Similar presentations:
Physical and chemical properties of oil
1.
Physical and ChemicalProperties of Oil
Novosibirsk, 2019
2.
Physical and Chemical Properties of OilOil is described by the physical properties of density, color,
viscosity, thermal expansion and other properties related to the
number of carbon atoms in the molecules.
2
3.
ColourPetroleum can be of different colors. Oil colors vary in a very
wide range from oilfield to oilfield: from pale yellow, yellow and
even colourless to dark grey, green and dark brown shades.
3
4.
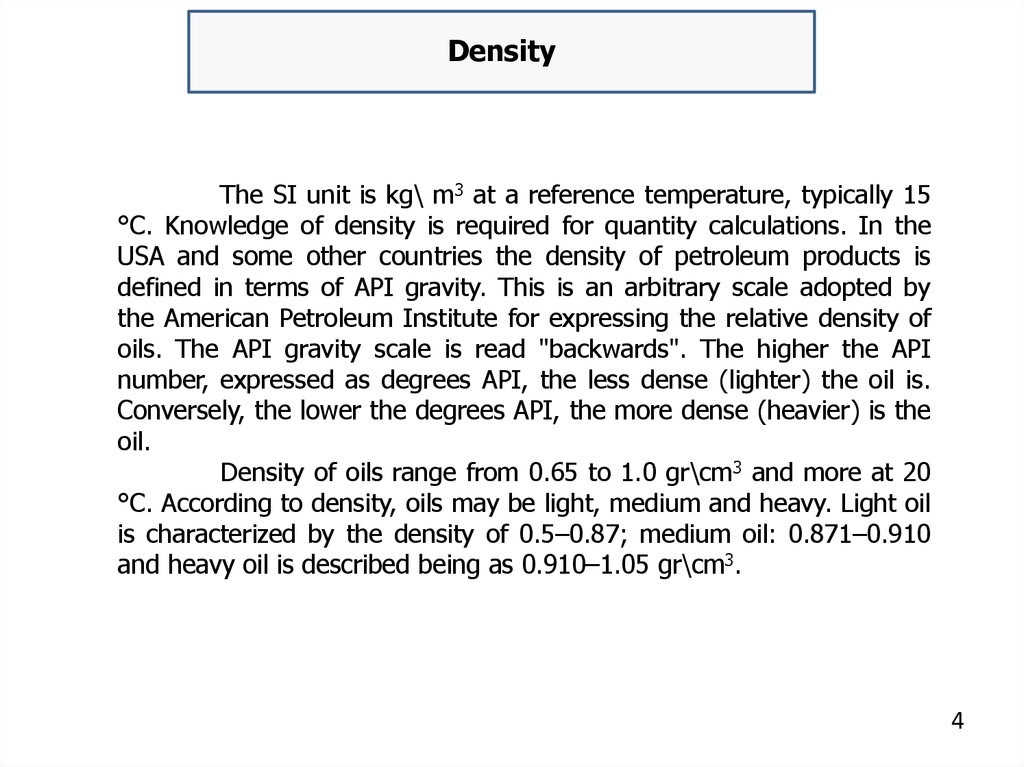
DensityThe SI unit is kg\ m3 at a reference temperature, typically 15
°C. Knowledge of density is required for quantity calculations. In the
USA and some other countries the density of petroleum products is
defined in terms of API gravity. This is an arbitrary scale adopted by
the American Petroleum Institute for expressing the relative density of
oils. The API gravity scale is read "backwards". The higher the API
number, expressed as degrees API, the less dense (lighter) the oil is.
Conversely, the lower the degrees API, the more dense (heavier) is the
oil.
Density of oils range from 0.65 to 1.0 gr\cm3 and more at 20
°C. According to density, oils may be light, medium and heavy. Light oil
is characterized by the density of 0.5–0.87; medium oil: 0.871–0.910
and heavy oil is described being as 0.910–1.05 gr\cm3.
4
5.
Density5
6.
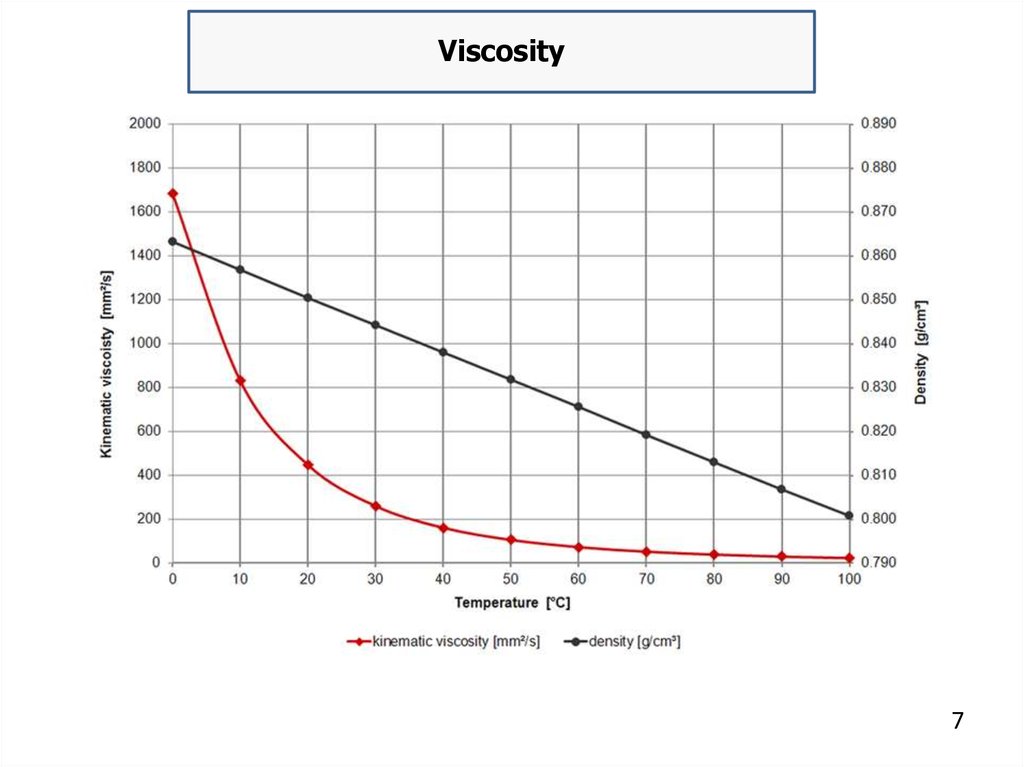
ViscosityViscosity is a property of fluids that indicates their resistance
to flow, defined as ratio of shear stress to shear rate. Crude oils range
in consistency from water-like to tar-like solids. Fluid with a high
viscosity such as syrup deforms more slowly than fluid with a low
viscosity such as water. Absolute viscosity is measured in Poise.
The oil specific viscosity is usually defined as ratio of absolute
viscosity of a given fluid to absolute viscosity of water at the same
temperature. The viscosity of oil is dependent upon temperature,
pressure and shear rate. Viscosity decreases as temperature increases
because molecules vibrate and interact less.
Conversely, the viscosity of oil increases as temperature
decreases and it can become grease-like at very low temperature. The
volume of given oil mass increases with temperature, therefore, its
density decreases.
6
7.
Viscosity7
8.
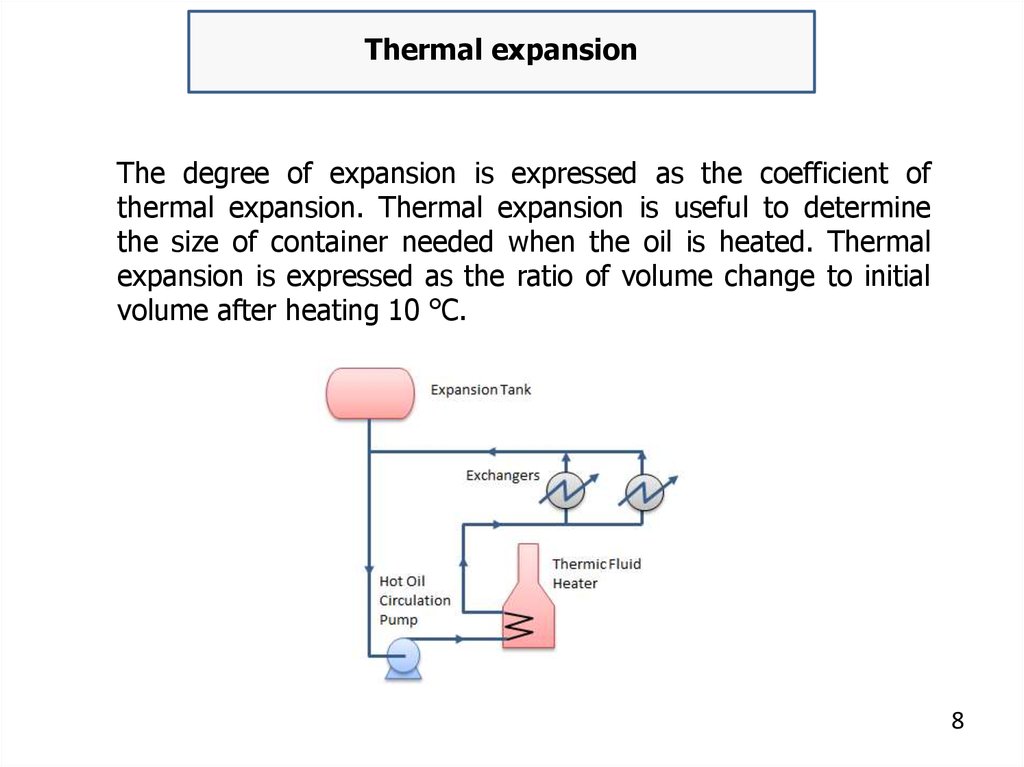
Thermal expansionThe degree of expansion is expressed as the coefficient of
thermal expansion. Thermal expansion is useful to determine
the size of container needed when the oil is heated. Thermal
expansion is expressed as the ratio of volume change to initial
volume after heating 10 °C.
8
9.
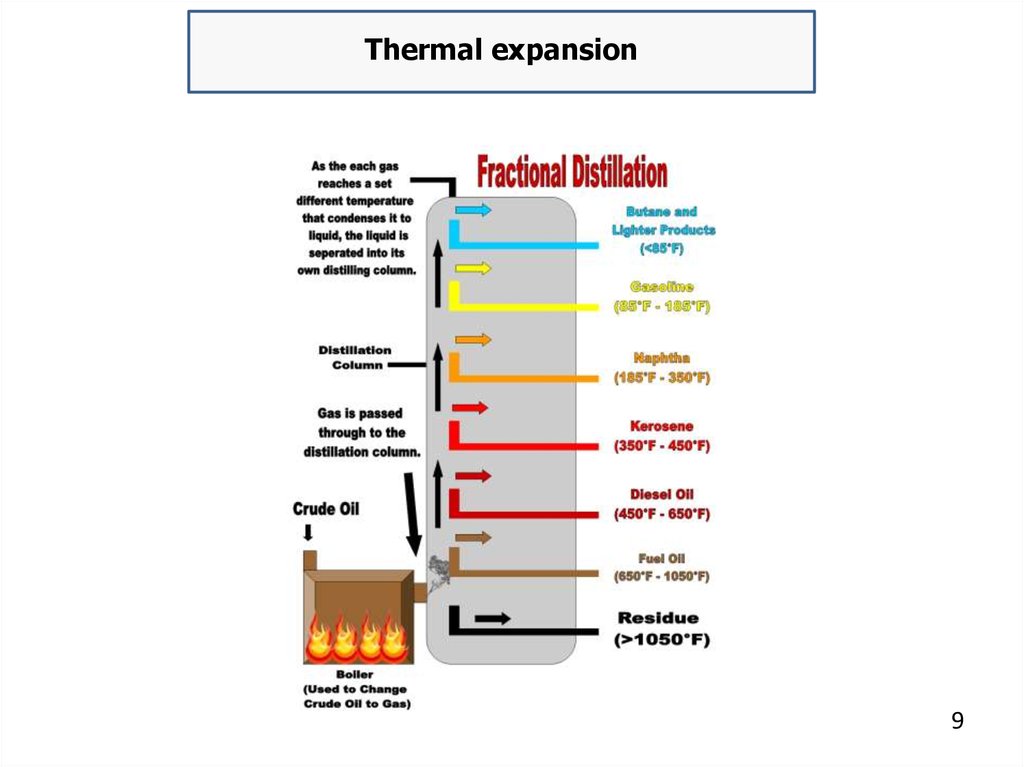
Thermal expansion9
10.
Crude oilCrude oils are complex mixtures containing hundreds of
different hydrocarbon compounds that vary in appearance and
composition from oil field to oil field, therefore, in various oil fields the
oil composition can vary significantly.
All hydrocarbons are divided into two groups: saturated
hydrocarbons and unsaturated hydrocarbons. Saturated hydrocarbons
are not capable of attaching atoms and molecules while unsaturated
hydrocarbons are capable of attaching atoms and molecules. The
latter take part in chemical reactions easier. Hydrocarbons can be as
simple as methane, but many are highly complex molecules and can
occur as gases, liquids or solids.
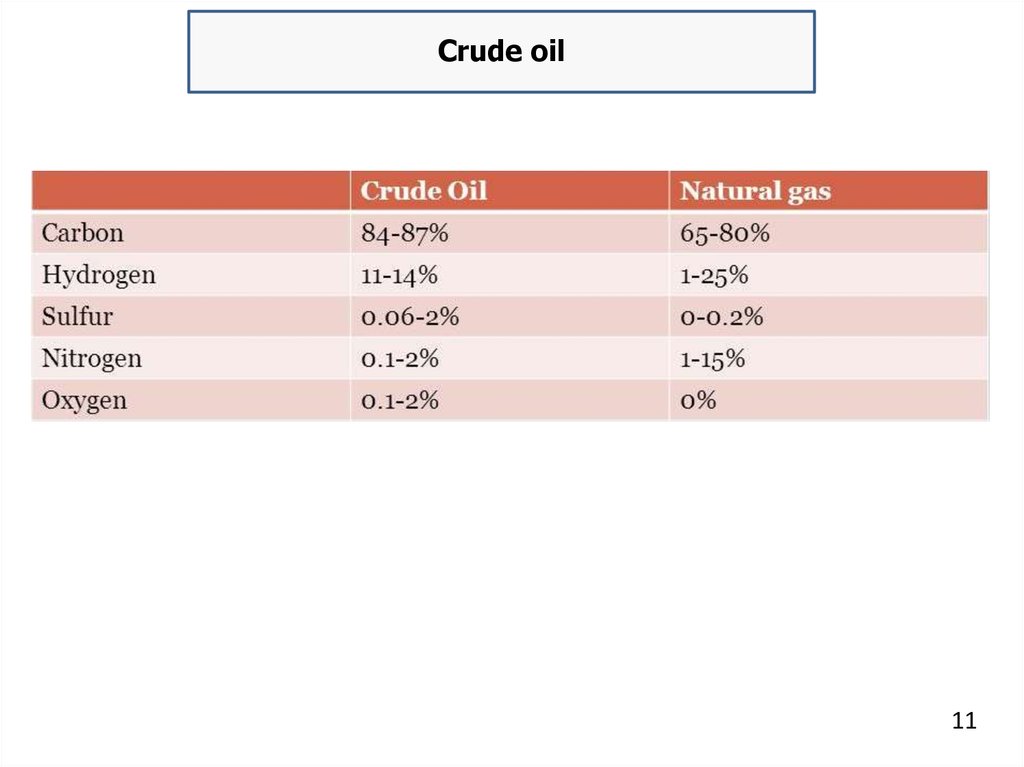
An "average" crude oil contains about 84 % carbon, 14 %
hydrogen, 1–5 % sulfur, and less than 1 % of nitrogen, oxygen, metals
and salts. In the refinery, most of these non-hydrocarbon substances
are removed and the oil is broken down into various compounds and
blended into useful products.
10
11.
Crude oil11
12.
Crude oilAll petroleum hydrocarbons are divided into three groups:
• Alkanes (methane group) with the general formula CnH2n + 2.
This group represents saturated hydrocarbons, since all their
valence bonds are involved. From a chemical point of view, they are
the most inert, in other words, unable to react with other chemical
compounds. The structure of alkanes can be either linear (normal
alkanes) or branched (isoalkanes).
• Cyclanes (naphthenic group) with the general formula CnH2n.
Their main attribute is a five- or six-membered ring consisting of
carbon atoms. In other words, cyclanes, unlike alkanes, have a
cyclic structure closed in a chain. This group also represents the
limiting (saturated) compounds, and they also hardly enter into
reactions with other chemical elements.
• Arenas (aromatic group) with the general formula CnH2n-6. Their
structure is six-membered cycles, which are based on the aromatic
benzene core (C6H6). They are distinguished by the presence of
double bonds between the atoms. Arenas are monocyclic (one
benzene ring), bicyclic (double benzene rings) and polycyclic (rings
are connected according to the principle of honeycombs).
12
13.
Thanks for attention13




 chemistry
chemistry industry
industry








