Similar presentations:
Pathomorphology of lungs lesions in new coronavirus infection Covid-19
1.
Pathomorphology of lungs lesions innew coronavirus infection Covid-19
review
Reporter: Markin Aleksei, 3rd-year student IKM
2.
First Autopsy Case ReportA 50-year-old man was admitted to a fever clinic on Jan 21,
2020, with symptoms of fever, chills, cough, fatigue and
shortness of breath. He reported a travel history to Wuhan Jan
8–12, and that he had initial symptoms of mild chills and dry
cough on Jan 14 (day 1 of illness) but did not see a doctor and
kept working until Jan 21. Chest x-ray showed multiple patchy
shadows in both lungs, and a throat swab sample was taken.
On Jan 22 (day 9 of illness), the Beijing Centers for Disease
Control (CDC) confirmed by reverse real-time PCR assay that
the patient had COVID-19.
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia
P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress
syndrome. Lancet Respir Med. 2020 Apr;8(4):420-422.
3.
First Autopsy Case Report• Bilateral diffuse alveolar damage with cellular
fibromyxoid exudates. The right lung showed
evident desquamation of pneumocytes and
hyaline membrane formation, indicating acute
respiratory distress syndrome (ARDS; figure 2A).
The left lung tissue displayed pulmonary oedema
with hyaline membrane formation, suggestive of
early-phase ARDS (figure 2B). In both lungsinterstitial mononuclear infiltrates, dominated by
lymphocytes. Multinucleated syncytial cells with
atypical enlarged pneumocytes characterised by
large nuclei, amphophilic granular cytoplasm, and
prominent nucleoli were identified in the intraalveolar spaces, showing viral cytopathic-like
changes
4.
Macroscopic features(A)The lungs are heavy, with bilateral interstitial
edema and congestion. The cut surfaces show
tan-grey consolidation and/or patchy
hemorrhagic areas.
(B)Grossly visible pulmonary emboli, and a
peculiar patchy gross appearance of the lung
parenchyma (both externally as well as on the
cut sections), and thrombosis of the prostatic
vein. Pleural adhesions were identified in one
complete autopsy.
5.
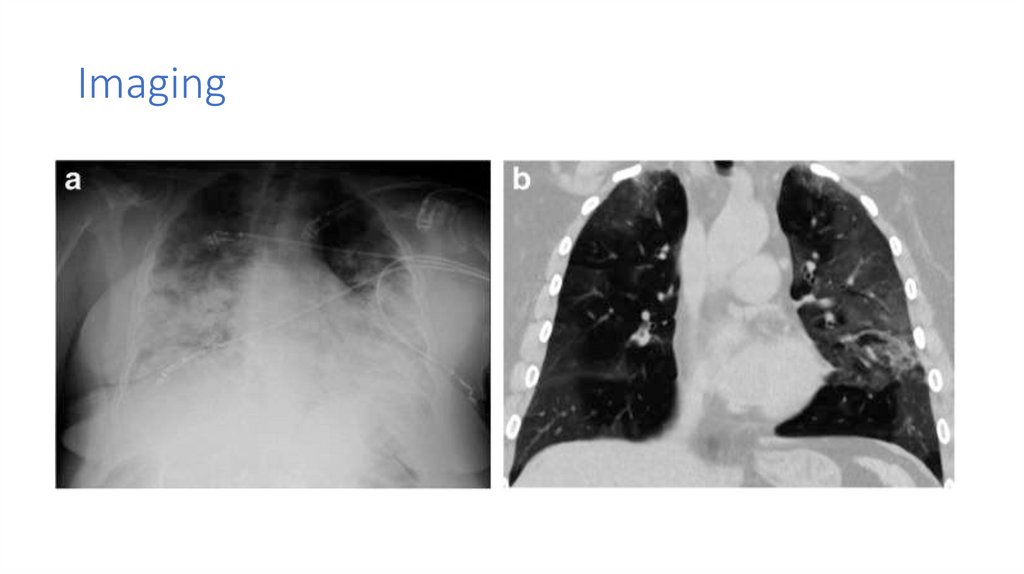
Imaging6.
EtiologyDu, Lanying et al. “The spike protein of SARS-CoV--a target for vaccine and therapeutic
development.” Nature reviews. Microbiology vol. 7,3 (2009): 226-36. doi:10.1038/nrmicro2090
7.
Patogenesis8.
SARS-COV-2 inflammatory response• A - Recruited monocytes secretes proinflammatory cytokines, inducing
pneumocytes apoptosis;
• B - Recruited macrophages releases cytokines
causing capillary permeability increase and
consequent neutrophils recruitment;
• C - Neutrophils migrate and degranulate,
culminating in permanent damage to cells,
resulting in alveolar-capillary barrier
disruption;
• D - Interstitial and alveolar edema.
Batah, S. S., & Fabro, A. T. (2021). Pulmonary pathology of ARDS in COVID-19: A pathological
review for clinicians. Respiratory medicine, 176, 106239.
https://doi.org/10.1016/j.rmed.2020.106239
9.
Diffuse alveolar damage• A - The first or exudative phase
constitutes alveolar edema,
neutrophil infiltration in the intraalveolar space and mainly by hyaline
membrane formed by fibrin
polymerization contained in the
plasma liquid, being recognized as
DAD hallmark;
• B - The second or proliferative phase:
intense fibroblast/myofibroblast
recruitment and proliferation, with
subsequent extracellular matrix
deposition. Over time and together
with the fibrotic deposition, there is
also the reepithelization by type I and
II pneumocytes.
10.
Histology• the exudative phase(10 days of viral infection):
hyaline membrane formation (A – green arrow);
alveolar-capillary barrier injury with red blood cell
extravasation (B – green arrows); intense
inflammatory cells infiltration into the intra-alveolar
space.
• the proliferative phase: an exacerbated
fibroblast/myofibroblast proliferation which can form
acute fibrinous organizing pneumonia (C - dark blue
circle) or organizing pneumonia (C – dark green circle)
with subsequent extracellular matrix
deposition=>parenchymal remodeling and pulmonary
fibrosis; pneumocytes squamous metaplasia and
proliferation of multinucleated giant cells.
• Thrombotic events in pulmonary small arteries (D)
may occur in this phase due to NET's influence.
11.
HistologyDiffuse alveolar damage in COVID-19.
with hyaline membranes. (Hematoxylin &
eosin, original magnification × 200).
Microthombi casting the capillaries
of the alveoli (Fibrin stain, original
magnification × 200)
Interstitial inflammation in COVID19. The inflammatory cells are
predominantly lymphocytes
(Hematoxylin & eosin, original
magnification × 200)
12.
Airway inflammation in COVID-19: a Chronic inflammationcomposed mainly of lymphocytes, involving the bronchial
mucosa (Hematoxylin & eosin, original magnification × 200).
13.
Endothelial cell dysfunction• Post-mortem lung specimen showed thickened
lung septa, including a large arterial vessel with
mononuclear and neutrophilic infiltration (arrow
in upper inset).
• The lower inset shows an immunohistochemical
staining of caspase 3 on the same lung
specimen; these staining patterns were
consistent with apoptosis of endothelial cells
and mononuclear cells observed in the
haematoxylin-eosin-stained sections.
Varga, Zsuzsanna et al. “Endothelial cell infection and endotheliitis in COVID-19.” Lancet (London,
England) vol. 395,10234 (2020): 1417-1418. doi:10.1016/S0140-6736(20)30937-5
14.
Neutrophil extracellular trapsBarnes, B. J., Adrover, J. M et al. (2020). Targeting potential drivers of COVID19: Neutrophil extracellular traps. The Journal of experimental
medicine, 217(6)
15.
Complement associated microvascular injuryand thrombosis
16.
Demonstration of co-localization ofcomplement components with SARS-CoV2
spike glycoprotein in the lung
• (A)Deposition of C4d within the
interalveolar septa demonstrated by DAB
staining.
• C4d image appears green (B) while the
SARS-CoV2 spike protein appears red (C).
D -merged image shows a significant
degree of C4d and SARS-CoV2 colocalization, as revealed by intense yellow
staining.
• E–H, A similar pattern was observed using
an anti-C5b-9 reagent whose image
appears green, with a significant degree
of C5b-9 and SARS-CoV2 co-localization,
as revealed by intense yellow staining.
17.
Is it “typical” ARDS?Li X, Ma X. Acute respiratory failure in
COVID-19: is it "typical" ARDS? Crit
Care. 2020 May 6;24(1):198.
18.
Litrature• Xu Z, Shi L et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020
Apr;8(4):420-422.
• Du, Lanying et al. “The spike protein of SARS-CoV--a target for vaccine and therapeutic development.” Nature reviews.
Microbiology vol. 7,3 (2009): 226-36.
• Batah, S. S., & Fabro, A. T. (2021). Pulmonary pathology of ARDS in COVID-19: A pathological review for clinicians. Respiratory
medicine, 176, 106239. Barnes, et al. (2020). Targeting potential drivers of COVID-19: Neutrophil extracellular traps. The Journal of
experimental medicine, 217(6)
• Varga, Zsuzsanna et al. “Endothelial cell infection and endotheliitis in COVID-19.” Lancet (London, England) vol. 395,10234 (2020):
1417-1418.
• Mohanty SK et al. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19) anatomic pathology perspective on current knowledge. Diagn Pathol. 2020 Aug 14;15(1):103.
• Du, Lanying et al. “The spike protein of SARS-CoV--a target for vaccine and therapeutic development.” Nature reviews.
Microbiology vol. 7,3 (2009): 226-36
• Barnes, B. J., Adrover, J. M et al. (2020). Targeting potential drivers of COVID-19: Neutrophil extracellular traps. The Journal of
experimental medicine, 217(6)
• Li X, Ma X. Acute respiratory failure in COVID-19: is it "typical" ARDS? Crit Care. 2020 May 6;24(1):198.

















 medicine
medicine








