and Electricity.
It burns in air at 900°C
It is colorless
Its density is 3.51 g/cm3
At 20000C it readilly changes to graphite. Each atom is bonded to 4 other C atoms in a
tetrahedron. These tetrahedrons together
form a 3-d covalent network. All valence
electrons are used in bonding.
25.
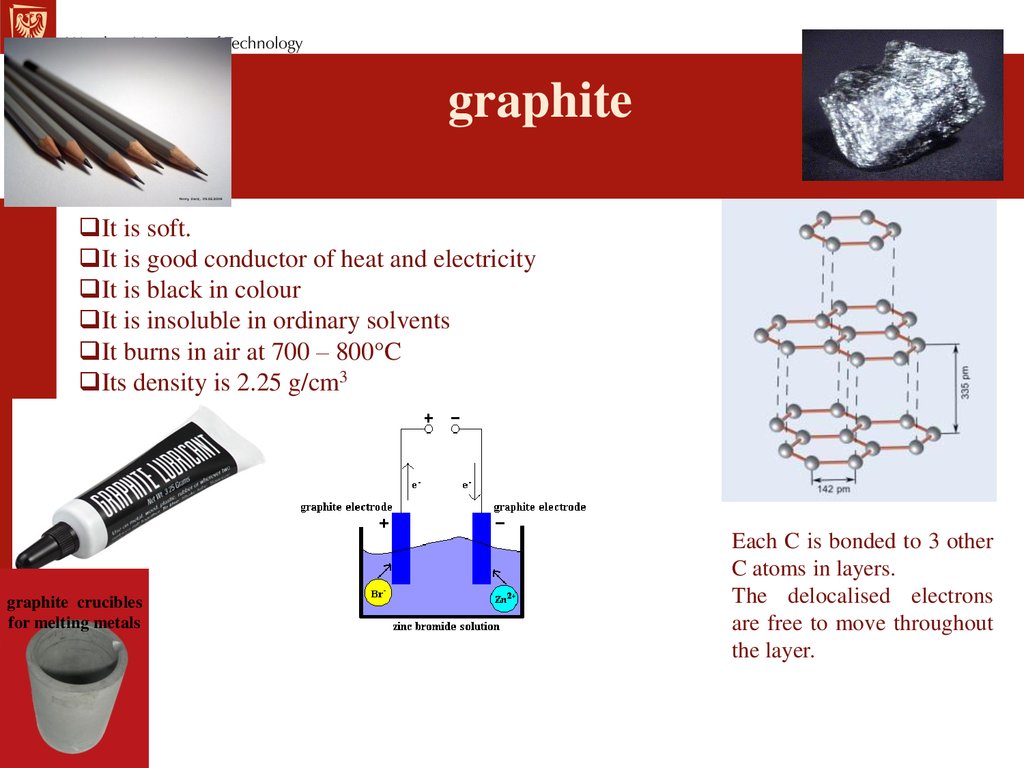
graphiteIt is soft.
It is good conductor of heat and electricity
It is black in colour
It is insoluble in ordinary solvents
It burns in air at 700 – 800°C
Its density is 2.25 g/cm3
graphite crucibles
for melting metals
Each C is bonded to 3 other
C atoms in layers.
The delocalised electrons
are free to move throughout
the layer.
26.
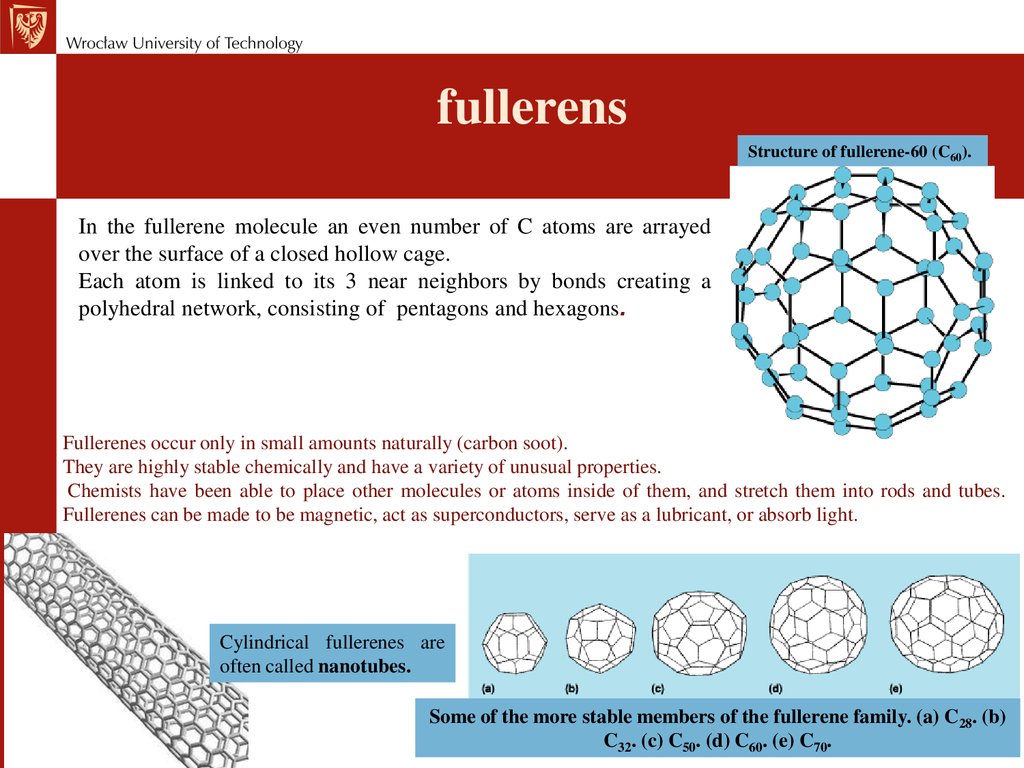
fullerensStructure of fullerene-60 (C60).
In the fullerene molecule an even number of C atoms are arrayed
over the surface of a closed hollow cage.
Each atom is linked to its 3 near neighbors by bonds creating a
polyhedral network, consisting of pentagons and hexagons.
Fullerenes occur only in small amounts naturally (carbon soot).
They are highly stable chemically and have a variety of unusual properties.
Chemists have been able to place other molecules or atoms inside of them, and stretch them into rods and tubes.
Fullerenes can be made to be magnetic, act as superconductors, serve as a lubricant, or absorb light.
Cylindrical fullerenes are
often called nanotubes.
Some of the more stable members of the fullerene family. (a) C28. (b)
C32. (c) C50. (d) C60. (e) C70.
27.
Real crystals = Imperfect crystalsDefects are responsible for many of the important properties of materials such as
mechanical strength, crystal growth, magnetic hysteresis, dielectric strength…
Crystalline defects:
(i) Point imperfections (Vacancy atoms, Interstitial atoms, Substitutional atoms)
(ii) Line imperfections (Dislocations)
(iii) Surface and grain boundary imperfections
28.
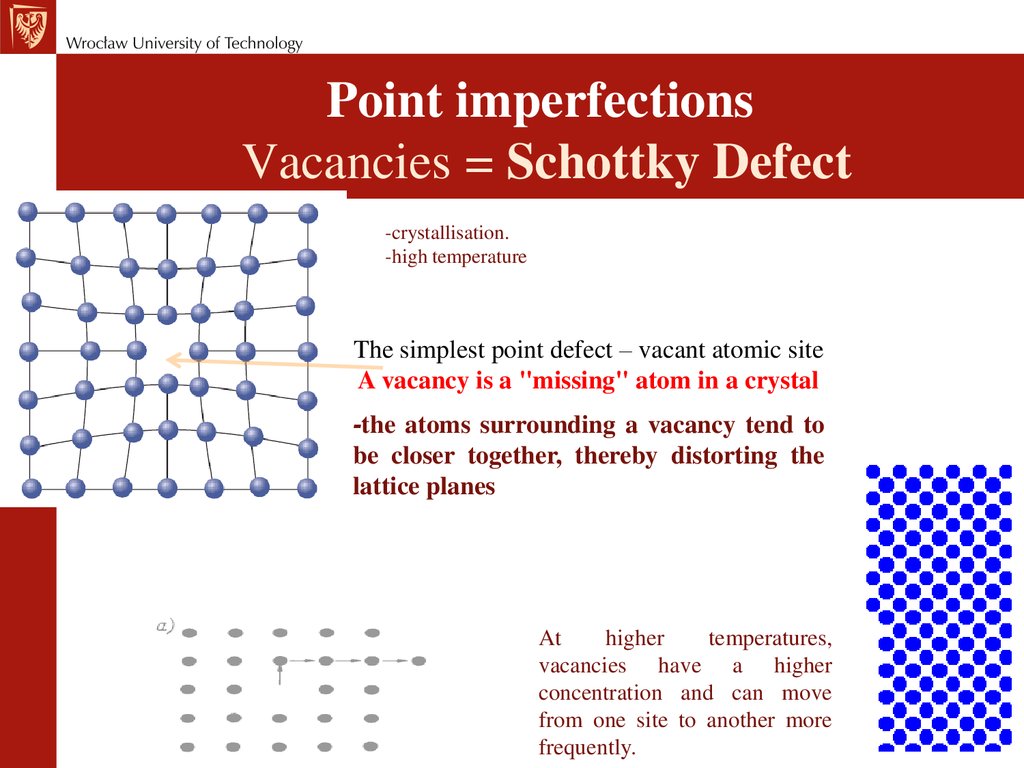
Point imperfectionsVacancies = Schottky Defect
-crystallisation.
-high temperature
The simplest point defect – vacant atomic site
A vacancy is a "missing" atom in a crystal
-the atoms surrounding a vacancy tend to
be closer together, thereby distorting the
lattice planes
At
higher
temperatures,
vacancies have a higher
concentration and can move
from one site to another more
frequently.
29.
Point imperfectionsInterstitial Imperfections = Frenkel Defect
interstitial position or void
between regularly positioned
atoms
an atom on a non-lattice site
30.
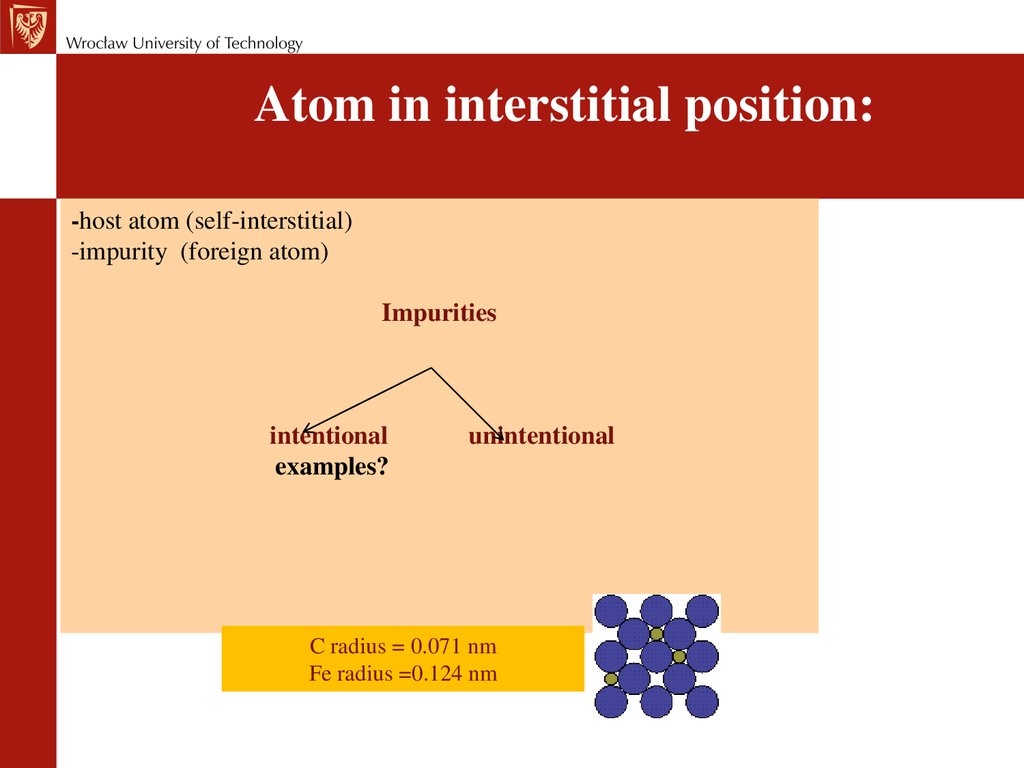
Atom in interstitial position:-host atom (self-interstitial)
-impurity (foreign atom)
Impurities
intentional
examples?
unintentional
C radius = 0.071 nm
Fe radius =0.124 nm
31.
Alloys = Metals (solids) with impurities - Solid SolutionsSolid solutions are made of a host (the solvent) which dissolves the minor component (solute).




























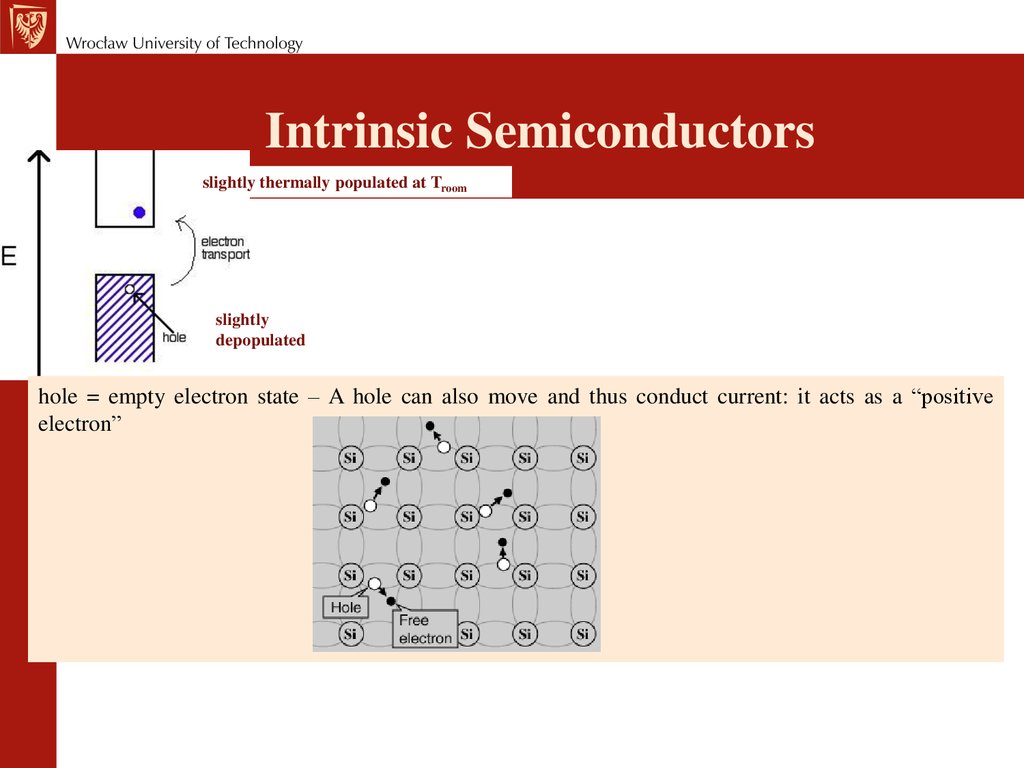


 chemistry
chemistry








