Similar presentations:
Qualification and maintenance on GMP Air Handling Systems
1.
Supplementary Training Moduleson GMP
Air Handling Systems
Heating
Ventilation and
Air Conditioning (HVAC)
Part 3: Design, qualification
and maintenance
Module 3, Part 3: Qualification and maintenance
Slide 1 of 27
WHO - EDM
2.
Air Handling SystemsCharacteristics of air handling systems
In the following slides, we will study alternatives in air
handling systems
Turbulent or uni-directional airflows
Filter position
Air re-circulation vs fresh air
Return air systems (positions)
Overpressure requirements
Module 3, Part 3: Design, qualification and maintenance
Slide 2 of 27
WHO - EDM
3.
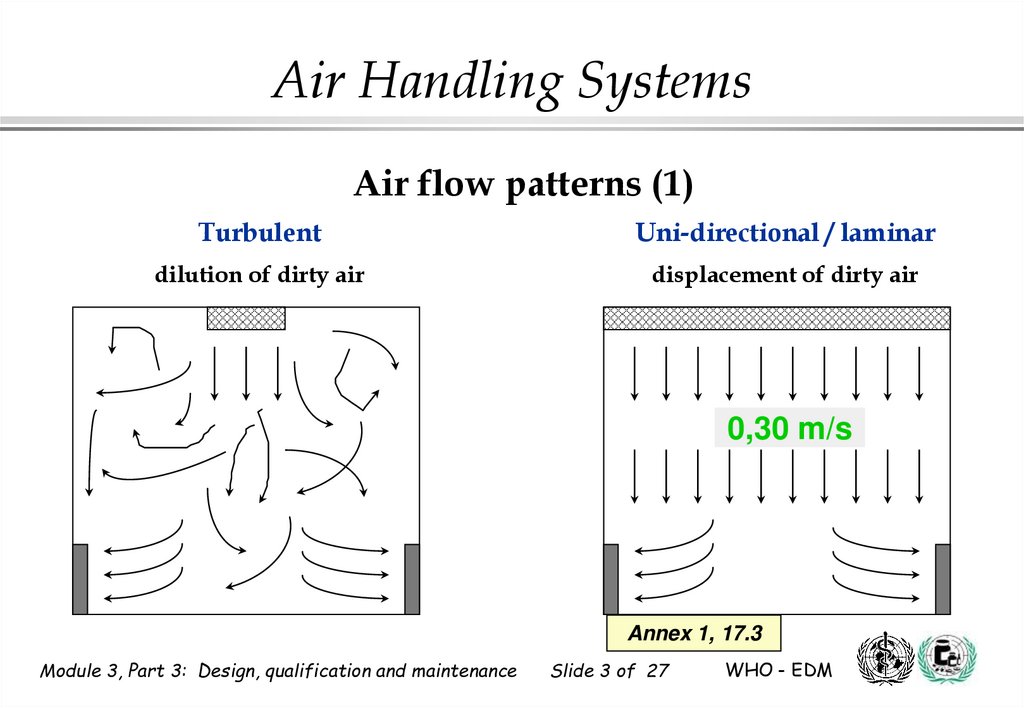
Air Handling SystemsAir flow patterns (1)
Turbulent
Uni-directional / laminar
dilution of dirty air
displacement of dirty air
0,30 m/s
Annex 1, 17.3
Module 3, Part 3: Design, qualification and maintenance
Slide 3 of 27
WHO - EDM
4.
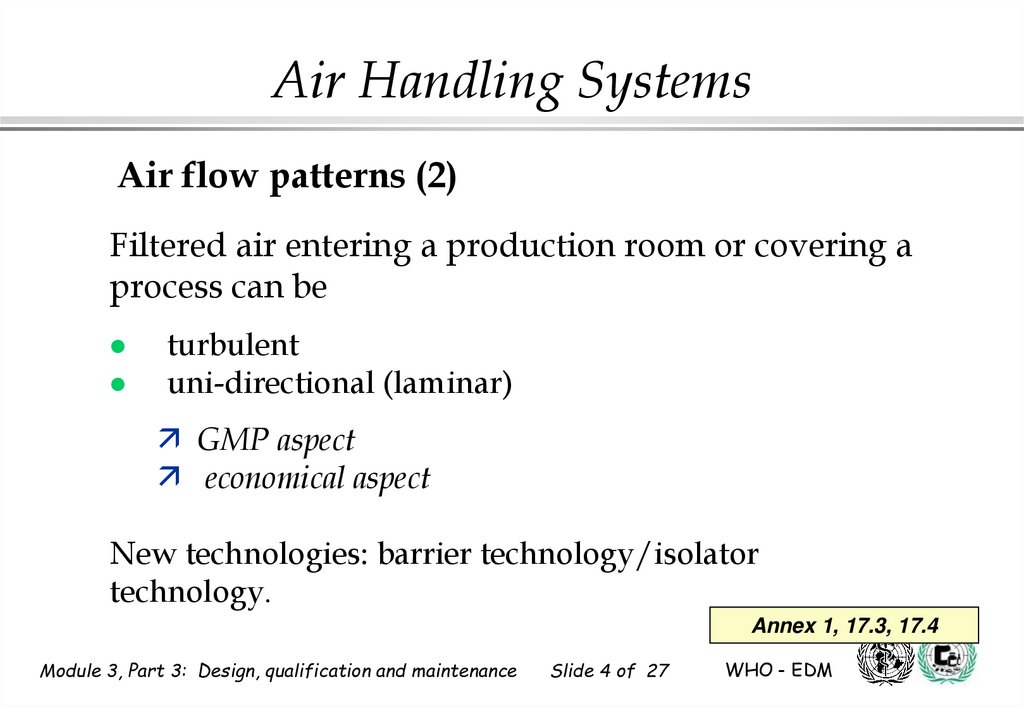
Air Handling SystemsAir flow patterns (2)
Filtered air entering a production room or covering a
process can be
turbulent
uni-directional (laminar)
GMP aspect
economical aspect
New technologies: barrier technology/isolator
technology.
Annex 1, 17.3, 17.4
Module 3, Part 3: Design, qualification and maintenance
Slide 4 of 27
WHO - EDM
5.
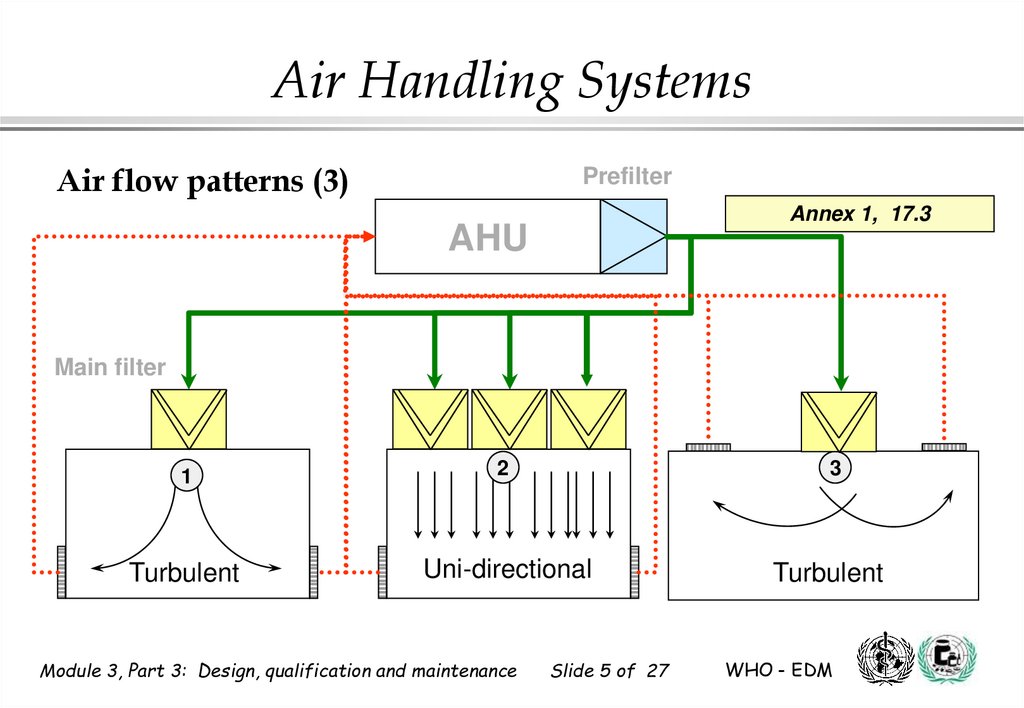
Air Handling SystemsAir flow patterns (3)
Prefilter
Annex 1, 17.3
AHU
Main filter
1
2
Turbulent
Uni-directional
Module 3, Part 3: Design, qualification and maintenance
3
Slide 5 of 27
Turbulent
WHO - EDM
6.
Air Handling SystemsAir flow patterns (4)
Workbench (vertical)
Cabin/ booth
Module 3, Part 3: Design, qualification and maintenance
Slide 6 of 27
Ceiling
WHO - EDM
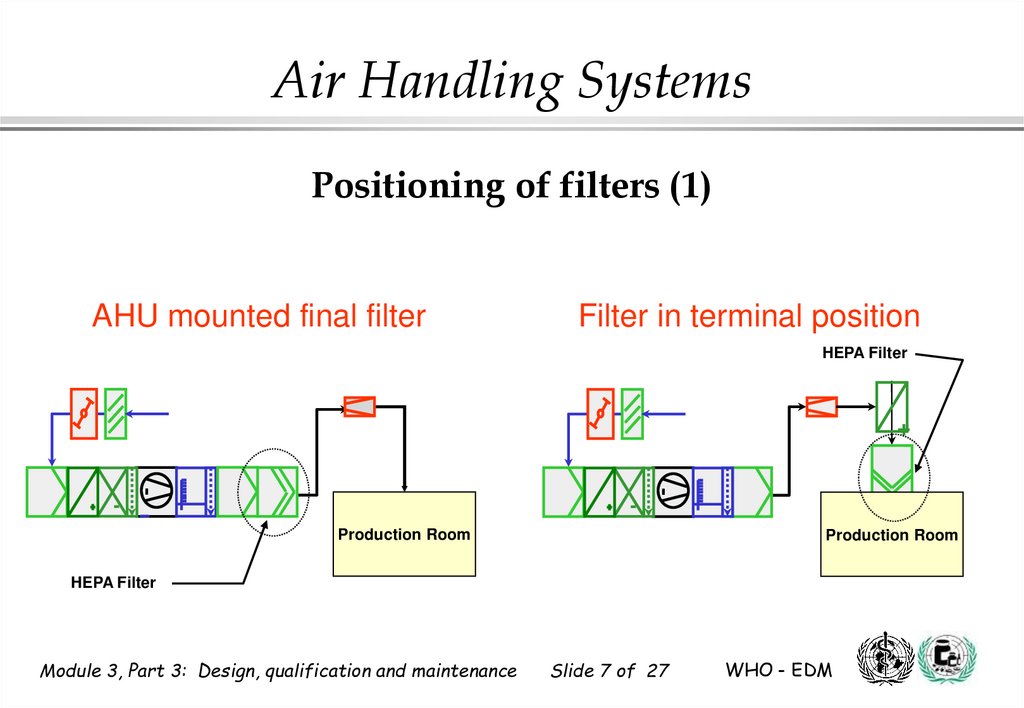
7.
Air Handling SystemsPositioning of filters (1)
AHU mounted final filter
Filter in terminal position
HEPA Filter
+
Production Room
Production Room
HEPA Filter
Module 3, Part 3: Design, qualification and maintenance
Slide 7 of 27
WHO - EDM
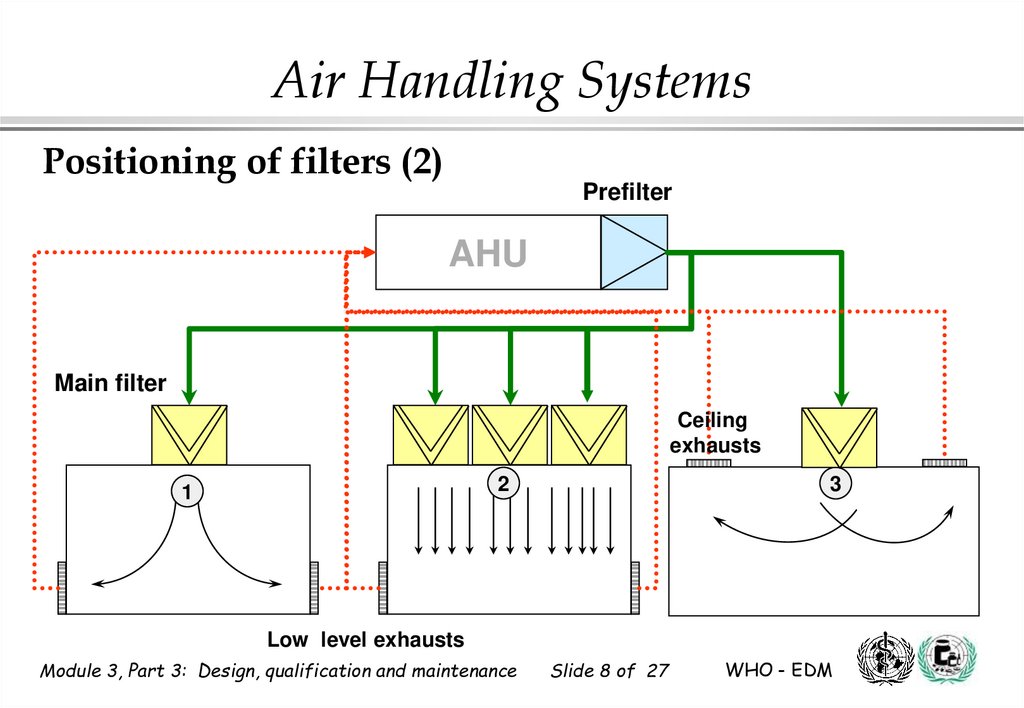
8.
Air Handling SystemsPositioning of filters (2)
Prefilter
AHU
Main filter
Ceiling
exhausts
2
1
3
Low level exhausts
Module 3, Part 3: Design, qualification and maintenance
Slide 8 of 27
WHO - EDM
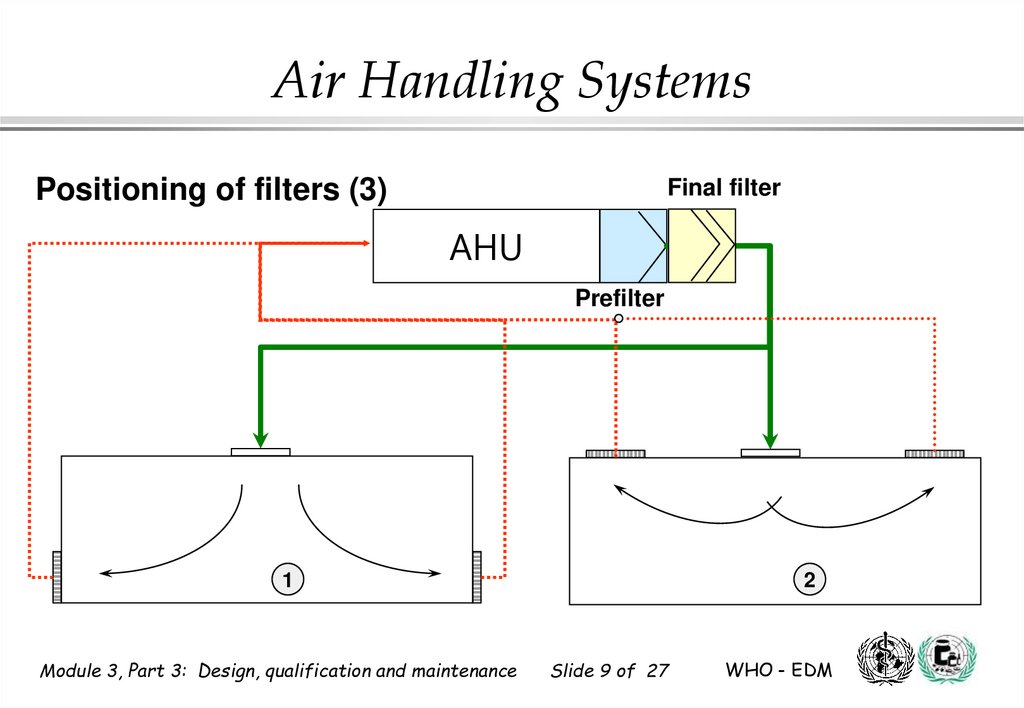
9.
Air Handling SystemsPositioning of filters (3)
Final filter
AHU
Prefilter
1
Module 3, Part 3: Design, qualification and maintenance
2
Slide 9 of 27
WHO - EDM
10.
Air Handling SystemsAir re-circulation
The filtered air entering a production room can be
100% exhausted or
a proportion re-circulated
GMP aspect
economical reasons
Annex 1, 15.10, 17.24
Module 3, Part 3: Design, qualification and maintenance
Slide 10 of 27
WHO - EDM
11.
Air Handling SystemsVentilation with 100% fresh air (no air re-circulation)
Washer (optional)
Exhaust Unit
W
Central Air Handling Unit
Production Rooms
Annex 1, 17.24
Module 3, Part 3: Design, qualification and maintenance
Slide 11 of 27
WHO - EDM
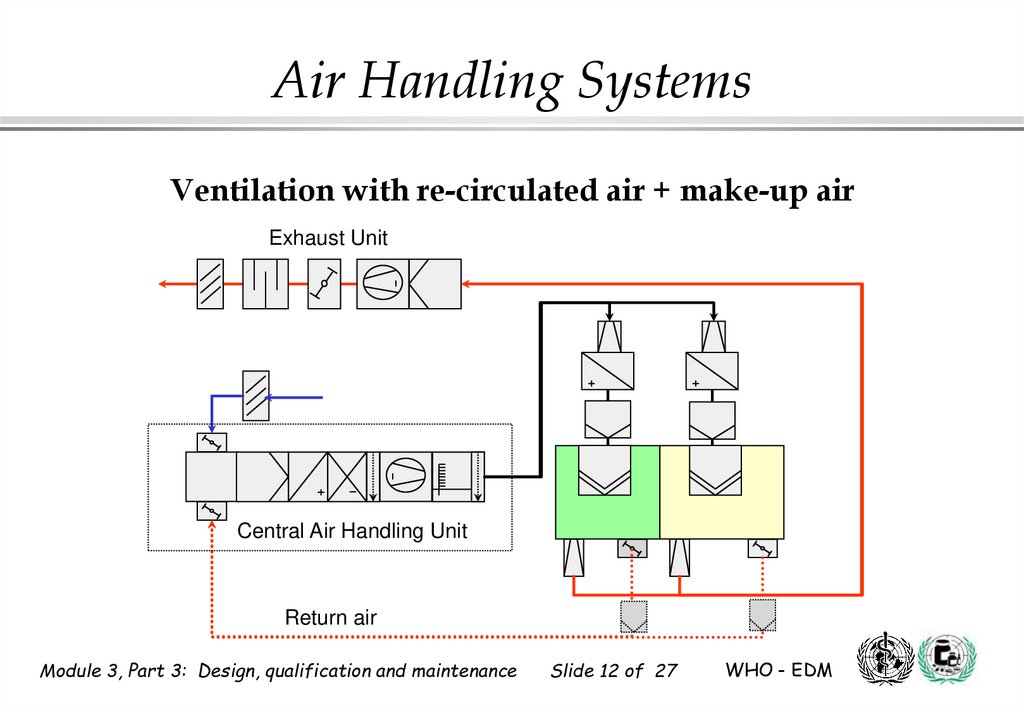
12.
Air Handling SystemsVentilation with re-circulated air + make-up air
Exhaust Unit
Central Air Handling Unit
Return air
Module 3, Part 3: Design, qualification and maintenance
Slide 12 of 27
WHO - EDM
13. Definition of Conditions
Air Handling SystemsDefinition of Conditions
as built
at rest
in operation
air
air
air
Module 3, Part 3: Design, qualification and maintenance
Slide 13 of 27
WHO - EDM
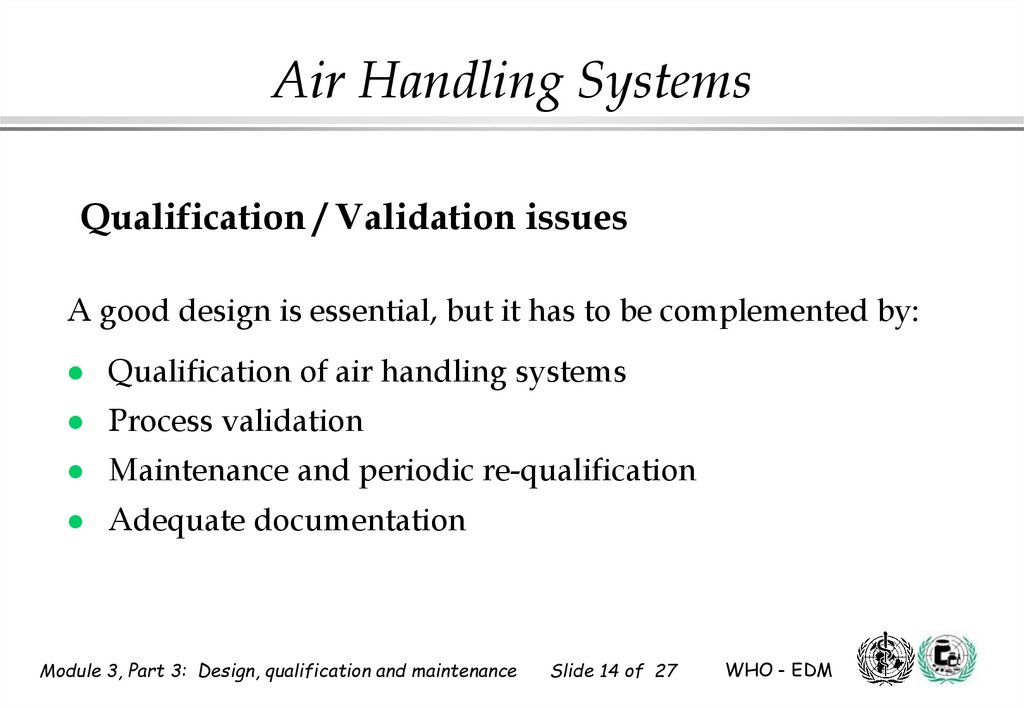
14. Qualification / Validation issues
Air Handling SystemsQualification / Validation issues
A good design is essential, but it has to be complemented by:
Qualification of air handling systems
Process validation
Maintenance and periodic re-qualification
Adequate documentation
Module 3, Part 3: Design, qualification and maintenance
Slide 14 of 27
WHO - EDM
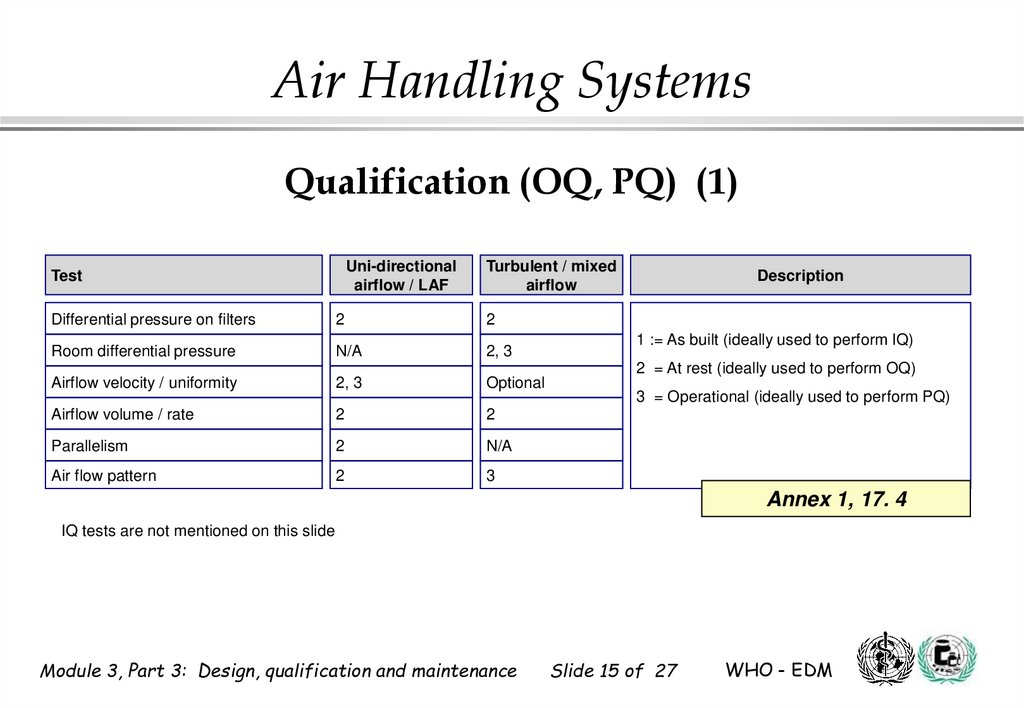
15. Qualification (OQ, PQ) (1)
Air Handling SystemsQualification (OQ, PQ) (1)
Uni-directional
airflow / LAF
Test
Turbulent / mixed
airflow
Differential pressure on filters
2
2
Room differential pressure
N/A
2, 3
Airflow velocity / uniformity
2, 3
Optional
Airflow volume / rate
2
2
Parallelism
2
N/A
Air flow pattern
2
3
Description
1 := As built (ideally used to perform IQ)
2 = At rest (ideally used to perform OQ)
3 = Operational (ideally used to perform PQ)
Annex 1, 17. 4
IQ tests are not mentioned on this slide
Module 3, Part 3: Design, qualification and maintenance
Slide 15 of 27
WHO - EDM
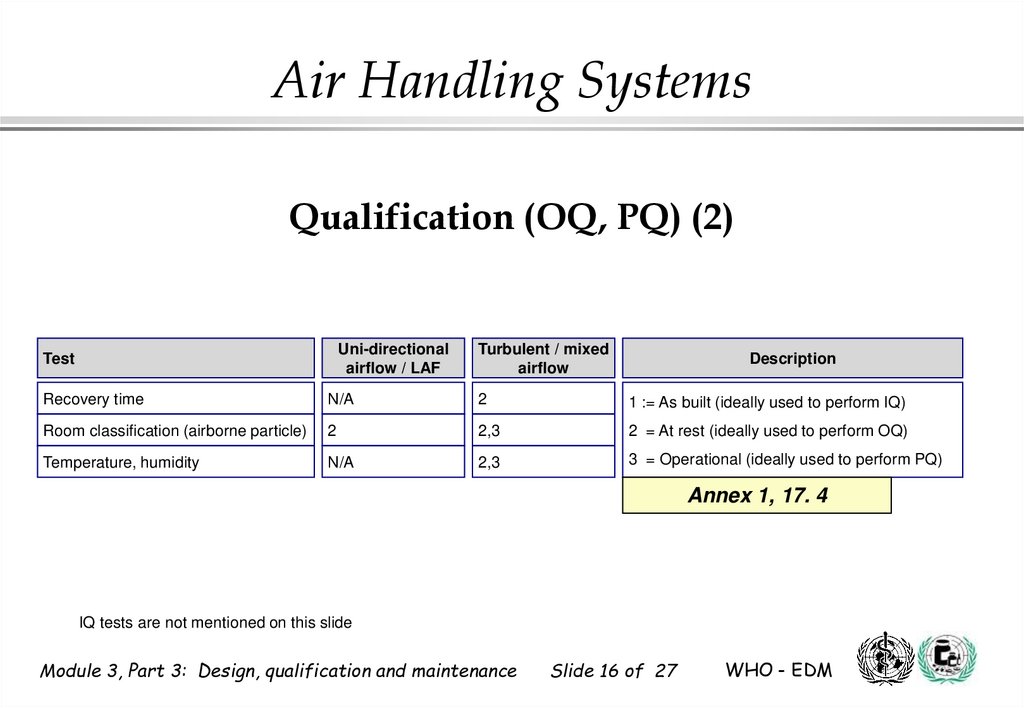
16. Qualification (OQ, PQ) (2)
Air Handling SystemsQualification (OQ, PQ) (2)
Uni-directional
airflow / LAF
Test
Turbulent / mixed
airflow
Description
Recovery time
N/A
2
1 := As built (ideally used to perform IQ)
Room classification (airborne particle)
2
2,3
2 = At rest (ideally used to perform OQ)
Temperature, humidity
N/A
2,3
3 = Operational (ideally used to perform PQ)
Annex 1, 17. 4
IQ tests are not mentioned on this slide
Module 3, Part 3: Design, qualification and maintenance
Slide 16 of 27
WHO - EDM
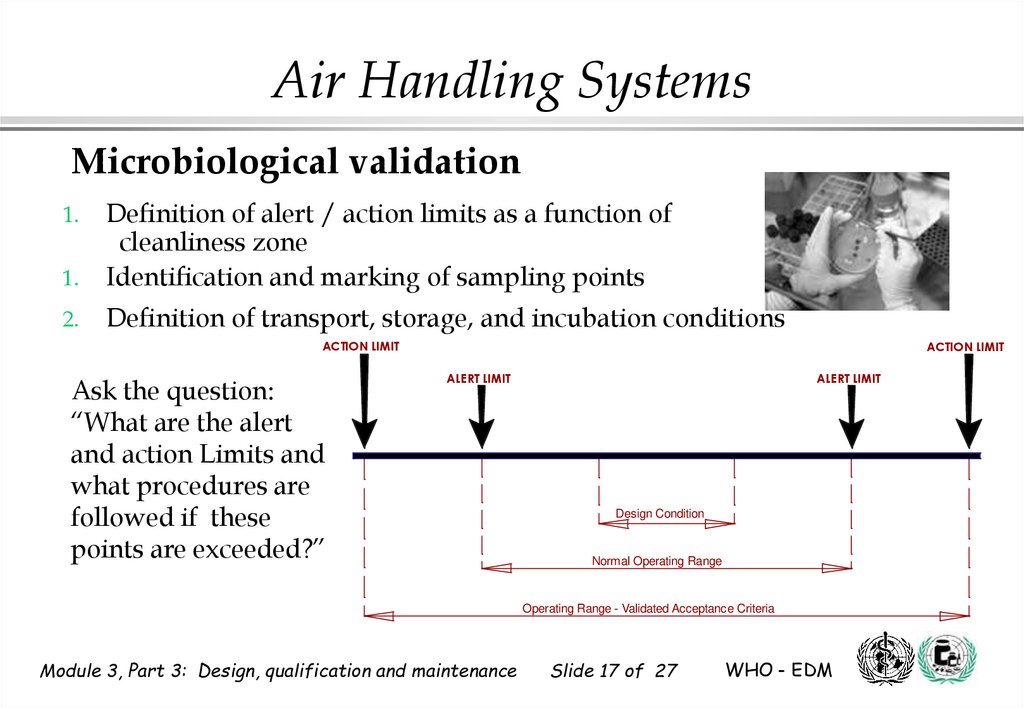
17. Microbiological validation
Air Handling SystemsMicrobiological validation
1.
Definition of alert / action limits as a function of
cleanliness zone
Identification and marking of sampling points
2.
Definition of transport, storage, and incubation conditions
1.
ACTION LIMIT
Ask the question:
“What are the alert
and action Limits and
what procedures are
followed if these
points are exceeded?”
ACTION LIMIT
ALERT LIMIT
ALERT LIMIT
Design Condition
Normal Operating Range
Operating Range - Validated Acceptance Criteria
Module 3, Part 3: Design, qualification and maintenance
Slide 17 of 27
WHO - EDM
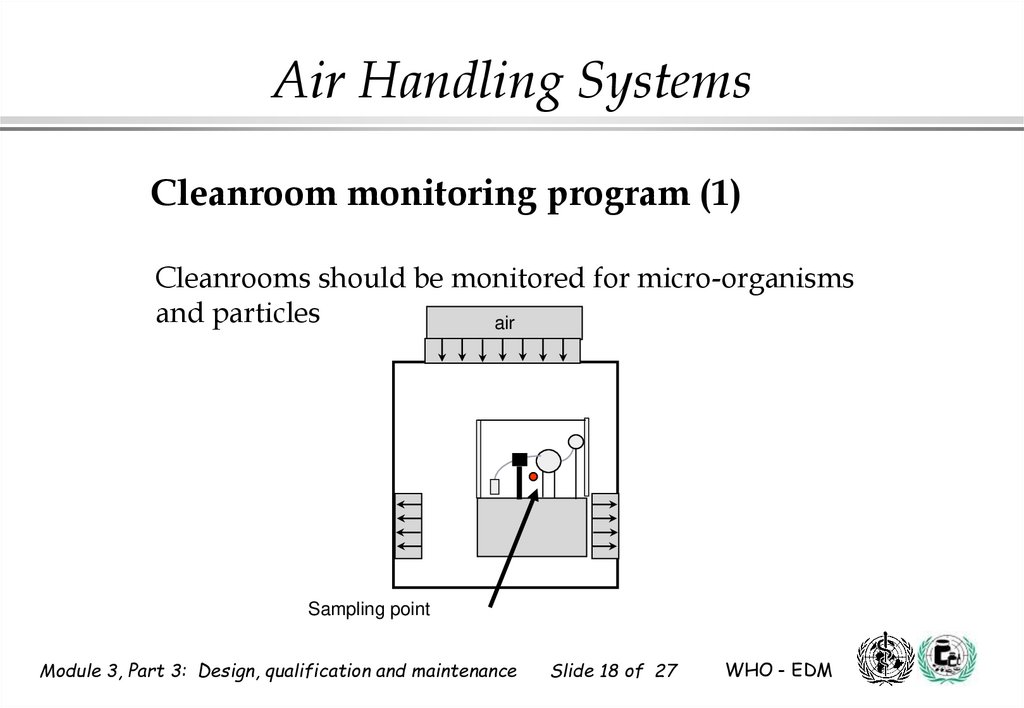
18.
Air Handling SystemsCleanroom monitoring program (1)
Cleanrooms should be monitored for micro-organisms
and particles
air
Sampling point
Module 3, Part 3: Design, qualification and maintenance
Slide 18 of 27
WHO - EDM
19. Cleanroom monitoring program (2)
Air Handling SystemsCleanroom monitoring program (2)
Routine monitoring program as part of quality assurance
Additional monitoring and triggers
1.
2.
3.
4.
Shutdown
Replacement of filter elements
Maintenance of air handling systems
Exceeding of established limits
Annex 1, 17.37
Module 3, Part 3: Design, qualification and maintenance
Slide 19 of 27
WHO - EDM
20.
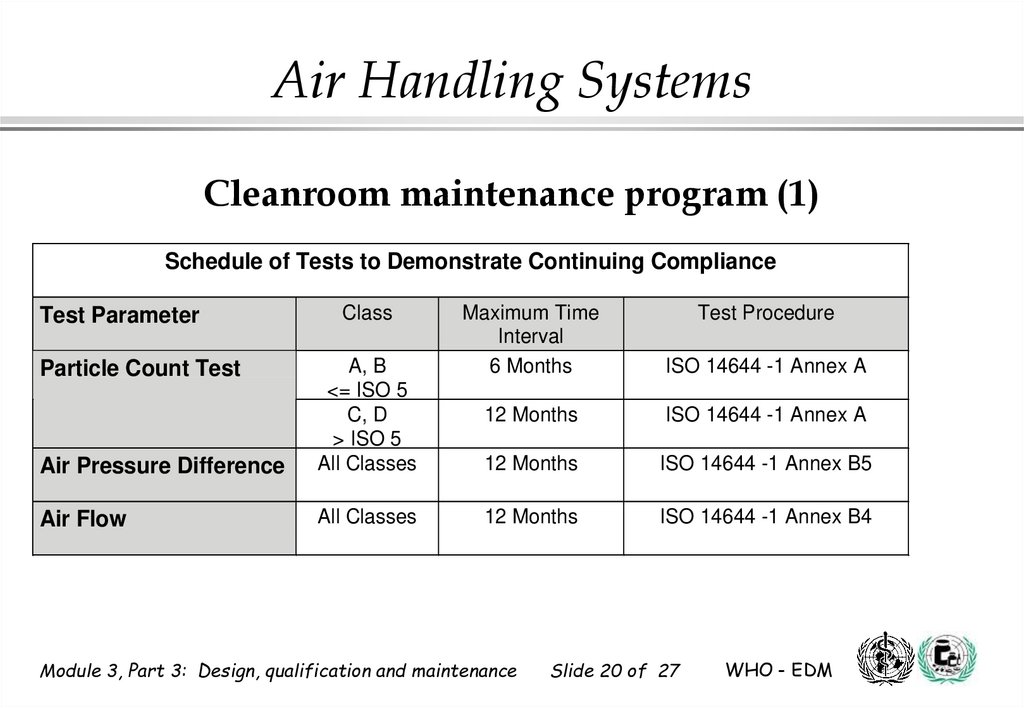
Air Handling SystemsCleanroom maintenance program (1)
Schedule of Tests to Demonstrate Continuing Compliance
Test Parameter
Class
Air Pressure Difference
A, B
<= ISO 5
C, D
> ISO 5
All Classes
Air Flow
All Classes
Particle Count Test
Maximum Time
Interval
6 Months
ISO 14644 -1 Annex A
12 Months
ISO 14644 -1 Annex A
12 Months
ISO 14644 -1 Annex B5
12 Months
ISO 14644 -1 Annex B4
Module 3, Part 3: Design, qualification and maintenance
Test Procedure
Slide 20 of 27
WHO - EDM
21.
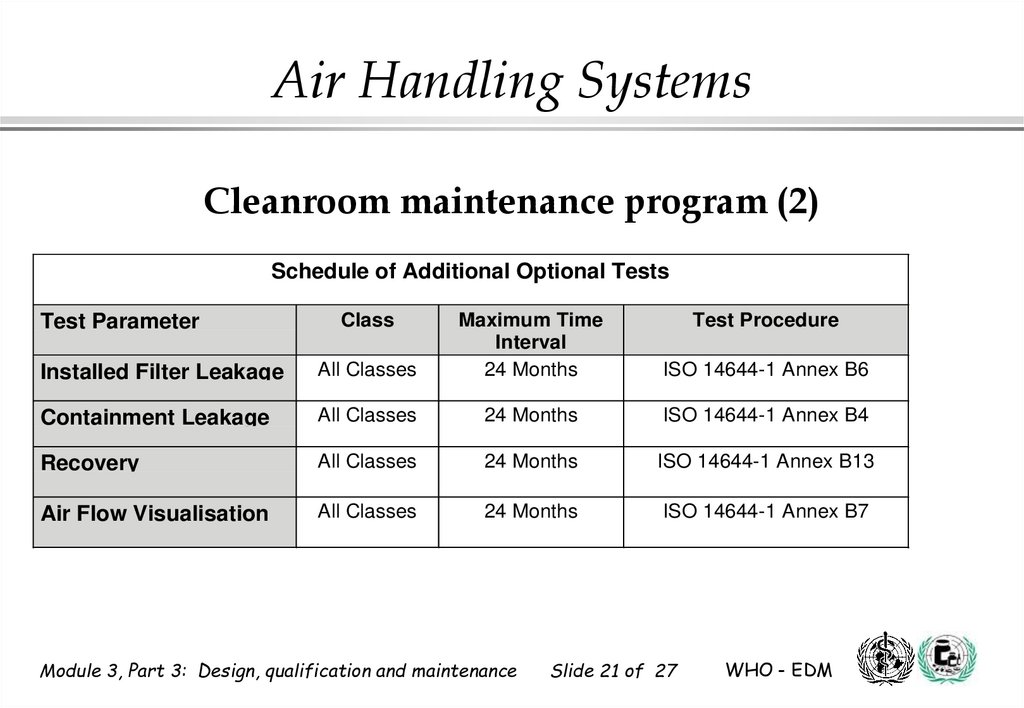
Air Handling SystemsCleanroom maintenance program (2)
Schedule of Additional Optional Tests
Installed Filter Leakage
All Classes
Maximum Time
Interval
24 Months
Containment Leakage
All Classes
24 Months
ISO 14644-1 Annex B4
Recovery
All Classes
24 Months
ISO 14644-1 Annex B13
Air Flow Visualisation
All Classes
24 Months
ISO 14644-1 Annex B7
Test Parameter
Class
Module 3, Part 3: Design, qualification and maintenance
Test Procedure
ISO 14644-1 Annex B6
Slide 21 of 27
WHO - EDM
22.
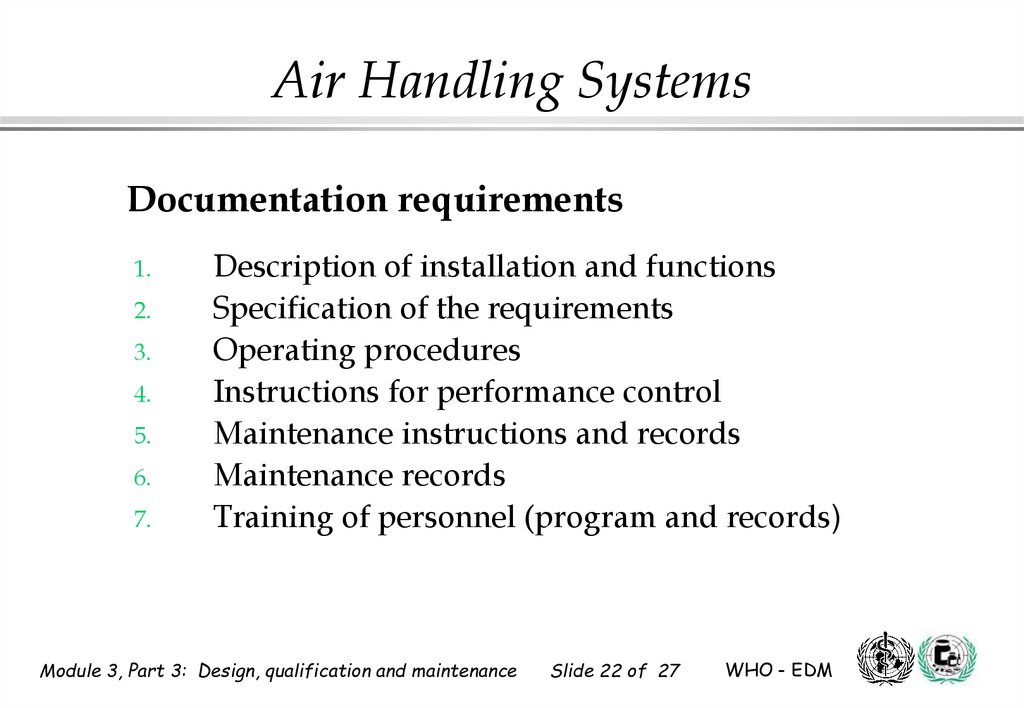
Air Handling SystemsDocumentation requirements
1.
2.
3.
4.
5.
6.
7.
Description of installation and functions
Specification of the requirements
Operating procedures
Instructions for performance control
Maintenance instructions and records
Maintenance records
Training of personnel (program and records)
Module 3, Part 3: Design, qualification and maintenance
Slide 22 of 27
WHO - EDM
23.
Air Handling SystemsInspecting the air handling plant
1.
Verification of design documentation, including
description of installation and functions
specification of the requirements
2.
3.
4.
5.
6.
7.
8.
Operating procedures
Maintenance instructions
Maintenance records
Training logs
Environmental records
Discussion on actions if OOS values
Walking around the plant
Module 3, Part 3: Design, qualification and maintenance
Slide 23 of 27
WHO - EDM
24.
Air Handling SystemsConclusion
Air handling systems:
1.
2.
3.
Play a major role in the quality of pharmaceuticals
Must be designed properly, by professionals
Must be treated as a critical system
Module 3, Part 3: Design, qualification and maintenance
Slide 24 of 27
WHO - EDM
25.
Air Handling SystemsFurther proceedings
This series of explanations will now be followed by:
Group discussion, with a simple exercise
Short test
Module 3, Part 3: Design, qualification and maintenance
Slide 25 of 27
WHO - EDM
26.
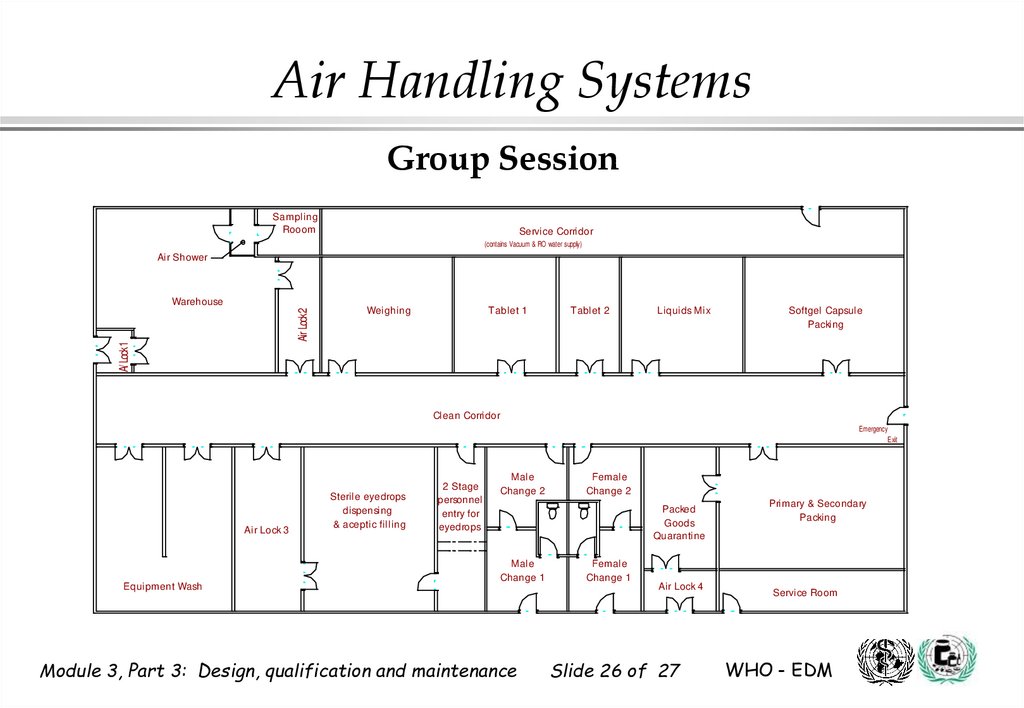
Air Handling SystemsGroup Session
Sampling
Rooom
Service Corridor
(contains Vacuum & RO water supply)
Air Shower
A/ Lock1
Air Lock2
Warehouse
Weighing
Tablet 1
Tablet 2
Liquids Mix
Softgel Capsule
Packing
Clean Corridor
Emergency
Exit
Air Lock 3
Equipment Wash
Sterile eyedrops
dispensing
& aceptic filling
2 Stage
personnel
entry for
eyedrops
Male
Change 2
Female
Change 2
Packed
Goods
Quarantine
Male
Change 1
Module 3, Part 3: Design, qualification and maintenance
Female
Change 1
Air Lock 4
Slide 26 of 27
Primary & Secondary
Packing
Service Room
WHO - EDM
27.
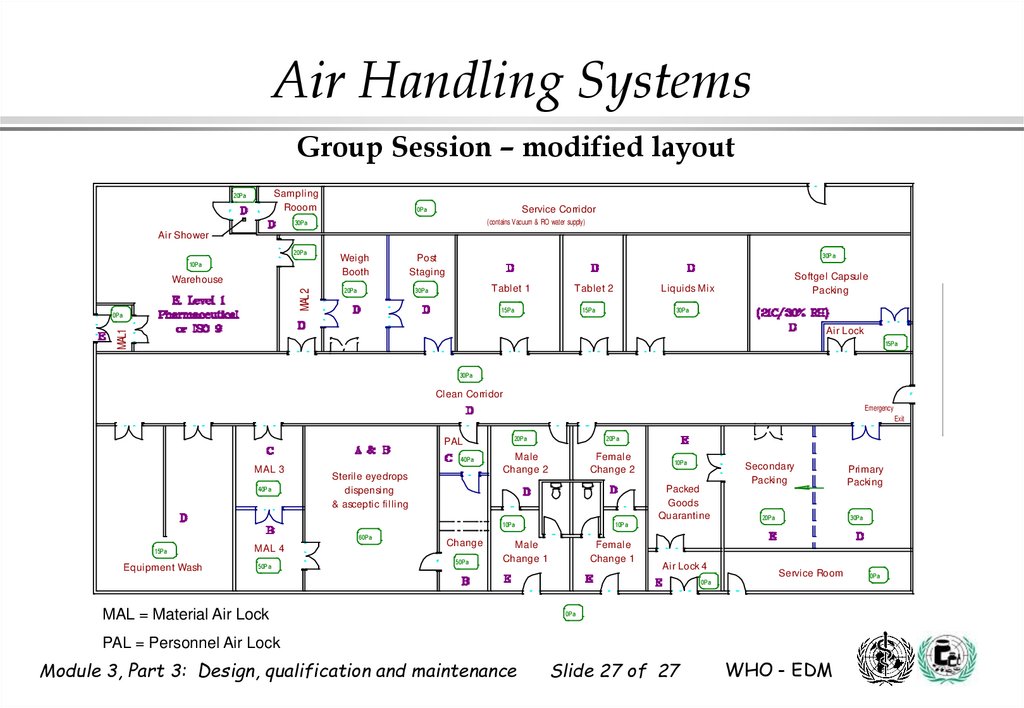
Air Handling SystemsGroup Session – modified layout
Sampling
Rooom
20Pa
0Pa
Service Corridor
(contains Vacuum & RO water supply)
30Pa
Air Shower
20Pa
10Pa
MAL 2
Warehouse
0Pa
Weigh
Booth
20Pa
30Pa
Post
Staging
Tablet 1
30Pa
Tablet 2
15Pa
Softgel Capsule
Packing
Liquids Mix
15Pa
30Pa
MAL1
Air Lock
15Pa
30Pa
Clean Corridor
Emergency
Exit
PAL
40Pa
MAL 3
40Pa
Sterile eyedrops
dispensing
& asceptic filling
20Pa
20Pa
Male
Change 2
Female
Change 2
10Pa
60Pa
15Pa
Equipment Wash
MAL 4
50Pa
Change
50Pa
10Pa
Male
Change 1
Female
Change 1
10Pa
Packed
Goods
Quarantine
Air Lock 4
0Pa
MAL = Material Air Lock
Secondary
Packing
20Pa
30Pa
Service Room
0Pa
PAL = Personnel Air Lock
Module 3, Part 3: Design, qualification and maintenance
Slide 27 of 27
Primary
Packing
WHO - EDM
0Pa







 industry
industry








