Similar presentations:
c_electron_config
1. Electron Configuration
Chemistry2.
Learning objectives11.1.3.1 understand and be able to work
with a shell model of the atom: shell, subshell, orbital
11.1.3.2 recall the shapes of s, p, d, and f
orbital (sets)
11.1.3.3 understand the rules for the filling
of shells and sub-shells
11.1.3.4 recall the Aufbau (Kletchkovsky)
principle as a mnemonic for the
arrangement of electrons
11.1.3.5 be able to draw the electronic
3. Success criteria
explain the shell - subshell - orbital structure of theatom and relate it to quantum numbers
describe and sketch the shapes of s and p orbitals
identify the main principles of atomic orbital filling
with electrons
state the electronic configuration of atoms and ions
given the proton number and charge, using the
convention 1s22s22p6 , etc.
construct the electronic configuration of atoms and
ions in full and shorthand form
4. Electron Configuration
The way electrons arearranged around the
nucleus.
5. Quantum Mechanical Model
1920’sWerner Heisenberg (Uncertainty
Principle)
Louis de Broglie (electron has wave
properties)
Erwin Schrodinger (mathematical
equations using probability, quantum
numbers)
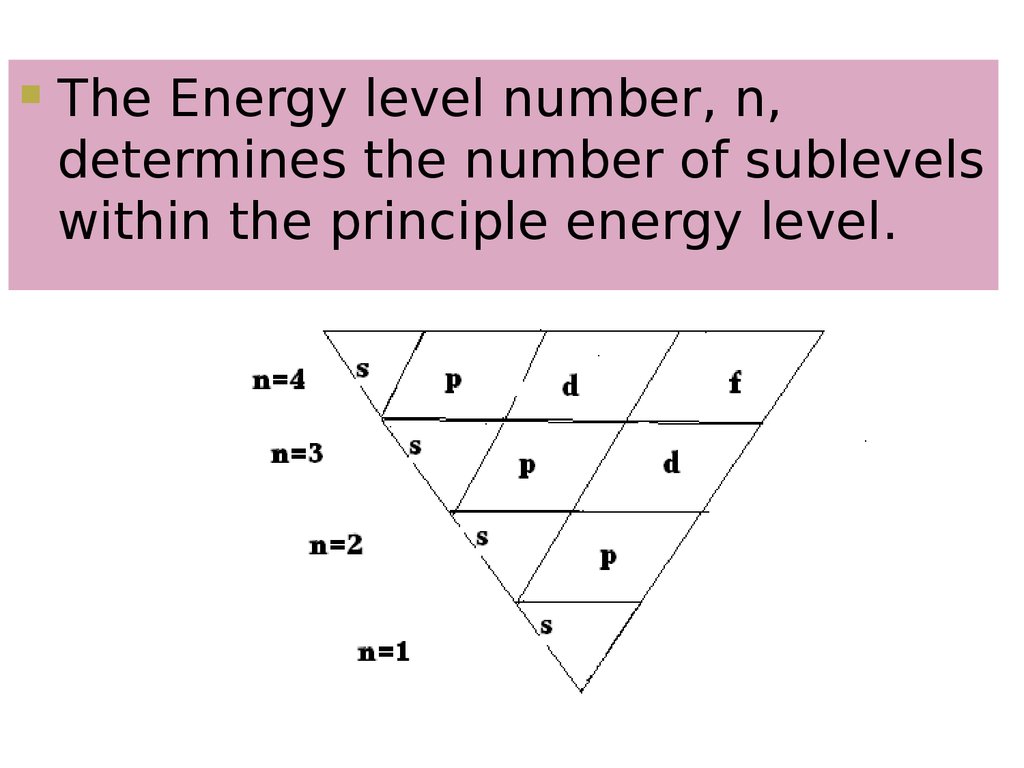
6. Energy Levels
Indicates main energy levelsn = 1, 2, 3, 4…
Farther from nucleus = higher
number
Each main energy level has sublevels
s p d f
7.
The Energy level number, n,determines the number of sublevels
within the principle energy level.
8. Orbital Quantum Number, ℓ (Angular Momentum Quantum Number)
OrbitalThe space where there is a high
probability that it is occupied by a
pair of electrons.
Orbitals are solutions of
Schrodinger’s equations.
9. Orbital
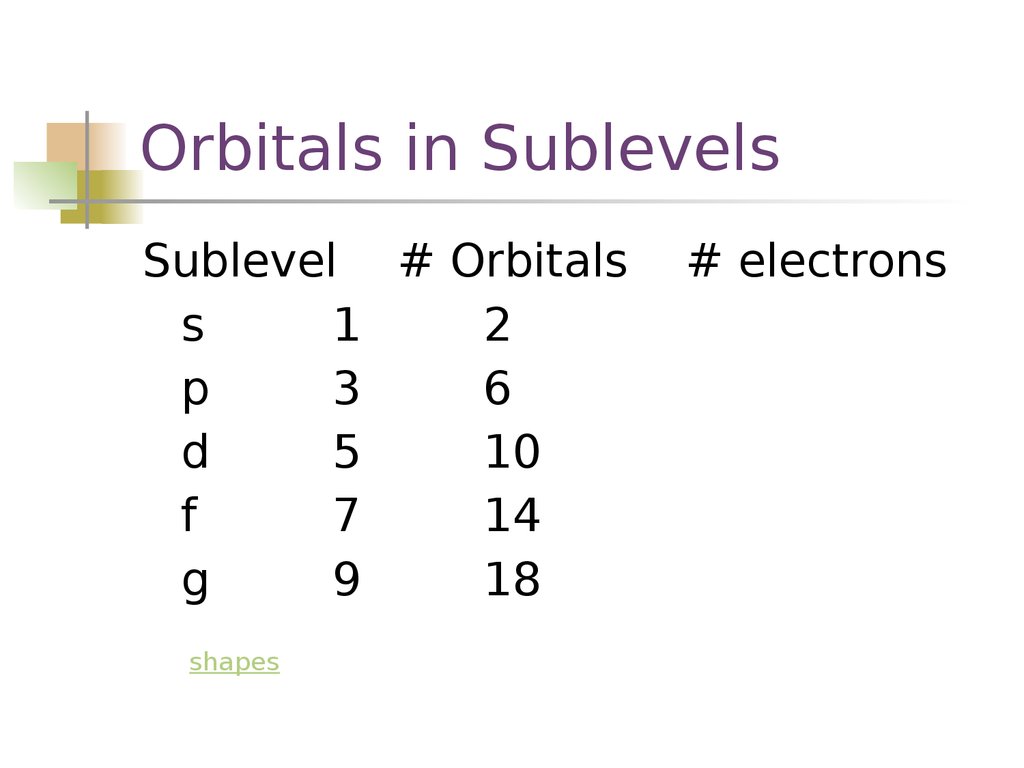
Orbitals in SublevelsSublevel # Orbitals
s
1
2
p
3
6
d
5
10
f
7
14
g
9
18
shapes
# electrons
10. Orbitals in Sublevels
Three rules are used tobuild the electron
configuration:
Aufbau principle
Pauli Exclusion Principle
Hund’s Rule
11. Three rules are used to build the electron configuration:
Aufbau PrincipleElectrons occupy orbitals of
lower energy first.
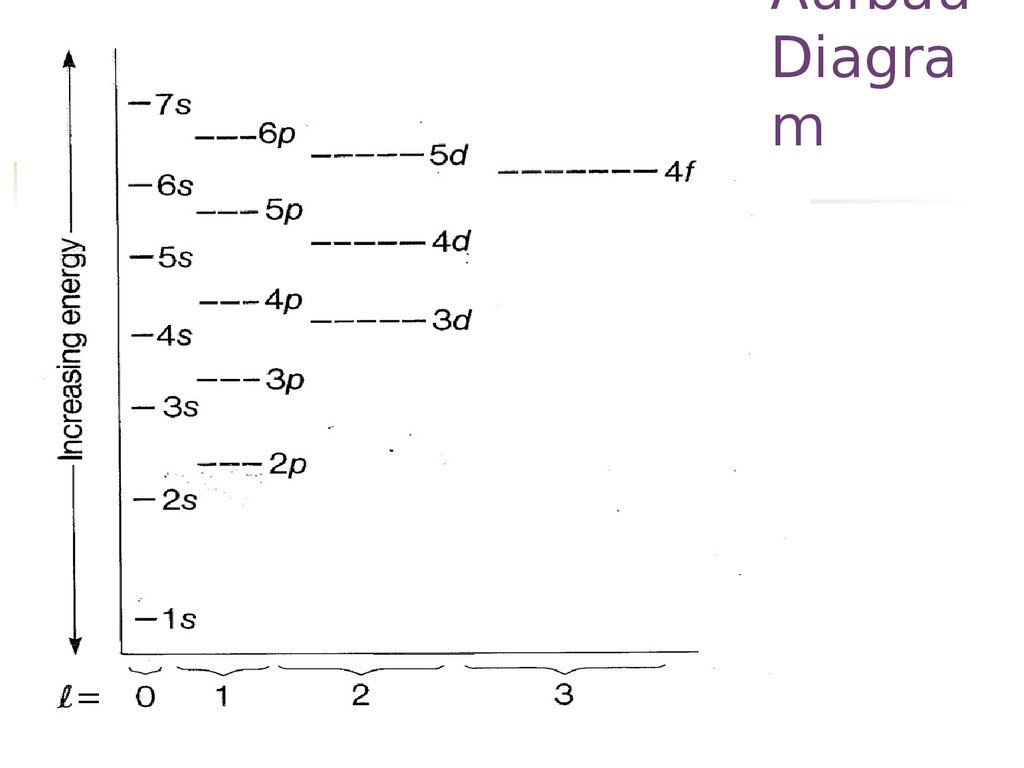
12. Aufbau Principle
AufbauDiagra
m
13. Aufbau Diagram
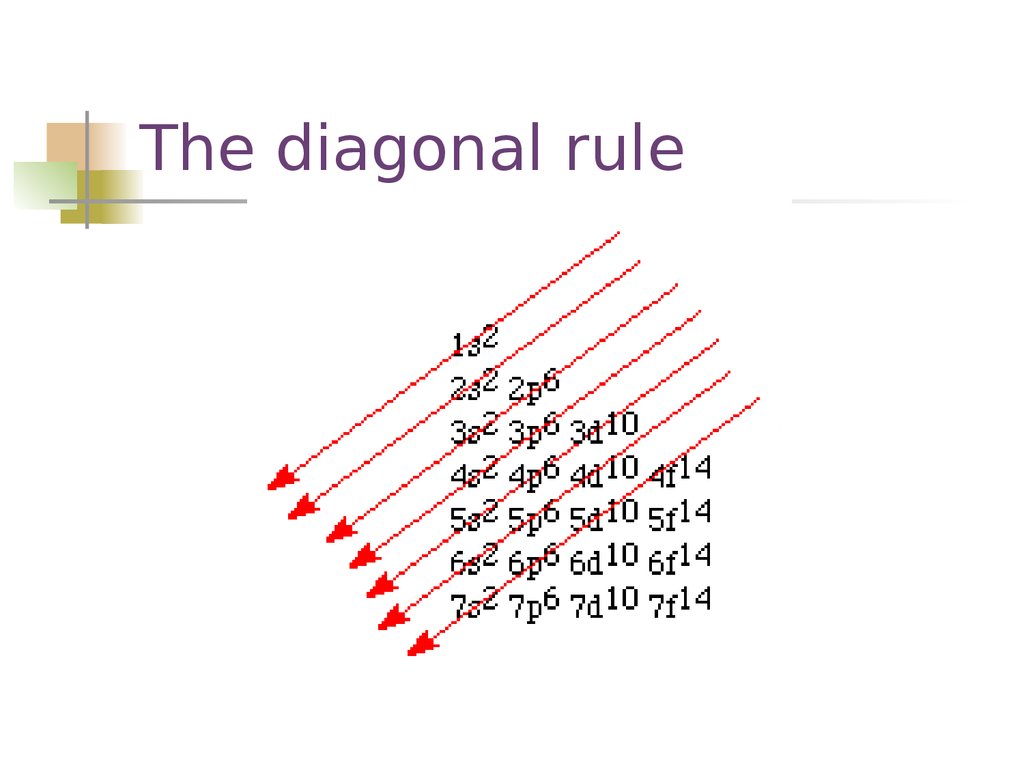
The diagonal rule14. The diagonal rule
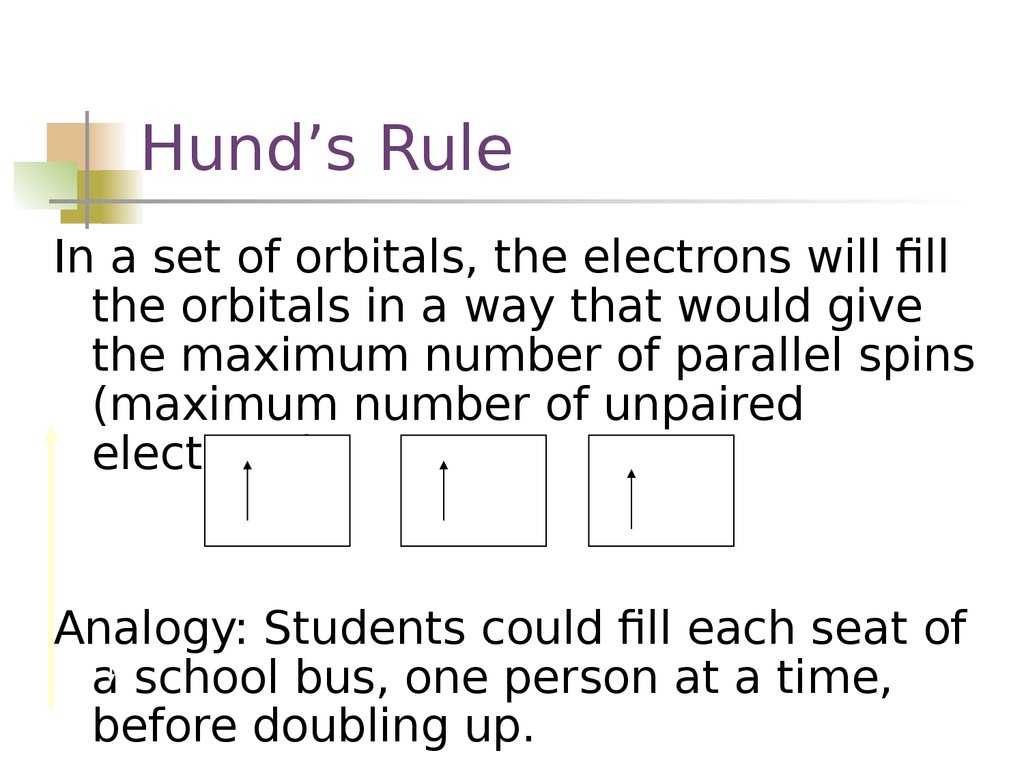
Hund’s RuleIn a set of orbitals, the electrons will fill
the orbitals in a way that would give
the maximum number of parallel spins
(maximum number of unpaired
electrons).
Analogy: Students could fill each seat of
a school bus, one person at a time,
before doubling up.
15. Hund’s Rule
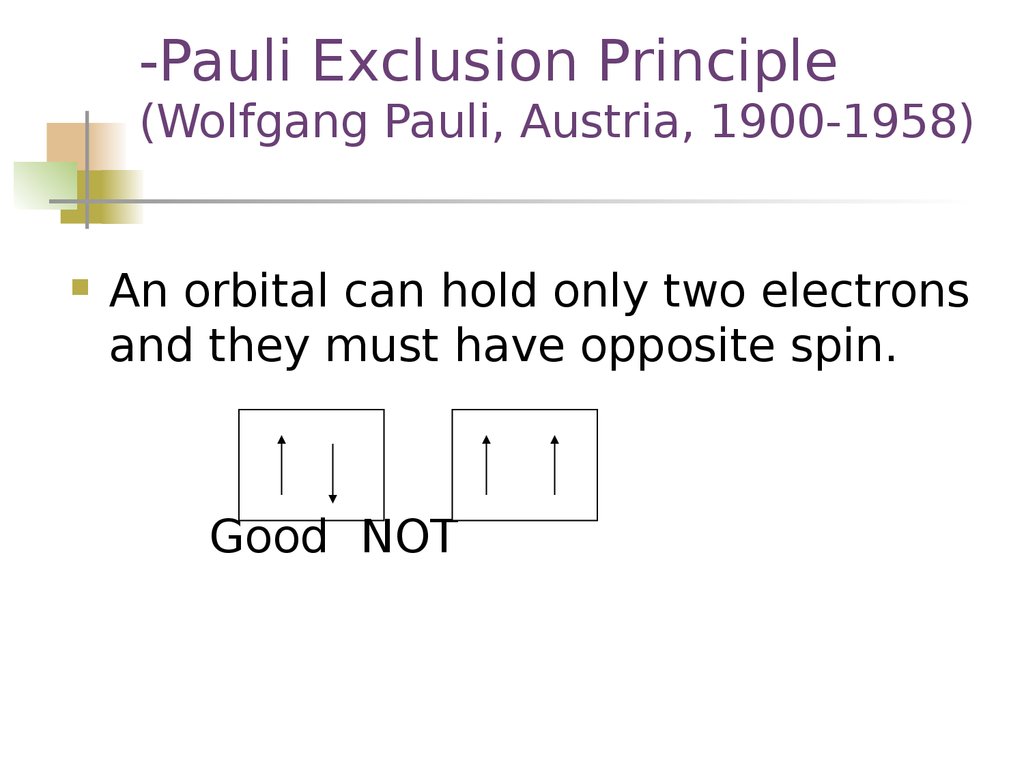
-Pauli Exclusion Principle(Wolfgang Pauli, Austria, 1900-1958)
An orbital can hold only two electrons
and they must have opposite spin.
Good NOT
16. -Pauli Exclusion Principle (Wolfgang Pauli, Austria, 1900-1958)
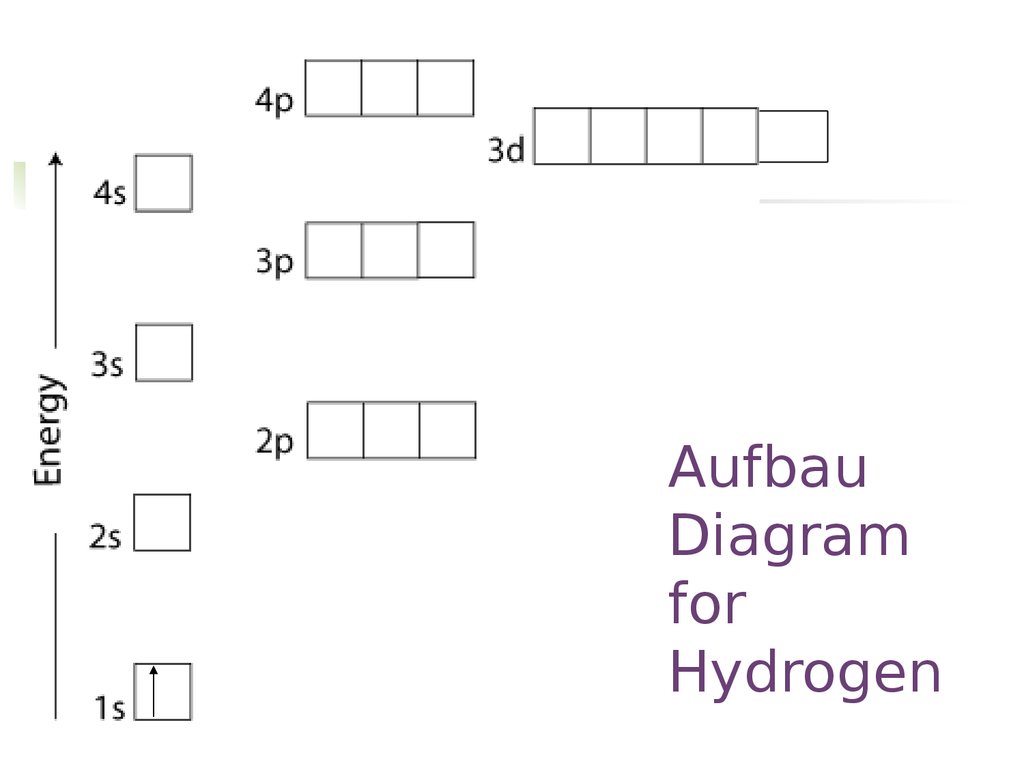
AufbauDiagram
for
Hydrogen
17. Aufbau Diagram for Hydrogen
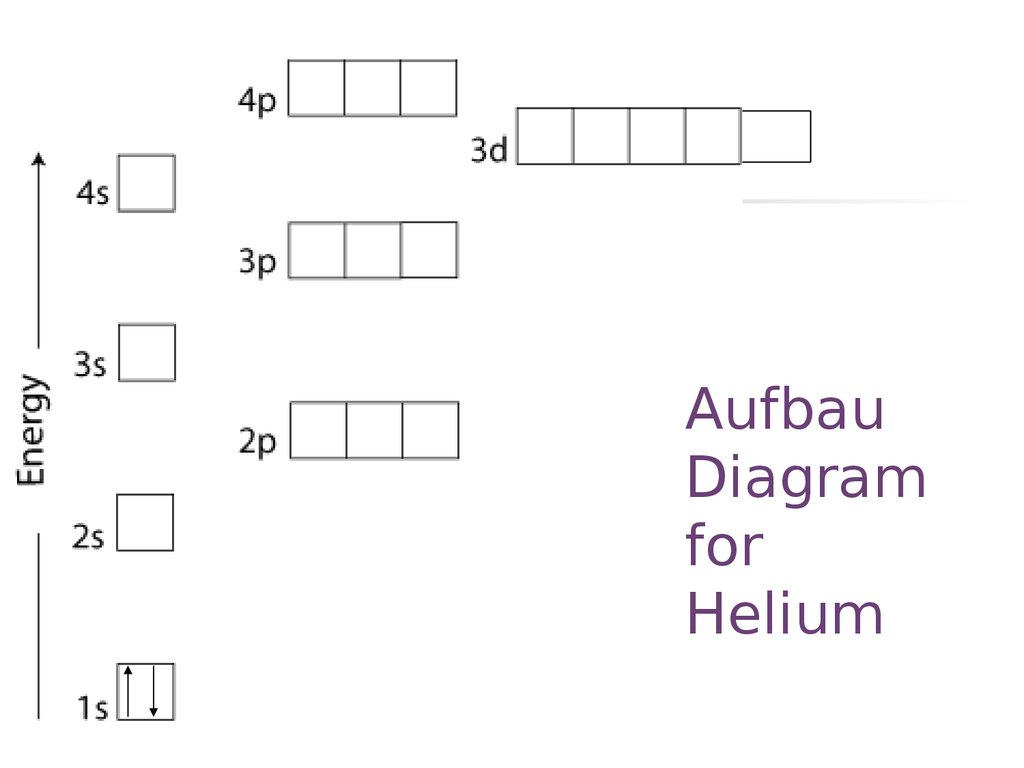
AufbauDiagram
for
Helium
18. Aufbau Diagram for Helium
AufbauDiagram
for
Lithium
19. Aufbau Diagram for Lithium
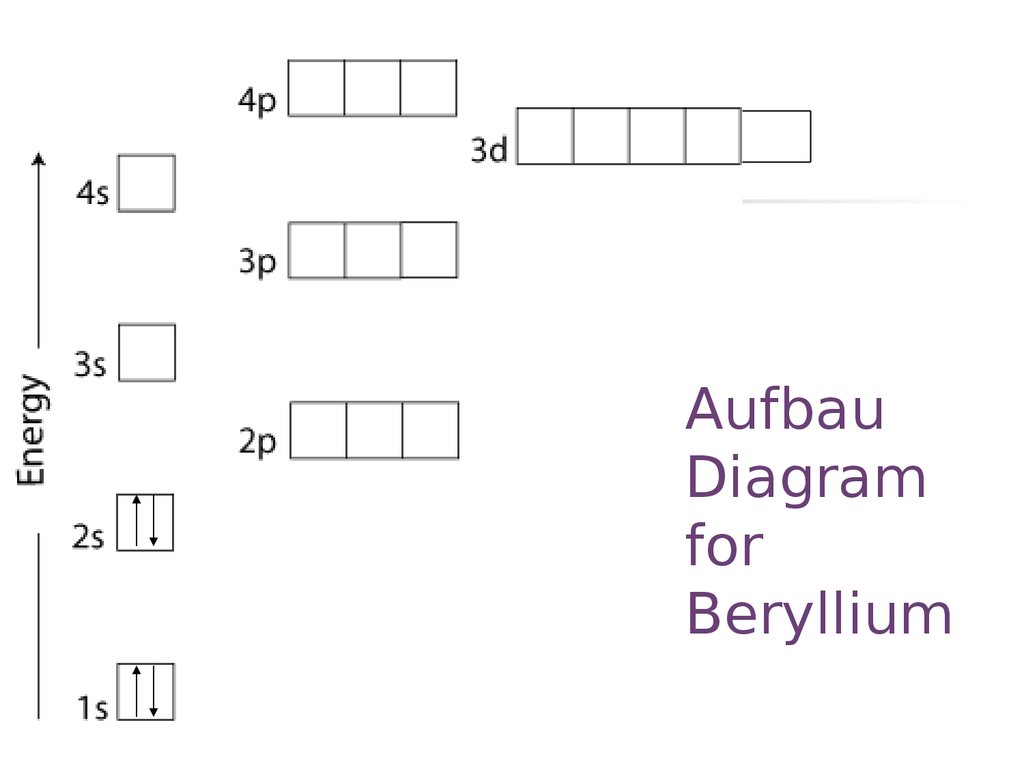
AufbauDiagram
for
Beryllium
20. Aufbau Diagram for Beryllium
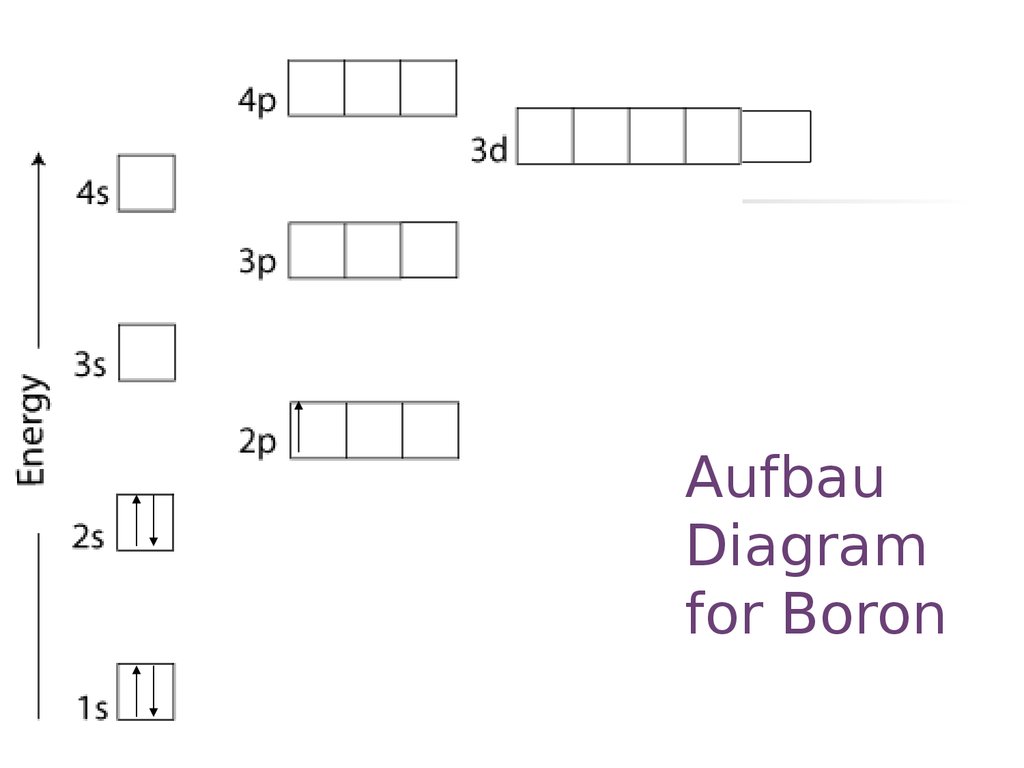
AufbauDiagram
for Boron
21. Aufbau Diagram for Boron
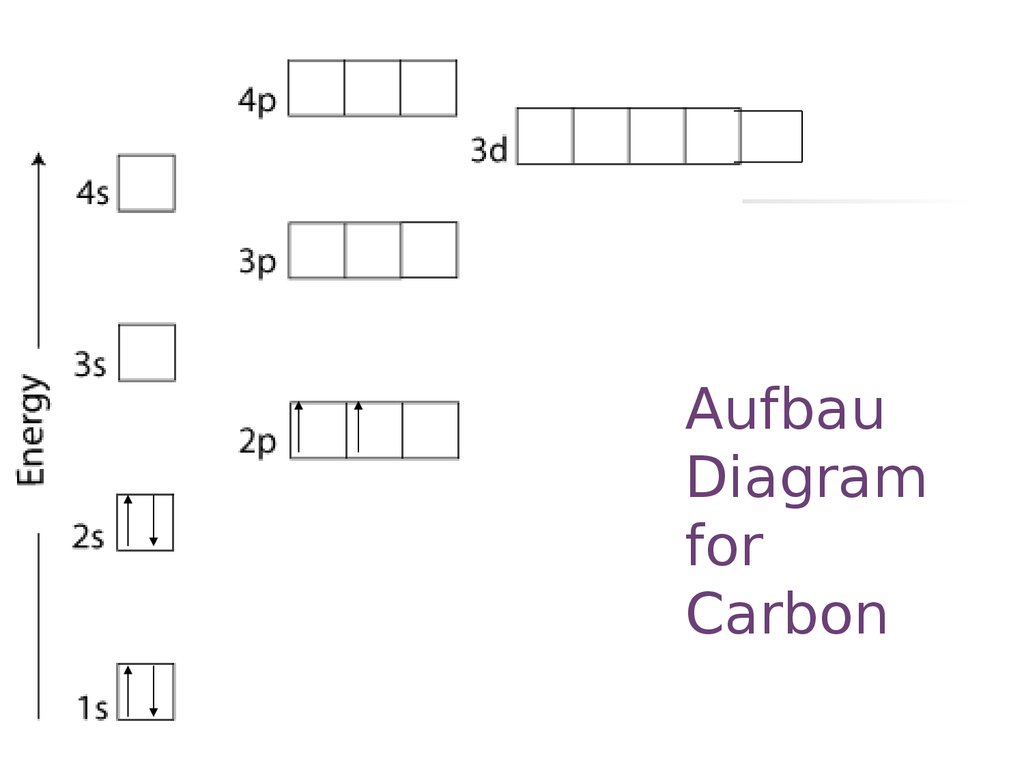
AufbauDiagram
for
Carbon
22. Aufbau Diagram for Carbon
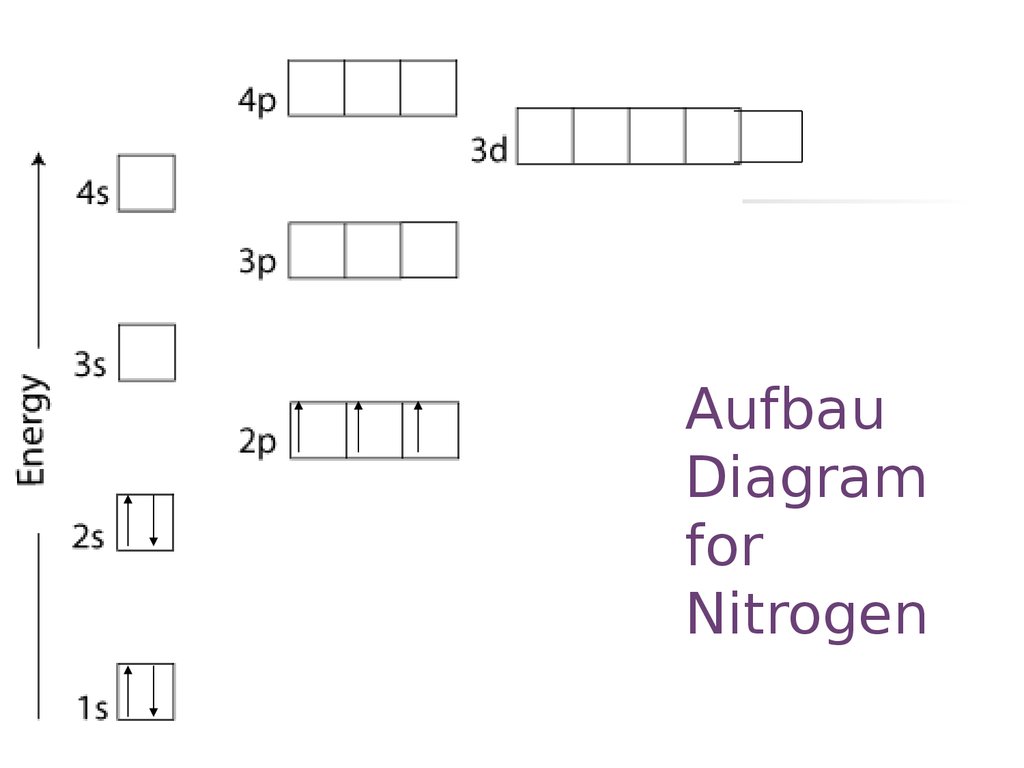
AufbauDiagram
for
Nitrogen
23. Aufbau Diagram for Nitrogen
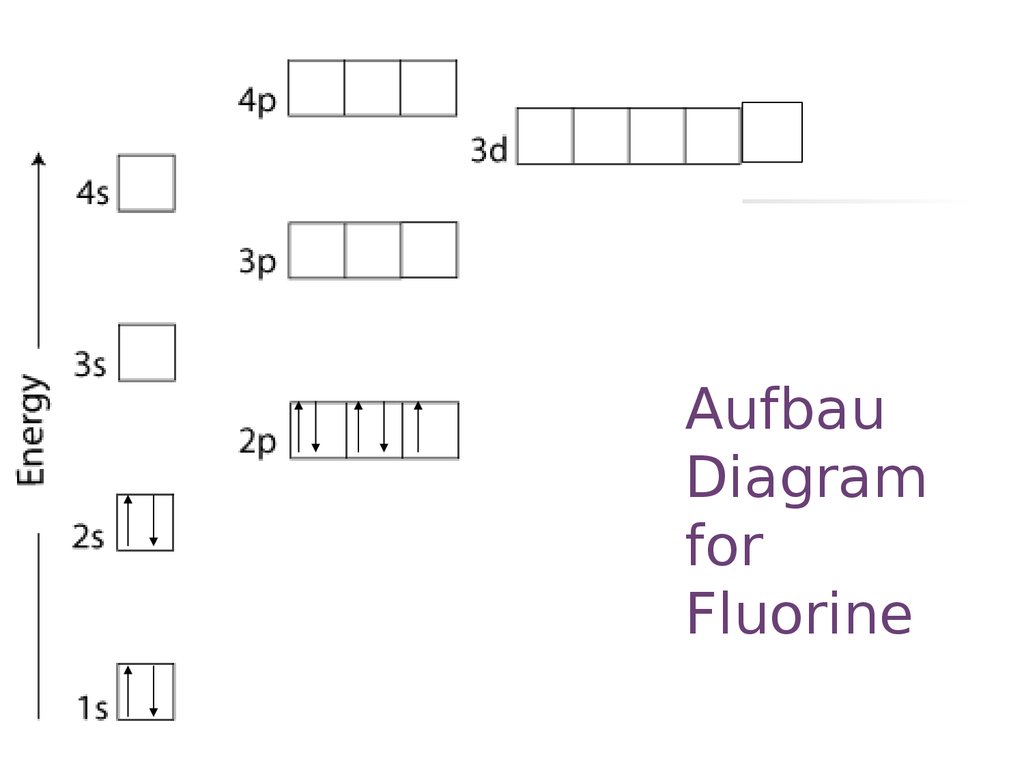
AufbauDiagram
for
Fluorine
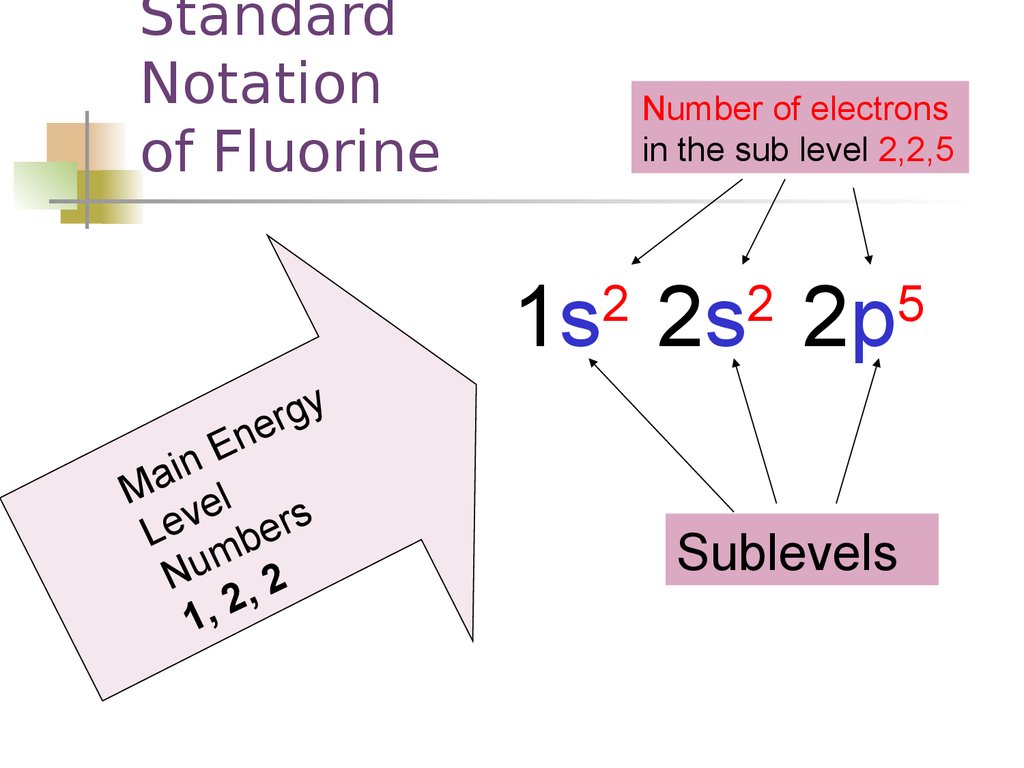
24. Notations of Electron Configurations
StandardNotation
of Fluorine
Number of electrons
in the sub level 2,2,5
1s 2s 2p
2
y
g
r
e
n
E
n
i
Ma el
v
s
r
e
e
L
b
m
Nu , 2
1, 2
2
5
Sublevels
25. Aufbau Diagram for Fluorine
Shorthand NotationUse the last noble gas that is
located in the periodic table right
before the element.
Write the symbol of the noble gas
in brackets.
Write the remaining configuration
after the brackets.
Ex: Fluorine: [He] 2s2 2p5
26. Standard Notation of Fluorine
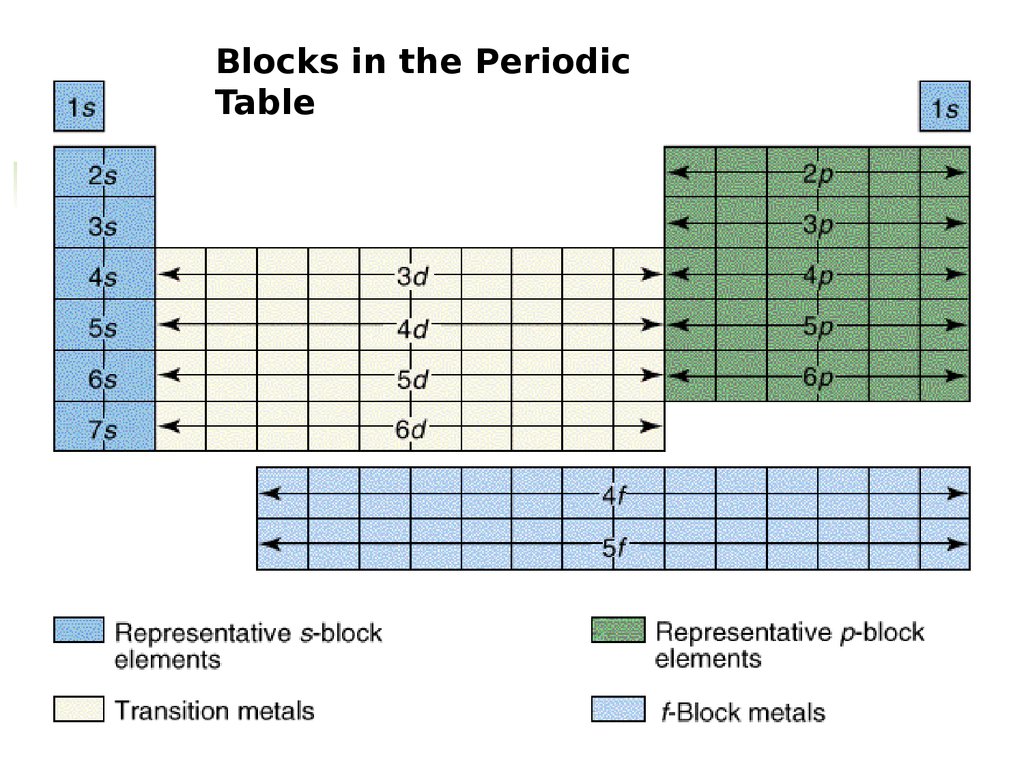
Blocks in the PeriodicTable








 english
english








