Similar presentations:
Defect in solid
1.
DEFECT IN SOLID2017
2.
What is a defect in a solid?Solids consist of crystal. Crystals are
described by scientists using an idealized
model, but such crystals are not found in
nature. The deviation of the crystal
structure from the periodic crystal structure
is defects.
The real crystal are not perfect. A real
crystal always has a large number of
imperfections in the lattice crystal. One can
reduce crystal defect considerably, but can
never eliminate them entirely.
Perfect model of crystal
2
3.
DictionarySolid – твердое тело, твердое состояние
Sufficiently – достаточно
Impurities – примеси
Invariably – неизменно
Interstitial – междуузлье
Mechanical treatment – механическая обработка
Imperfections – несовершенство, дефект
Grain boundary – граница зерна
Porosity - пористость
Cracks - трещины
Inclusions – включение
Discontinuities – разрывы
Grinding – шлифование
Enriched uranium – обагащенный уран
3
4.
Point defectsDefects are divided into four
classes, if we consider the
geometric of defects
Line defects
Surface defects
Volume defects
Defects occur in a solid at
any temperature. The number
of defects increases with
increasing temperature and
under the influence of
ionizing radiation and
mechanical treatment
4
5.
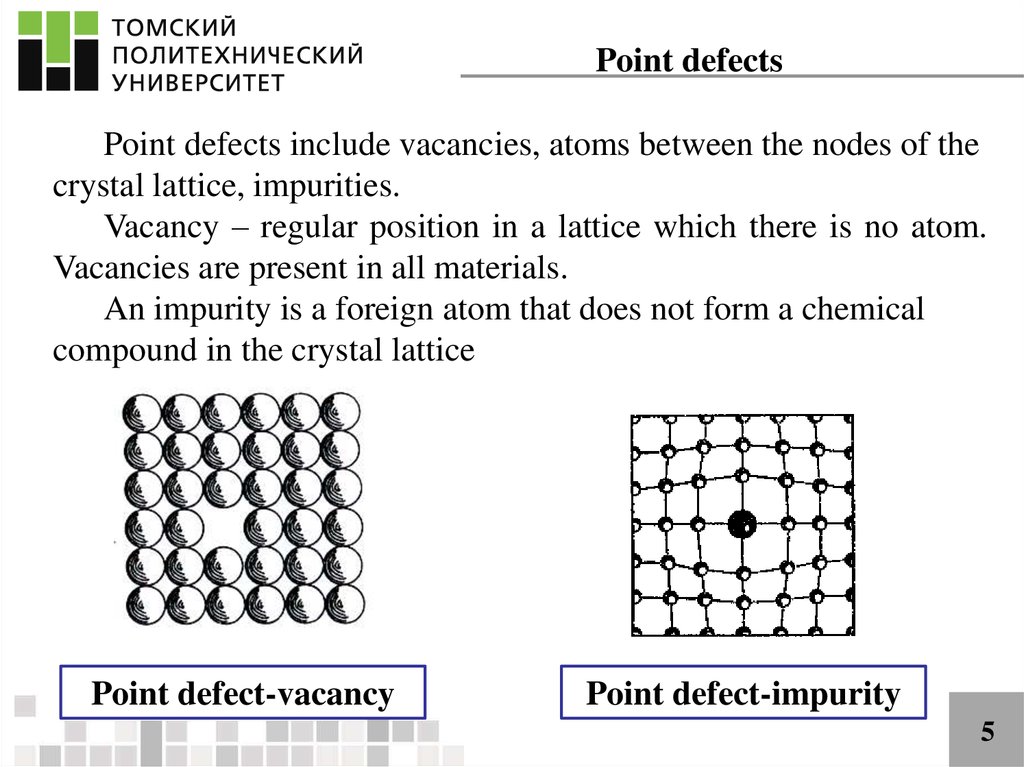
Point defectsPoint defects include vacancies, atoms between the nodes of the
crystal lattice, impurities.
Vacancy – regular position in a lattice which there is no atom.
Vacancies are present in all materials.
An impurity is a foreign atom that does not form a chemical
compound in the crystal lattice
Point defect-vacancy
Point defect-impurity
5
6.
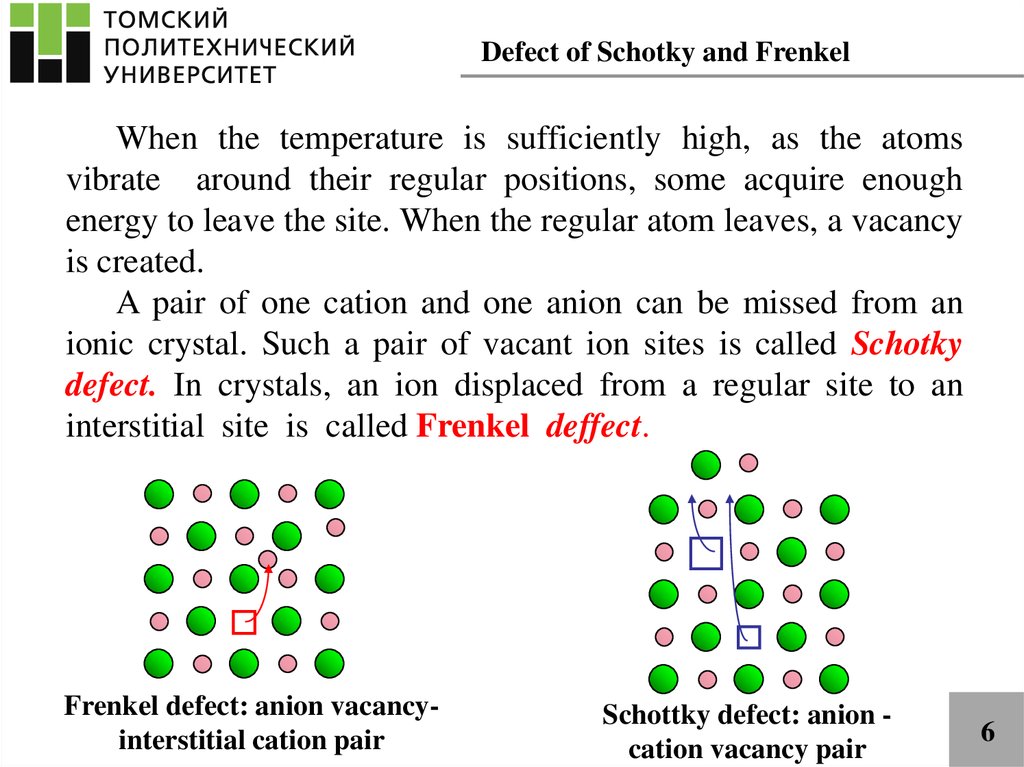
Defect of Schotky and FrenkelWhen the temperature is sufficiently high, as the atoms
vibrate around their regular positions, some acquire enough
energy to leave the site. When the regular atom leaves, a vacancy
is created.
A pair of one cation and one anion can be missed from an
ionic crystal. Such a pair of vacant ion sites is called Schotky
defect. In crystals, an ion displaced from a regular site to an
interstitial site is called Frenkel deffect.
Frenkel defect: anion vacancyinterstitial cation pair
Schottky defect: anion cation vacancy pair
6
7.
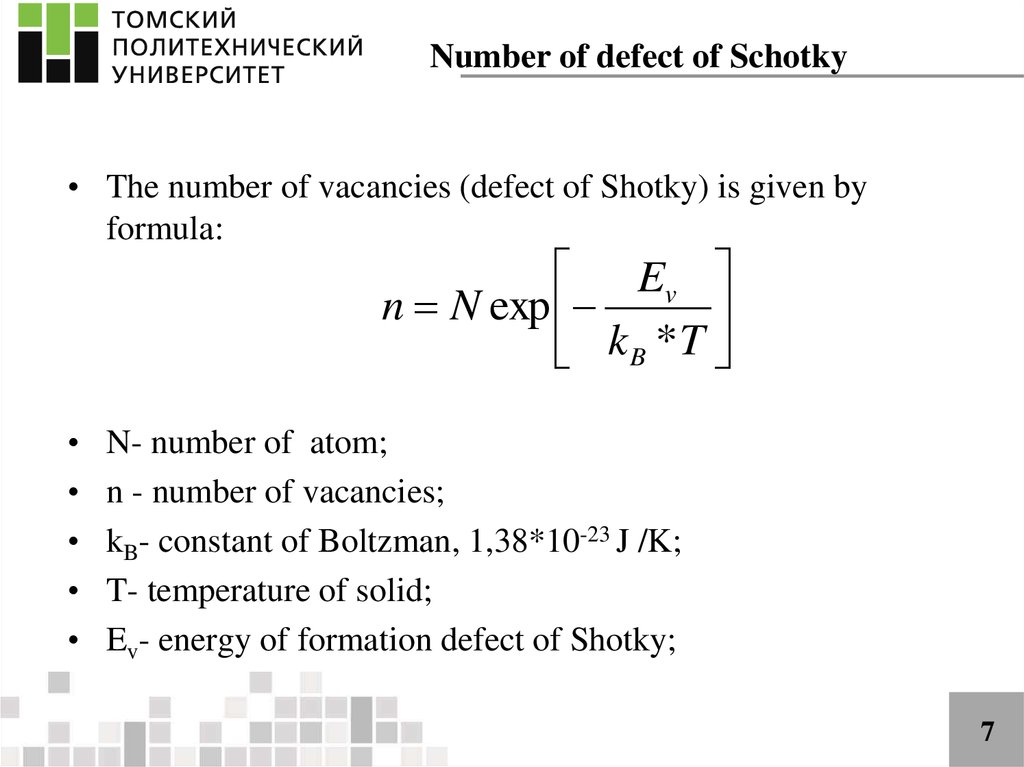
Number of defect of Schotky• The number of vacancies (defect of Shotky) is given by
formula:
Ev
n N exp
kB *T
N- number of atom;
n - number of vacancies;
kB- constant of Boltzman, 1,38*10-23 J /K;
T- temperature of solid;
Ev- energy of formation defect of Shotky;
7
8.
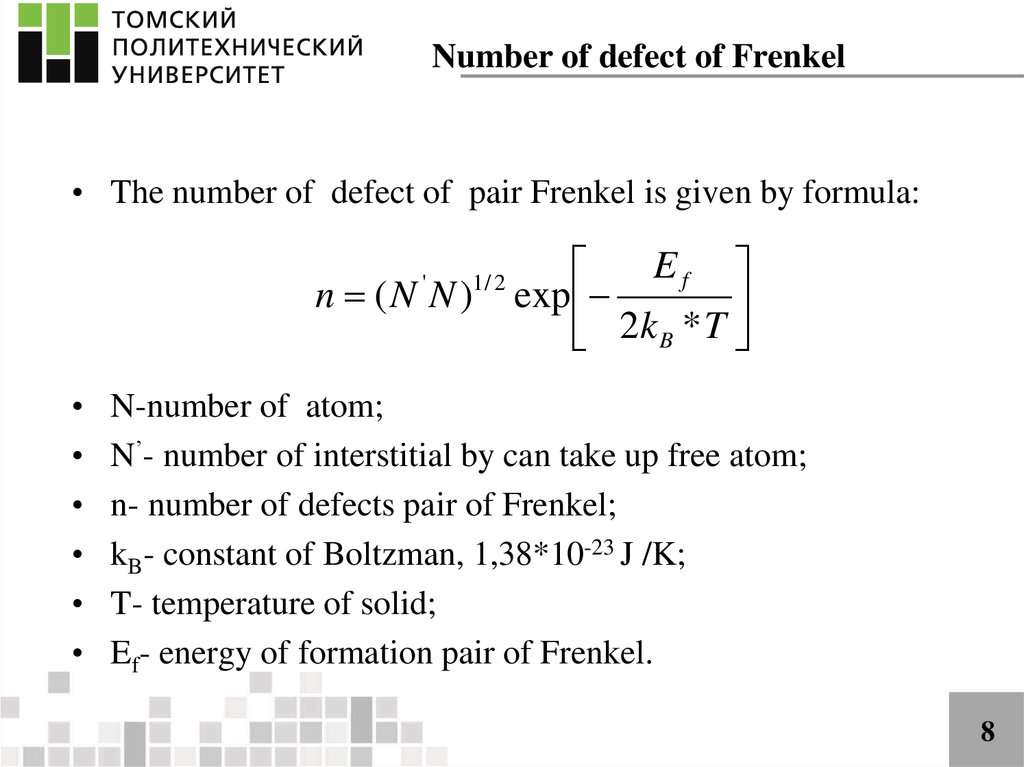
Number of defect of Frenkel• The number of defect of pair Frenkel is given by formula:
n (N N )
'
1/ 2
Ef
exp
2k B * T
N-number of atom;
N’- number of interstitial by can take up free atom;
n- number of defects pair of Frenkel;
kB- constant of Boltzman, 1,38*10-23 J /K;
T- temperature of solid;
Ef- energy of formation pair of Frenkel.
8
9.
Line defect - dislocationThe experimental data do not show that the observed values of
the yield strength are much smaller than the theoretical values. To
explain this effect, a new type of defect was introduced, which the
scientists called a dislocation.
Dislocation is the region of localized lattice distortion which
separates the slipped and unslipped portion of the crystal. The upper
region of the crystal over the slip plane has slipped relative to the
bottom portion.
The line (AD) between the
slipped and unslipped portions
is the dislocation. The
magnitude and direction of
slip produced by dislocation
(pink shaded) is the Burger
vector of the dislocation.
9
10.
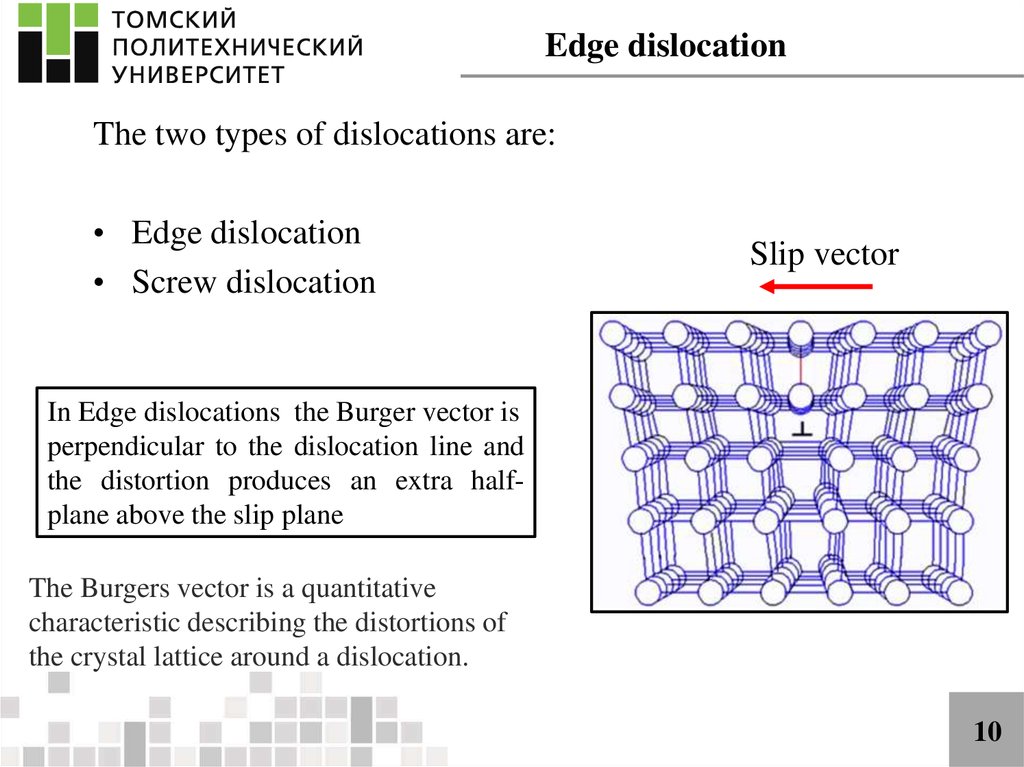
Edge dislocationThe two types of dislocations are:
• Edge dislocation
• Screw dislocation
Slip vector
In Edge dislocations the Burger vector is
perpendicular to the dislocation line and
the distortion produces an extra halfplane above the slip plane
The Burgers vector is a quantitative
characteristic describing the distortions of
the crystal lattice around a dislocation.
10
11.
Screw dislocationThe other type of dislocation is the screw dislocation
where the Burger vector is parallel to the dislocation line (AD).
The trace of the atomic planes around the screw dislocation
makes a spiral or helical path (pink shade) like a screw and
hence, the name.
Atomic
positions along
a screw
dislocation
11
12.
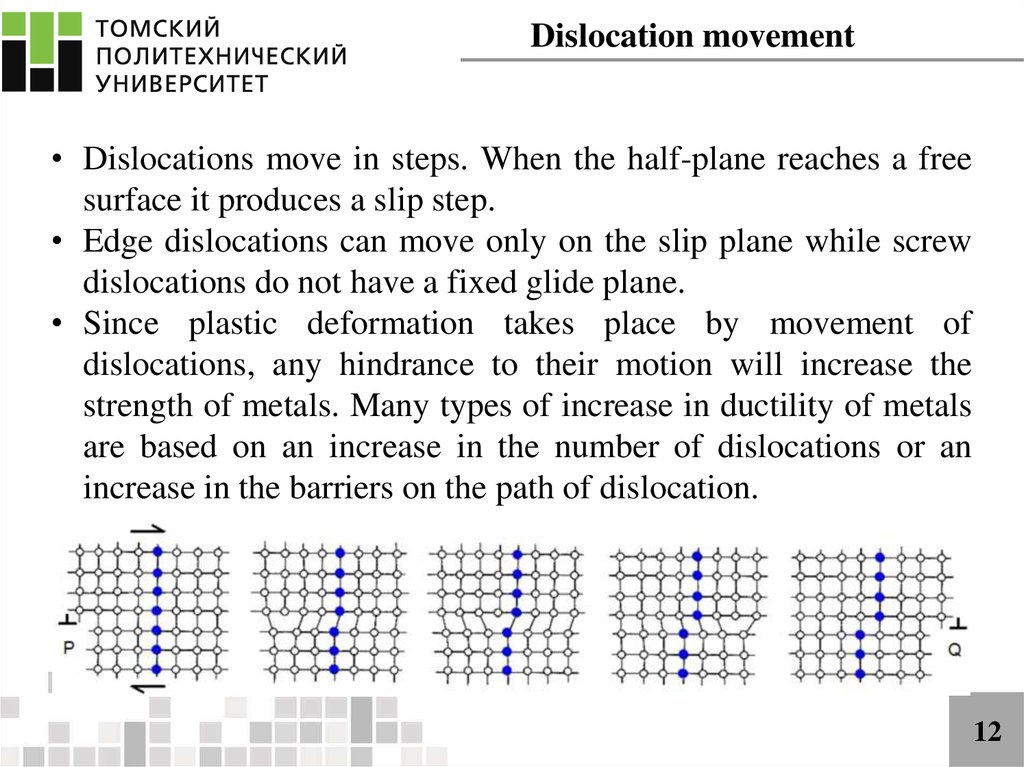
Dislocation movement• Dislocations move in steps. When the half-plane reaches a free
surface it produces a slip step.
• Edge dislocations can move only on the slip plane while screw
dislocations do not have a fixed glide plane.
• Since plastic deformation takes place by movement of
dislocations, any hindrance to their motion will increase the
strength of metals. Many types of increase in ductility of metals
are based on an increase in the number of dislocations or an
increase in the barriers on the path of dislocation.
12
13.
Observing dislocationsDislocations appear as lines when observed under
transmission electron microscope (TEM)
13
14.
Grain boundaries• Grain boundaries is surface defect.
• Most crystalline solids are an aggregate of several crystals.
Such materials are called polycrystalline. Each crystal is
known as a grain. The boundary between the grains is the
grain boundary.
Grain
boundaries
Optical micrograph of
a polycrystalline material
Schematic of orientation change
across the grain boundary
14
15.
Volume defect• Porosity
• Cracks
• Inclusions
• These defects form during manufacturing processes for various
reasons and often harmful to the material.
• A lot of inclusions fall into the material during welding, thermal,
mechanical
or
other
processing.
For
example
during grinding.
• Example porosity reduces thermal conductivity of material, but
sometimes porosity specifically increase for increase thermal
isolate.
15
16.
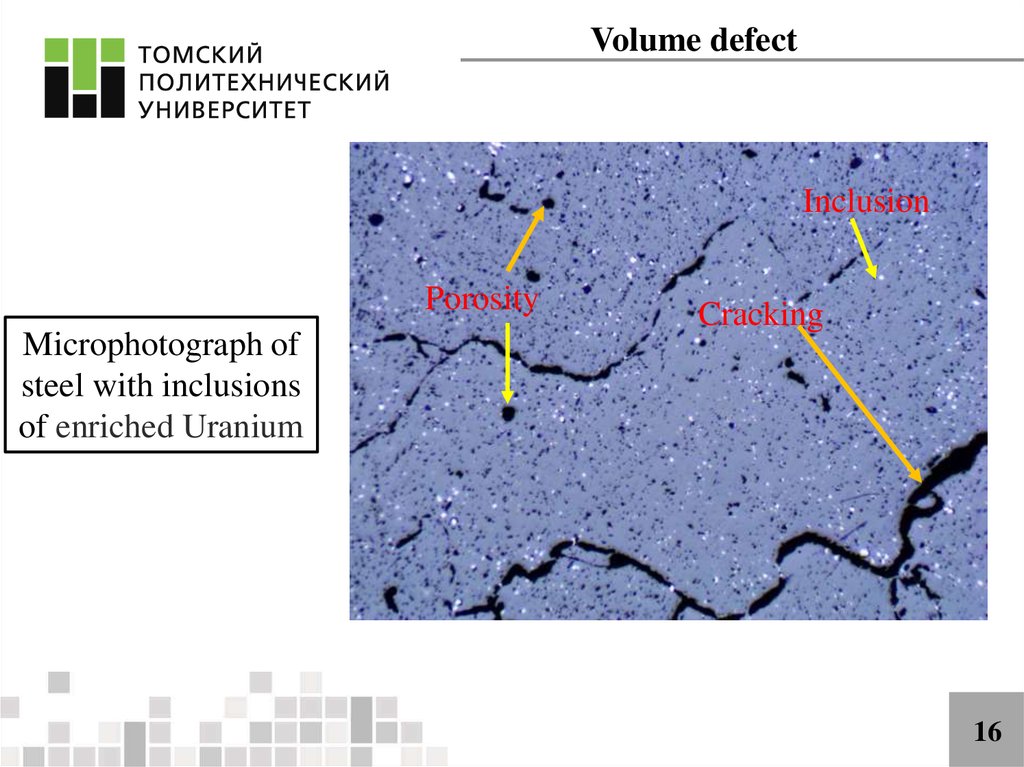
Volume defectInclusion
Porosity
Microphotograph of
steel with inclusions
of enriched Uranium
Cracking
16
17.
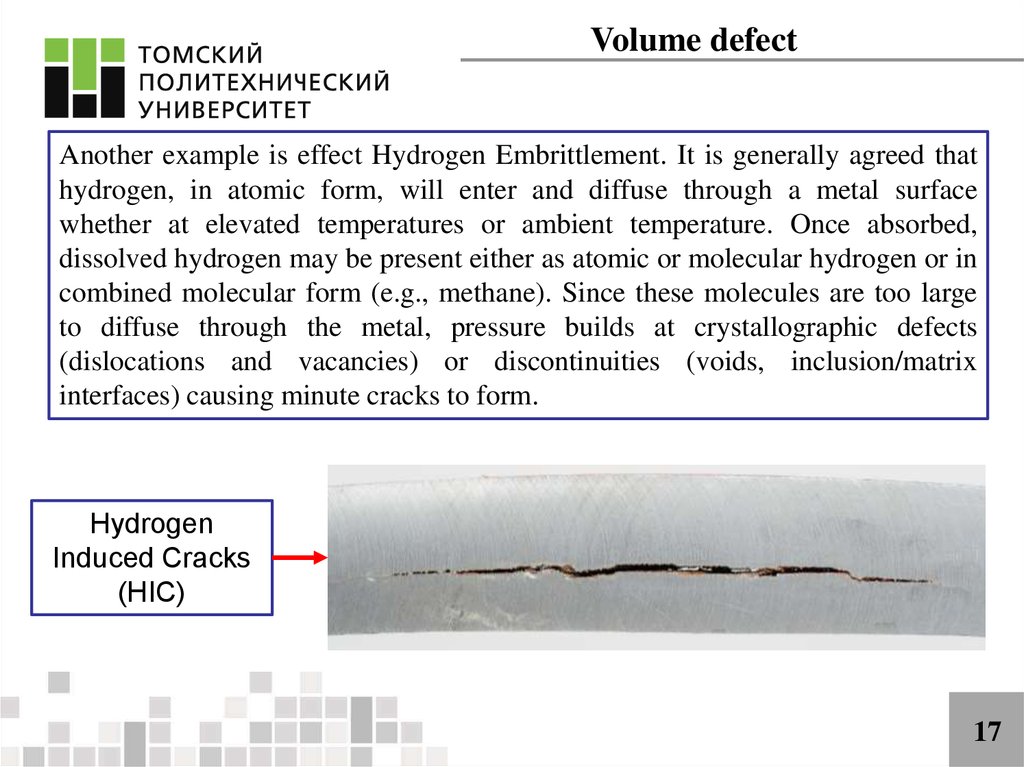
Volume defectAnother example is effect Hydrogen Embrittlement. It is generally agreed that
hydrogen, in atomic form, will enter and diffuse through a metal surface
whether at elevated temperatures or ambient temperature. Once absorbed,
dissolved hydrogen may be present either as atomic or molecular hydrogen or in
combined molecular form (e.g., methane). Since these molecules are too large
to diffuse through the metal, pressure builds at crystallographic defects
(dislocations and vacancies) or discontinuities (voids, inclusion/matrix
interfaces) causing minute cracks to form.
Hydrogen
Induced Cracks
(HIC)
17
18.
Methods for studying defects in solidThe best known methods for studying defects in solid:
• Measurement of electrical resistance
• Positron annihilation
• Studying of diffusion properties
18











 chemistry
chemistry








