Similar presentations:
Metallic bonding
1. CHEMICAL BONDING
Ms Sarah Ang2.
Why won’t I falloff the wall?
I’m a survivor!!! (in a frozen pond)
I’m bendy!
Pencil as electrodes?
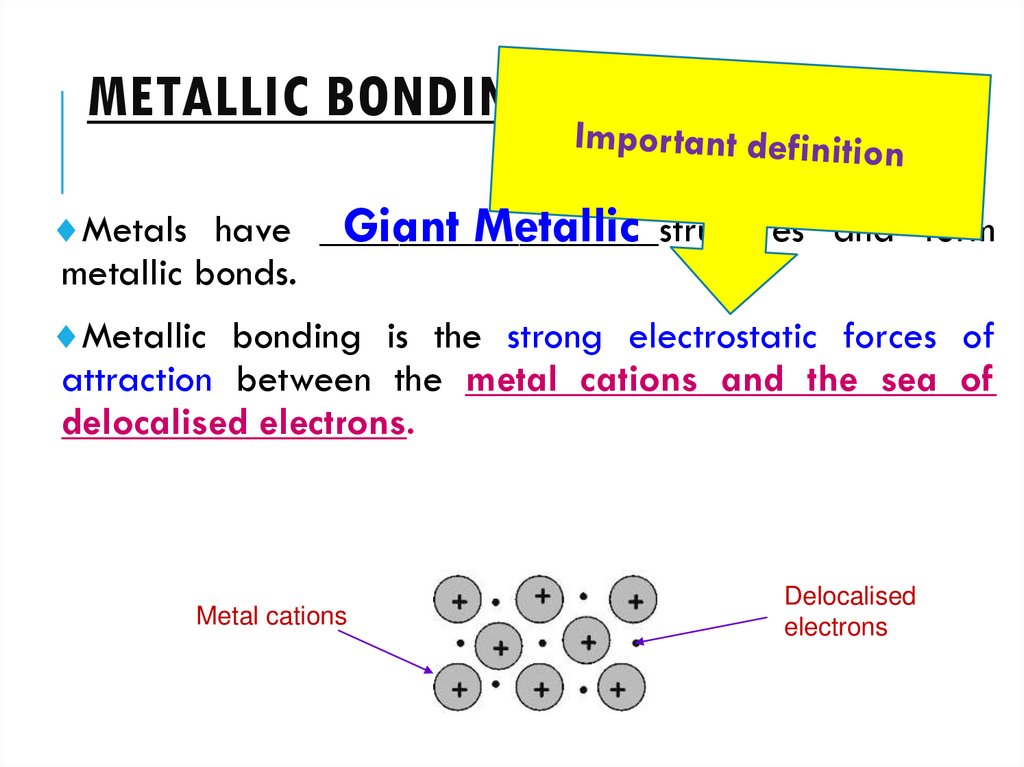
3. METALLIC BONDING
Giant MetallicMetals have _________________structures
and form
metallic bonds.
Metallic bonding is the strong electrostatic forces of
attraction between the metal cations and the sea of
delocalised electrons.
Metal cations
Delocalised
electrons
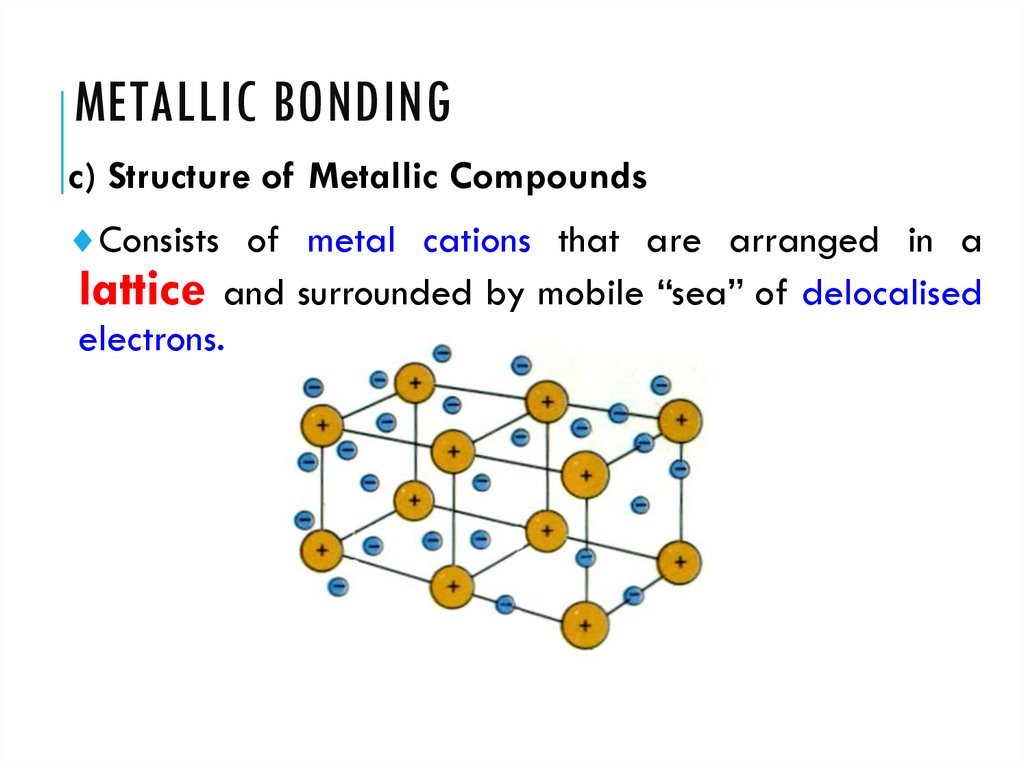
4. Metallic bonding
METALLIC BONDINGc) Structure of Metallic Compounds
Consists of metal cations that are arranged in a
lattice and surrounded by mobile “sea” of delocalised
electrons.
5. What are the PROPERTIES OF METALLIC COMPOUNDS?
6. PROPERTIES OF METALLIC COMPOUNDS
(i) High melting and boiling pointA large amount of energy is required to overcome the
strong metallic bonds.
Hence metallic compounds have generally high melting
points and boiling points e.g Hg.
(ii) Good conductors of electricity
When a potential difference is applied, the
___________________________
delocalised mobile electrons flow towards the
positive potential.
Hence, metallic compounds are good conductors of
electricity
7. PROPERTIES OF METALLIC COMPOUNDS
(iii) Good conductors of heatWhen heat is supplied, the kinetic energy of the particles
is increased.
The mobile sea of delocalised electrons transmits the
increase in energy to other parts of the metal
conduction of heat.
Hence, metallic compounds are good conductors of heat.
8. PROPERTIES OF METALLIC COMPOUNDS
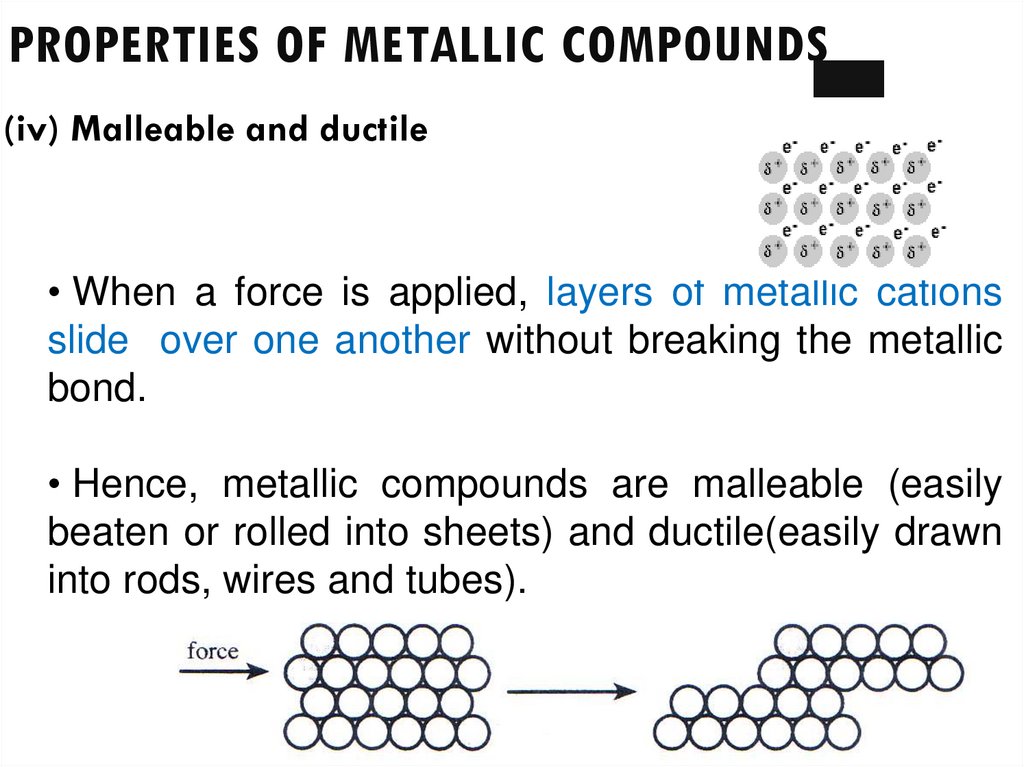
(iv) Malleable and ductile• When a force is applied, layers of metallic cations
slide over one another without breaking the metallic
bond.
• Hence, metallic compounds are malleable (easily
beaten or rolled into sheets) and ductile(easily drawn
into rods, wires and tubes).
9.
Examples of malleability of metals10. Metallic bonding
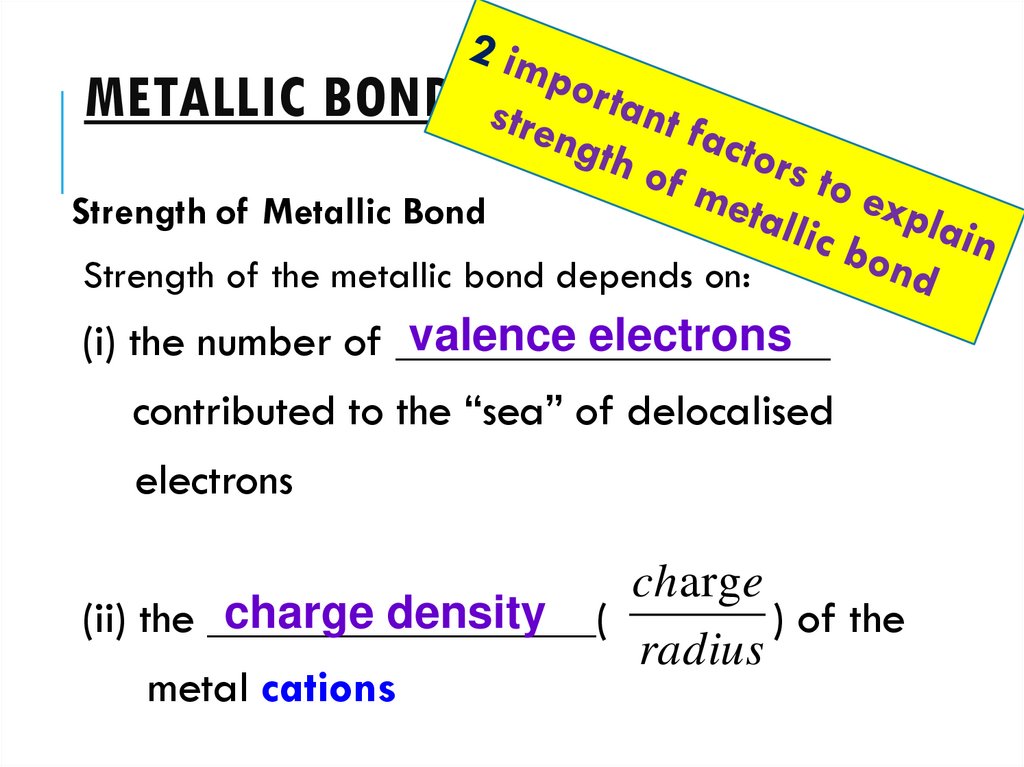
METALLIC BONDINGStrength of Metallic Bond
Strength of the metallic bond depends on:
valence electrons
(i) the number of ___________________
contributed to the “sea” of delocalised
electrons
charg e
charge density
(ii) the _________________(
) of the
radius
metal cations
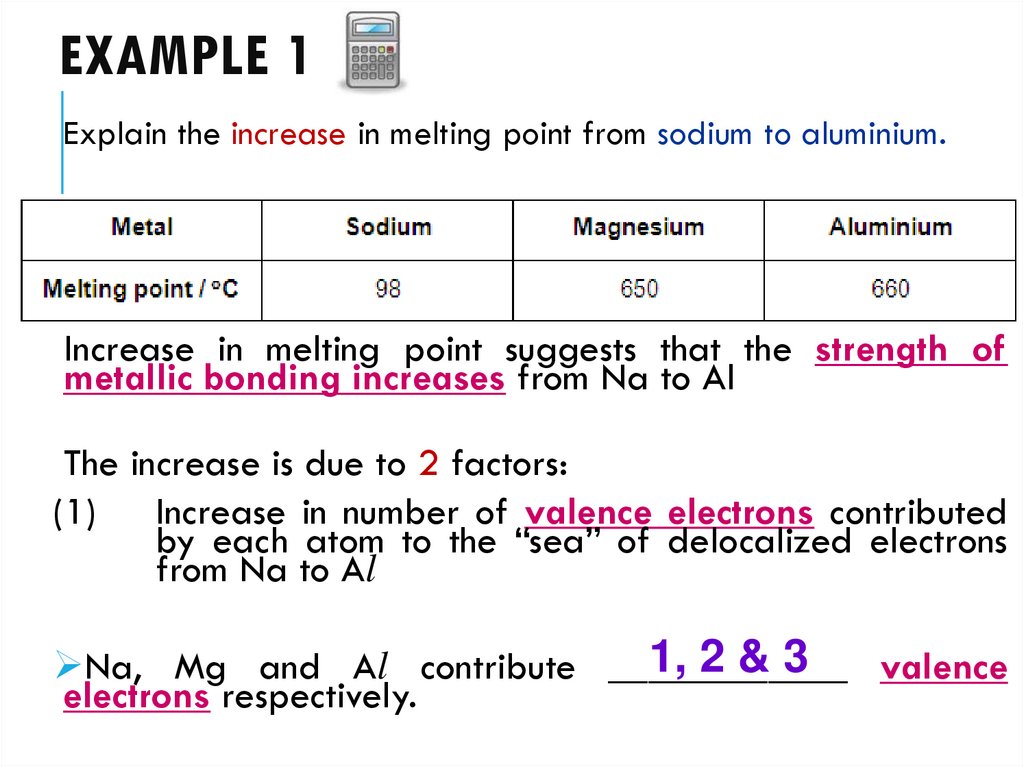
11. EXAMPLE 1
Explain the increase in melting point from sodium to aluminium.Increase in melting point suggests that the strength of
metallic bonding increases from Na to Al
The increase is due to 2 factors:
(1) Increase in number of valence electrons contributed
by each atom to the “sea” of delocalized electrons
from Na to Al
1, 2 & 3
Na, Mg and Al contribute ____________
valence
electrons respectively.
12.
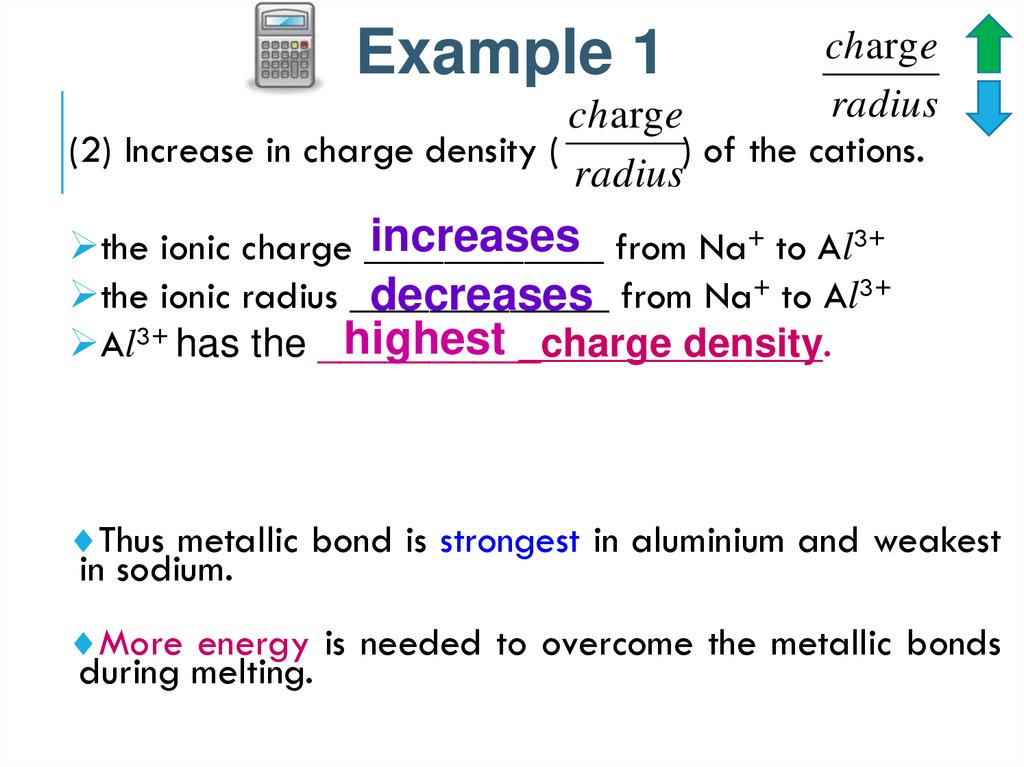
Example 1charg e
radius
charg e
(2) Increase in charge density (
) of the cations.
radius
increases from Na+ to Al3+
the ionic charge ____________
the ionic radius _____________
decreases from Na+ to Al3+
highest
Al3+ has the __________charge
density.
Thus metallic bond is strongest in aluminium and weakest
in sodium.
More energy is needed to overcome the metallic bonds
during melting.
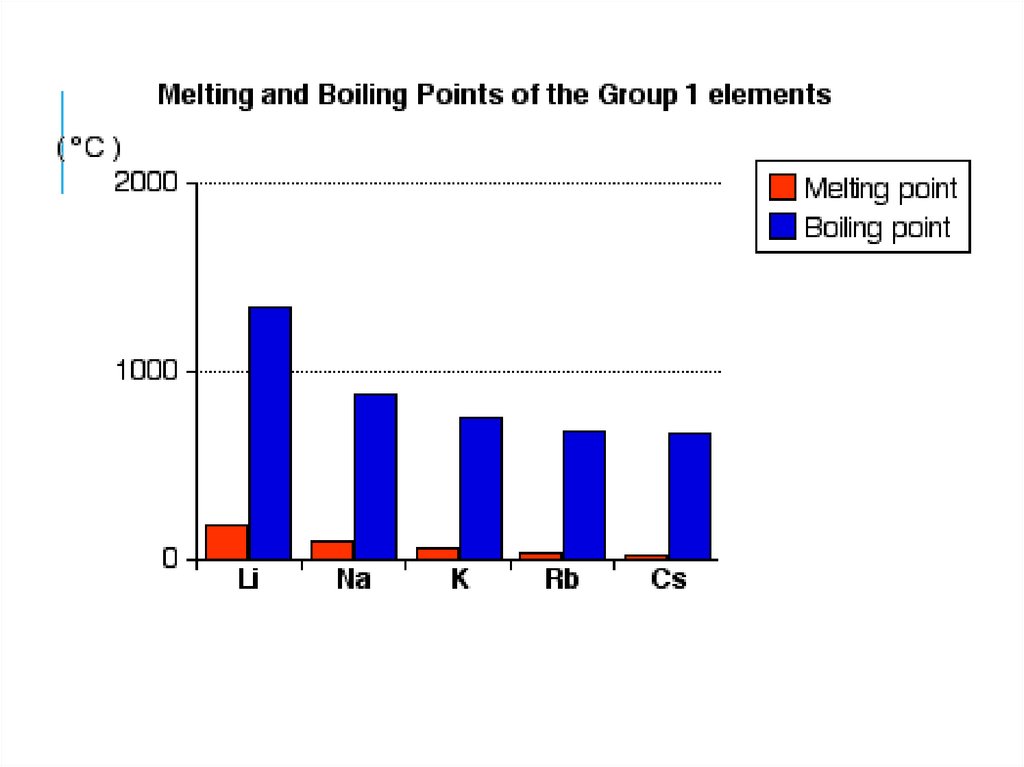
13. How about melting points of metals down the group?
14.
15. 1. No of valence electrons are the same 2. Charge density of the metals decreases down the group due to increasing atomic
radius3. Metallic bond strength decreases down the
group
16.
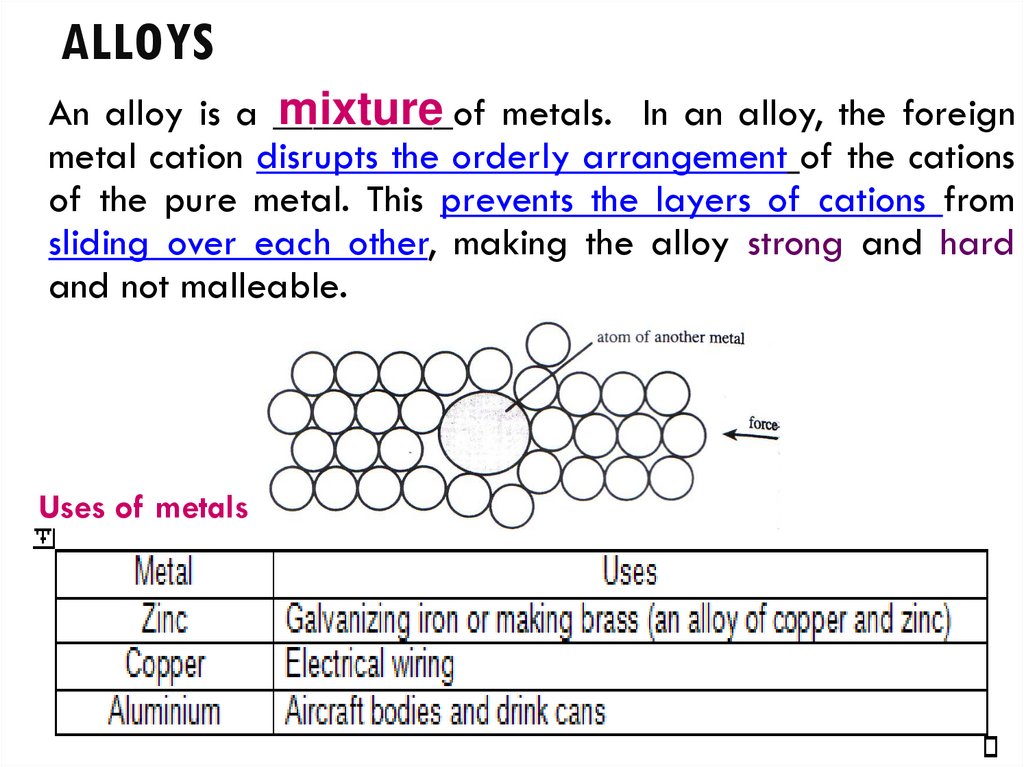
17. ALLOYS
mixture metals. In an alloy, the foreignAn alloy is a _________of
metal cation disrupts the orderly arrangement of the cations
of the pure metal. This prevents the layers of cations from
sliding over each other, making the alloy strong and hard
and not malleable.
Uses of metals
18.
_____ _________
_____
____
____ _____
______
___________
___________
______
______ ______
19.
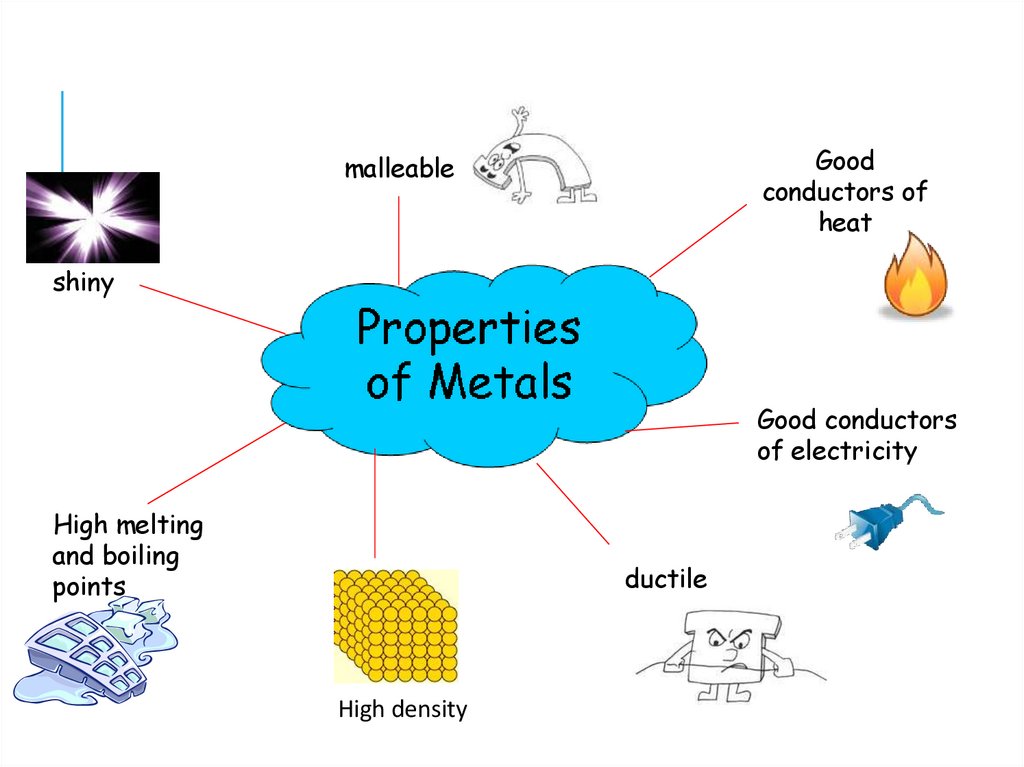
Goodconductors of
heat
malleable
shiny
Good conductors
of electricity
High melting
and boiling
points
ductile
High density
20. Covalent bond
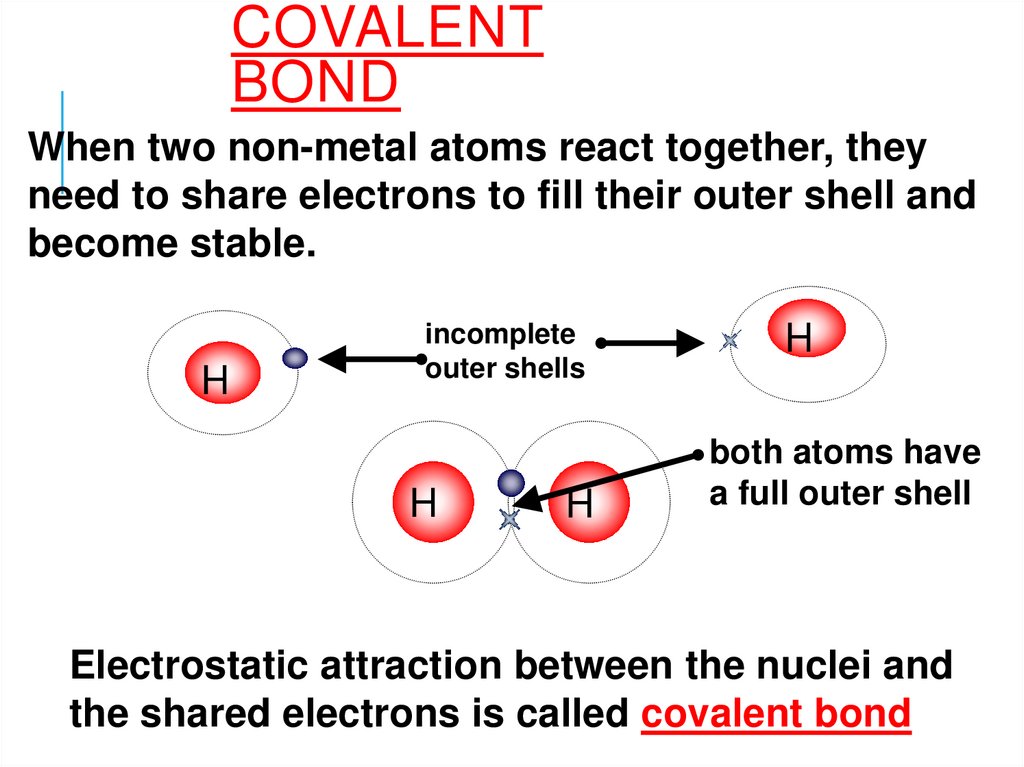
COVALENTBOND
When two non-metal atoms react together, they
need to share electrons to fill their outer shell and
become stable.
H
incomplete
outer shells
H
H
H
both atoms have
a full outer shell
Electrostatic attraction between the nuclei and
the shared electrons is called covalent bond
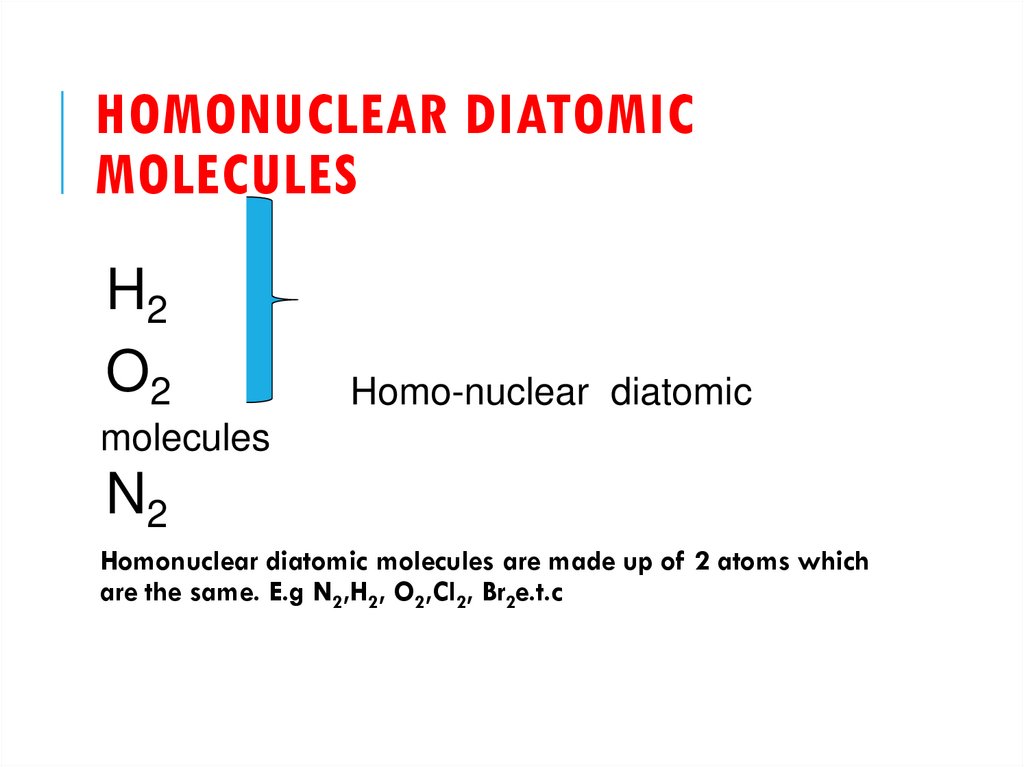
21. HOMONUCLEAR DIATOMIC MOLECULES
H2O2
Homo-nuclear diatomic
molecules
N2
Homonuclear diatomic molecules are made up of 2 atoms which
are the same. E.g N2,H2, O2,Cl2, Br2e.t.c
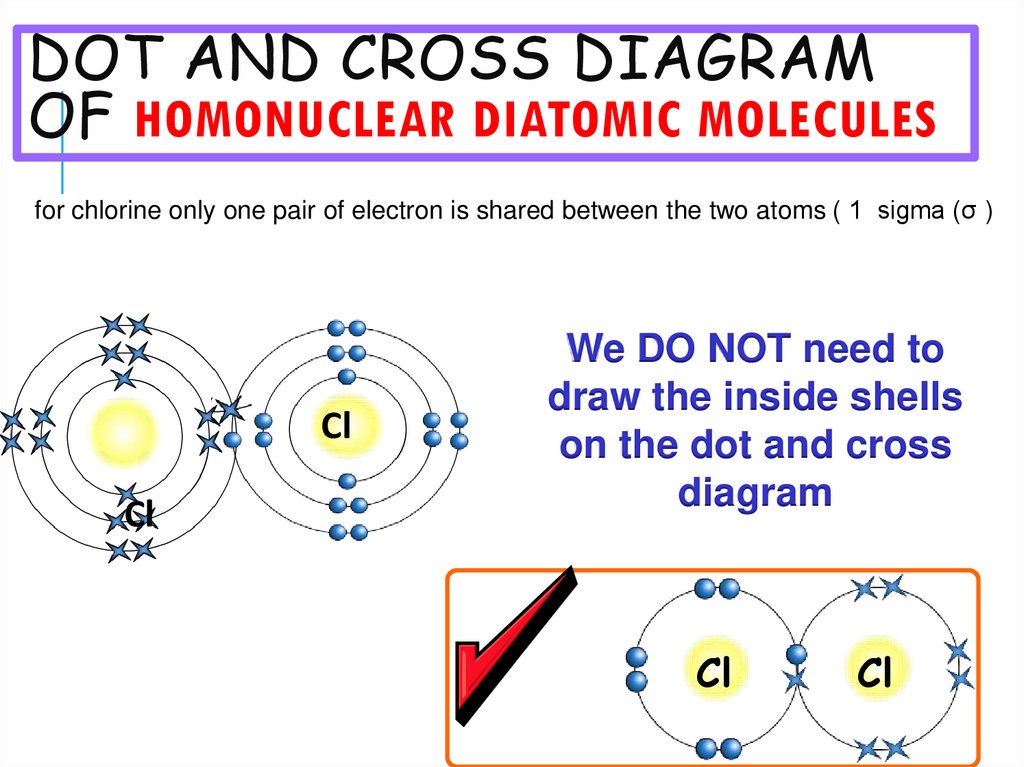
22. Dot and cross diagram of HOMONUCLEAR DIATOMIC MOLECULES
DOT AND CROSS DIAGRAMOF HOMONUCLEAR DIATOMIC MOLECULES
for chlorine only one pair of electron is shared between the two atoms ( 1 sigma (σ )
Cl
Cl
We DO NOT need to
draw the inside shells
on the dot and cross
diagram
Cl
Cl
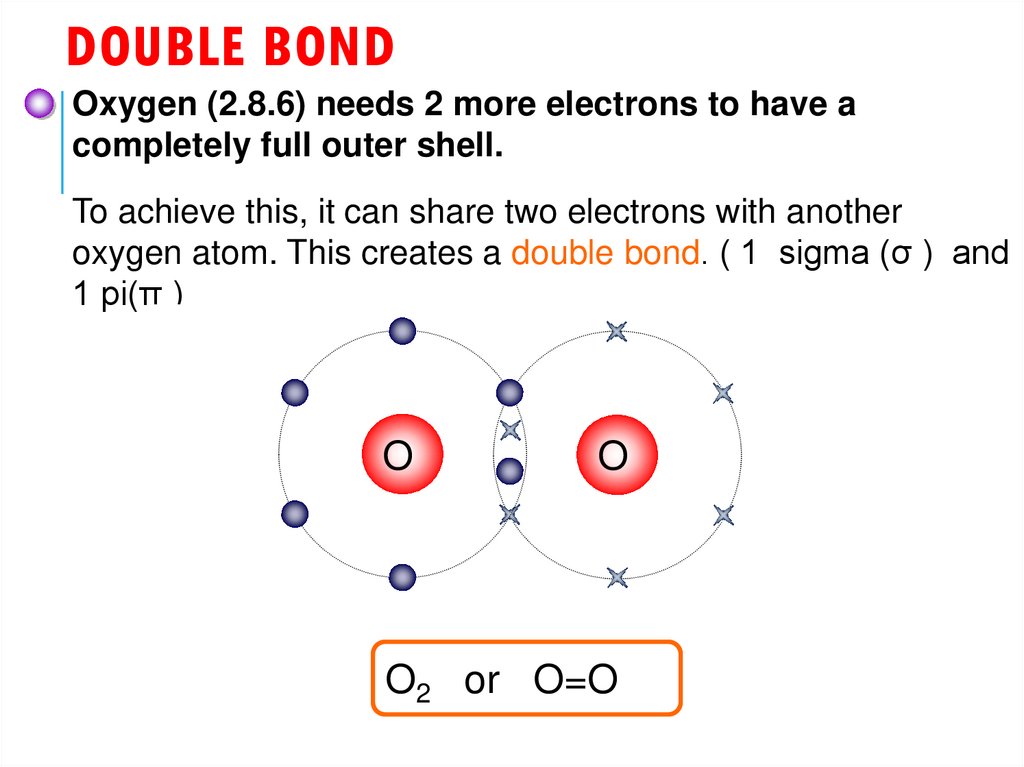
23. Double bond
DOUBLE BONDOxygen (2.8.6) needs 2 more electrons to have a
completely full outer shell.
To achieve this, it can share two electrons with another
oxygen atom. This creates a double bond. ( 1 sigma (σ ) and
1 pi(π )
O
O
O
O2 or O=O
O
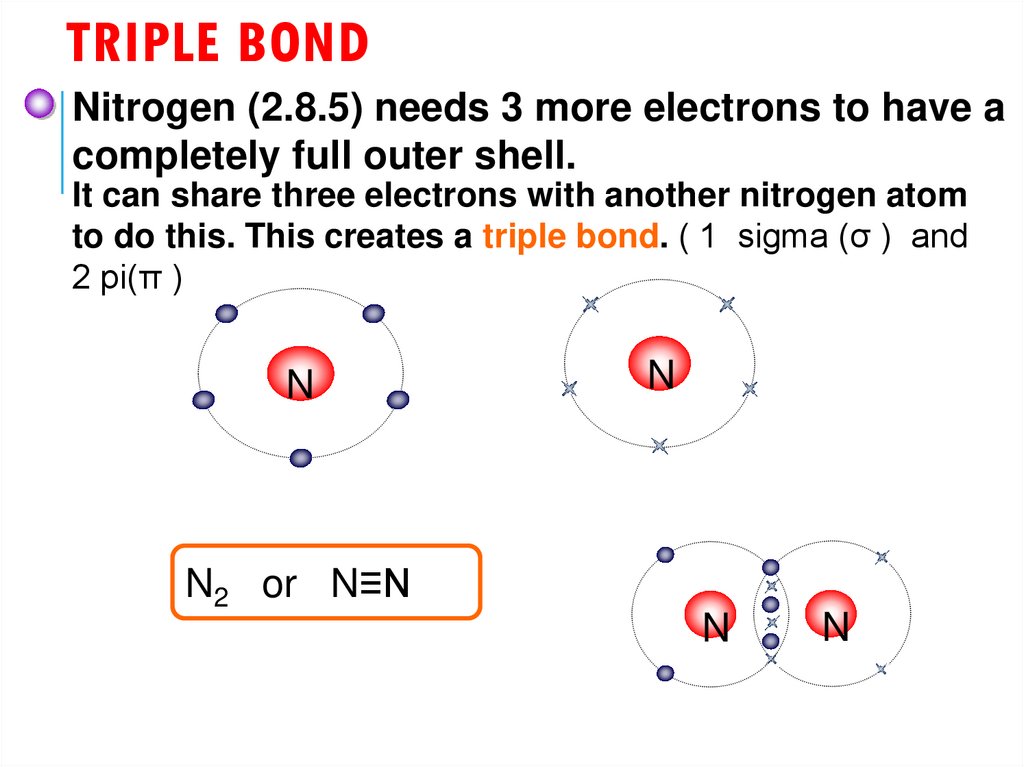
24. Triple bond
TRIPLE BONDNitrogen (2.8.5) needs 3 more electrons to have a
completely full outer shell.
It can share three electrons with another nitrogen atom
to do this. This creates a triple bond. ( 1 sigma (σ ) and
2 pi(π )
N
N
N2 or N≡N
N
N
25. Summary
SUMMARYSingle bond- 1 pair of electrons is shared
Double bond- 2 pairs of electrons are shared
Triple bond-3 pairs of electrons
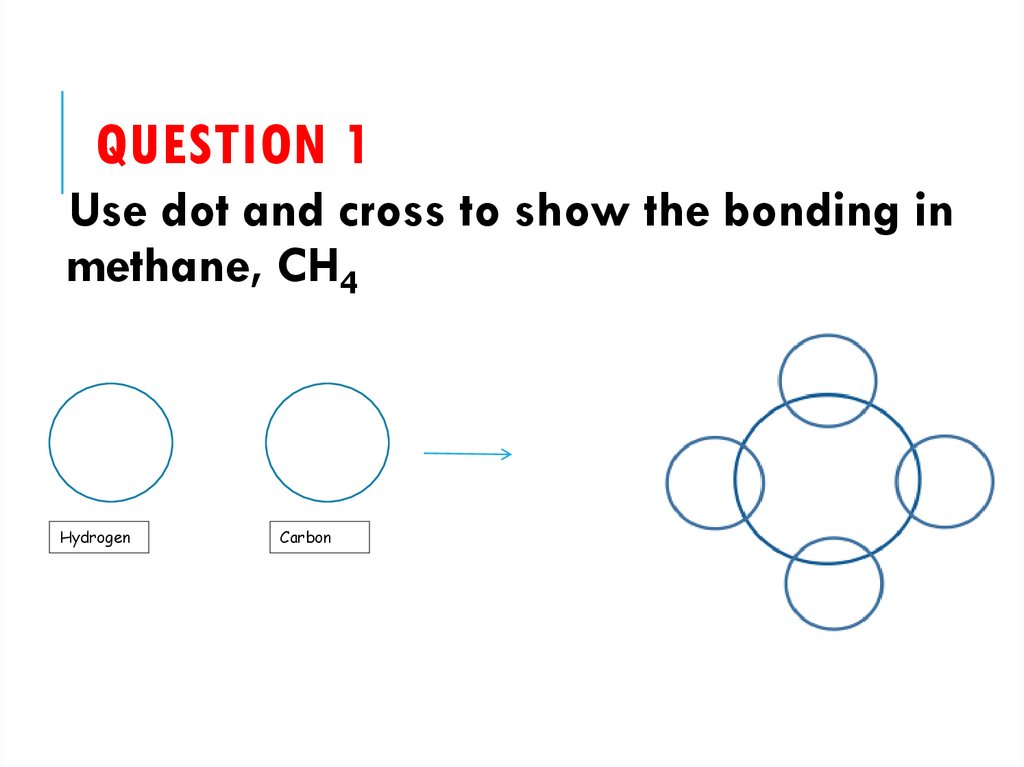
26. Question 1
QUESTION 1Use dot and cross to show the bonding in
methane, CH4
Hydrogen
Carbon
27. Question 2
QUESTION 2Use dot and cross to show the bonding in
ammonia, NH3
Hydrogen
Nitrogen
28. Question 3
QUESTION 3Use dot and cross to show the bonding in
water, H2O.
Hydrogen
Oxygen
29.
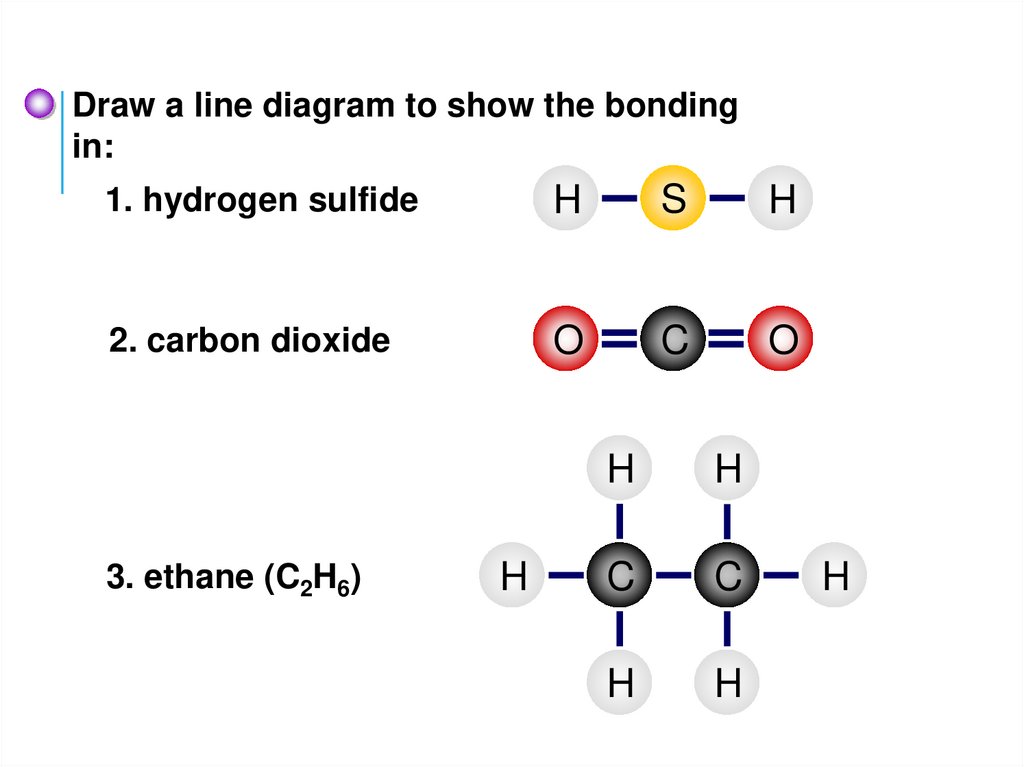
Draw a line diagram to show the bondingin:
1. hydrogen sulfide
H
S
H
2. carbon dioxide
O
C
O
3. ethane (C2H6)
H
H
H
C
C
H
H
H






 chemistry
chemistry








