Similar presentations:
Laboratory tests in Rheumatology
1. Laboratory tests in Rheumatology
Dr Katya DolnikovD_katya@rambam.health.gov.il
2017
2. Introduction
• In rheumatic disease lab test contribute todiagnosis
• Laboratory investigation should be guided by
clinical picture
• Measurement of biomarkers can be useful to
monitor treatment efficacy and safety
• Stratification of patients to predict prognosis
3. Utility of Lab Tests
Aims of lab test:1. Identification of pathological process in the body &
evaluation of its severity
2. Support or negation of specific diagnosis
3. Follow up of disease & complications
4. Detection of adverse reactions of drug therapy
• Interpretation of lab tests should be done only in
relation to certain clinical context.
• Without the clinical picture most lab tests are useless.
4. Diagnostic vs. Evaluative Tests
• Need to determine which test isappropriate
• Diagnostic tests accurately distinguish a
group of patients with a specific disease
from a non-disease group
• Evaluative tests monitor disease activity
over time
5. Blood Panel - Hemoglobin
• Anemia of chronic disease – usually normocyticand normochromic, but sometimes hypochromic
microcytic
• Should be differentiated from iron deficiency
• Macrocytic anemia – not common in
rheumatology, except for methotrexate
treatment
• Hemolytic anemia – LDH, Bilirubin, haptoglobin
• Due to Gastrointestinal bleeding (acute or
chronic)
6. Blood panel - WBC
• White blood cells – neutrophils, lymphocytes,eosinophils:
– Neutrophils are acute phase reactants
– Neutropenia – in patients undergoing
immunosupressive treatment
– Neutropenia can be associated with splenomegaly
– Lymphopenia – active phases of SLE
– Eosinophilia – Churg-Strauss (EGPA)
7. Platelets
• Thrombocytosis can accompany active phasesof autoimmune diseases – RA (APR)
• Thrombocytopenia –
– can be related to the presence of
antithrombocyte antibodies, as in SLE
– Drug induced toxicity
8. Examples
What CBC abnormalities do you expext in this patient?9. Biochemical testing- liver
• Synthetic activity (albumin, coagulationfactors, Glucose, Bil)
• Liver enzymes – hepatocellular, cholestatic
• Should be ordered before and after initiation
of treatment (NSAIDS, DMARDS, including
MTX, biological)
10. Kidney function tests
• Connective tissue diseases and systemicvasculitides are frequently associated with
kidney involvement –
vascular/glomerular/tubular-interstitial
• Creatinine/ Creatinine Clearance provide
sufficient information
• Urinalysis – always part of investigation
(hematuria, leukocyturia, proteins)
• Monitor for adverse effects of treatment
11. Uric acid
• Commonly included in the workup of patientswith arthritis
• Elevated in 90% of patients with Gout
• Healthy population can also have increased
levels of uric acid
• Important to monitor urate lowering therapy –
goal of <5-6 mg/dL
12. Acute-phase reactants
• Are not specific for rheumatic disorders• AP response occurs in a variety of
inflammatory conditions – infection, trauma,
malignancy.
• The most widely used APR
– ESR – erythrocyte sedimentation rate
– CRP
– Ferritin
13. Acute phase reactants
• Produced by hepatocytes upon stimulation bycytokines (IL-1, IL -6, TNF – tumor necrosis factor)
• Examples – CRP, fibrinogen, ferritin, haptoglobin,
ceruloplasmin, amyloid protein A, complement
(C3, immunoglobilins
• ESR and CRP are useful for monitoring the level of
inflammation, however sometimes are not
sensitive enough and sometimes are “slow” and
should not guide the clinical decisions
14. Example
What lab abnormalities do you expect in this patient?15. Example
What lab abnormalities do you expect in this patient?16. Serologic testing
• Testing for autoantibodies is frequently usedin the diagnoses of rheumatic conditions and
sometimes for monitoring of disease activity.
• An adjunct to diagnosis and management
rather than precise clinical guide.
17. Rheumatoid Factor
• Autoantibodies directed against Fc– chains of IgGmolecules
• Laboratories test only for IgM RF
• The main immunoglobilin classes of RF that can
be easily detected are IgA and IgM RF
• IgM RF is produced in many chronic inflammatory
conditions – endocarditis, hepatitis B/C,
tuberculosis, Idiopathic pulmonary fibrosis, mixed
connective tissue disease, SLE, cryoglobulinemia
• Can be present in 5% of normal elderly
population
18. Rheumatoid Factor
• Not specific for Rheumatoid Arthritis• The main indication for RF testing – suspicion
for RA and Sjogren syndrome
• The specificity of RF increases with higher
titers
• Higher titers are associated with more
aggressive and erosive disease
19. Antibodies to citrullinated protein and peptide ACPA- antigens
Antibodies to citrullinated protein and peptideantigens -ACPA
• Citrullination of proteins (arginine – citrullin)
occurs as the result of synovial inflammation and
inflammation induced apoptosis
• RA patients react to such modified proteins by
creating ACPAs
• ACPAs are especially prevalent in RF patients but
can be found in 25% of RF negative patients
• ACPAs predict later development of erosive RA
• Helpful in discriminating between RA and
psoriasis with erosive arthritis
20. Anti-nuclear Antibodies (ANA)
• Immunoglobulins directed against structures withinthe cell ( i.e. DNA, ribonuclear proteins, histones, and
centromere)
• Screening tool
• Titer/ pattern
• Found in a variety of autoimmune diseases such as
SLE, MCTD, JRA, scleroderma, Sjogren’s syndrome in
high titres (>1:320)
• Almost always present in SLE (95-98%)
• High titer increases the likelihood that the presence
of ANA is related to autoimmune disease
21. ANA
• ANAs do not correlate with disease activity• Consider using as a screening test in only
symptomatic patients (arthritis, rash, serositis,
proteinuria)
• Must measure ANAs in patients with JIA (esp.
oligoarticular) to assess risk of uveitis
22. ANA
• Low titres (<= 1:160) found in:– Infections (EBV, CMV, Hepatitis B, bacterial
endocarditis, HIV)
– Drugs (hydralazine, INH, dilantin, tegretol, ETX,
PCN, and sulfas)
– Neoplasias (lymphoma)
• It is sensitive but not specific
• ~ 10% of the population have a positive low
titer ANA and can be asymptomatic
• As one ages, ANA titers increase
23. ANA detection and measurement
• IIF - the indirect immunofluorescence test isthe most widely used assay for the detection
of ANA and remains the reference method of
choice for the detection of these antibodies
• Nuclear staining patterns include:
homogeneous, speckled, centromere, and
nucleolar
24. ANA patterns
• In the homogeneous staining pattern, the entirenucleus is diffusely stained. EX: Antibodies to histone
proteins, DNA, and DNA-histone complexes.
• In the speckled staining pattern, fine or coarse
speckles are seen throughout the nucleus. Ex:
Antibodies against U1 RNP, Sm, and La antigens.
• The centromere pattern - anti centromere
• The nucleolar pattern refers to homogeneous or
speckled staining of the nucleolus; Ex: fibrillarin, RNA
polymerase I and III, Th, PM-Scl, and RNA helicase.
25.
homogenouscenromere
speckled
nucleolar
26. ELISA method
• Solid phase assays - enzyme-linked immunoabsorbantassays (ELISA)
• A panel of purified native or recombinant autoantigens is
prepared and each antigen is immobilized on a solid surface
• The panel of antigens used in solid phase assays may
include all or some of the following: Ro, La, Sm, U1 RNP, Scl70, PM-Scl, Jo-1, centromere, histone, ribosomal P, and
DNA.
• Diluted human serum is incubated with the immobilized
antigen and, as with the indirect immunofluorescence
assay, a secondary antibody is used to detect bound
autoantibodies.
27. Advantages and Disadvantages
• The major advantage of indirectimmunofluorescence is the large number of
autoantibodies that can be detected.
• Some autoantigens may not be present in the
HEp-2 cell substrate
– The Ro60 antigen, (SLE, Sjogren’s)
– Anti-ribosomal P antibodies (SLE)
28. Advantages and Disadvantages
• The number of autoantigens that are includedin solid phase (ELIZA) assays is limited
compared with the number that are present in
the HEp-2 cell substrate. As an example, most
solid phase assays do not contain antigens
found in the nucleolus; patients with
autoantibodies directed against these
structures will have a falsely negative solid
phase ANA result
29. Anti-dsDNA antibodies
• Antibodies that target DNA• Produce homogenous pattern in ANA IIF
• Positive result for anti-dsDNA screening
should be confirmed by additional assays
• Anti-dsDNA antibody testing is very specific
(95%), but less sensitive (70%) for SLE
• Are associated with disease activity in lupus
nephritis
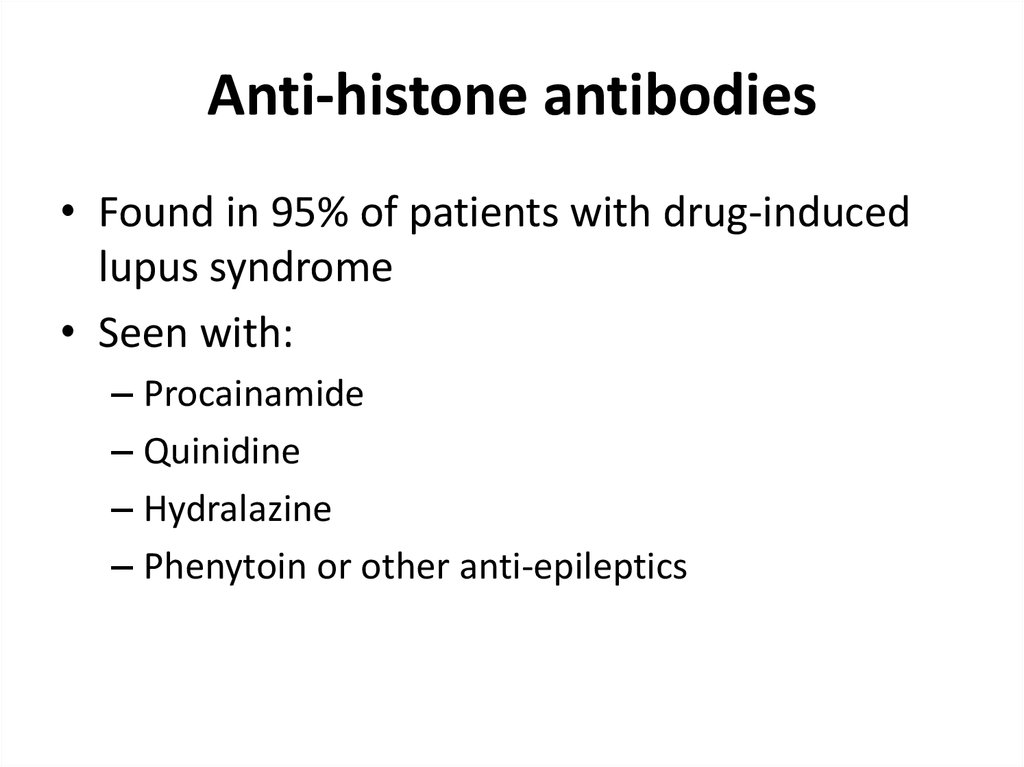
30. Anti-histone antibodies
• Found in 95% of patients with drug-inducedlupus syndrome
• Seen with:
– Procainamide
– Quinidine
– Hydralazine
– Phenytoin or other anti-epileptics
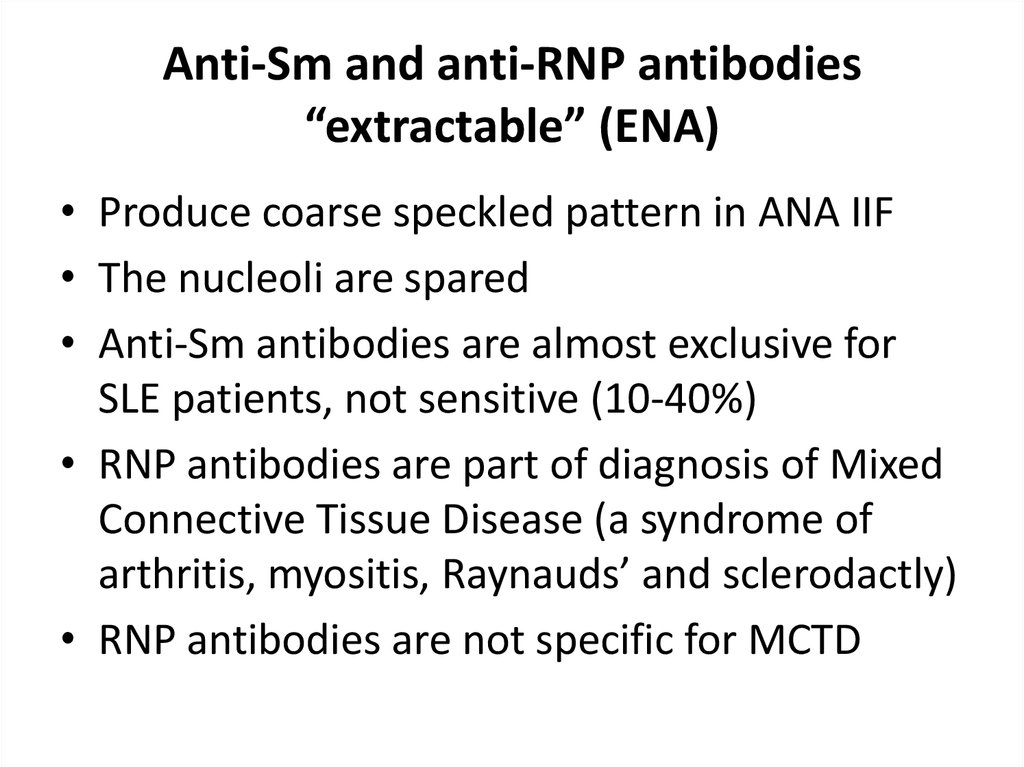
31. Anti-Sm and anti-RNP antibodies “extractable” (ENA)
• Produce coarse speckled pattern in ANA IIF• The nucleoli are spared
• Anti-Sm antibodies are almost exclusive for
SLE patients, not sensitive (10-40%)
• RNP antibodies are part of diagnosis of Mixed
Connective Tissue Disease (a syndrome of
arthritis, myositis, Raynauds’ and sclerodactly)
• RNP antibodies are not specific for MCTD
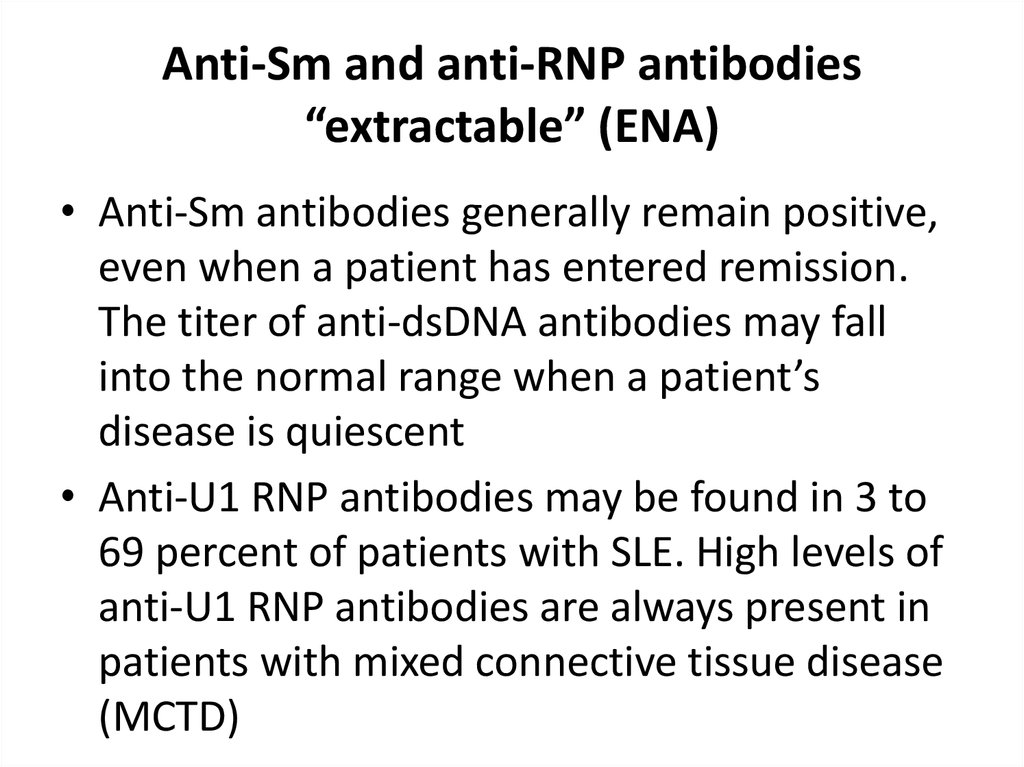
32. Anti-Sm and anti-RNP antibodies “extractable” (ENA)
• Anti-Sm antibodies generally remain positive,even when a patient has entered remission.
The titer of anti-dsDNA antibodies may fall
into the normal range when a patient’s
disease is quiescent
• Anti-U1 RNP antibodies may be found in 3 to
69 percent of patients with SLE. High levels of
anti-U1 RNP antibodies are always present in
patients with mixed connective tissue disease
(MCTD)
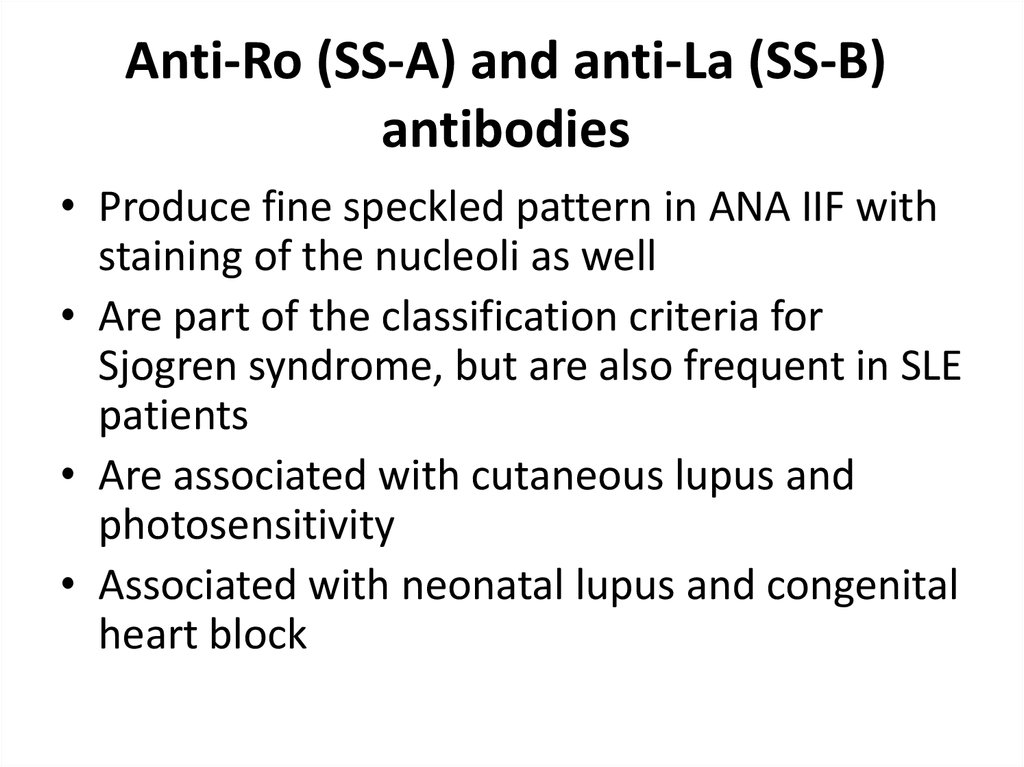
33. Anti-Ro (SS-A) and anti-La (SS-B) antibodies (ENAs)
• Two sets of names assigned by two different groups;first seen in Sjogren’s patients and then seen in SLE
patients
• Anti Ro/SS-A antibodies seen in:
– 5-15% of normals
– 50% of Sjogren’s patients
– 30% of SLE patients (many have negative ANA or subacute
cutaneous lupus)
– Correlates with active nephritis and cytopenias
34. Anti-Ro (SS-A) and anti-La (SS-B) antibodies
• Produce fine speckled pattern in ANA IIF withstaining of the nucleoli as well
• Are part of the classification criteria for
Sjogren syndrome, but are also frequent in SLE
patients
• Are associated with cutaneous lupus and
photosensitivity
• Associated with neonatal lupus and congenital
heart block
35. Anticentomere and anti-SCL-70
• Anticentromere antibodies (ACA) produce a typicalpattern in ANA IIF by staining the centromere region of
the chromosomes – this pattern is pathognomonic
• The presence of anti-Scl-70 antibodies should be
confirmed using ELIZA
• These two antibodies are associated with distinct
clinical pictures and are mutually exclusive
• Anti-Scl-70 antibodies (also known as antitopoisomerase I) are associated with increased risk of
pulmonary fibrosis in both limited and diffuse
cutaneous systemic sclerosis
36. Antineutrophil cytoplasmic antibodies - ANCA
• Subgroup of neutrophil specific antibodies• Commonly directed to myeloperoxidase (MPO) - and
proteinase 3 (PR3)
• P-ANCA – perinuclear staining (MPO)
• C-ANCA – cytoplasmic staining (PR3)
• Positive result on IIF should be confirmed using ELIZA
• ANCA is useful in diagnosis of ANCA – associated
vasculitides (GPA, EGPA, microscopic polyangiitis)
• C-ANCA – GPA
• P-ANCA – EGPA, microscopic polyangiitis
37. ANCA
• c-ANCA is seen in 90% of GPA (Wegener’sgranulomatosis)
• p-ANCA is associated with microscopic polyangiitis,
EGPA (Churg-Strauss), and Ulcerative Colitis
• Consider vasculitis (ANCA) if patient has:
– Fever of unknown origin
– Palpable purpura, vasculitis urticaria, or dermal
necrosis
– Mononeuritis multiplex
– Unexplained arthritis, myositis, or serositis
– Unexplained pulmonary, CV or renal disease
38. Complement
• The most frequent clinical parameters usedfor judging complement activation – C3, C4
• C3, C4 are APR
• C3, C4 consumption is associated with
immune complexes diseases
• In SLE low levels reflect disease activity and in
lupus nephritis normalization is associated
with better outcomes
39. Antiphospholipid antibodies (APLA)
• Anti-cardiolipin antibodies (ELIZA)– IgG – better related to procoagulant activity
compared to IgM/IgA
• β2glycoprotein-1 IgG and IgM
• Lac - functional assay for the lupus
anticoagulant (LA) phenomenon (prolonged
aPTT/dRVVT) not corrected by control plasma
but shortened by adding excess phospholipid
40. Examples
• 24y woman presents with weakness, nausea,ptechia and echymozes
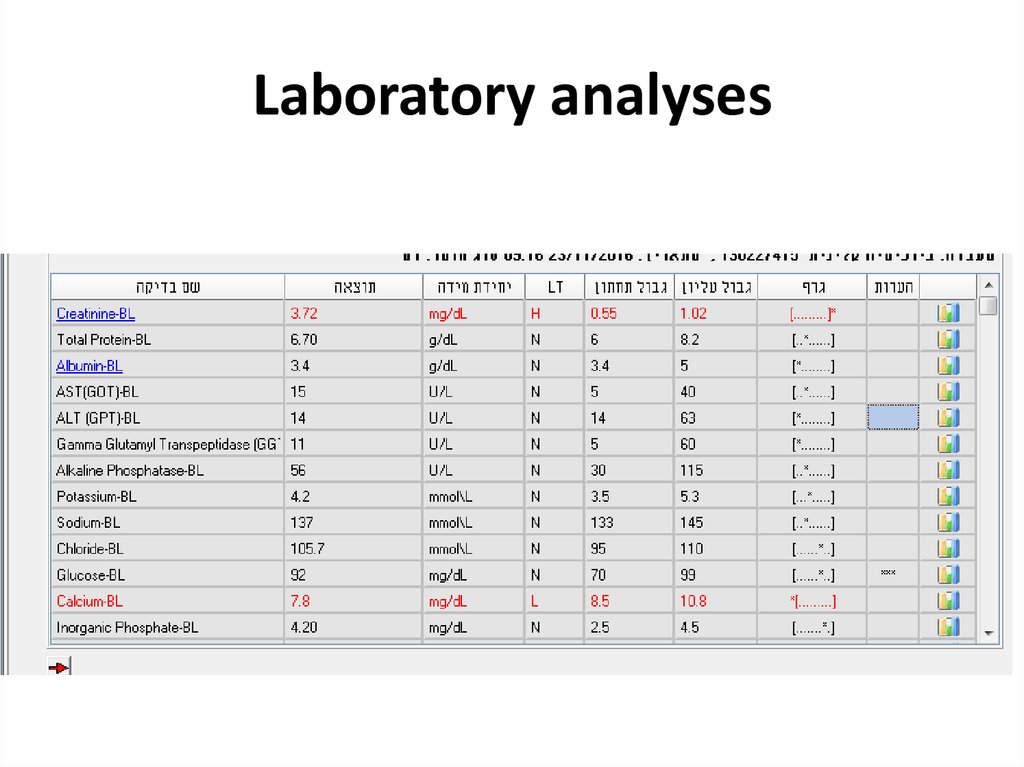
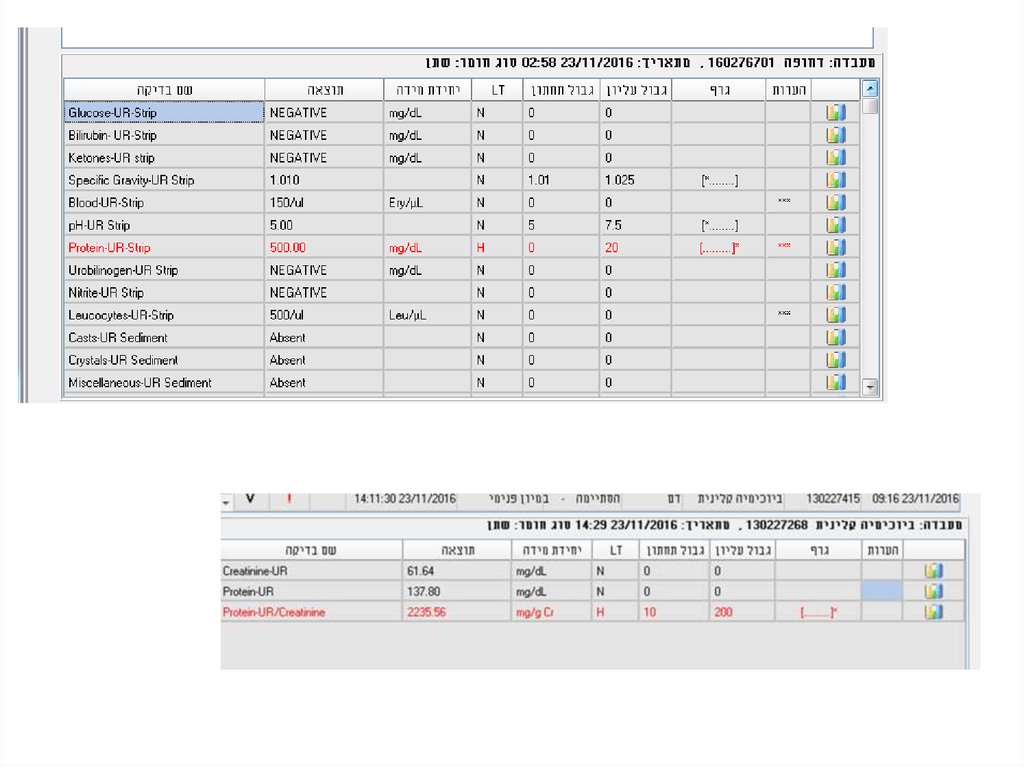
41. Laboratory analyses
42. Laboratory analyses
43.
44.
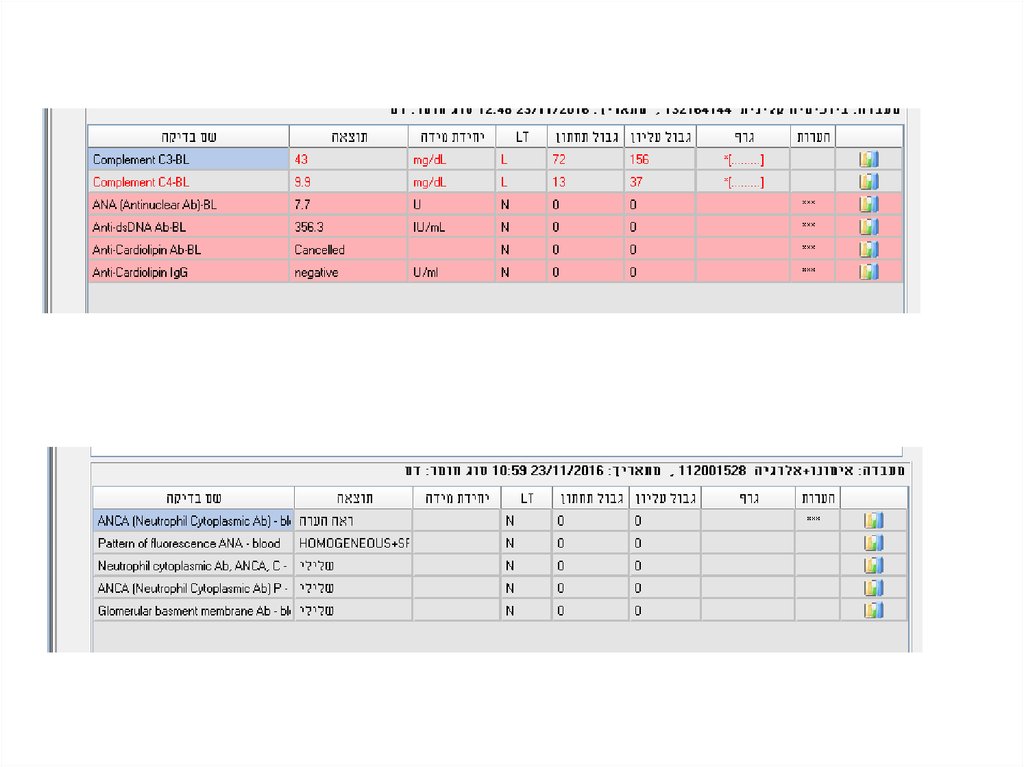
45. Examples
• 28y old woman presents with cough, fever,dyspnea, fatigue
46. Laboratory analyses
47. Laboratory analyses
48.
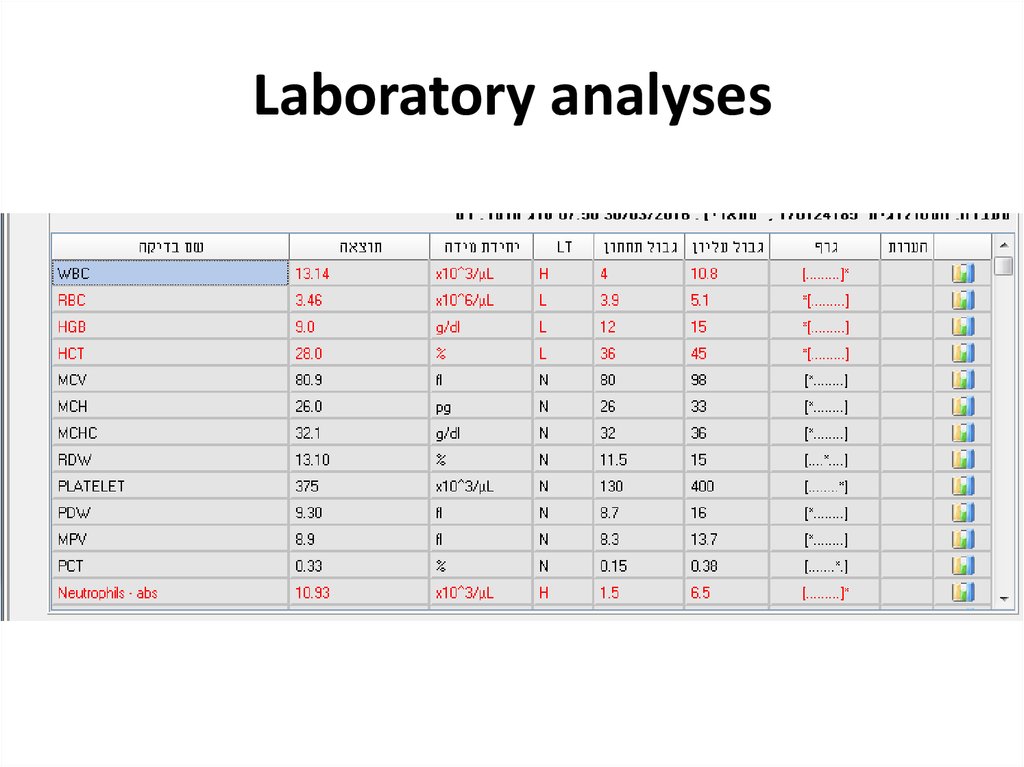
49. Laboratory analyses
50. Example
• 42y old woman with SLE presents with fatigue,hair loss and new digital ulcer
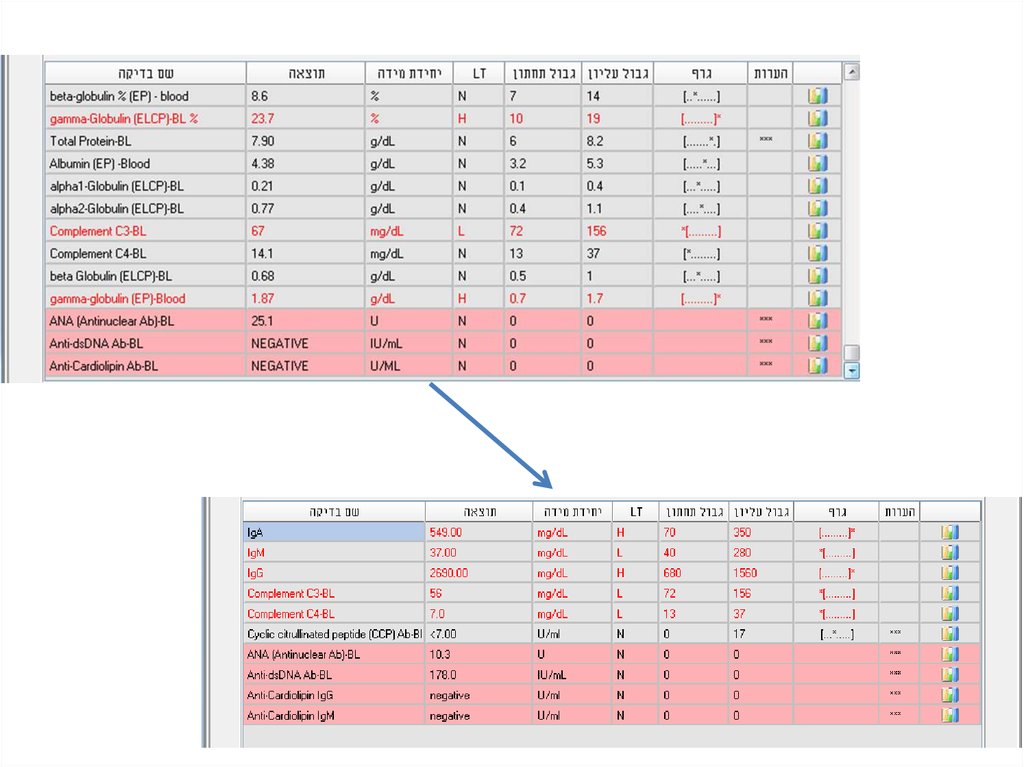
51. Examples
52.
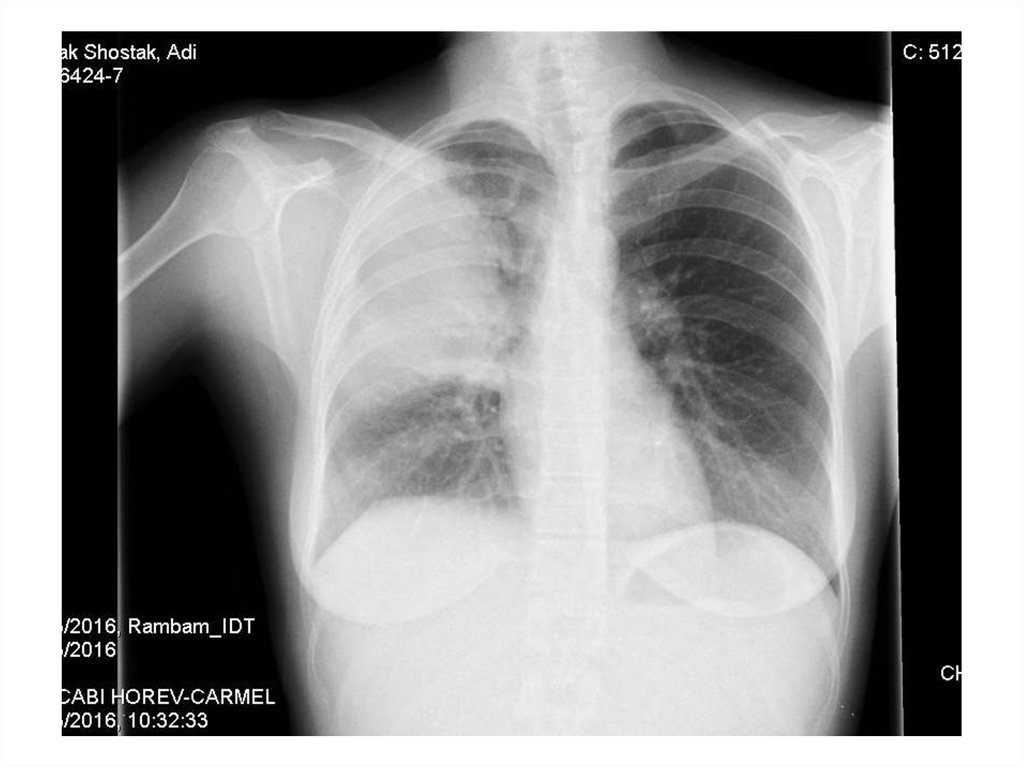
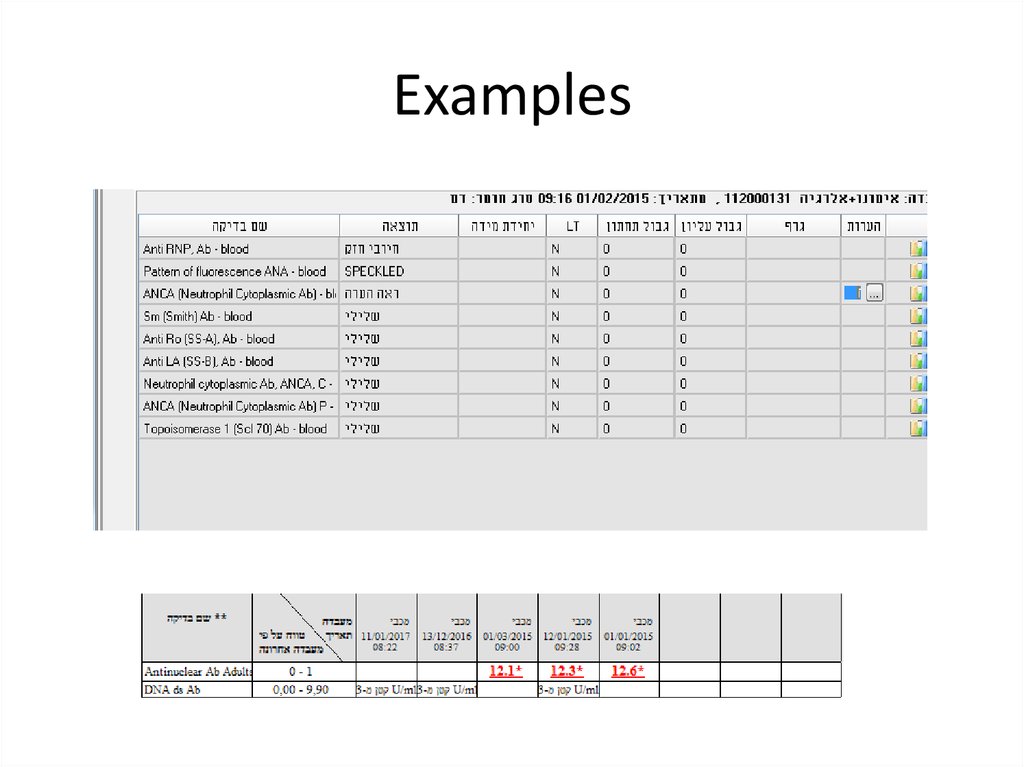
53. Examples
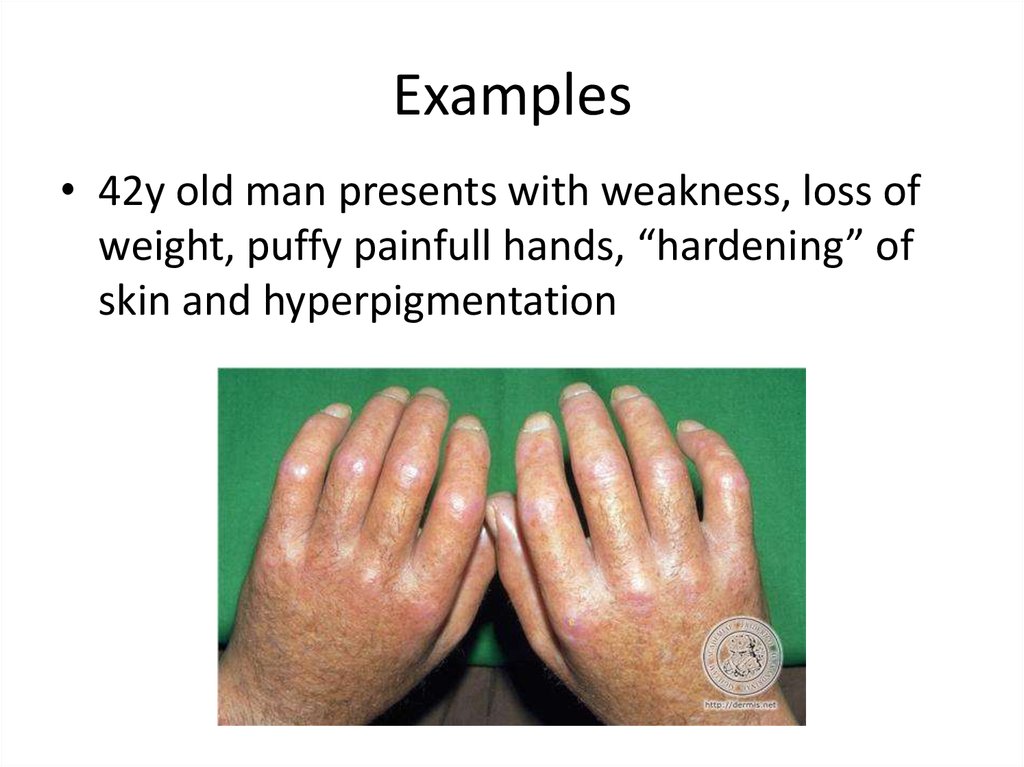
54. Examples
• 42y old man presents with weakness, loss ofweight, puffy painfull hands, “hardening” of
skin and hyperpigmentation
































 medicine
medicine








