Similar presentations:
Halogens and their compounds (lecture 5)
1.
HALOGENS AND THEIRCOMPOUNDS: FLOURINE AND
ITS COMPOUNDS. CHLOREALKALI INDUSTRIES:
ELECTROLYTIC PROCESSES
LYAZZAT
DANIYARKYZY
2.
3.
INTRODUCTION TOHALOGENS
Halogens are a group of non-metal elements that include
fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and
astatine (At). They are located in Group 17 of the periodic
table.
Properties:
Halogens are highly reactive due to their seven valence
electrons, needing one more electron to achieve a stable
electronic configuration.
• As you move down the group, the reactivity decreases:
fluorine being the most reactive, and astatine the least.
• Halogens exist in various states at room temperature:
fluorine and chlorine are gases, bromine is a liquid, and
iodine is a solid.
Importance in Industry:
Halogens and their compounds have a wide range of
industrial applications, from manufacturing chemicals to
pharmaceuticals and materials.
4.
INDUSTRIAL APPLICATIONS OF HALOGENSFluorine (F):
Properties:
• Fluorine is the most reactive of all the halogens.
• It is a pale yellow gas under standard conditions.
Industrial Applications:
1. Fluorine Gas:
• Used in the production of uranium hexafluoride for the nuclear industry.
•Utilized in the synthesis of various fluorides for pharmaceuticals and agrochemicals.
2. Hydrofluoric Acid (HF):
• HF is a key raw material in the production of fluorinated compounds.
• It is used in the etching of glass and in the semiconductor industry.
3. Fluorine-Containing Polymers:
•Examples
include
polytetrafluoroethylene
(PTFE,
known
as
Teflon)
and
polyvinylidene fluoride (PVDF).
• These polymers are valued for their non-stick and chemical-resistant properties.
5.
INDUSTRIAL APPLICATIONS OF HALOGENSChlorine (Cl):
Properties:
• Chlorine is a greenish-yellow gas with a distinct odor.
• It is highly reactive and a powerful oxidizing agent.
Industrial Applications:
1. Water Treatment:
• Chlorine is widely used to disinfect water, making it safe for consumption.
• It helps kill bacteria, viruses, and other pathogens.
2. PVC Production:
• Chlorine is a key ingredient in the production of polyvinyl chloride (PVC) plastics.
• PVC is used in construction, healthcare, and various consumer products.
3. Organic Chemistry:
• Chlorine is used in the synthesis of a wide range of organic compounds, including
solvents and pesticides.
• It is also utilized in the production of chlorinated rubber for coatings and adhesives.
6.
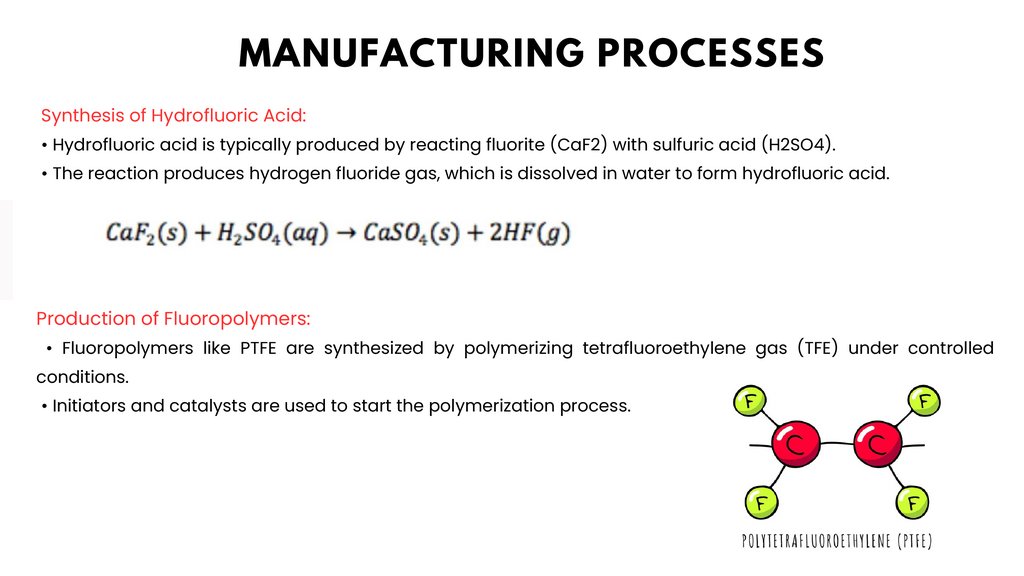
MANUFACTURING PROCESSESSynthesis of Hydrofluoric Acid:
• Hydrofluoric acid is typically produced by reacting fluorite (CaF2) with sulfuric acid (H2SO4).
• The reaction produces hydrogen fluoride gas, which is dissolved in water to form hydrofluoric acid.
Production of Fluoropolymers:
• Fluoropolymers like PTFE are synthesized by polymerizing tetrafluoroethylene gas (TFE) under controlled
conditions.
• Initiators and catalysts are used to start the polymerization process.
7.
CHLORINE PRODUCTIONElectrolysis of Sodium Chloride:
•In the electrolysis of brine (sodium chloride
solution), chlorine gas is produced at the
anode.
• Sodium hydroxide (NaOH) and hydrogen gas
are produced at the cathode.
Mercury Cell Process:
• Involves the electrolysis of brine in a mercury
cathode cell to produce chlorine and sodium
hydroxide.
• This process has environmental concerns due
to mercury usage.
8.
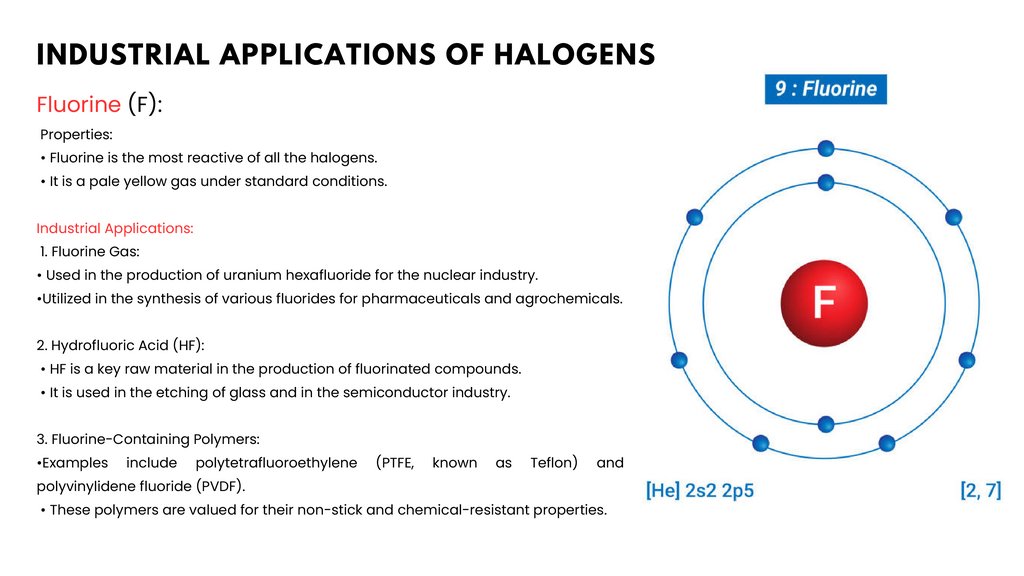
FLUORINE AND ITS COMPOUNDSProperties of Fluorine
• Chemical Symbol: F
• Atomic Number: 9
• Atomic Mass: 18.9984 g/mol
• State at Room Temperature: Pale yellow gas
Reactivity:
•Fluorine is the most reactive of all the halogens.
•It readily forms compounds with almost all other
elements.
Electronegativity:
•Fluorine has the highest electronegativity of all
elements.
•This means it strongly attracts electrons in a chemical
bond.
9.
INDUSTRIAL APPLICATIONS OFFLUORINE AND ITS COMPOUNDS
Fluorine Gas:
Production:
•Fluorine gas is not usually produced for commercial
purposes due to its extreme reactivity and hazardous
nature.
• It is typically generated in situ for specific reactions.
Applications:
•Used in the production of uranium hexafluoride (UF6) for
the nuclear industry.
•Key in the synthesis of various fluorides for
pharmaceuticals,
agrochemicals,
and
specialty
chemicals.
10.
Hydrofluoric Acid (HF):Production:
•HF is commonly produced by reacting fluorite
(calcium fluoride, CaF2) with sulfuric acid
(H2SO4).
•The reaction produces hydrogen fluoride gas
(HF), which is then dissolved in water to form
hydrofluoric acid.
Applications:
•Etching and cleaning of glass and silicon
wafers in the semiconductor industry.
•Catalyst in the alkylation process in
petroleum refining.
•Production of fluorinated compounds for
pharmaceuticals and agrochemicals.
11.
Safety ConsiderationsFluorine and HF Handling:
• Both fluorine gas and hydrofluoric acid are highly toxic and corrosive.
• Proper safety equipment, including gloves, goggles, and protective clothing, must be worn
when handling these substances.
12.
Chlor-Alkali Industries13.
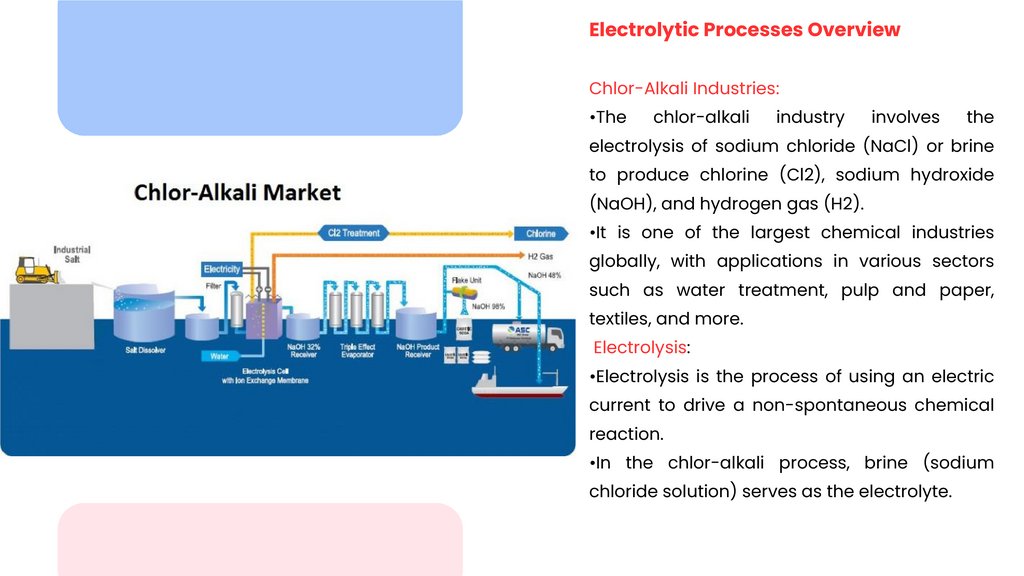
Electrolytic Processes OverviewChlor-Alkali Industries:
•The
chlor-alkali
industry
involves
the
electrolysis of sodium chloride (NaCl) or brine
to produce chlorine (Cl2), sodium hydroxide
(NaOH), and hydrogen gas (H2).
•It is one of the largest chemical industries
globally, with applications in various sectors
such as water treatment, pulp and paper,
textiles, and more.
Electrolysis:
•Electrolysis is the process of using an electric
current to drive a non-spontaneous chemical
reaction.
•In the chlor-alkali process, brine (sodium
chloride solution) serves as the electrolyte.
14.
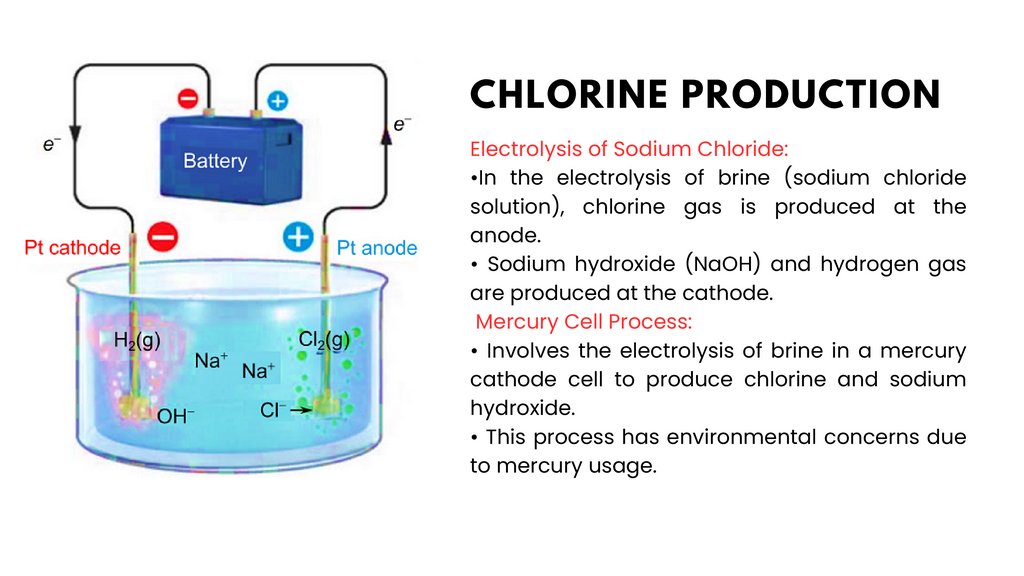
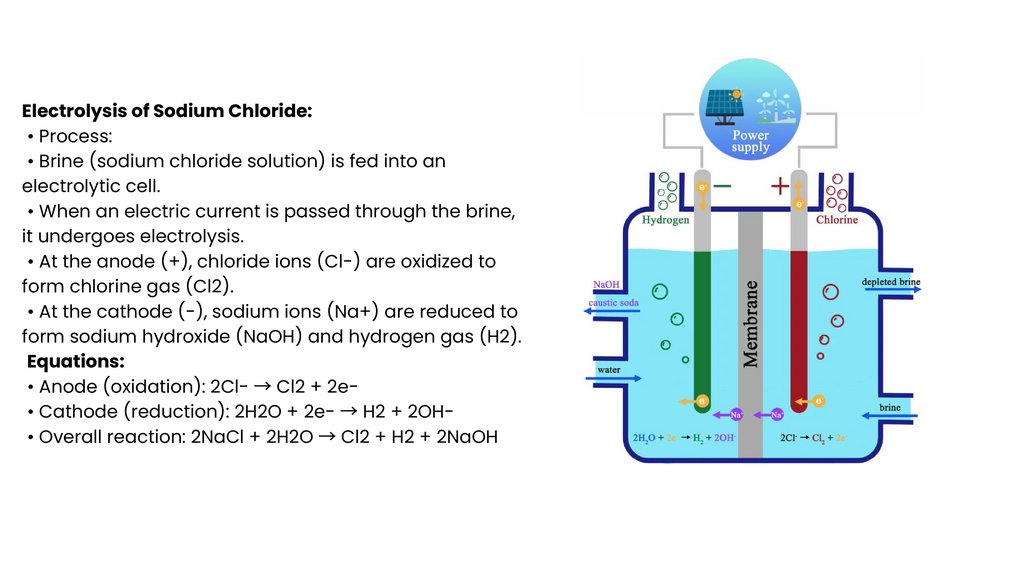
Electrolysis of Sodium Chloride:• Process:
• Brine (sodium chloride solution) is fed into an
electrolytic cell.
• When an electric current is passed through the brine,
it undergoes electrolysis.
• At the anode (+), chloride ions (Cl-) are oxidized to
form chlorine gas (Cl2).
• At the cathode (-), sodium ions (Na+) are reduced to
form sodium hydroxide (NaOH) and hydrogen gas (H2).
Equations:
• Anode (oxidation): 2ClCl2 + 2e• Cathode (reduction): 2H2O + 2eH2 + 2OH• Overall reaction: 2NaCl + 2H2O
Cl2 + H2 + 2NaOH
→
→
→
15.
Co-Products and By-ProductsHydrogen Gas (H2):
• Produced as a by-product in the chlor-alkali process.
• Used in various industrial processes, including the production of ammonia
(for fertilizers) and in hydrogenation reactions.
Sodium Hypochlorite (Bleach):
• Produced by the reaction of chlorine with sodium hydroxide.
• Widely used as a disinfectant and bleaching agent in the household and
industrial settings.
Sodium Chlorate (NaClO3):
• Produced by the electrolysis of a sodium chloride solution.
• Used in the manufacture of chlorine dioxide (a bleaching agent) and in the
pulp and paper industry.
16.
Case Studies and Examples17.
18.
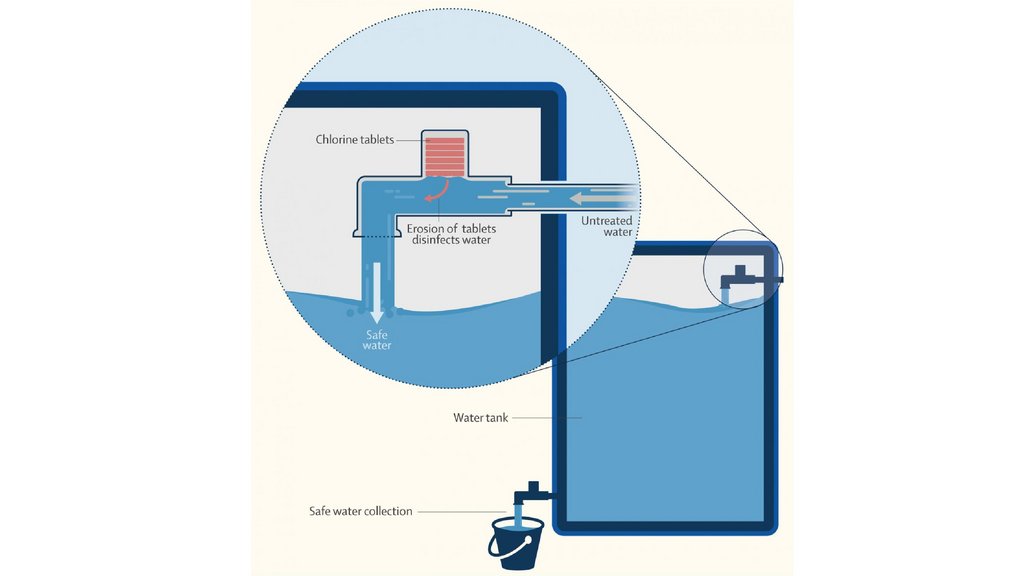
Chlorine Use in Water Treatment• Chlorine is widely used for disinfection in water treatment plants to ensure the safety of
drinking water.
Process:
• Chlorine is added to water in small amounts to kill harmful bacteria, viruses, and
parasites.
• It reacts with organic and inorganic matter to form disinfection by-products.
Benefits:
• Provides a cost-effective method for ensuring clean and safe drinking water.
• Helps prevent waterborne diseases and outbreaks.
Challenges:
• Formation of disinfection by-products such as trihalomethanes (THMs), which are
regulated due to potential health risks.
Regulations:
• Water treatment plants must adhere to strict regulations on chlorine levels and
disinfection by-product levels.
Examples:
• Many cities and municipalities use chlorine in their water treatment processes, ensuring
clean and safe drinking water for their residents.
19.
20.
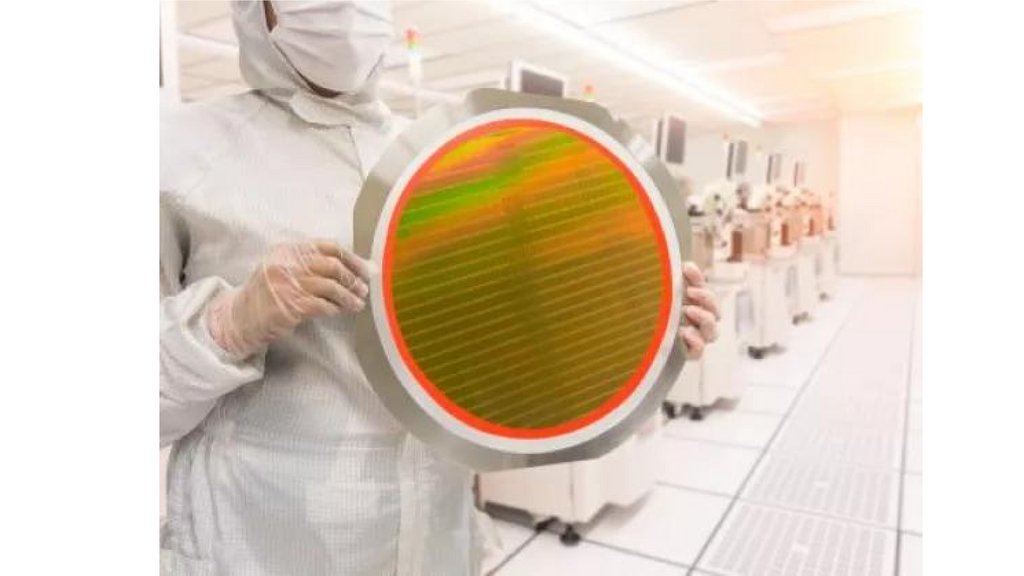
Fluorine in Semiconductor ManufacturingFluorine-containing compounds play a crucial role in semiconductor manufacturing
processes.
Applications:
• Etching: Fluorine gas (F2) is used for plasma etching of silicon and other materials in
semiconductor fabrication.
• Cleaning: Hydrofluoric acid (HF) is used for cleaning silicon wafers and removing oxide
layers.
• Deposition: Fluorocarbon gases are used in chemical vapor deposition (CVD) processes
to create thin films.
Benefits:
• Precise and controlled etching and cleaning processes essential for semiconductor
device manufacturing.
• Enables the creation of complex integrated circuits and microelectronics.
Challenges:
• Handling of highly reactive and hazardous fluorine-containing compounds requires strict
safety protocols.
• Environmental considerations for waste disposal and chemical management.
Examples:
• Major semiconductor manufacturers use fluorine-based processes in their fabrication
facilities to produce cutting-edge electronic components.
21.
EXAMPLES OF INDUSTRIAL APPLICATIONS2. Metal Cleaning:
1. PVC Production:
• Chlorine is a key raw material in the
production of polyvinyl chloride (PVC).
Process:
• Chlorine reacts with ethylene to produce
vinyl chloride monomer (VCM).
• Polymerization of VCM forms PVC resin,
which is then processed into various PVC
products.
Applications:
• PVC is used in construction for pipes,
window frames, flooring, and insulation.
• Also used in healthcare for IV tubing,
blood bags, and medical devices.
• Hydrofluoric acid (HF) is used for cleaning and etching
metal surfaces.
Process:
• HF dissolves oxides and contaminants from metal surfaces,
preparing them for further processing.
• Etching with HF creates precise patterns on metal surfaces
for electronic and decorative applications.
3. Glass Etching:
• Hydrofluoric acid (HF) is commonly used for glass etching.
process:
• HF reacts with the silica (SiO2) in glass to create a frosted or
etched appearance.
• Used in artistic glassware, signage, and electronics
manufacturing.
• Safety precautions are crucial due to the corrosive nature of
HF.
22.
THANK YOU!Feel free to contact me if you have any questions
lyazzatdaniyarkyzy01@gmail.com
LYAZZATDN







 chemistry
chemistry