Similar presentations:
Electrochemical Cells. You need
1.
Introduction Electrochemistry2.
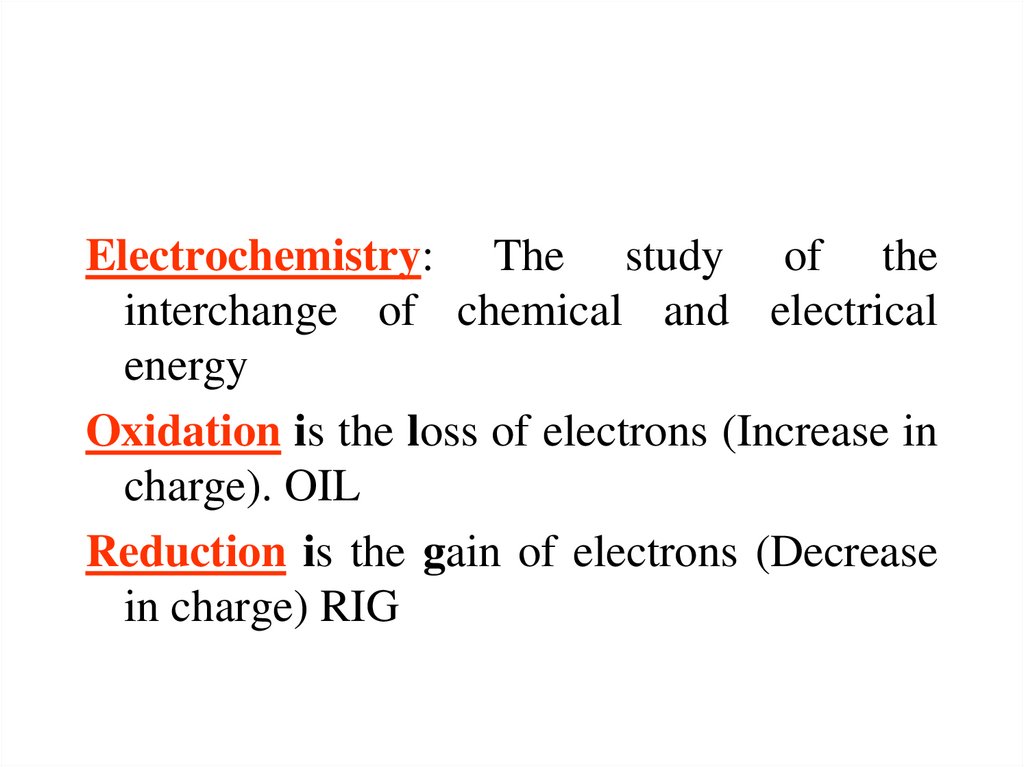
Electrochemistry: The study of theinterchange of chemical and electrical
energy
Oxidation is the loss of electrons (Increase in
charge). OIL
Reduction is the gain of electrons (Decrease
in charge) RIG
3.
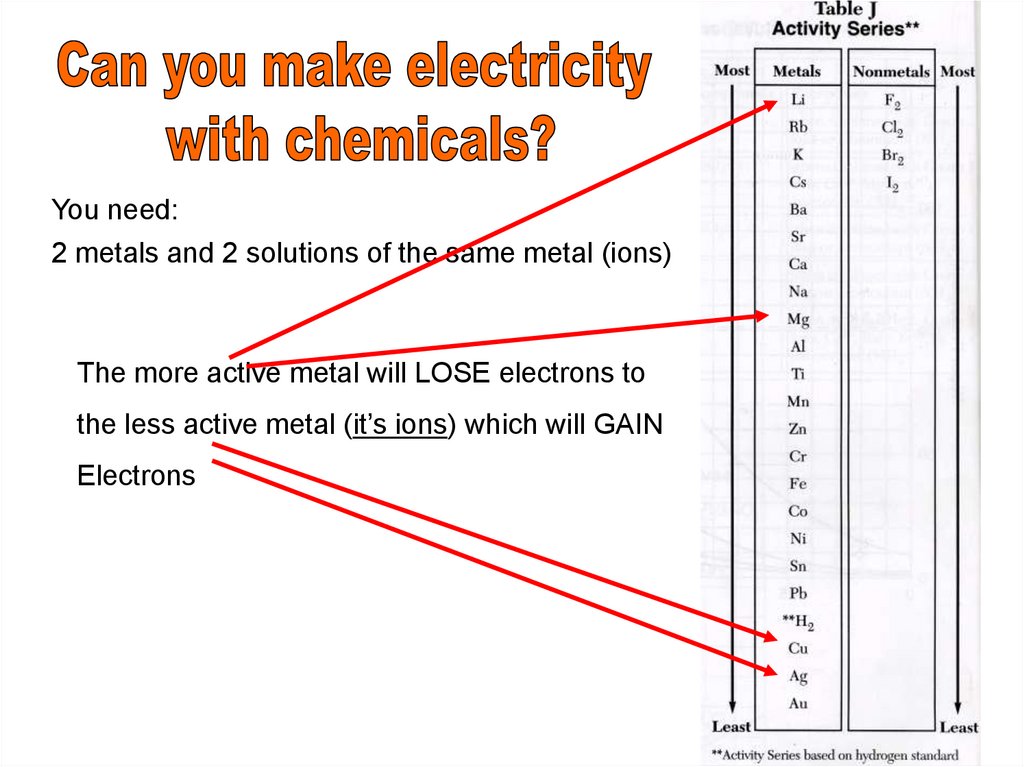
You need:2 metals and 2 solutions of the same metal (ions)
The more active metal will LOSE electrons to
the less active metal (it’s ions) which will GAIN
Electrons
4.
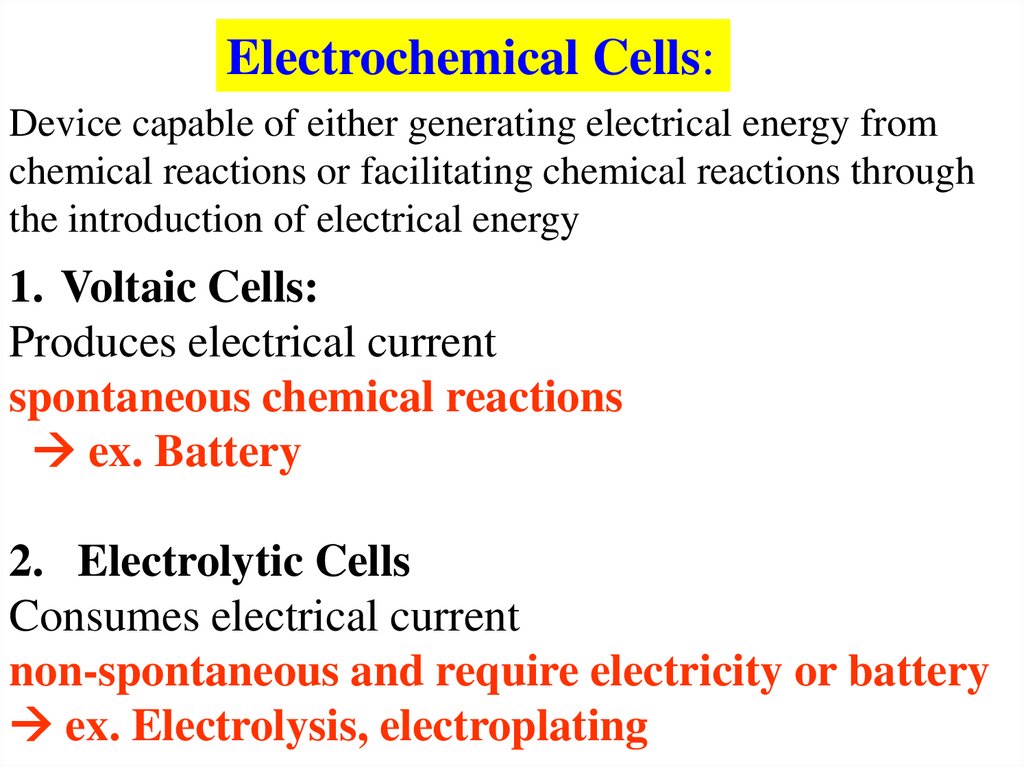
Electrochemical Cells:Device capable of either generating electrical energy from
chemical reactions or facilitating chemical reactions through
the introduction of electrical energy
1. Voltaic Cells:
Produces electrical current
spontaneous chemical reactions
ex. Battery
2. Electrolytic Cells
Consumes electrical current
non-spontaneous and require electricity or battery
ex. Electrolysis, electroplating
5.
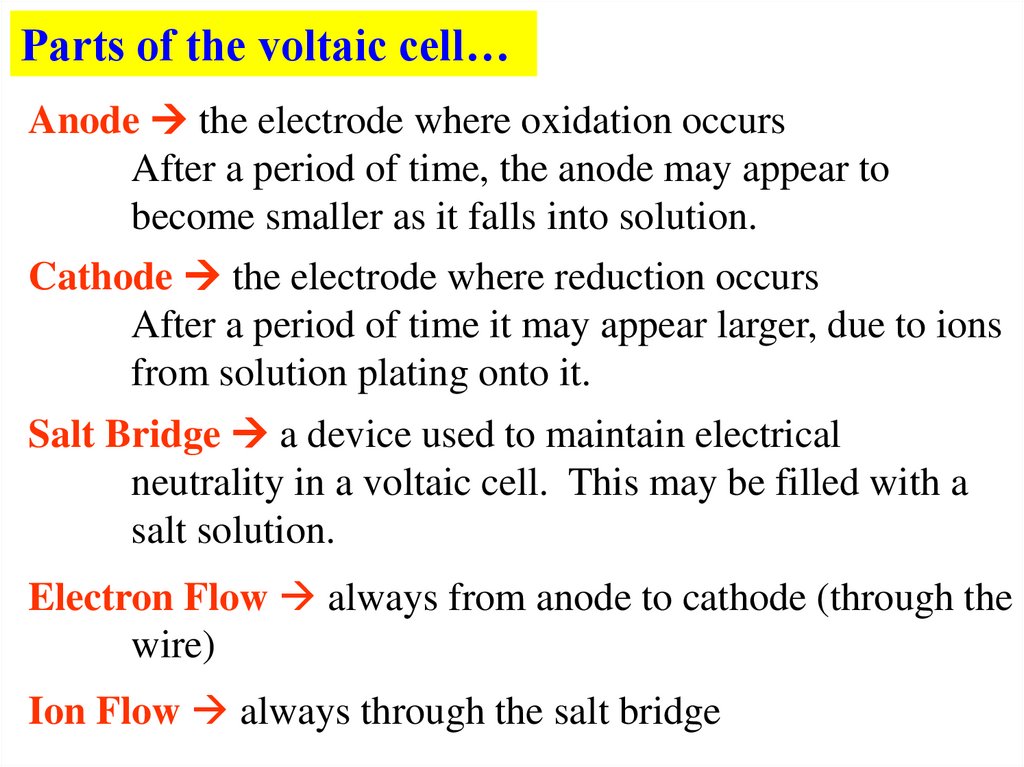
Parts of the voltaic cell…Anode the electrode where oxidation occurs
After a period of time, the anode may appear to
become smaller as it falls into solution.
Cathode the electrode where reduction occurs
After a period of time it may appear larger, due to ions
from solution plating onto it.
Salt Bridge a device used to maintain electrical
neutrality in a voltaic cell. This may be filled with a
salt solution.
Electron Flow always from anode to cathode (through the
wire)
Ion Flow always through the salt bridge
6.
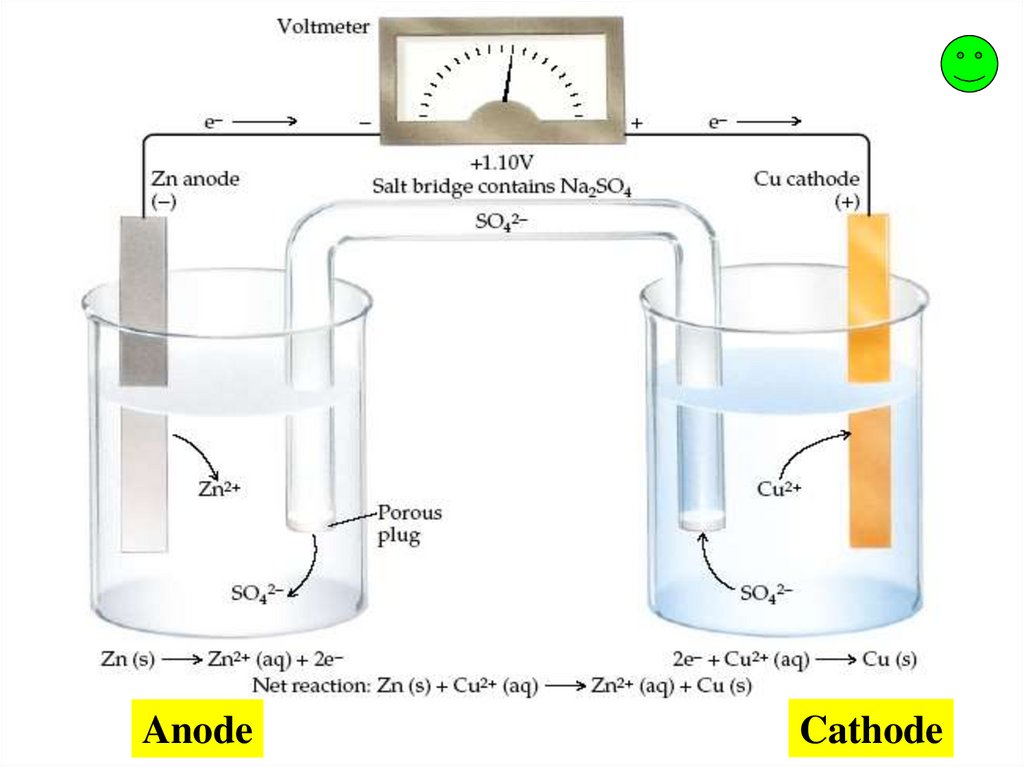
AnodeCathode
7.
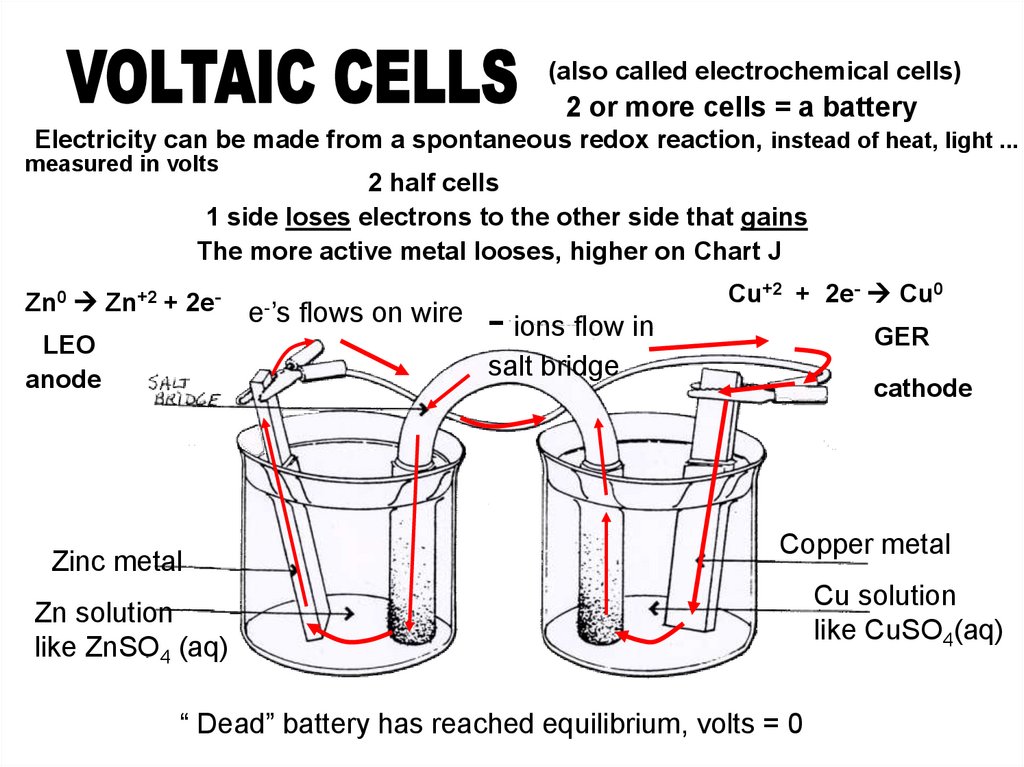
(also called electrochemical cells)2 or more cells = a battery
Electricity can be made from a spontaneous redox reaction, instead of heat, light ...
measured in volts
2 half cells
1 side loses electrons to the other side that gains
The more active metal looses, higher on Chart J
Zn0 Zn+2 + 2e-
LEO
anode
e-’s flows on wire
- ions flow in
Cu+2 + 2e- Cu0
GER
salt bridge
Zinc metal
cathode
Copper metal
Zn solution
like ZnSO4 (aq)
“ Dead” battery has reached equilibrium, volts = 0
Cu solution
like CuSO4(aq)
8.
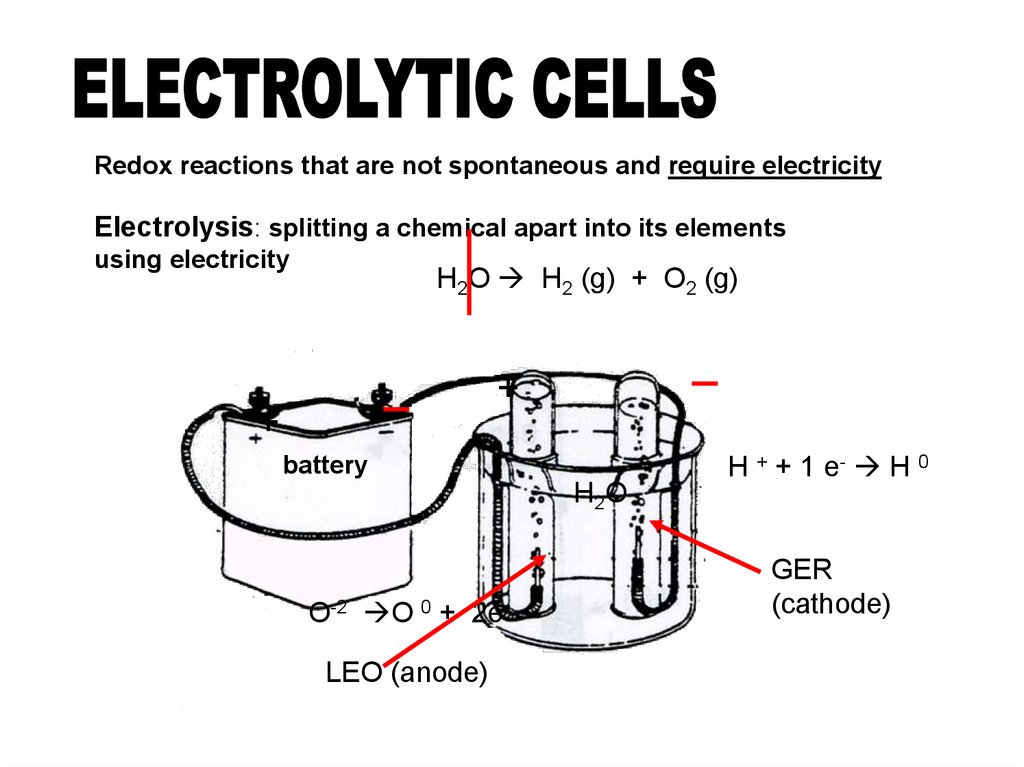
Redox reactions that are not spontaneous and require electricityElectrolysis: splitting a chemical apart into its elements
using electricity
H2O H2 (g) + O2 (g)
+
+
battery
H 2O
O-2 O 0 + 2eLEO (anode)
H + + 1 e- H 0
GER
(cathode)
9.
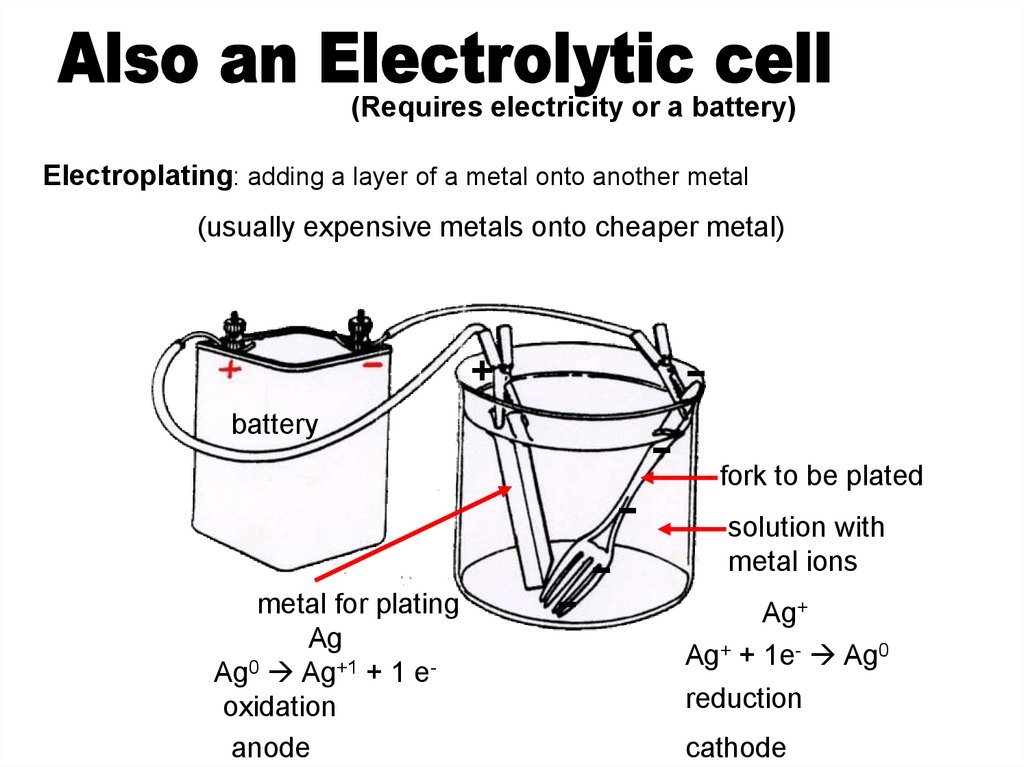
(Requires electricity or a battery)Electroplating: adding a layer of a metal onto another metal
(usually expensive metals onto cheaper metal)
-
+
battery
-
metal for plating
Ag
Ag0 Ag+1 + 1 eoxidation
anode
-
-
fork to be plated
solution with
metal ions
Ag+
Ag+ + 1e- Ag0
reduction
cathode
 chemistry
chemistry