Similar presentations:
Corrosion
1.
LECTURE № 13CORROSION
15.04.2015
2.
Corrosionis
the
gradual
destruction
of
materials (usually metals) by
chemical reaction with their
environment.
In the most common use
of the word, this means
electrochemical oxidation of
metals in reaction with an
oxidants such as oxygen.
Rusting, the formation of iron
oxides, is a well-known example
of electrochemical corrosion. This
type
of
damage
typically
produces oxide(s) or salt(s) of the
original metal.
3.
Corrosion can also occur inmaterials other than metals, such
as
ceramics
or
polymers,
although in this context, the term
degradation is more common.
Corrosion degrades the useful
properties of materials and
structures including strength,
appearance and permeability to
liquids and gases.
4.
Many structural alloys corrode merely fromexposure to moisture in air, but the process can be
strongly affected by exposure to certain substances.
Corrosion can be concentrated locally to form a pit or
crack, or it can extend across a wide area more or
less uniformly corroding the surface. Because
corrosion is a diffusion-controlled process, it occurs
on exposed surfaces. As a result, methods to reduce
the activity of the exposed surface, such as
passivation and chromate conversion, can increase a
material's corrosion resistance. However, some
corrosion mechanisms are less visible and less
predictable.
5.
Metal corrosion is a spontaneousthermodynamic
destruction
(anodic
oxidation) metal as a result of exposure to
chemical and electrochemical environment,
it is heterogeneous redox process that
occurs at the interface.
6.
In terms of redox reactions: nature of thisinteraction is reduced to the oxidation of the metal
and restore oxidant. When metal corrosion occur at
its surface take place simultaneously two independent
electrochemical reactions:
at anode: Me - ne = Me+n
at cathode: Ox + ne = Red
Corrosive environment called surfactants,
are present around the structural member, its
impact on the material and cause it to corrode.
Corrosive medium may be air, industrial
atmosphere, gases, water, sea climate, land - soil,
acids, alkalis, water and salt solutions.
7.
• Iron oxidation by atmospheric oxygen:4Fe + 3O2 = 2Fe2O3
• Corrosion of iron in aqueous solution
containing hydrogen ions (acid medium,
pH<7):
2Fe + 6H+ = 2Fe3+ + 3H2
• Corrosion of iron in water containing
oxygen (neutral medium, pH=7):
4Fe + 3O2 + 2H2О = 2Fe2O3·H2О
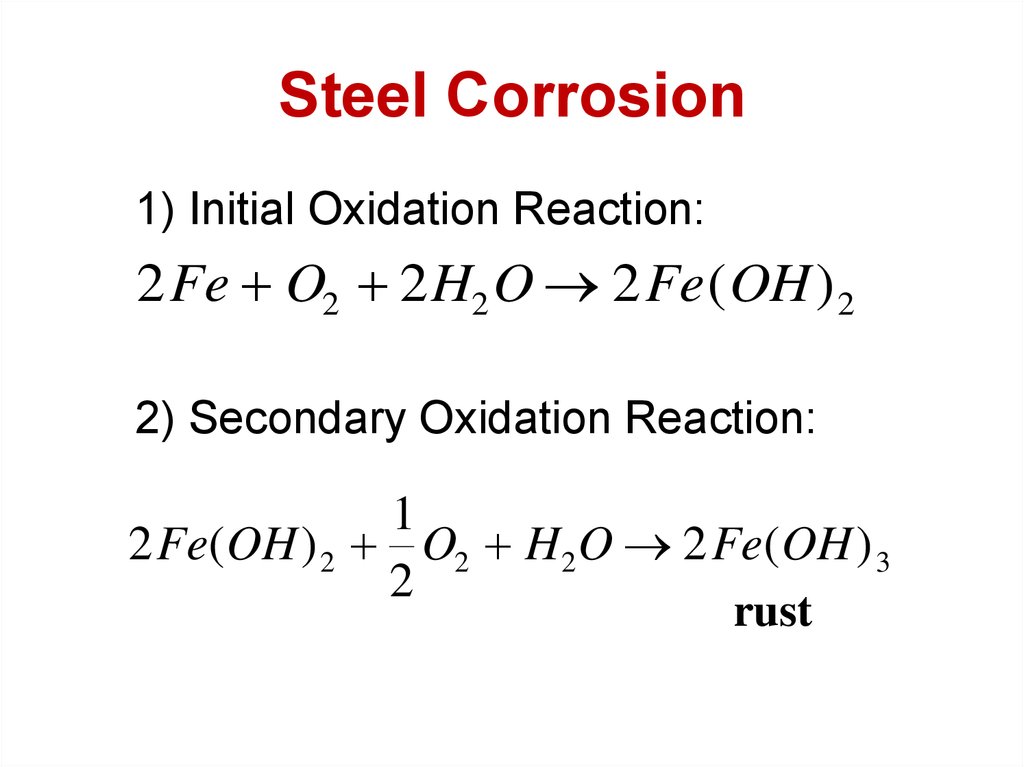
8. Steel Corrosion
1) Initial Oxidation Reaction:2 Fe O2 2 H2 O 2 Fe ( OH ) 2
2) Secondary Oxidation Reaction:
1
2 Fe(OH ) 2 O2 H2 O 2 Fe(OH ) 3
2
rust
9.
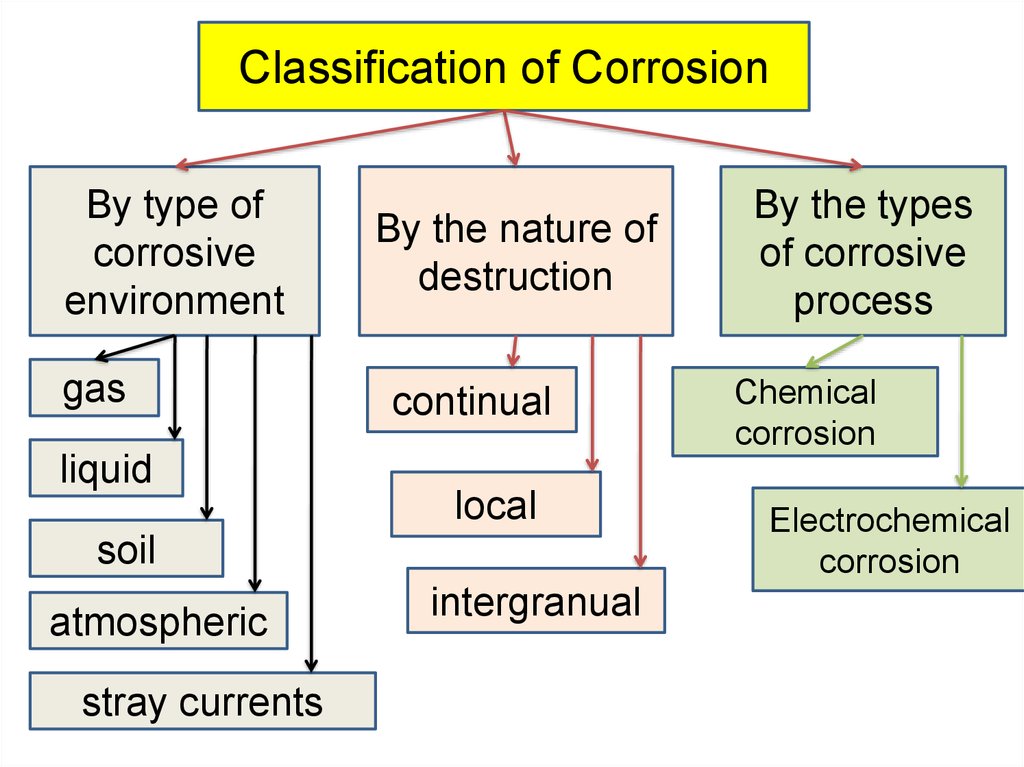
Classification of CorrosionBy type of
corrosive
environment
gas
liquid
By the nature of
destruction
continual
local
soil
atmospheric
stray currents
intergranual
By the types
of corrosive
process
Chemical
corrosion
Electrochemical
corrosion
10.
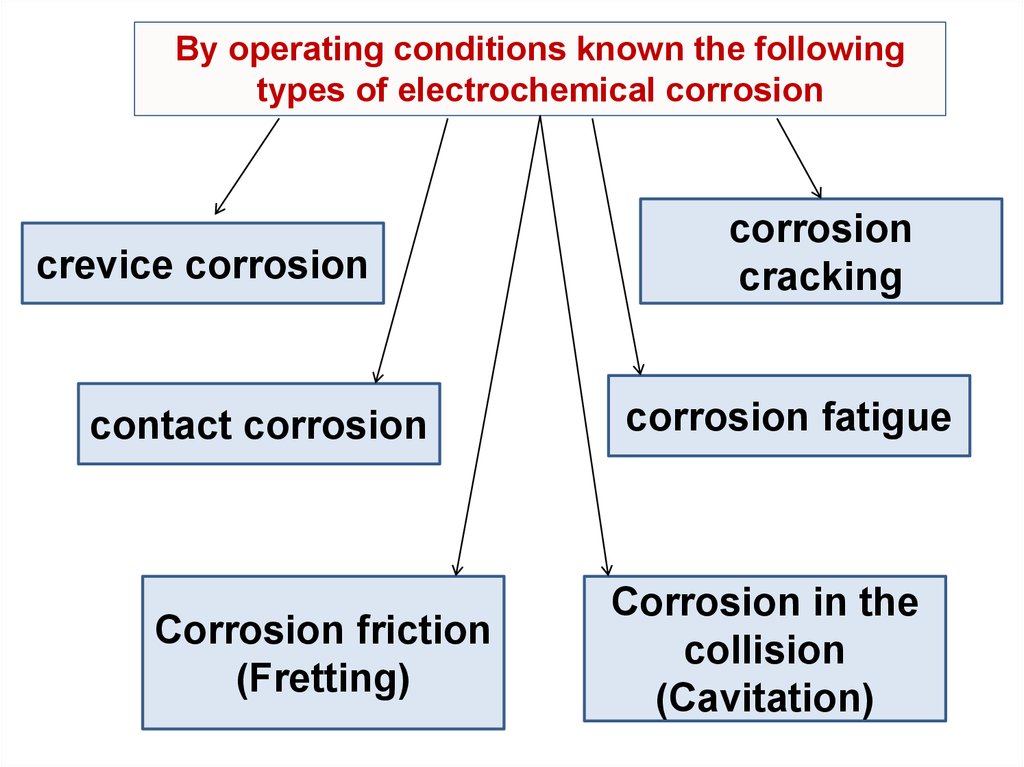
By operating conditions known the followingtypes of electrochemical corrosion
crevice corrosion
contact corrosion
Corrosion friction
(Fretting)
corrosion
cracking
corrosion fatigue
Corrosion in the
collision
(Cavitation)
11.
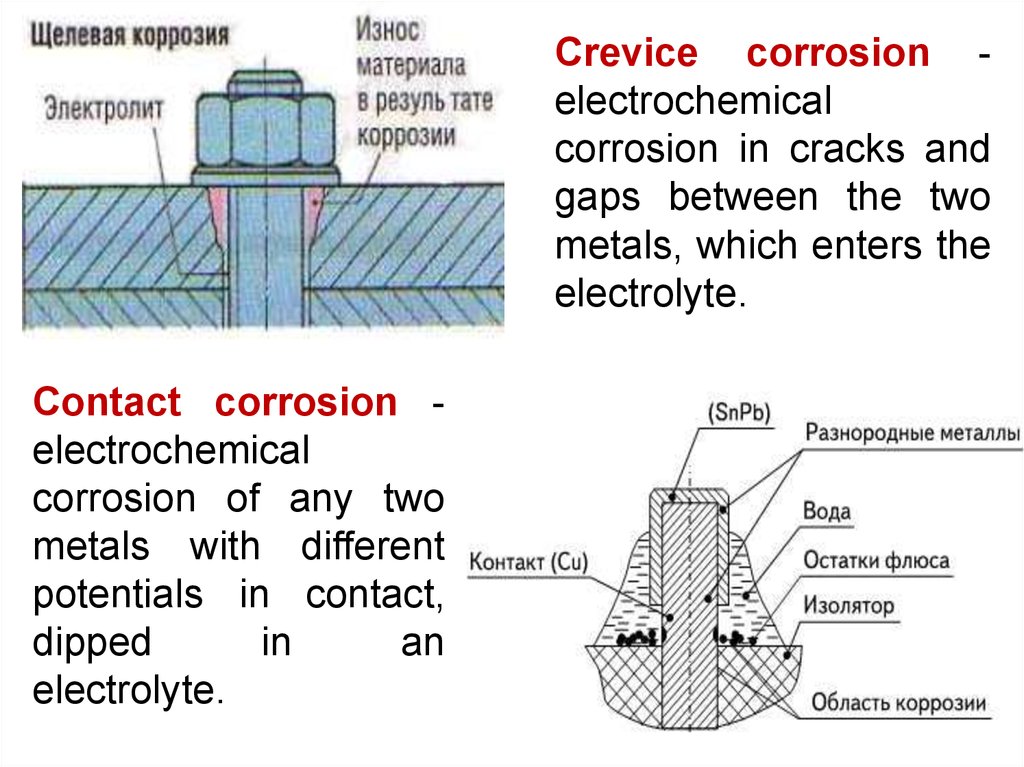
Crevice corrosion electrochemicalcorrosion in cracks and
gaps between the two
metals, which enters the
electrolyte.
Contact corrosion electrochemical
corrosion of any two
metals with different
potentials in contact,
dipped
in
an
electrolyte.
12.
Atmospheric corrosionGas corrosion
Liquid corrosion
Soil corrosion
Stray currents
13.
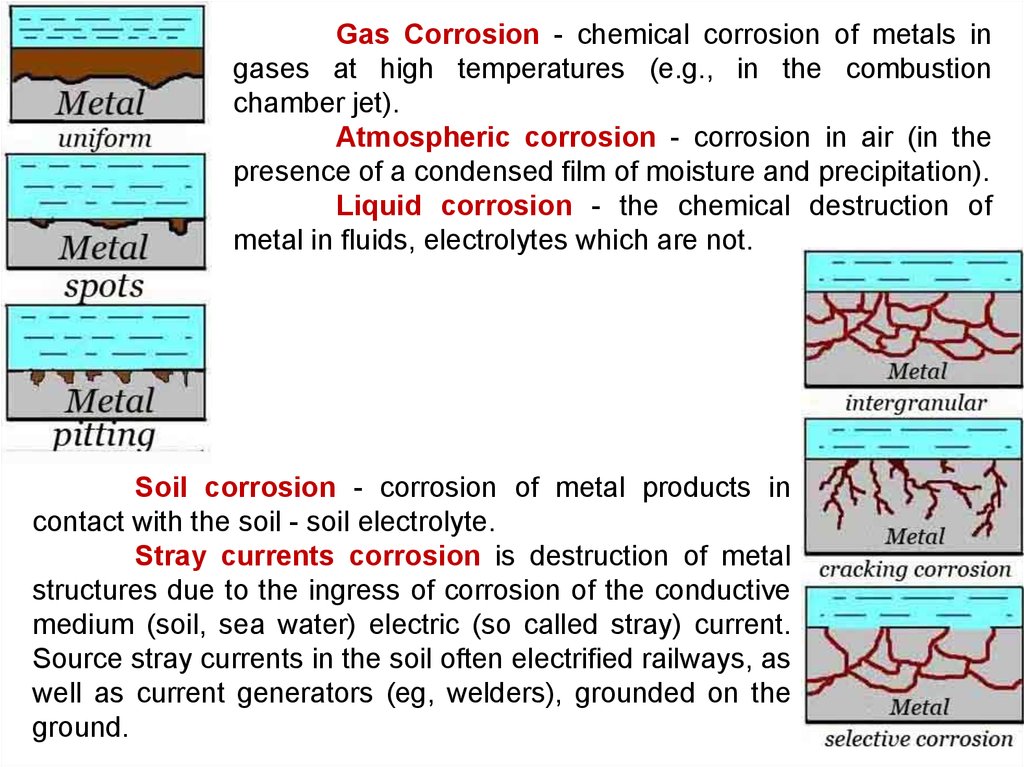
Gas Corrosion - chemical corrosion of metals ingases at high temperatures (e.g., in the combustion
chamber jet).
Atmospheric corrosion - corrosion in air (in the
presence of a condensed film of moisture and precipitation).
Liquid corrosion - the chemical destruction of
metal in fluids, electrolytes which are not.
Soil corrosion - corrosion of metal products in
contact with the soil - soil electrolyte.
Stray currents corrosion is destruction of metal
structures due to the ingress of corrosion of the conductive
medium (soil, sea water) electric (so called stray) current.
Source stray currents in the soil often electrified railways, as
well as current generators (eg, welders), grounded on the
ground.
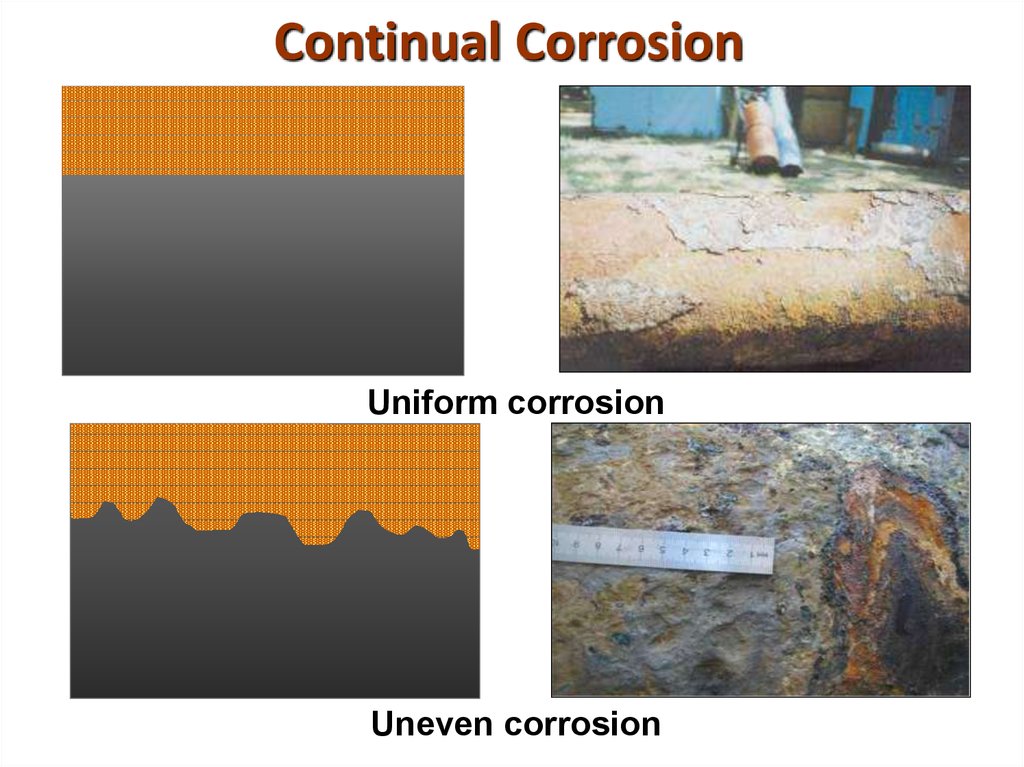
14. Continual Corrosion
Uniform corrosionUneven corrosion
15. Local corrosion
By ulcersBy points
By spots
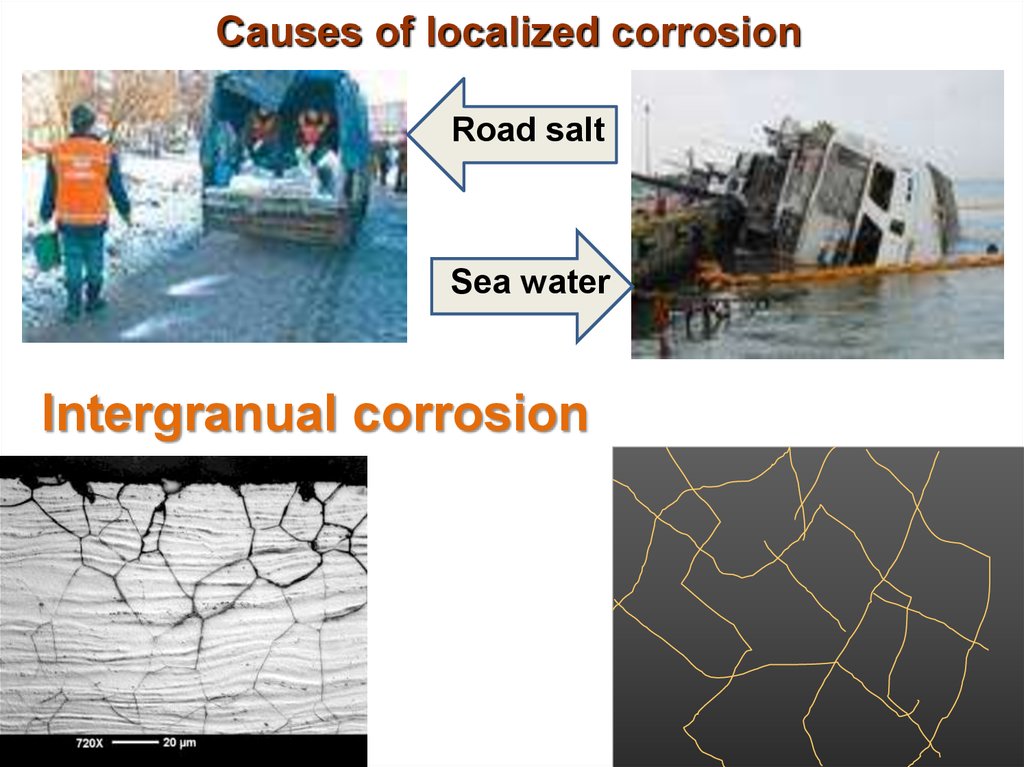
16. Causes of localized corrosion
Road saltSea water
Intergranual corrosion
17.
CHEMICAL CORROSIONis the interaction of the metal with the
environment in which the oxidation of the metal
and restoring the oxidant corrosive environment
occurs in one act without causing an electric
current.
This type of corrosion occurs due to direct chemical
attack of environment or atmospheric gases like oxygen
halogens, hydrogen sulphide, sulphur dioxide, nitrogen or
anhydrous inorganic liquid with metal surfaces in
immediate proximity.
Chemical corrosion is of three main types as:
• Oxidation Corrosion (with Oxygen)
• Corrosion by Other Gases
• In non-electrolyte Liquid Metal Corrosion
18.
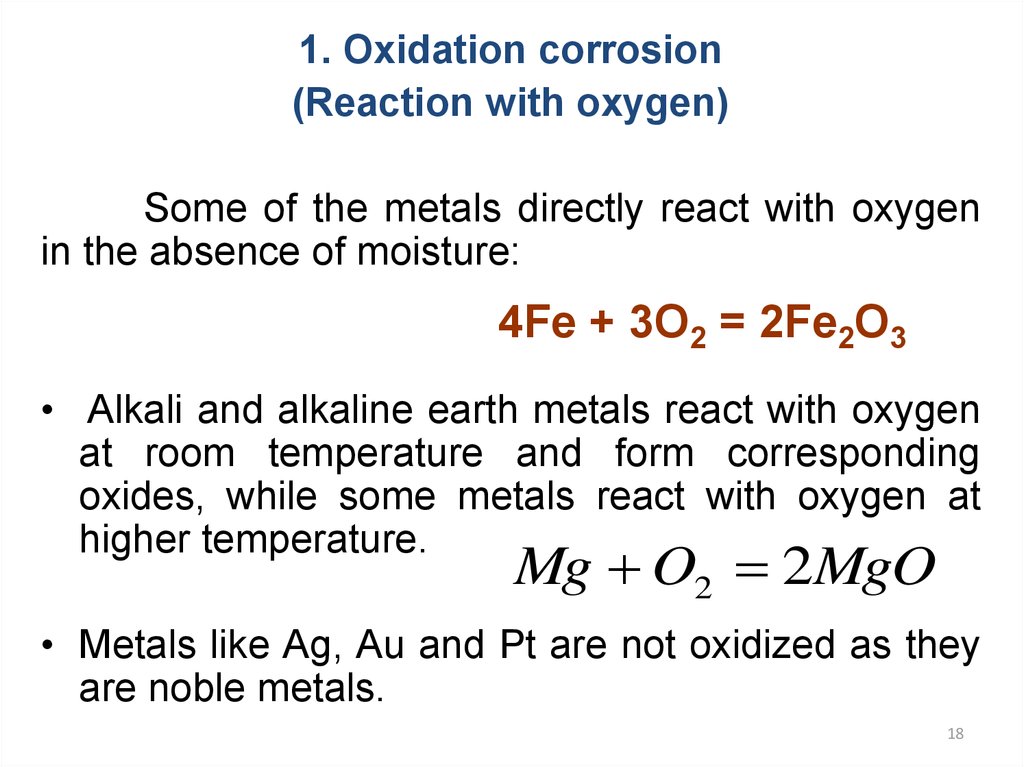
1. Oxidation corrosion(Reaction with oxygen)
Some of the metals directly react with oxygen
in the absence of moisture:
4Fe + 3О2 = 2Fe2О3
• Alkali and alkaline earth metals react with oxygen
at room temperature and form corresponding
oxides, while some metals react with oxygen at
higher temperature.
Mg O2 2MgO
• Metals like Ag, Au and Pt are not oxidized as they
are noble metals.
18
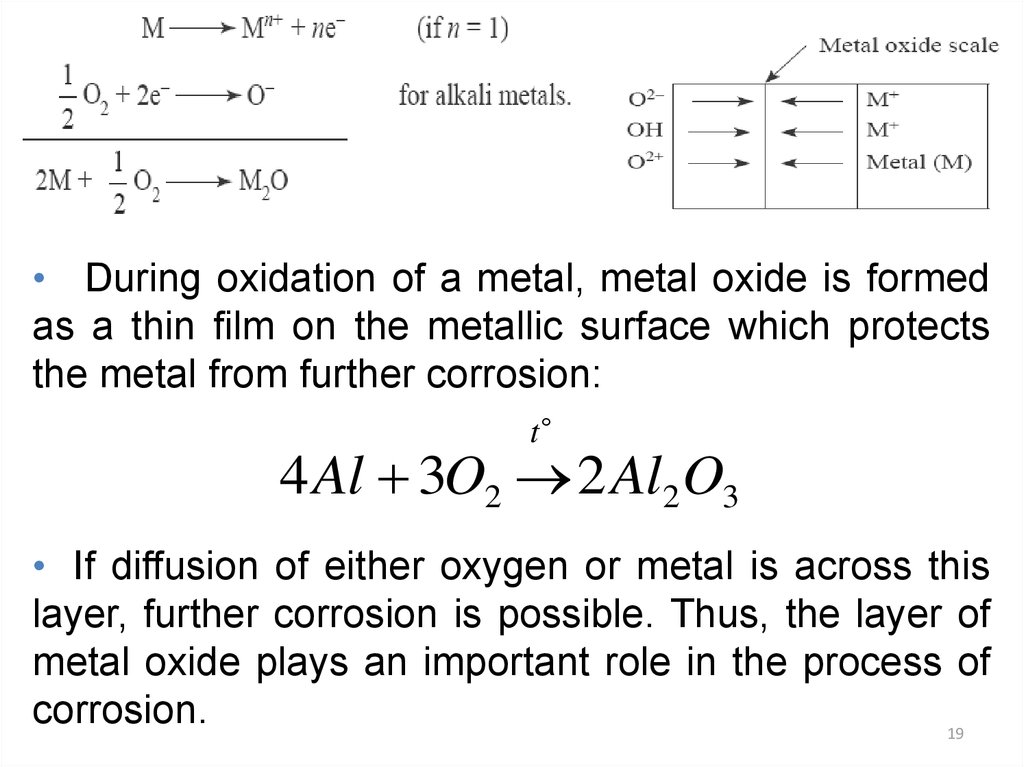
19.
• During oxidation of a metal, metal oxide is formedas a thin film on the metallic surface which protects
the metal from further corrosion:
t
4 Al 3O2 2 Al2 O3
• If diffusion of either oxygen or metal is across this
layer, further corrosion is possible. Thus, the layer of
metal oxide plays an important role in the process of
corrosion.
19
20.
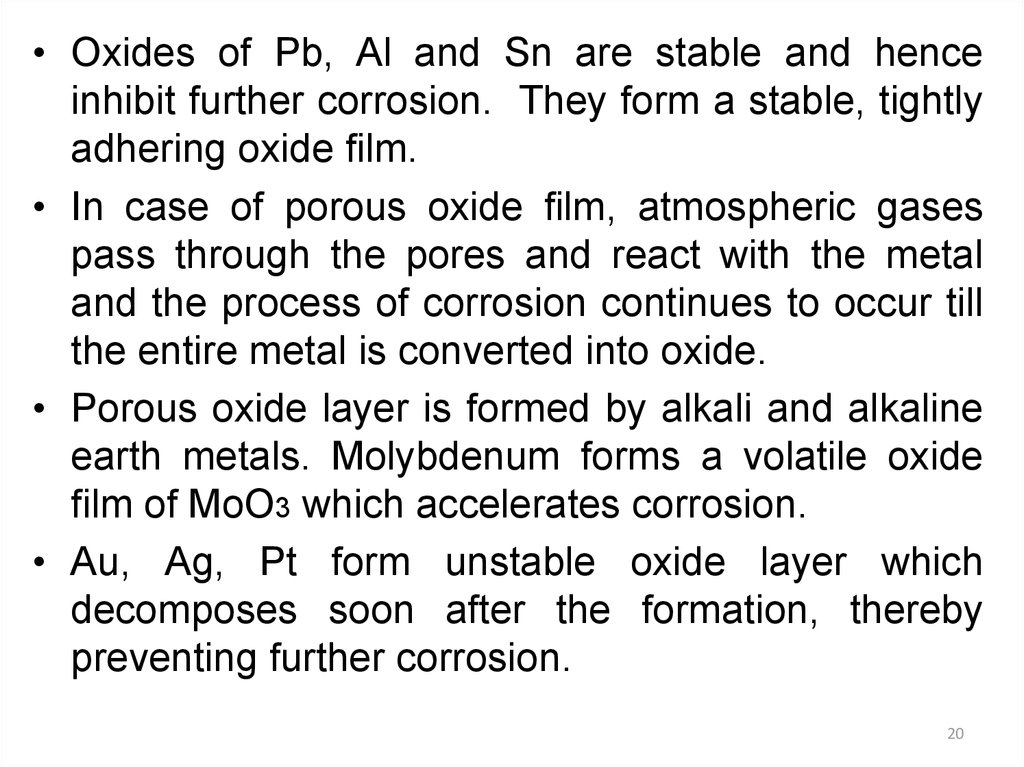
• Oxides of Pb, Al and Sn are stable and henceinhibit further corrosion. They form a stable, tightly
adhering oxide film.
• In case of porous oxide film, atmospheric gases
pass through the pores and react with the metal
and the process of corrosion continues to occur till
the entire metal is converted into oxide.
• Porous oxide layer is formed by alkali and alkaline
earth metals. Molybdenum forms a volatile oxide
film of MoO3 which accelerates corrosion.
• Au, Ag, Pt form unstable oxide layer which
decomposes soon after the formation, thereby
preventing further corrosion.
20
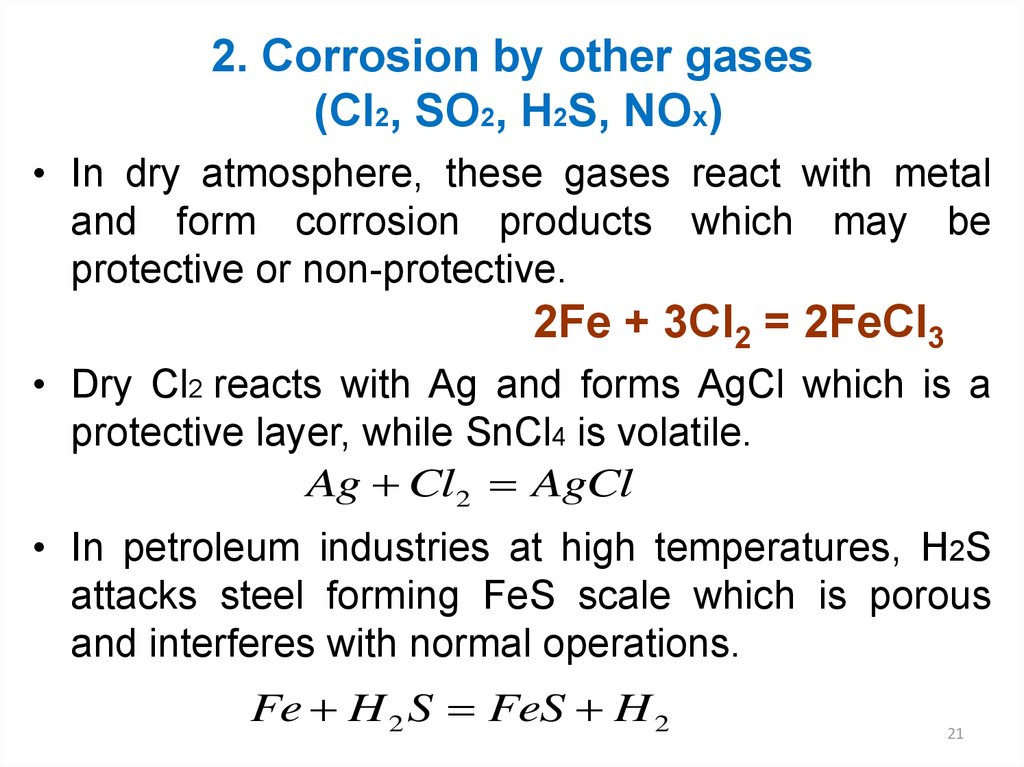
21. 2. Corrosion by other gases (Cl2, SO2, H2S, NOx)
• In dry atmosphere, these gases react with metaland form corrosion products which may be
protective or non-protective.
2Fe + 3Cl2 = 2FeCl3
• Dry Cl2 reacts with Ag and forms AgCl which is a
protective layer, while SnCl4 is volatile.
Ag Cl2 AgCl
• In petroleum industries at high temperatures, H2S
attacks steel forming FeS scale which is porous
and interferes with normal operations.
Fe H 2 S FeS H 2
21
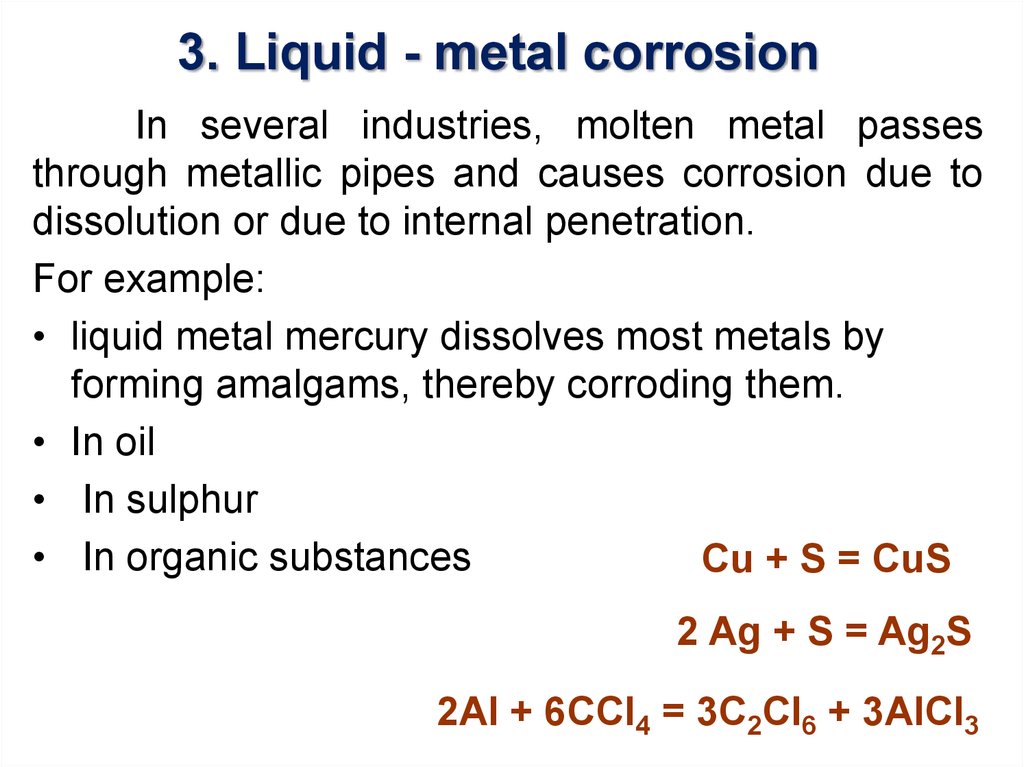
22. 3. Liquid - metal corrosion
In several industries, molten metal passesthrough metallic pipes and causes corrosion due to
dissolution or due to internal penetration.
For example:
• liquid metal mercury dissolves most metals by
forming amalgams, thereby corroding them.
• In oil
• In sulphur
• In organic substances
Cu + S = CuS
2 Ag + S = Ag2S
2Al + 6ССl4 = 3C2Cl6 + 3AlCl3
23.
ELECTROCHEMICAL CORROSIONis anodic oxidation of the metal by environment
elements in which the oxidation of the metal and
restoring the oxidant corrosive environment is a
result of the flow of several elementary acts,
accompanied by the appearance of a galvanic
couple and electric current.
This type of corrosion occurs when the metal comes in
contact with a conducting liquid or when two dissimilar metals
are immersed or dipped partly in a solution. There is the
formation of a galvanic cell on the surface of metals. Some
parts of the metal surface act as anode and rest act as
cathode. Water must be present to serve as a medium for the
transport of ions.
The most common depolarizers are oxygen, acids, and
the cations of less active metals.
24.
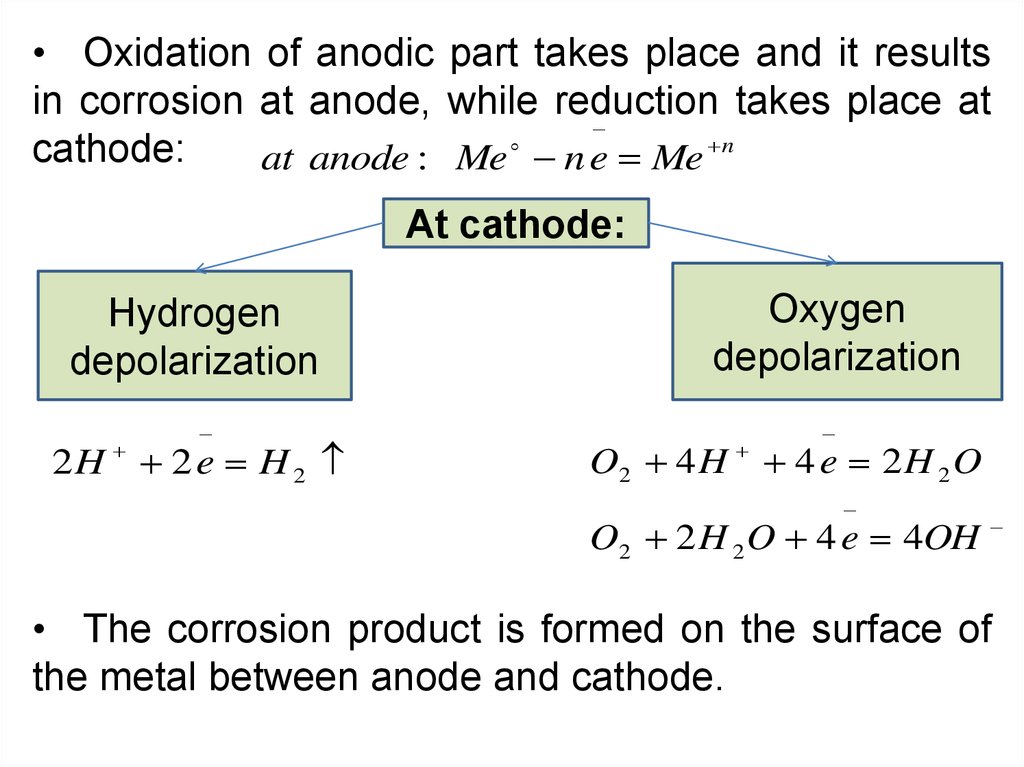
• Oxidation of anodic part takes place and it resultsin corrosion at anode, while reduction takes place at
cathode:
at anode : Me n e Me n
At cathode:
Hydrogen
depolarization
2H 2 e H 2
Oxygen
depolarization
O2 4 H
4 e 2H 2O
O2 2 H 2 O 4 e 4OH
• The corrosion product is formed on the surface of
the metal between anode and cathode.
25.
Mechanism: Electrochemical corrosion involves theflow of electron current between anodic and catholic regions.
The anodic reaction involves dissolution of the metal as
corresponding metallic tops with the liberation of tree
electrons.
Anodic area: Me -ne- = Me+n
(Oxidation)
Cathodic area: Reduction reaction consumes electrons
and depending on the nature, of corrosive environment the
following reactions may take place:
• Hydrogen evolution: 2H+ + 2e- = H2
• Oxygen reduction (acidic medium pH<7):
O2 + 4H+ + 4e- = 2H2O
• Oxygen reduction (neutral or alkaline medium):
O2 + 2H2O + 4e- = 4OH• Metal ion reduction: M3+ + e- = M2+
• Metal deposition:
M+ + e- = M
26.
Thus, electrochemical corrosion occurs:• where a conducting liquid (water, acid, salt
solutions) is in contact with a metal or
• when two dissimilar, metals or alloys are either
immersed or dipped partially in a aqueous solution.
In electrochemical or wet corrosion the
following observations are made:
1) Formation of anodic and catholic part or parts in
contact with each, other.
2) Presence of conducting medium
3) Corrosion of anodic areas only.
4) Formation of corrosion product between anodic
and catholic areas.
27.
Fe2
1 e Fe 3
• Oxygen reduction (acidic medium pH<7):
Fe + O2 + 4H+ = Fe2O3 * nH2O
• Oxygen reduction (neutral or alkaline medium):
2Fe + O2 + 3H2O = 2Fe(OH)3
28.
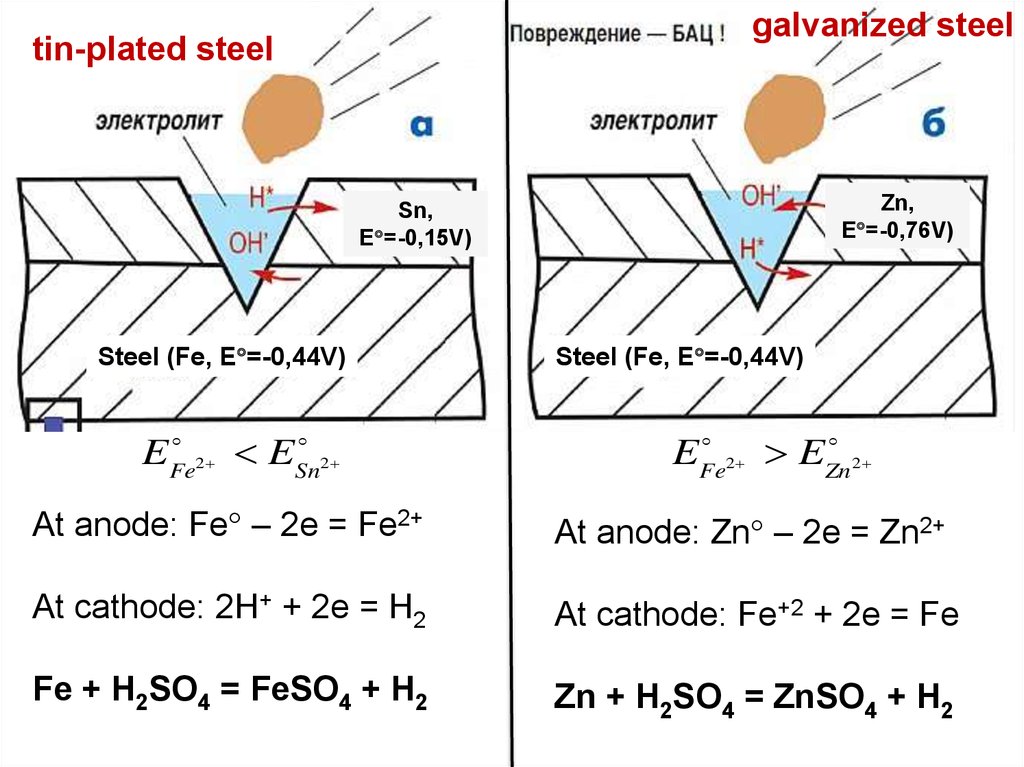
galvanized steeltin-plated steel
Zn,
E =-0,76V)
Sn,
E =-0,15V)
Steel (Fe, E =-0,44V)
EFe
E
2
Sn2
Steel (Fe, E =-0,44V)
EFe
E
2
Zn 2
At anode: Fe – 2e = Fe2+
At anode: Zn – 2e = Zn2+
At cathode: 2H+ + 2e = H2
At cathode: Fe+2 + 2e = Fe
Fe + H2SO4 = FeSO4 + H2
Zn + H2SO4 = ZnSO4 + H2
29. Factors Influencing Corrosion
The nature and extent of corrosiondepend on the metal and the environment. The
important factors which may influence the
corrosion process are:
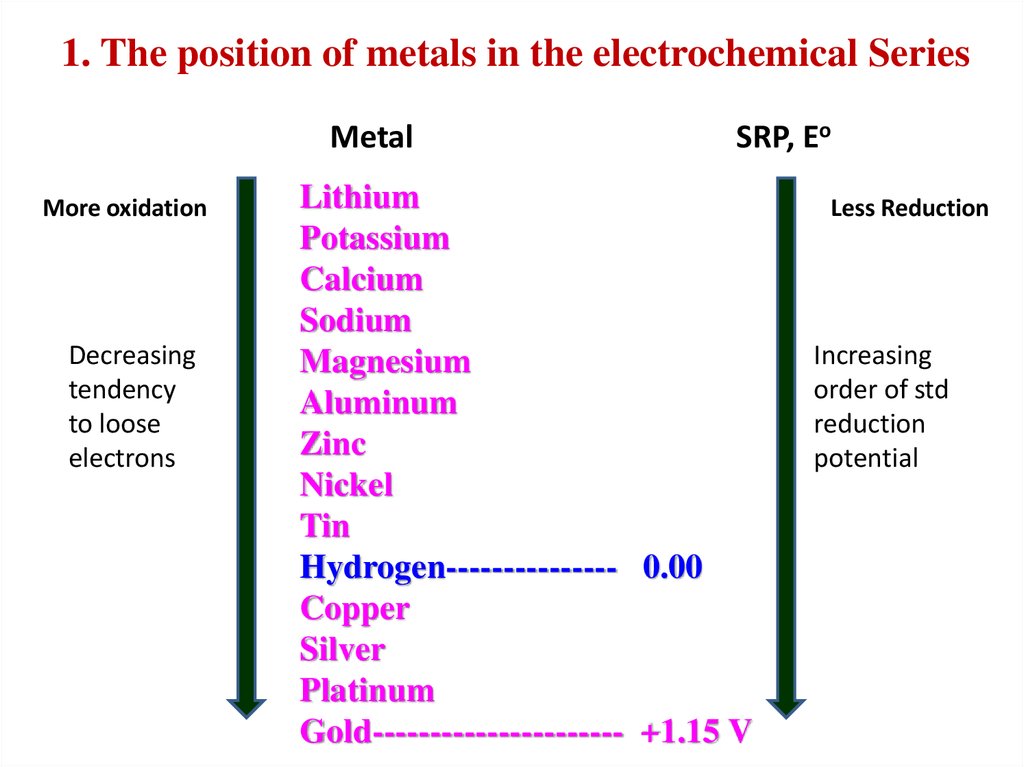
1) Nature of the metal (position Me in ECS)
2) Environment (oxidant type)
3) Concentration of electrolyte
4) Temperature
5) Hydrogen over voltage (pH)
30.
1. The position of metals in the electrochemical SeriesMetal
More oxidation
Decreasing
tendency
to loose
electrons
SRP, Eo
Lithium
Potassium
Calcium
Sodium
Magnesium
Aluminum
Zinc
Nickel
Tin
Hydrogen--------------- 0.00
Copper
Silver
Platinum
Gold---------------------- +1.15 V
Less Reduction
Increasing
order of std
reduction
potential
31.
E Me npotential area where any corrosion impossible,
example Аu (1,5 В)
+ 0,82 В
area of corrosion with oxygen depolarization
possible, example Cu (0,34 В)
-0,41 B
area of corrosion with hydrogen depolarization
possible, example Fe (-0,44 B)
32.
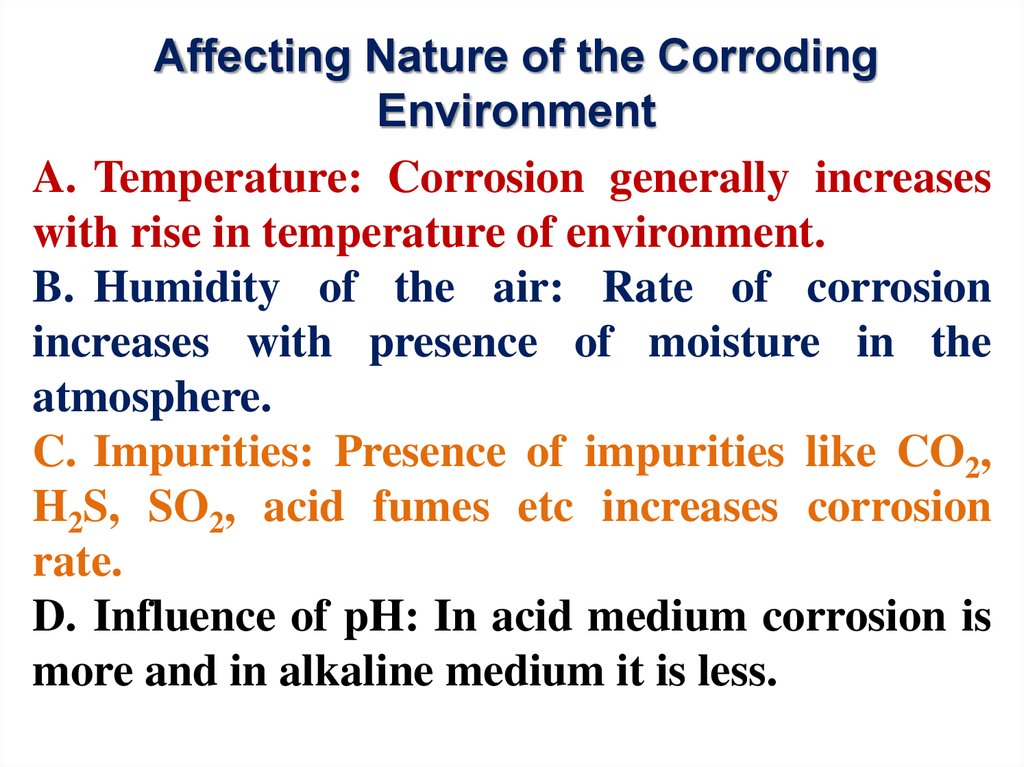
Affecting Nature of the CorrodingEnvironment
A. Temperature: Corrosion generally increases
with rise in temperature of environment.
B. Humidity of the air: Rate of corrosion
increases with presence of moisture in the
atmosphere.
C. Impurities: Presence of impurities like CO2,
H2S, SO2, acid fumes etc increases corrosion
rate.
D. Influence of pH: In acid medium corrosion is
more and in alkaline medium it is less.
33.
• Group 1 - alkali metals - thelowest corrosion resistance
• 1 group of the sub-groups very resistant metals
• Group 2 – are unstable,
• Group 2 sub-groups – are
more stable (in the presence of
oxygen to form a strong oxide
film protecting from further
destruction)
34.
• Group 3 - Aluminium - formingstable oxide film (but it is
destroyed in solutions of acids
and bases). In concentrated
nitric
and
sulfuric
acids
passivated aluminum.
• Group 4 – tin (Sn) and lead
(Pb)
–
corrosion-resistant
metals, thanks to strong oxide
film.
35.
• 5,6,7,8 group - by metals ofsubgroups have high ability to
passivate and hence high
resistance to corrosion.
• Osmium, iridium, platinum - the
most resistant to corrosion
• Iron passivated by concentrated
sulfuric and nitric acids
36. PROTECTION METHODS
Material selection
Improvements in material
Design of structures
Alteration of environment
Add inhibitors
Galvanic Cathodic or Anodic protection
Active electrochemical protection
Coatings
37.
38.
Make sure you thoroughly understand the following essential ideas whichhave been presented above. It is especially imortant that you know the precise
meanings of all the highlighted terms in the context of this topic.
• Electrochemical corrosion of metals occurs when electrons from atoms at the
surface of the metal are transferred to a suitable electron acceptor or depolarizer.
Water must be present to serve as a medium for the transport of ions.
• The most common depolarizers are oxygen, acids, and the cations of less active
metals.
• Because the electrons flow through the metallic object itself, the anodic and
cathodic regions (the two halves of the electrochemical cell) can be at widely
separated locations.
• Anodic regions tend to develop at locations where the metal is stressed or is
protected from oxygen.
• Contact with a different kind of metal, either direct or indirect, can lead to
corrosion of the more active one.
• Corrosion of steel can be inhibited by galvanizing, that is, by coating it with zinc,
a more active metal whose dissolution leaves a negative charge on the metal which
inhibits the further dissolution of Fe2+.
• Cathodic protection using an external voltage source is widely used to protect
underground structures such as tanks, pipelines and piers. The source can be a
sacrificial anode of zinc or aluminum, or a line-operated or photovoltaic power
supply.



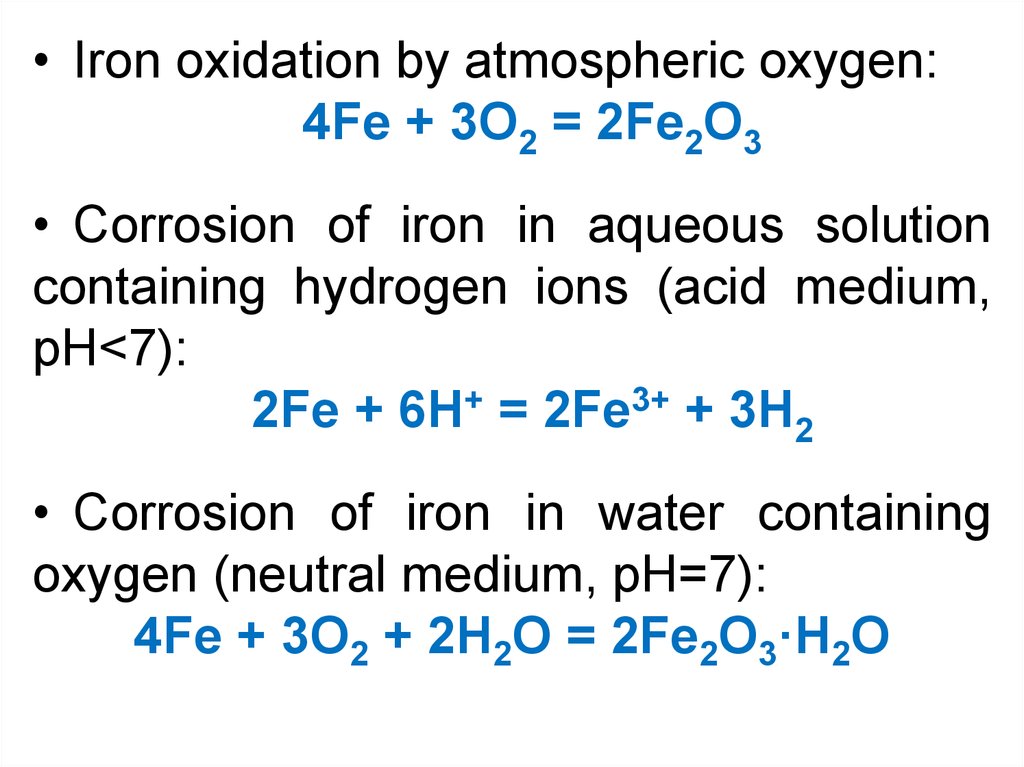













 chemistry
chemistry








