Similar presentations:
EdExcel Unit C2 – Discovering Chemistry
1. EdExcel Unit C2 – Discovering Chemistry
04/12/2017EdExcel Unit C2 –
Discovering Chemistry
N Smith
St. Aidan’s
2. Topic 1 – Atomic Structure and the Periodic Table
04/12/2017Topic 1 – Atomic Structure and the
Periodic Table
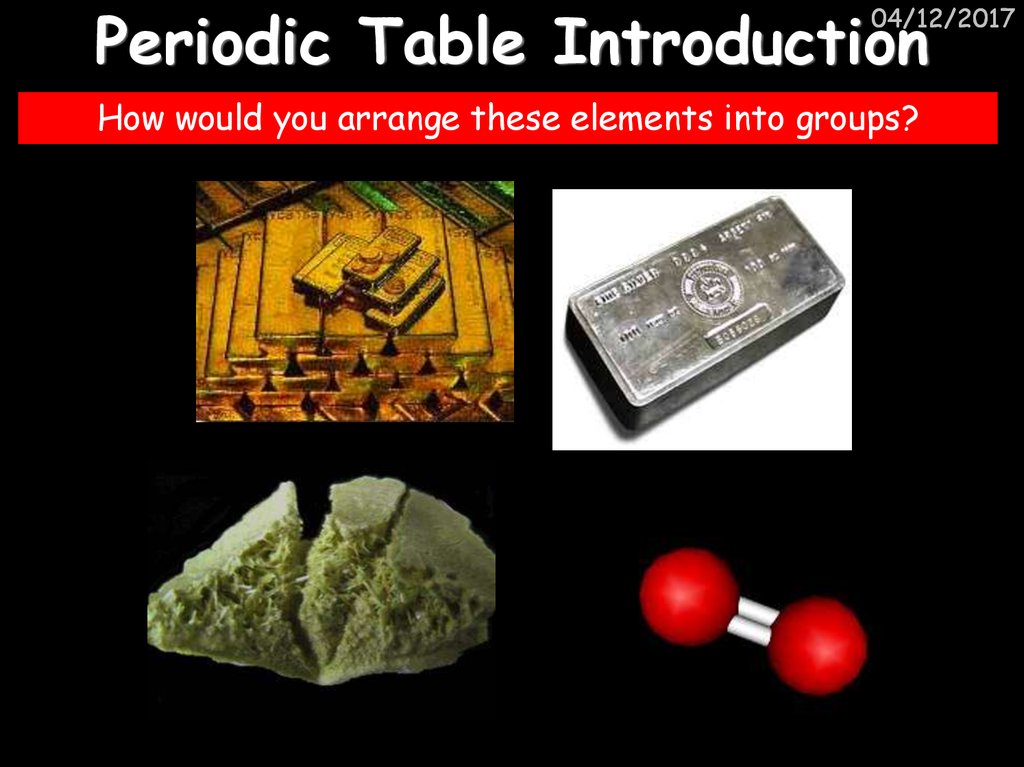
3. Periodic Table Introduction
04/12/2017How would you arrange these elements into groups?
4. Development of the Periodic Table
04/12/20171817: Johann Dobereiner developed the law of “triads” –
he put elements together in groups of 3 according to
their properties.
1864: John Newlands arranged the known
elements in order of atomic mass and found out
that every 8th element had similar properties:
Li
Be
B
C
N
O
F
Na Mg Al
1869: Dimitri Mendeleev arranged the known elements in order
of mass but he also left in gaps and was able to predict the
properties of unknown elements:
Li
Be
B
C
N
O
F
Na Mg Al
1913: Henry Moseley proposed the use of
atomic number rather than atomic mass.
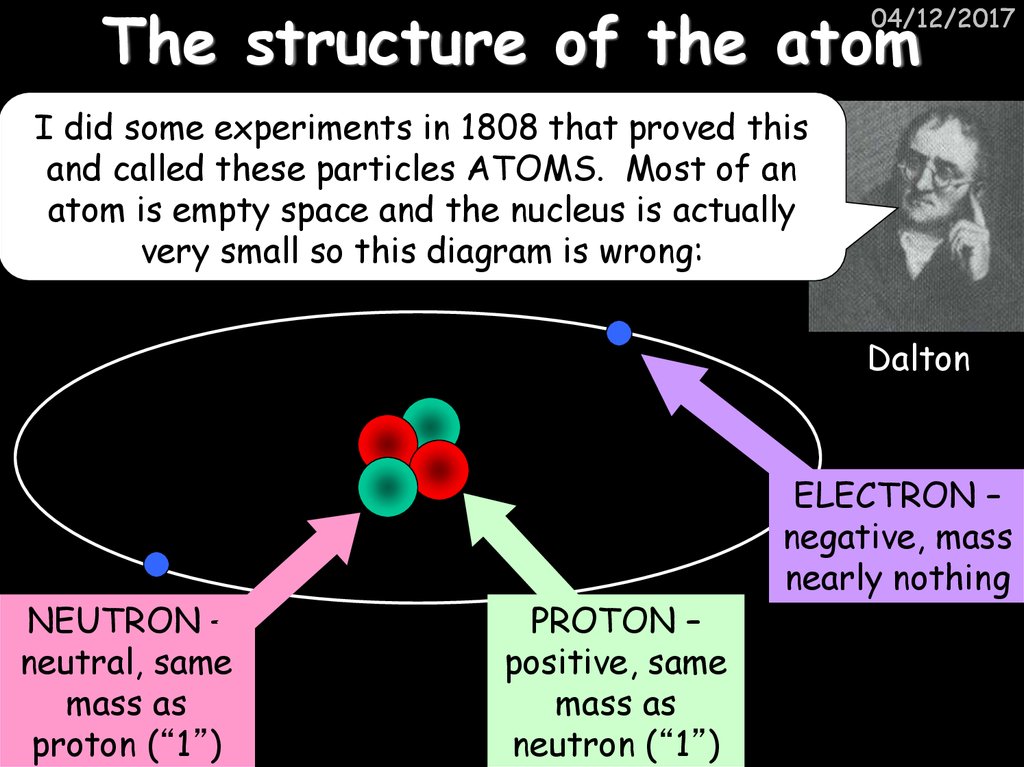
5. The structure of the atom
04/12/2017I did some experiments in 1808 that proved this
and called these particles ATOMS. Most of an
atom is empty space and the nucleus is actually
very small so this diagram is wrong:
Dalton
NEUTRON –
neutral, same
mass as
proton (“1”)
PROTON –
positive, same
mass as
neutron (“1”)
ELECTRON –
negative, mass
nearly nothing
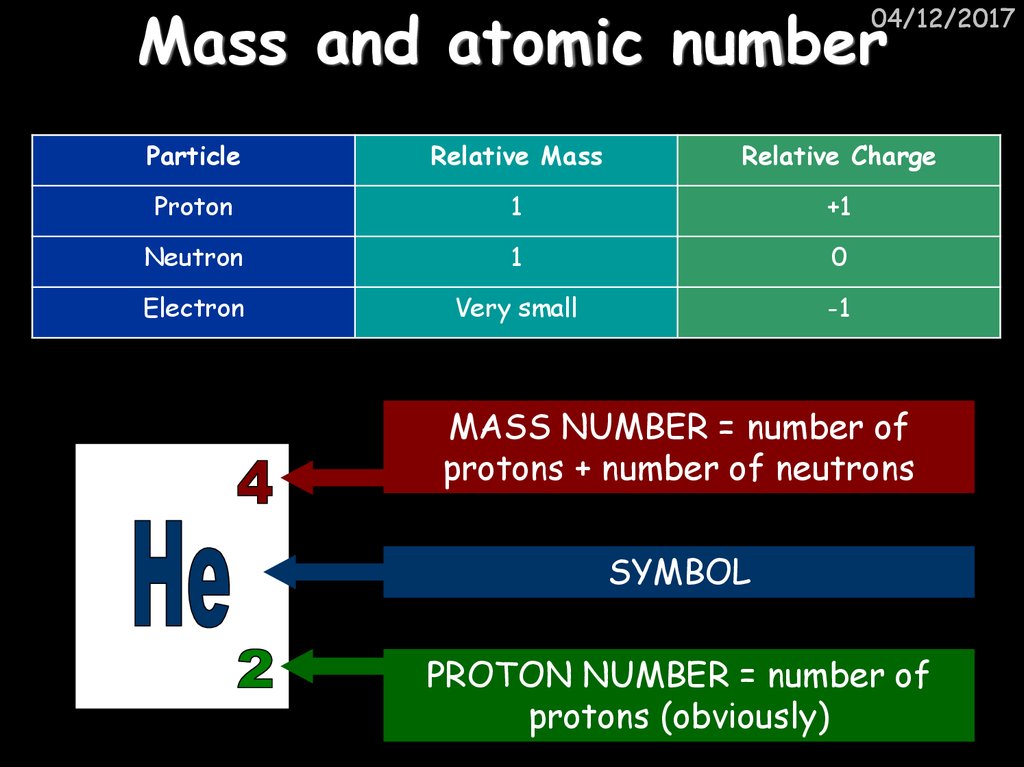
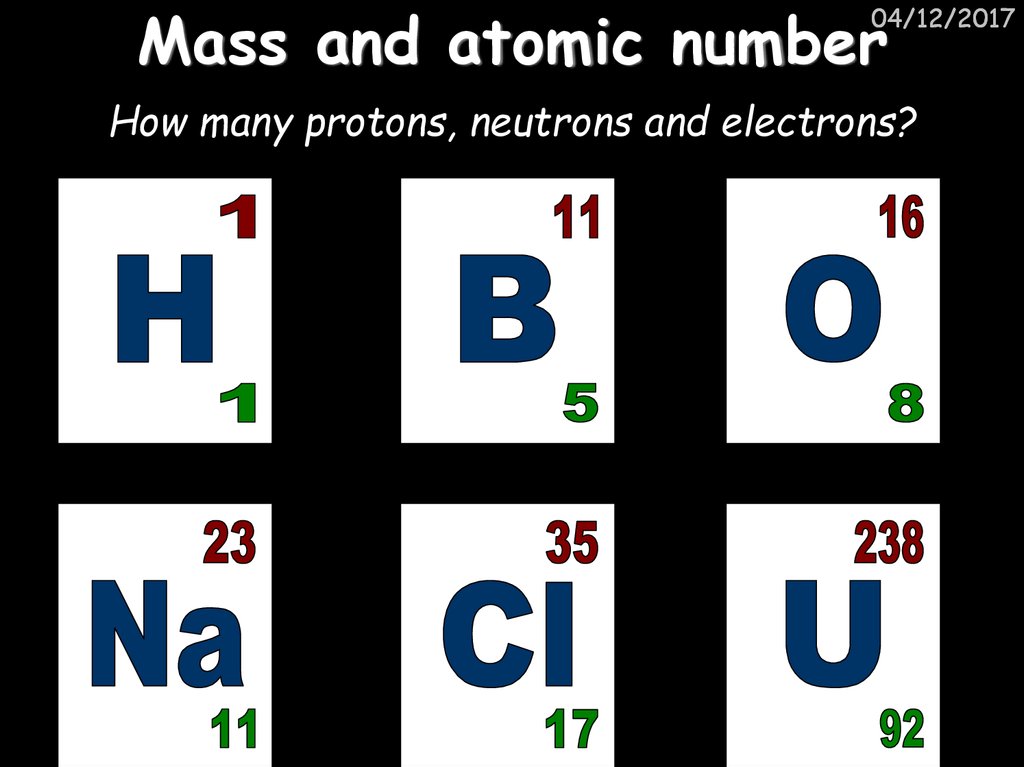
6. Mass and atomic number
04/12/2017Particle
Relative Mass
Relative Charge
Proton
1
+1
Neutron
1
0
Electron
Very small
-1
MASS NUMBER = number of
protons + number of neutrons
SYMBOL
PROTON NUMBER = number of
protons (obviously)
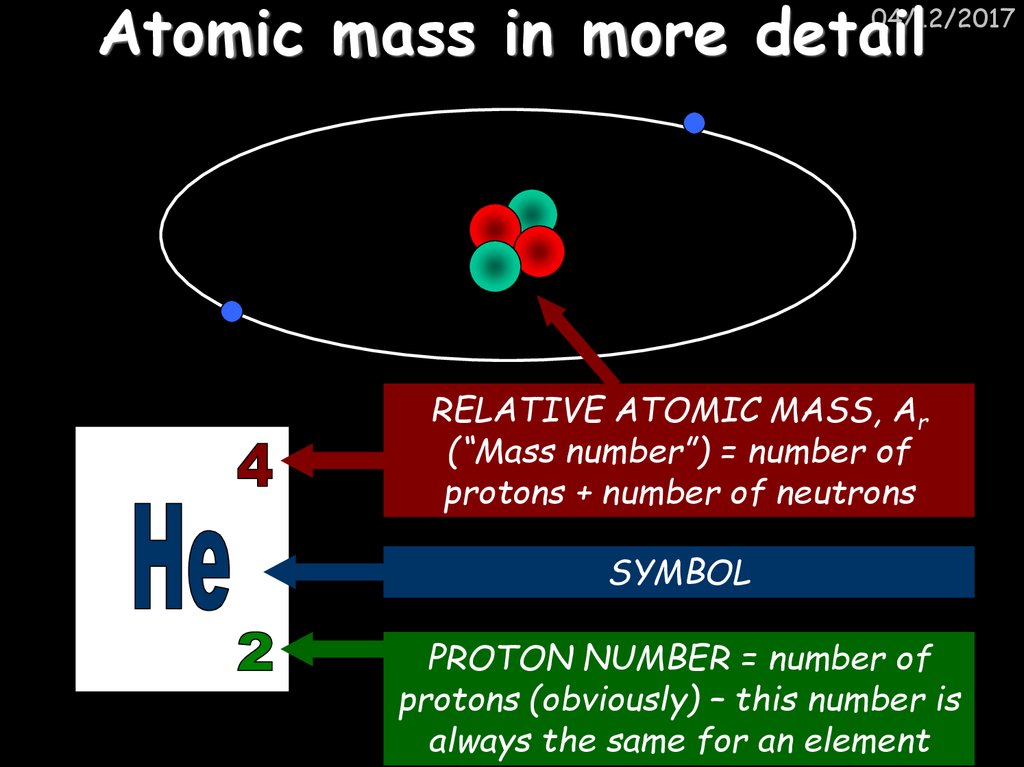
7. Atomic mass in more detail
04/12/2017RELATIVE ATOMIC MASS, Ar
(“Mass number”) = number of
protons + number of neutrons
SYMBOL
PROTON NUMBER = number of
protons (obviously) – this number is
always the same for an element
8. Mass and atomic number
04/12/2017How many protons, neutrons and electrons?
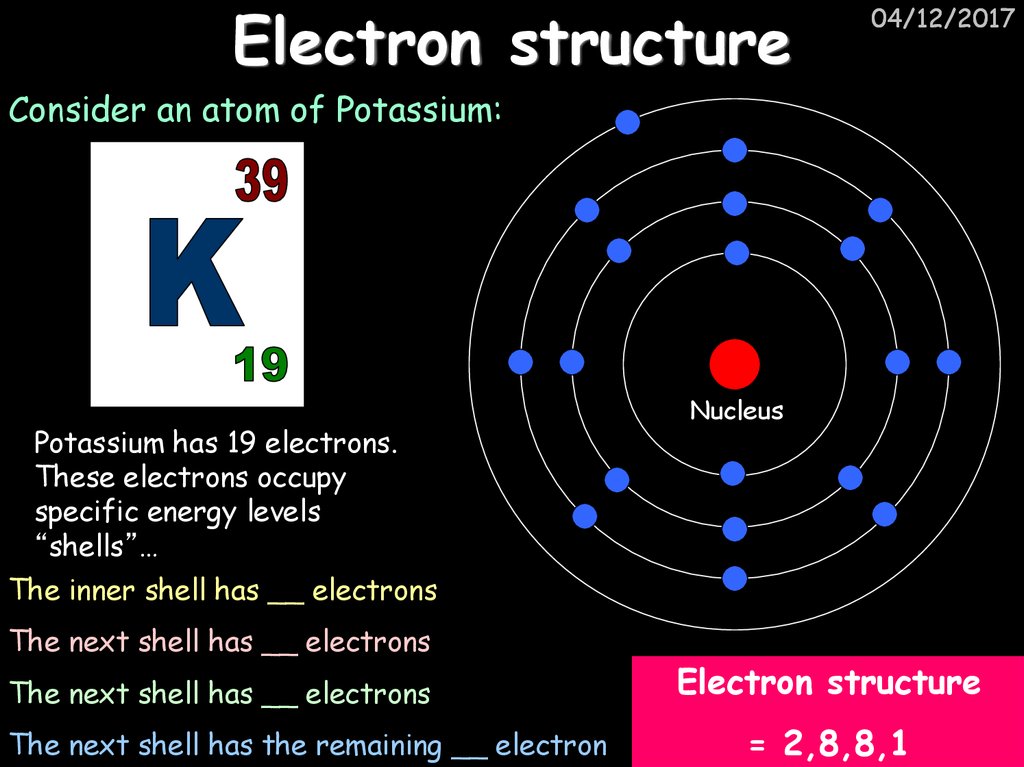
9. Electron structure
04/12/2017Consider an atom of Potassium:
Potassium has 19 electrons.
These electrons occupy
specific energy levels
“shells”…
Nucleus
The inner shell has __ electrons
The next shell has __ electrons
The next shell has __ electrons
The next shell has the remaining __ electron
Electron structure
= 2,8,8,1
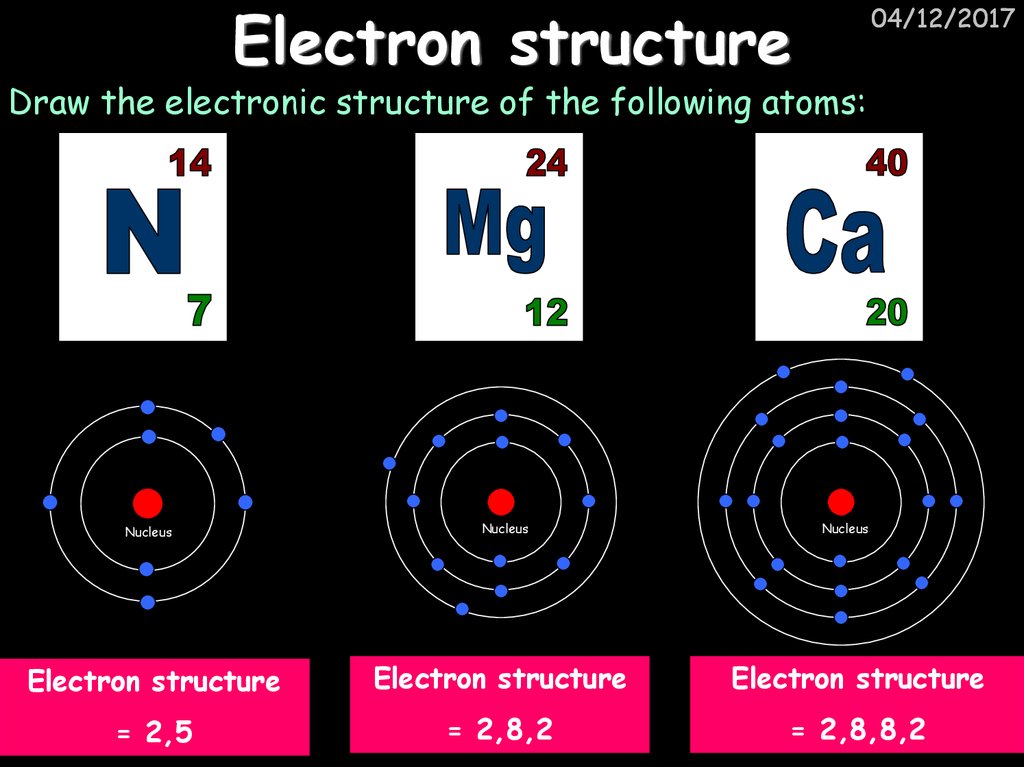
10. Electron structure
04/12/2017Draw the electronic structure of the following atoms:
Nucleus
Nucleus
Nucleus
Electron structure
Electron structure
Electron structure
= 2,5
= 2,8,2
= 2,8,8,2
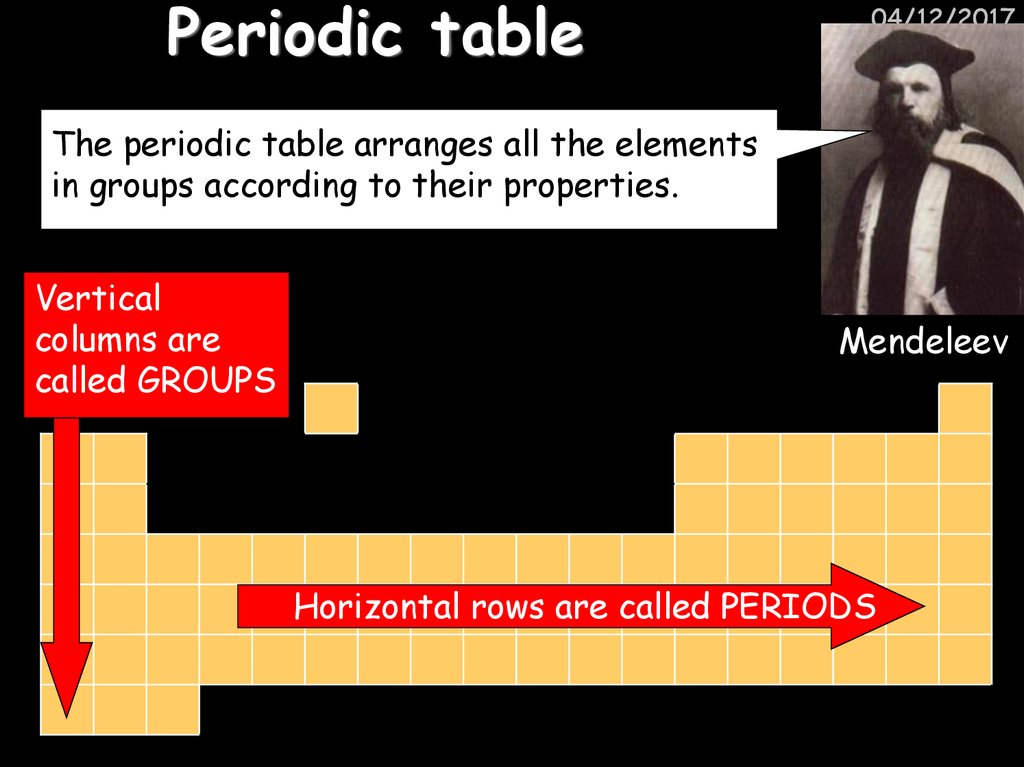
11. Periodic table
04/12/2017The periodic table arranges all the elements
in groups according to their properties.
Vertical
columns are
called GROUPS
Mendeleev
Horizontal rows are called PERIODS
12. The Periodic Table
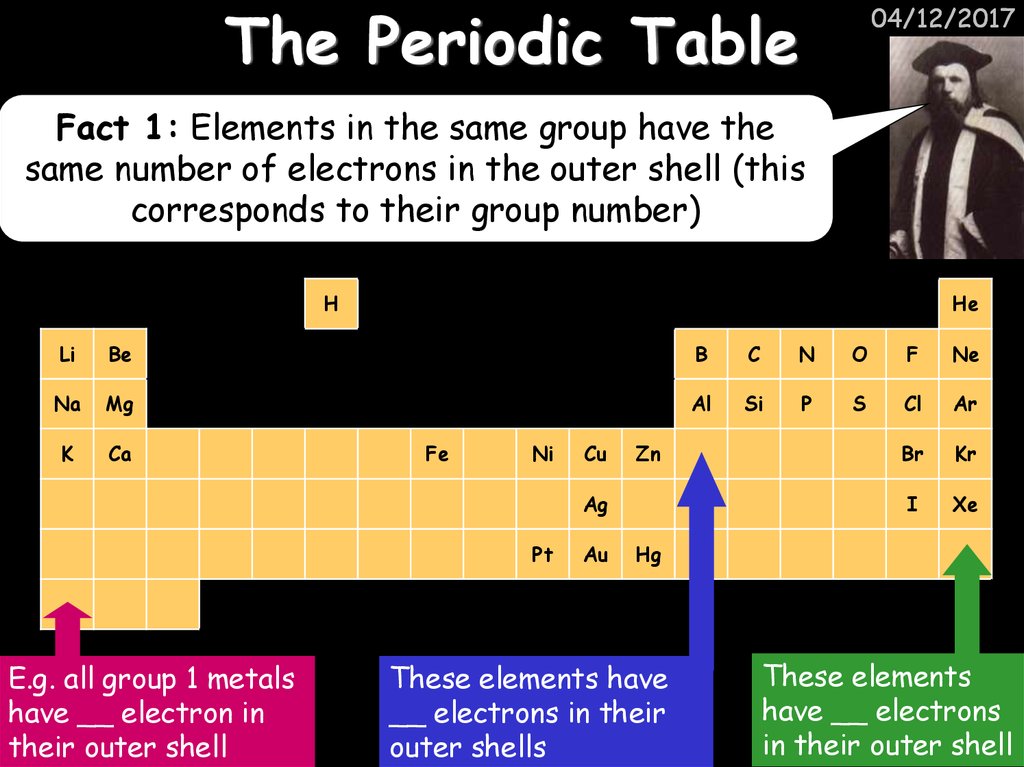
04/12/2017Fact 1: Elements in the same group have the
same number of electrons in the outer shell (this
corresponds to their group number)
H
He
Li
Be
B
C
N
O
F
Ne
Na
Mg
Al
Si
P
S
Cl
Ar
K
Ca
Br
Kr
I
Xe
Fe
Ni
Cu
Zn
Ag
Pt
E.g. all group 1 metals
have __ electron in
their outer shell
Au
Hg
These elements have
__ electrons in their
outer shells
These elements
have __ electrons
in their outer shell
13. The Periodic Table
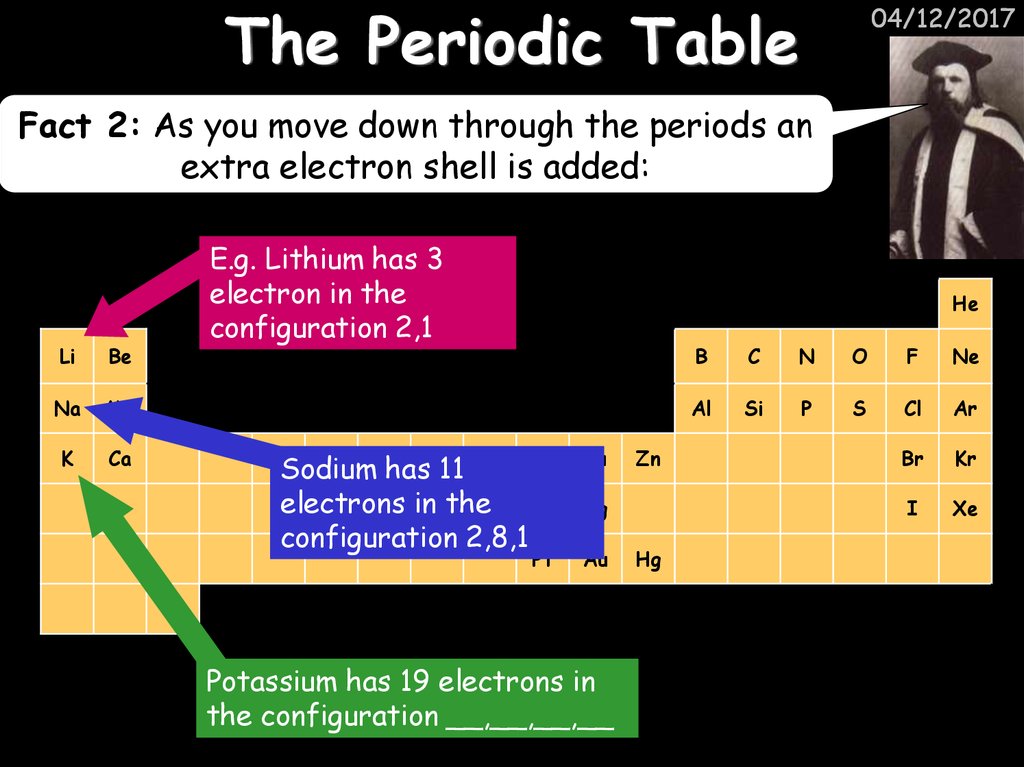
04/12/2017Fact 2: As you move down through the periods an
extra electron shell is added:
Li
Be
Na
Mg
K
Ca
E.g. Lithium has 3
electron Hin the
configuration 2,1
He
Ni
Sodium hasFe11
electrons in the
configuration 2,8,1
Pt
Cu
Zn
Ag
Au
Potassium has 19 electrons in
the configuration __,__,__,__
Hg
B
C
N
O
F
Ne
Al
Si
P
S
Cl
Ar
Br
Kr
I
Xe
14. The Periodic Table
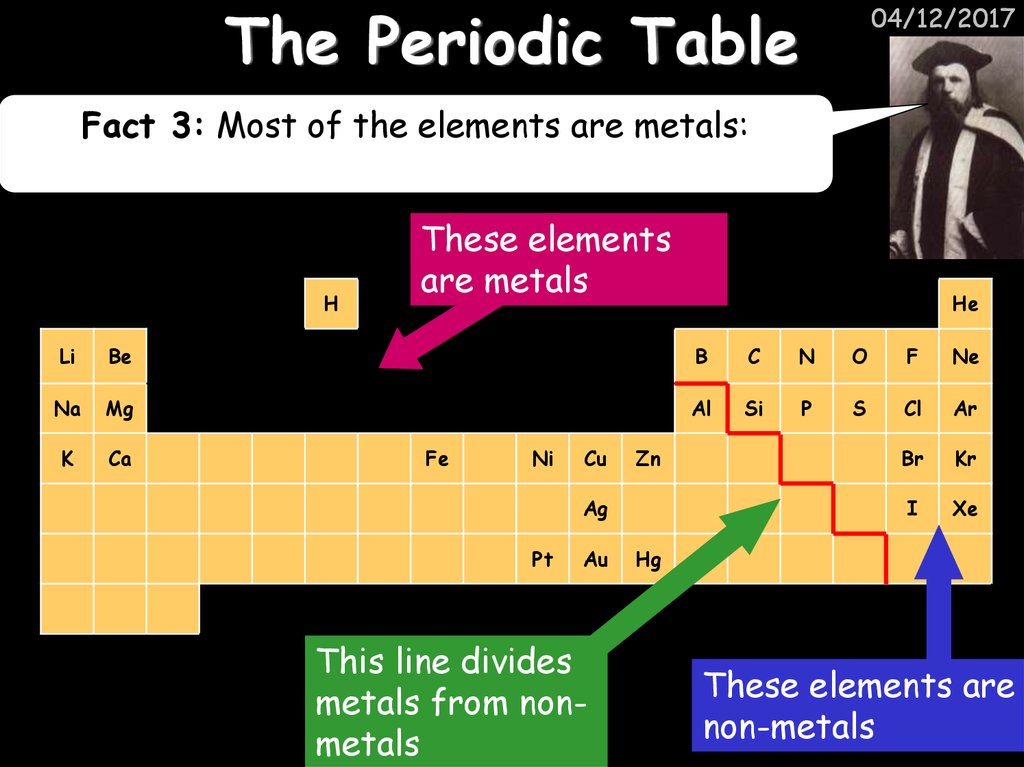
04/12/2017Fact 3: Most of the elements are metals:
H
These elements
are metals
He
Li
Be
B
C
N
O
F
Ne
Na
Mg
Al
Si
P
S
Cl
Ar
K
Ca
Br
Kr
I
Xe
Fe
Ni
Cu
Zn
Ag
Pt
Au
This line divides
metals from nonmetals
Hg
These elements are
non-metals
15. The Periodic Table
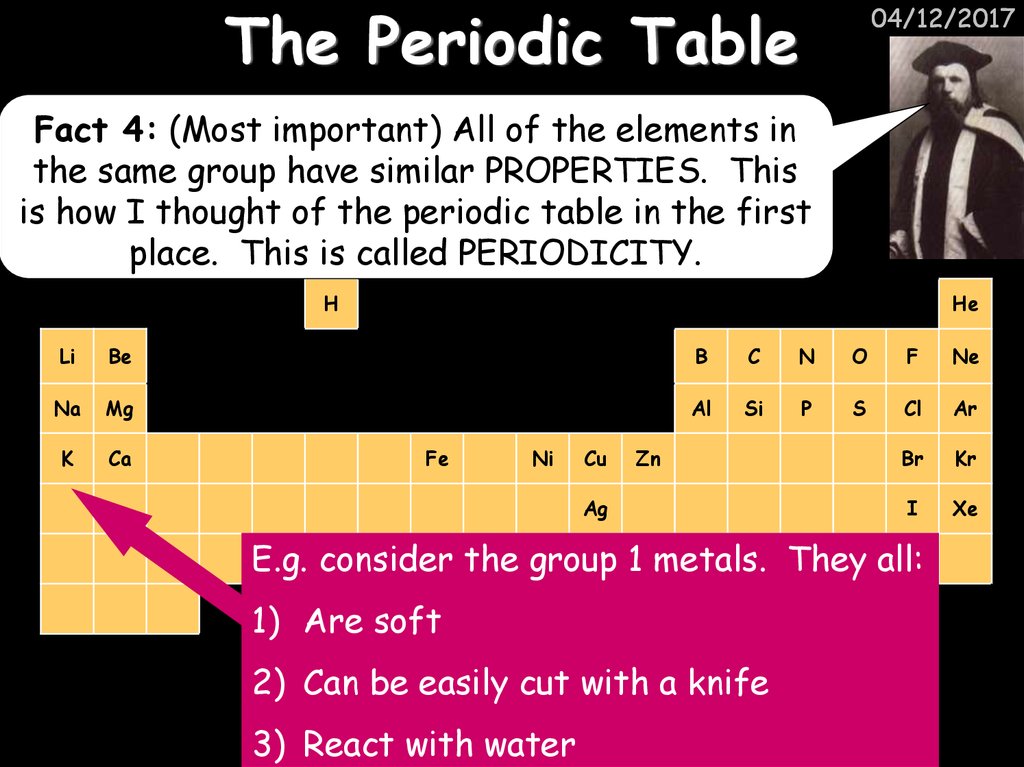
04/12/2017Fact 4: (Most important) All of the elements in
the same group have similar PROPERTIES. This
is how I thought of the periodic table in the first
place. This is called PERIODICITY.
H
He
Li
Be
B
C
N
O
F
Ne
Na
Mg
Al
Si
P
S
Cl
Ar
K
Ca
Br
Kr
I
Xe
Fe
Ni
Cu
Zn
Ag
Pt
Au 1Hgmetals. They all:
E.g. consider the group
1) Are soft
2) Can be easily cut with a knife
3) React with water
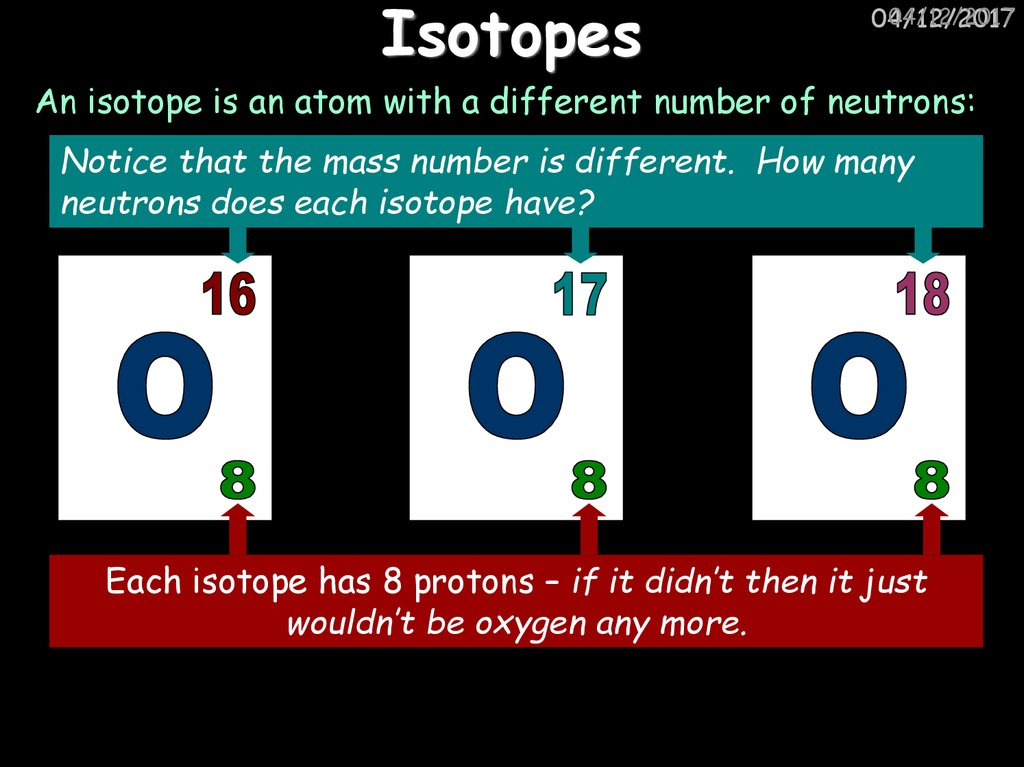
16. Isotopes
04/12/201704/12/2017
An isotope is an atom with a different number of neutrons:
Notice that the mass number is different. How many
neutrons does each isotope have?
Each isotope has 8 protons – if it didn’t then it just
wouldn’t be oxygen any more.
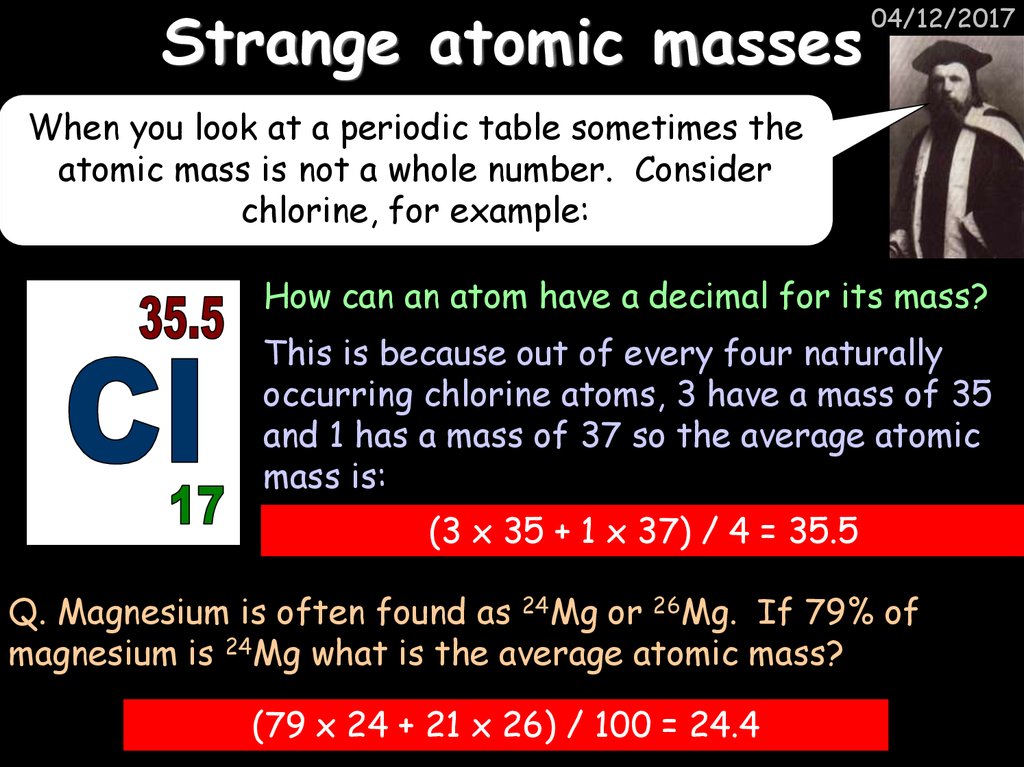
17. Strange atomic masses
04/12/2017When you look at a periodic table sometimes the
atomic mass is not a whole number. Consider
chlorine, for example:
How can an atom have a decimal for its mass?
This is because out of every four naturally
occurring chlorine atoms, 3 have a mass of 35
and 1 has a mass of 37 so the average atomic
mass is:
(3 x 35 + 1 x 37) / 4 = 35.5
Q. Magnesium is often found as 24Mg or 26Mg. If 79% of
magnesium is 24Mg what is the average atomic mass?
(79 x 24 + 21 x 26) / 100 = 24.4
18. Topic 2 – Ionic Compounds and Analysis
04/12/2017Topic 2 – Ionic Compounds and Analysis
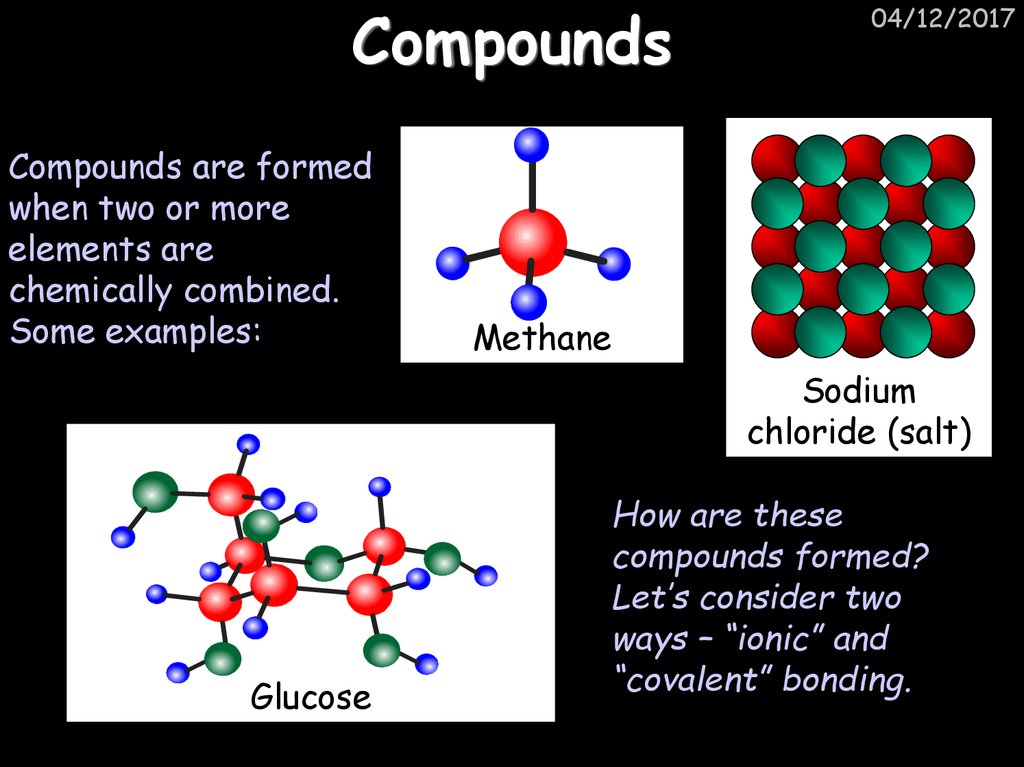
19. Compounds
Compounds are formedwhen two or more
elements are
chemically combined.
Some examples:
04/12/2017
Methane
Sodium
chloride (salt)
Glucose
How are these
compounds formed?
Let’s consider two
ways – “ionic” and
“covalent” bonding.
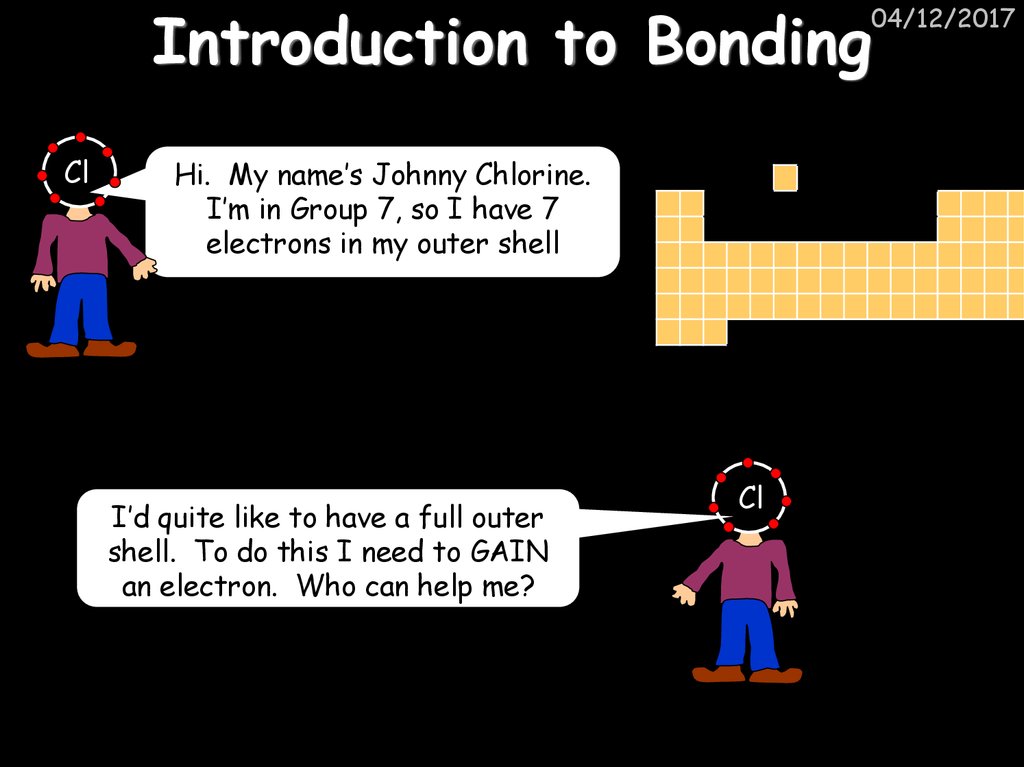
20. Introduction to Bonding
ClHi. My name’s Johnny Chlorine.
I’m in Group 7, so I have 7
electrons in my outer shell
I’d quite like to have a full outer
shell. To do this I need to GAIN
an electron. Who can help me?
Cl
04/12/2017
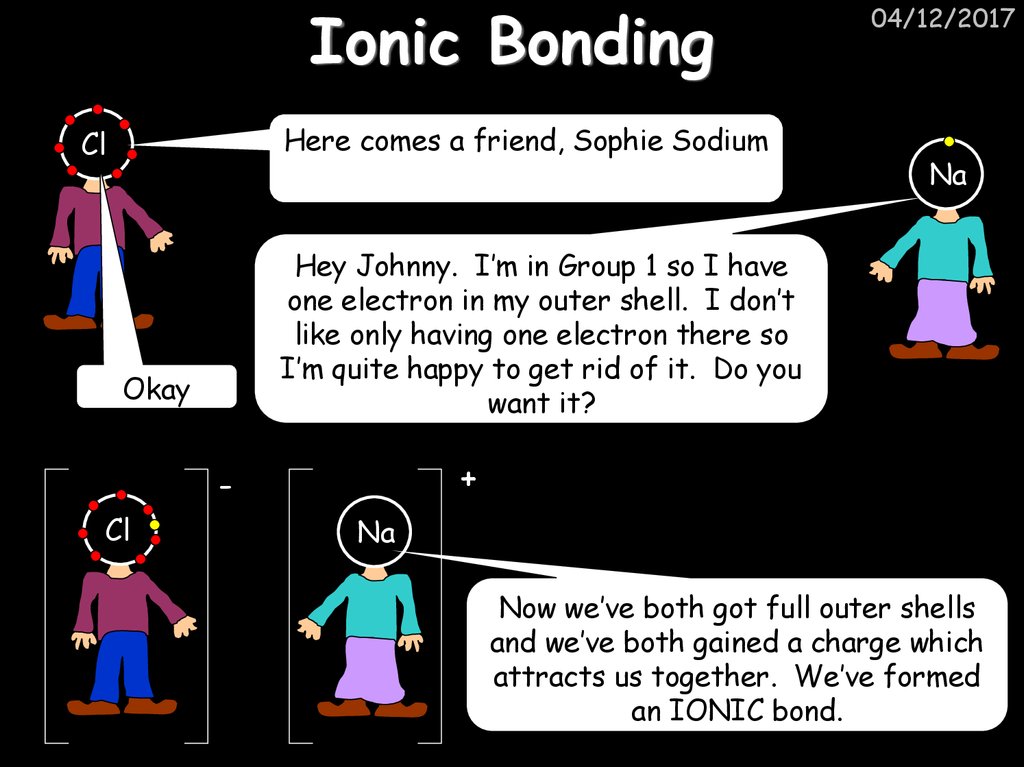
21. Ionic Bonding
Here comes a friend, Sophie SodiumCl
Na
Hey Johnny. I’m in Group 1 so I have
one electron in my outer shell. I don’t
like only having one electron there so
I’m quite happy to get rid of it. Do you
want it?
Okay
+
Cl
04/12/2017
Na
Now we’ve both got full outer shells
and we’ve both gained a charge which
attracts us together. We’ve formed
an IONIC bond.
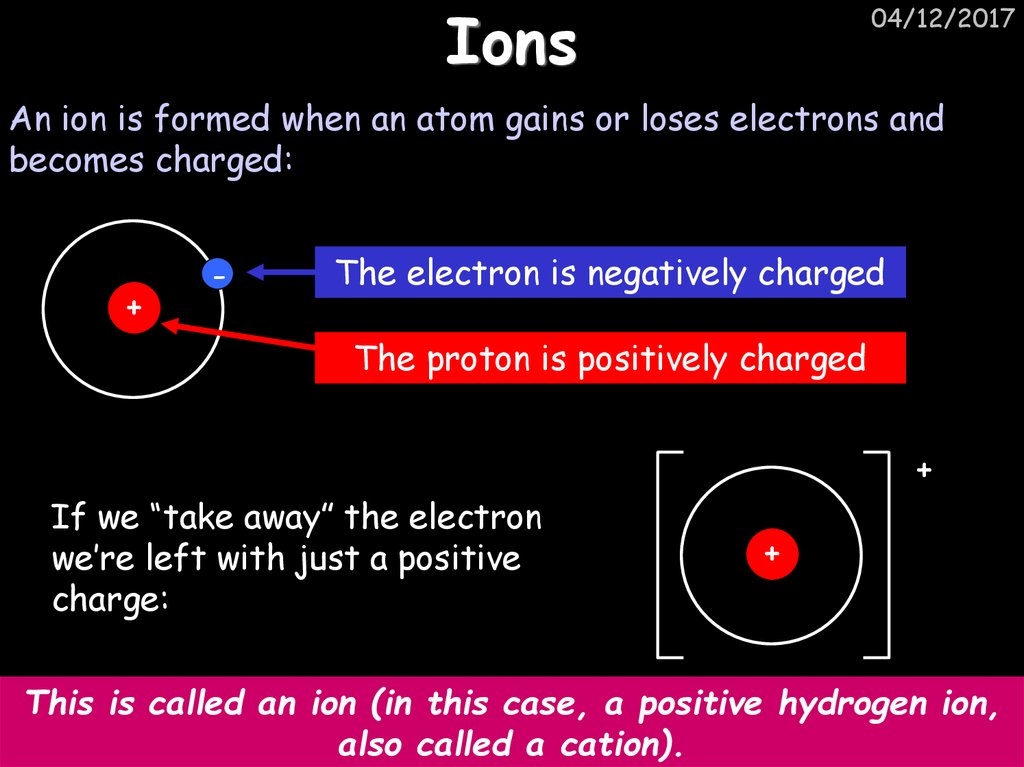
22. Ions
04/12/2017An ion is formed when an atom gains or loses electrons and
becomes charged:
+
-
The electron is negatively charged
The proton is positively charged
If we “take away” the electron
we’re left with just a positive
charge:
+
+
This is called an ion (in this case, a positive hydrogen ion,
also called a cation).
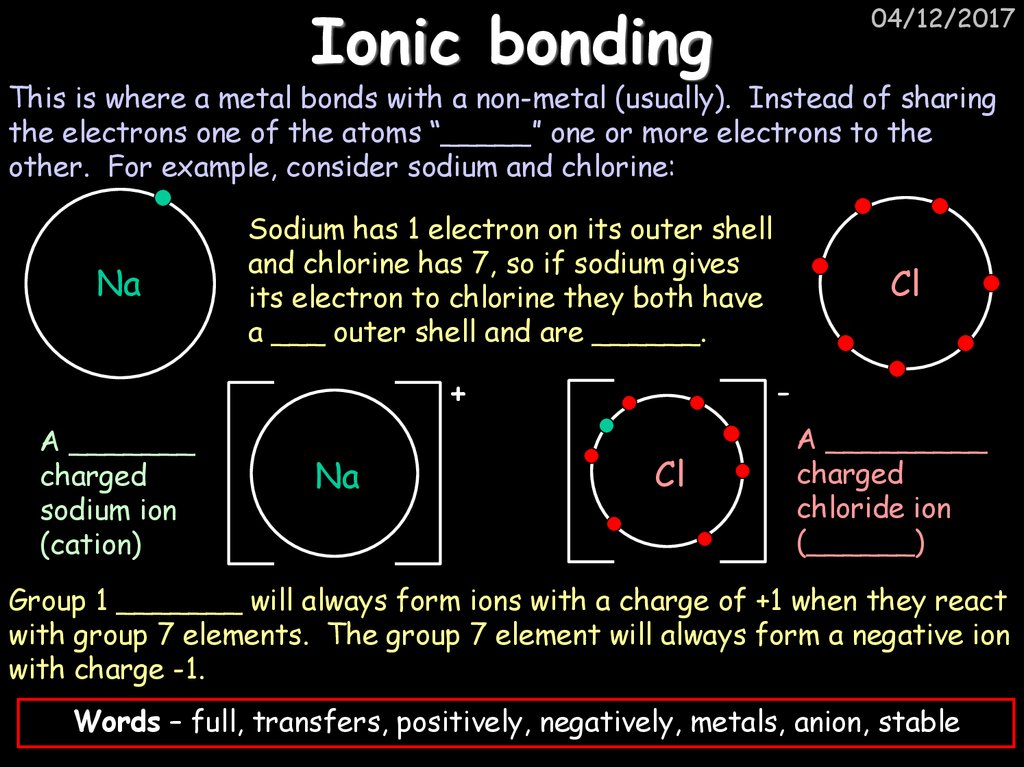
23. Ionic bonding
04/12/2017This is where a metal bonds with a non-metal (usually). Instead of sharing
the electrons one of the atoms “_____” one or more electrons to the
other. For example, consider sodium and chlorine:
Na
Sodium has 1 electron on its outer shell
and chlorine has 7, so if sodium gives
its electron to chlorine they both have
a ___ outer shell and are ______.
-
+
A _______
charged
sodium ion
(cation)
Na
Cl
Cl
A _________
charged
chloride ion
(______)
Group 1 _______ will always form ions with a charge of +1 when they react
with group 7 elements. The group 7 element will always form a negative ion
with charge -1.
Words – full, transfers, positively, negatively, metals, anion, stable
24. Naming compounds
04/12/2017Rule 1 – When two elements join and one is a halogen,
oxygen or sulphur the name ends with ____ide
e.g. Magnesium + oxygen
magnesium oxide
1) Sodium + chlorine
6) KBr
2) Magnesium + fluorine
7) LiCl
3) Lithium + iodine
8) CaO
4) Chlorine + copper
9) MgS
5) Oxygen + iron
10)KF
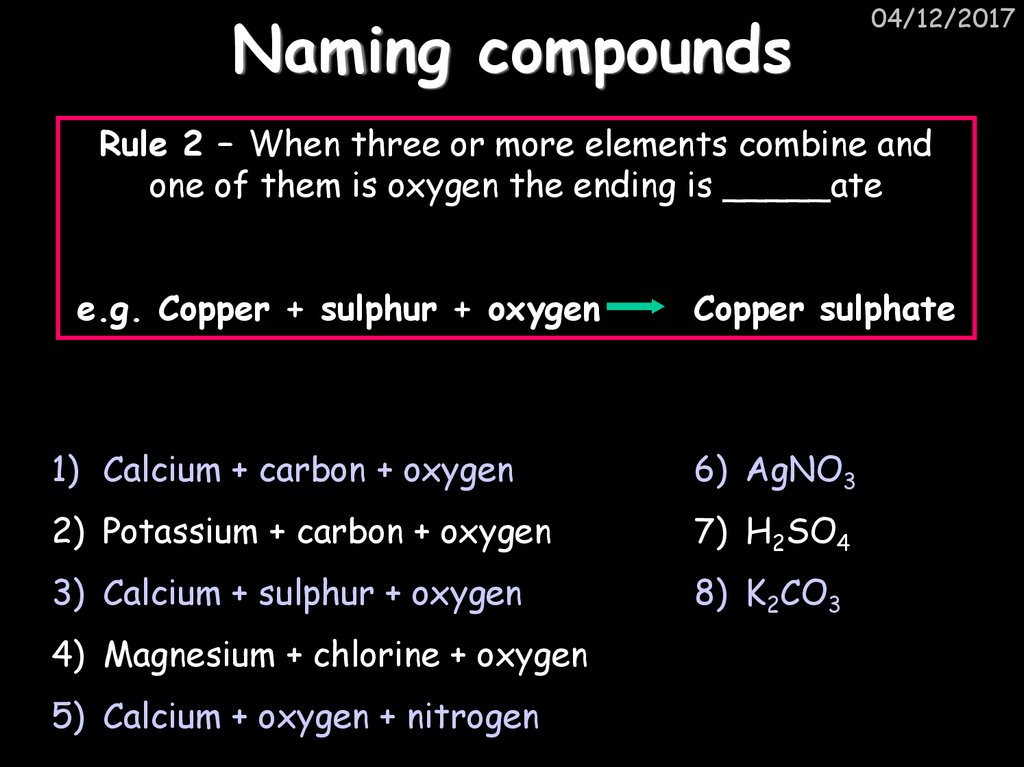
25. Naming compounds
04/12/2017Rule 2 – When three or more elements combine and
one of them is oxygen the ending is _____ate
e.g. Copper + sulphur + oxygen
Copper sulphate
1) Calcium + carbon + oxygen
6) AgNO3
2) Potassium + carbon + oxygen
7) H2SO4
3) Calcium + sulphur + oxygen
8) K2CO3
4) Magnesium + chlorine + oxygen
5) Calcium + oxygen + nitrogen
26. The Periodic Table
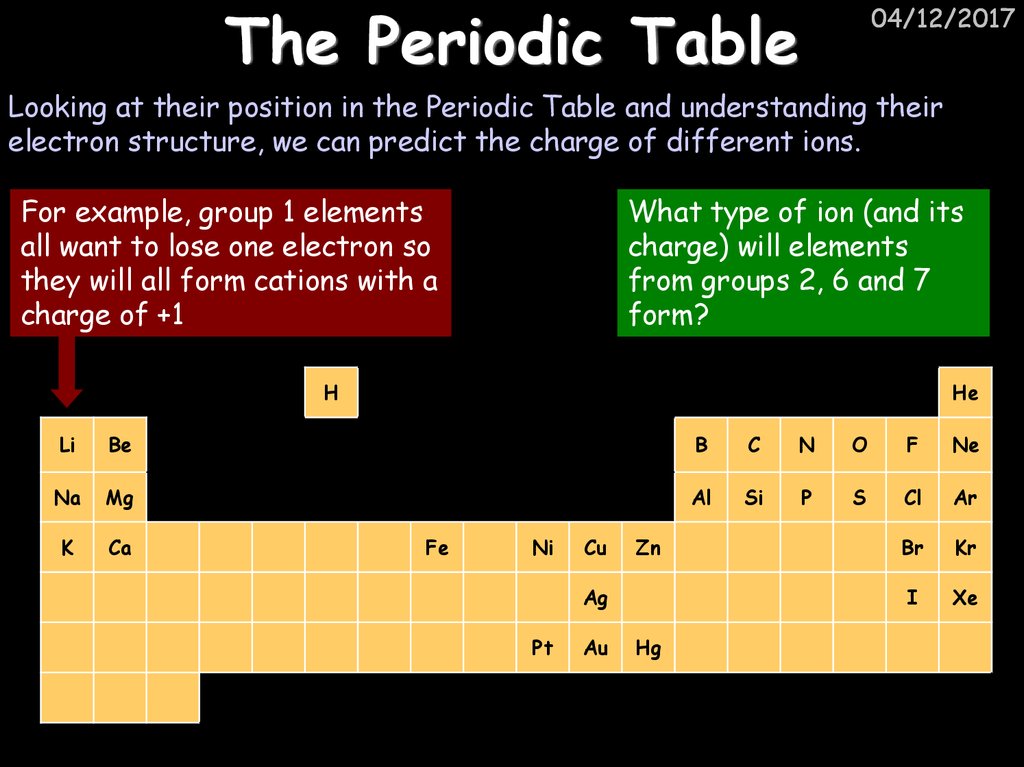
04/12/2017Looking at their position in the Periodic Table and understanding their
electron structure, we can predict the charge of different ions.
For example, group 1 elements
all want to lose one electron so
they will all form cations with a
charge of +1
What type of ion (and its
charge) will elements
from groups 2, 6 and 7
form?
H
He
Li
Be
B
C
N
O
F
Ne
Na
Mg
Al
Si
P
S
Cl
Ar
K
Ca
Br
Kr
I
Xe
Fe
Ni
Cu
Zn
Ag
Pt
Au
Hg
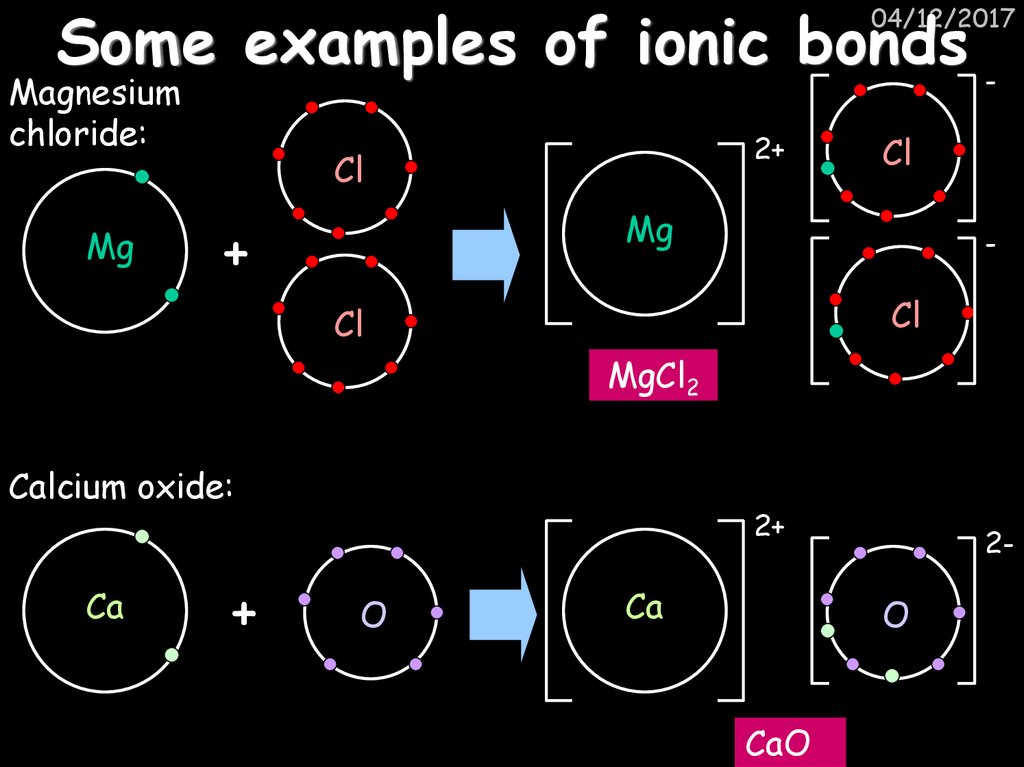
27. Some examples of ionic bonds
-04/12/2017
Magnesium
chloride:
Mg
2+
Cl
Cl
Mg
+
-
Cl
Cl
MgCl2
Calcium oxide:
Ca
+
2+
O
Ca
2-
O
CaO
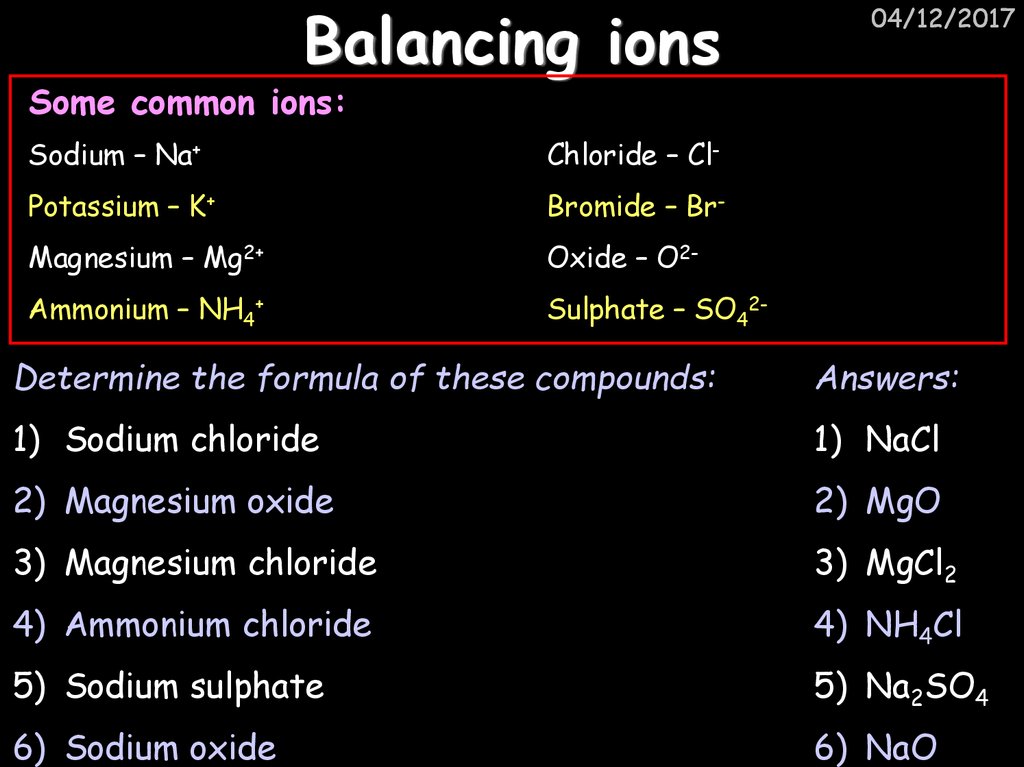
28. Balancing ions
04/12/2017Some common ions:
Sodium – Na+
Chloride – Cl-
Potassium – K+
Bromide – Br-
Magnesium – Mg2+
Oxide – O2-
Ammonium – NH4+
Sulphate – SO42-
Determine the formula of these compounds:
Answers:
1) Sodium chloride
1) NaCl
2) Magnesium oxide
2) MgO
3) Magnesium chloride
3) MgCl2
4) Ammonium chloride
4) NH4Cl
5) Sodium sulphate
5) Na2SO4
6) Sodium oxide
6) NaO
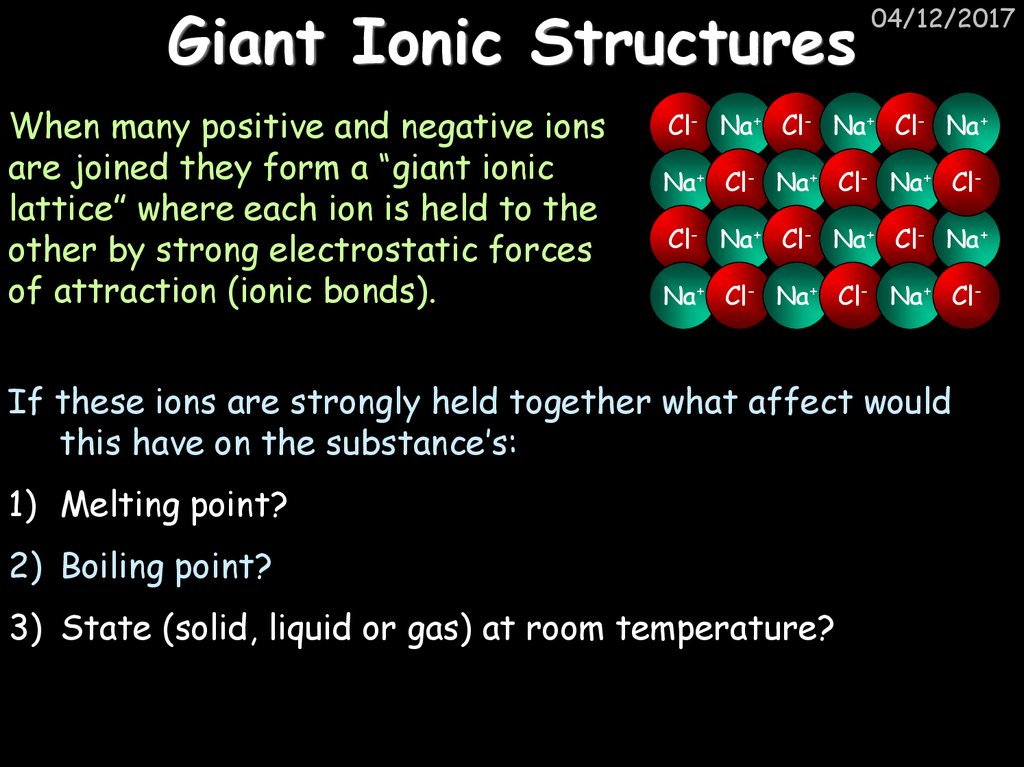
29. Giant Ionic Structures
When many positive and negative ionsare joined they form a “giant ionic
lattice” where each ion is held to the
other by strong electrostatic forces
of attraction (ionic bonds).
04/12/2017
Cl- Na+ Cl- Na+ Cl- Na+
Na+ Cl- Na+ Cl- Na+ ClCl- Na+ Cl- Na+ Cl- Na+
Na+ Cl- Na+ Cl- Na+ Cl-
If these ions are strongly held together what affect would
this have on the substance’s:
1) Melting point?
2) Boiling point?
3) State (solid, liquid or gas) at room temperature?
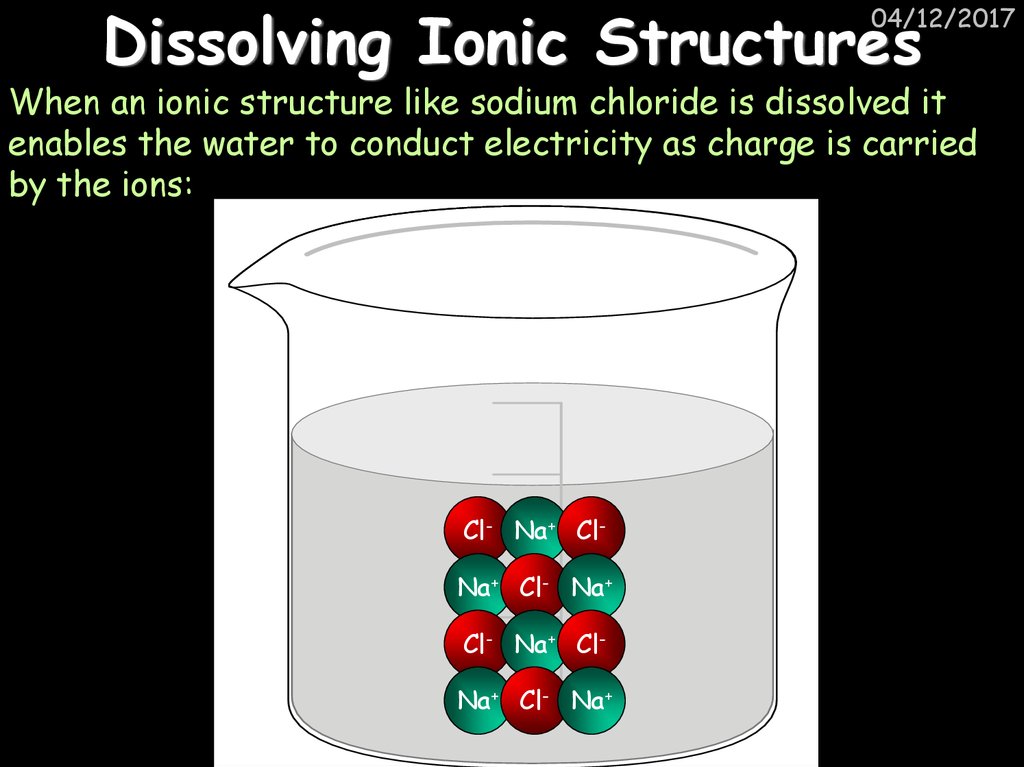
30. Dissolving Ionic Structures
04/12/2017When an ionic structure like sodium chloride is dissolved it
enables the water to conduct electricity as charge is carried
by the ions:
Cl- Na+ ClNa+ Cl- Na+
Cl- Na+ ClNa+ Cl- Na+
31. Solubility rules
04/12/2017The following guidelines are useful in working out if a
substance will dissolve:
• All common sodium, potassium and ammonium salts are soluble
• All nitrates are soluble
• Common chlorides are soluble but not silver and lead
• Common sulfates are soluble but not those of lead, barium
and calcium
• Common carbonates and hydroxides are insoluble except
those of sodium, potassium and ammonium
32. Precipitation Reactions
04/12/2017A precipitation reaction occurs when an insoluble solid is made
by mixing two ionic solutions together.
Method:
1) Mix the reactants together
2) Filter off the precipitate
3) Wash the residue
4) Dry the residue in an oven at 50OC
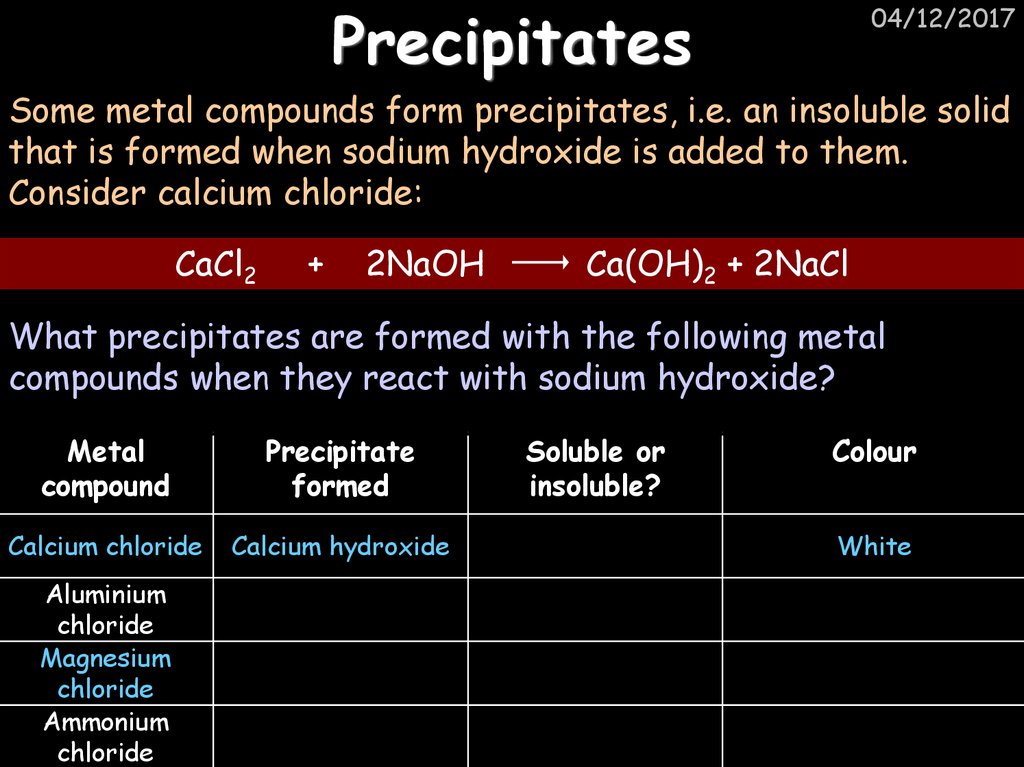
33. Precipitates
04/12/2017Some metal compounds form precipitates, i.e. an insoluble solid
that is formed when sodium hydroxide is added to them.
Consider calcium chloride:
CaCl2
+
2NaOH
Ca(OH)2 + 2NaCl
What precipitates are formed with the following metal
compounds when they react with sodium hydroxide?
Metal
compound
Precipitate
formed
Calcium chloride
Calcium hydroxide
Aluminium
chloride
Magnesium
chloride
Ammonium
chloride
Soluble or
insoluble?
Colour
White
34. Barium Sulfate
Barium sulfate can be used aspart of a “barium meal” to X-ray
patients. Why?
1) Barium sulfate is opaque
to X rays so they will
show up in an X ray
2) It’s insoluble so it won’t
pass into the bloodstream
04/12/2017
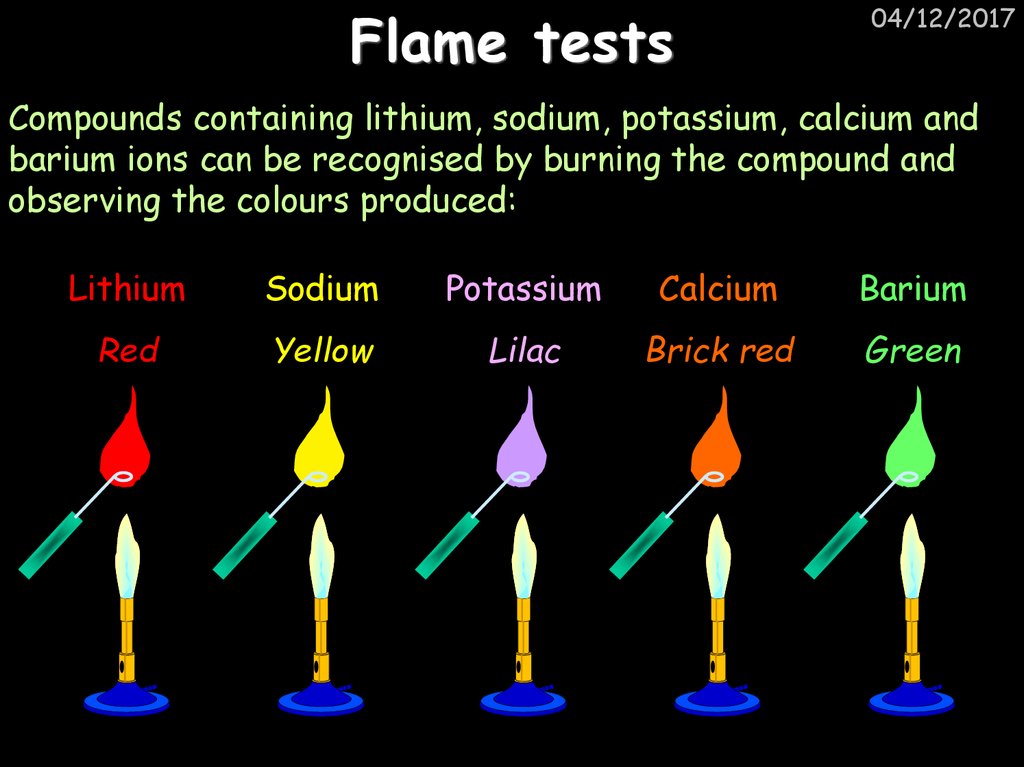
35. Flame tests
04/12/2017Compounds containing lithium, sodium, potassium, calcium and
barium ions can be recognised by burning the compound and
observing the colours produced:
Lithium
Sodium
Potassium
Calcium
Barium
Red
Yellow
Lilac
Brick red
Green
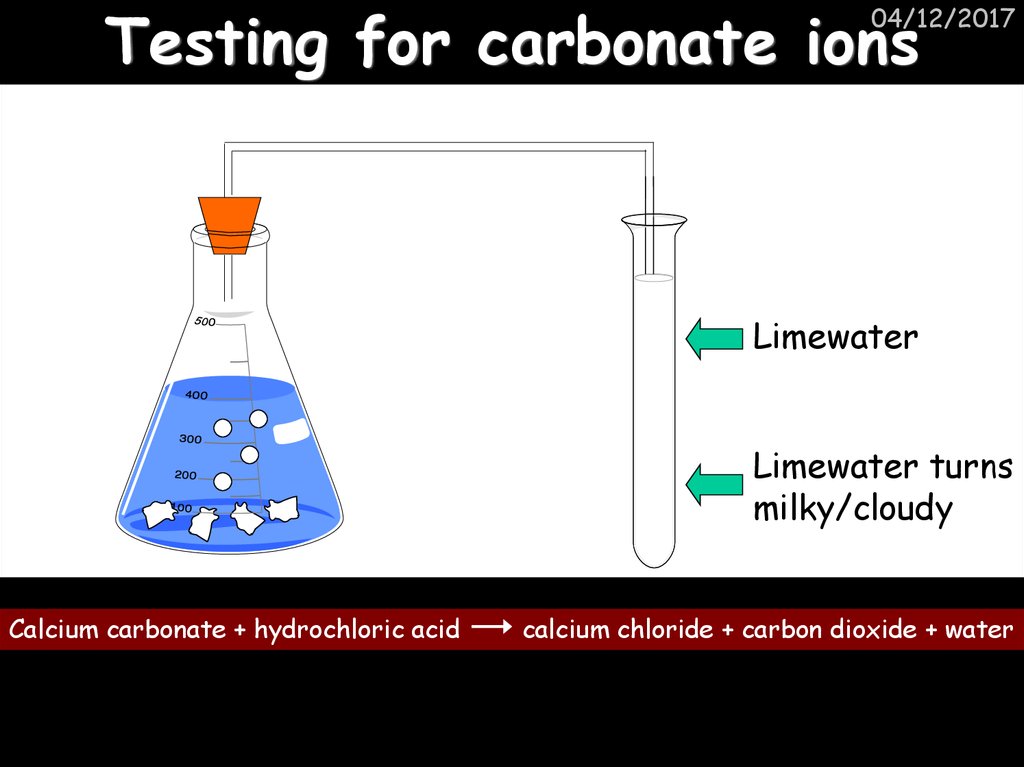
36. Testing for carbonate ions
04/12/2017Limewater
Limewater turns
milky/cloudy
Calcium carbonate + hydrochloric acid
calcium chloride + carbon dioxide + water
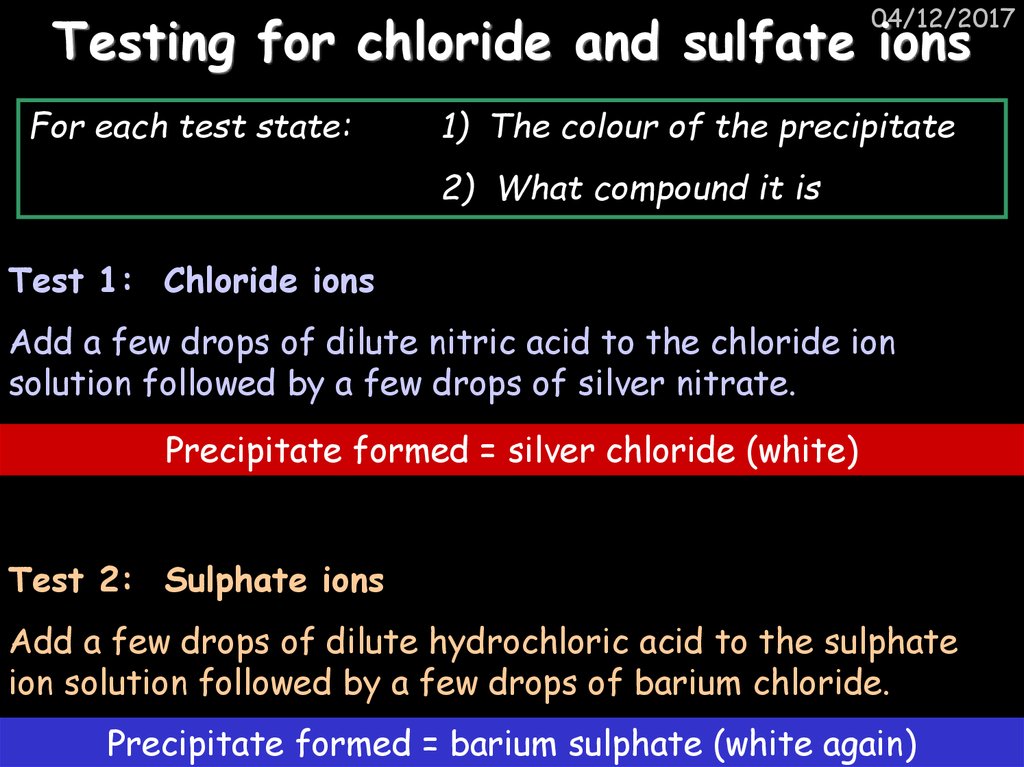
37. Testing for chloride and sulfate ions
04/12/2017Testing for chloride and sulfate ions
For each test state:
1) The colour of the precipitate
2) What compound it is
Test 1: Chloride ions
Add a few drops of dilute nitric acid to the chloride ion
solution followed by a few drops of silver nitrate.
Precipitate formed = silver chloride (white)
Test 2: Sulphate ions
Add a few drops of dilute hydrochloric acid to the sulphate
ion solution followed by a few drops of barium chloride.
Precipitate formed = barium sulphate (white again)
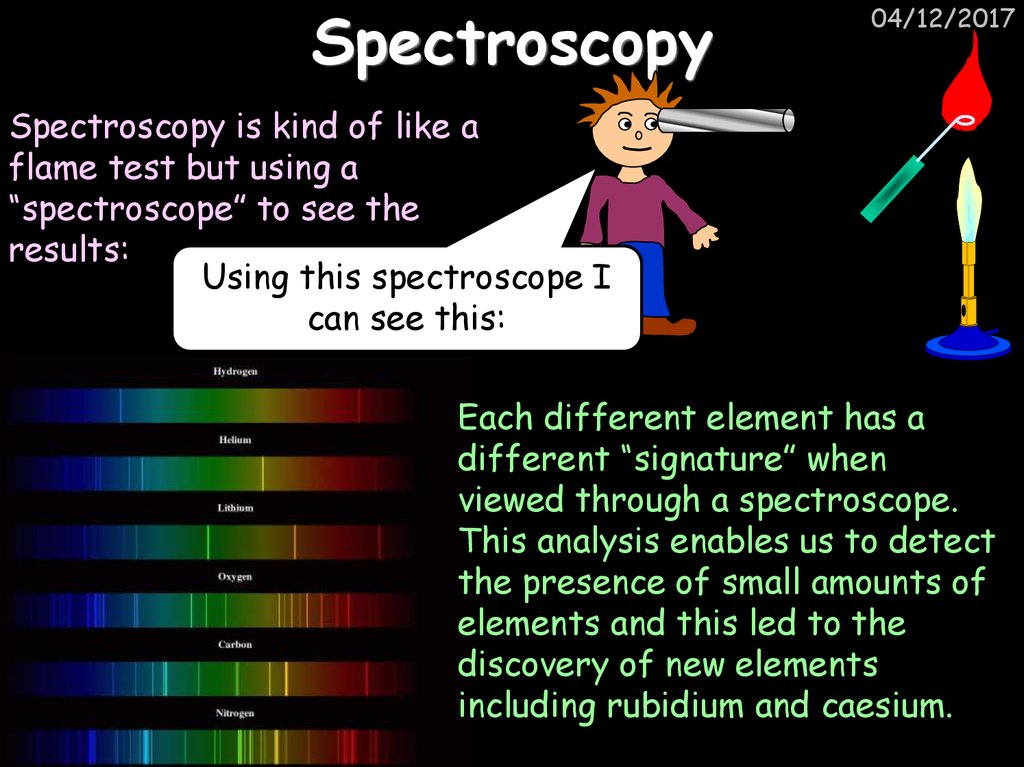
38. Spectroscopy
04/12/2017Spectroscopy is kind of like a
flame test but using a
“spectroscope” to see the
results:
Using this spectroscope I
can see this:
Each different element has a
different “signature” when
viewed through a spectroscope.
This analysis enables us to detect
the presence of small amounts of
elements and this led to the
discovery of new elements
including rubidium and caesium.
39. Topic 3 – Covalent Compounds and Separation Techniques
04/12/2017Topic 3 – Covalent Compounds and
Separation Techniques
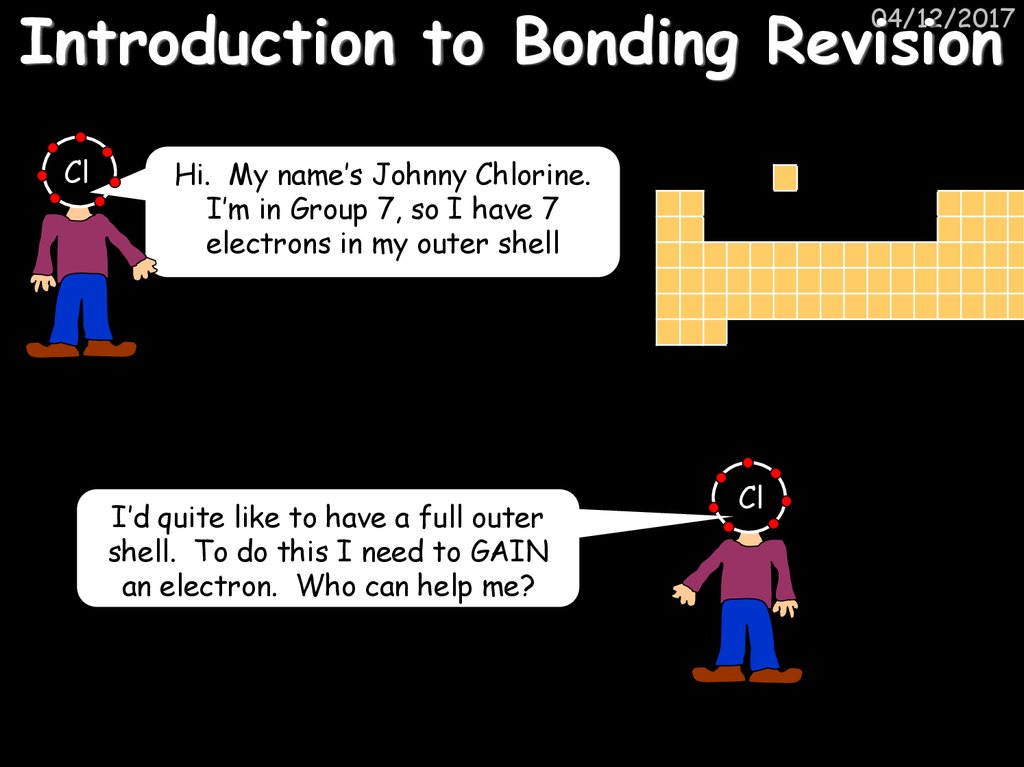
40. Introduction to Bonding Revision
04/12/2017Cl
Hi. My name’s Johnny Chlorine.
I’m in Group 7, so I have 7
electrons in my outer shell
I’d quite like to have a full outer
shell. To do this I need to GAIN
an electron. Who can help me?
Cl
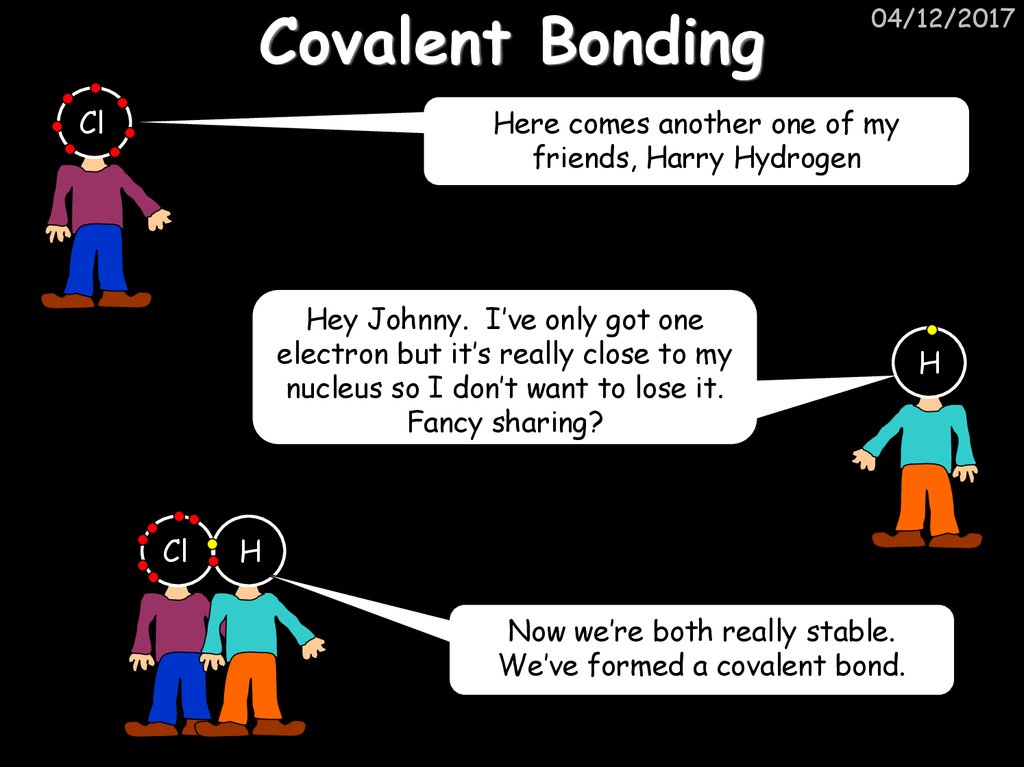
41. Covalent Bonding
Cl04/12/2017
Here comes another one of my
friends, Harry Hydrogen
Hey Johnny. I’ve only got one
electron but it’s really close to my
nucleus so I don’t want to lose it.
Fancy sharing?
Cl
H
Now we’re both really stable.
We’ve formed a covalent bond.
H
42. Covalent bonding
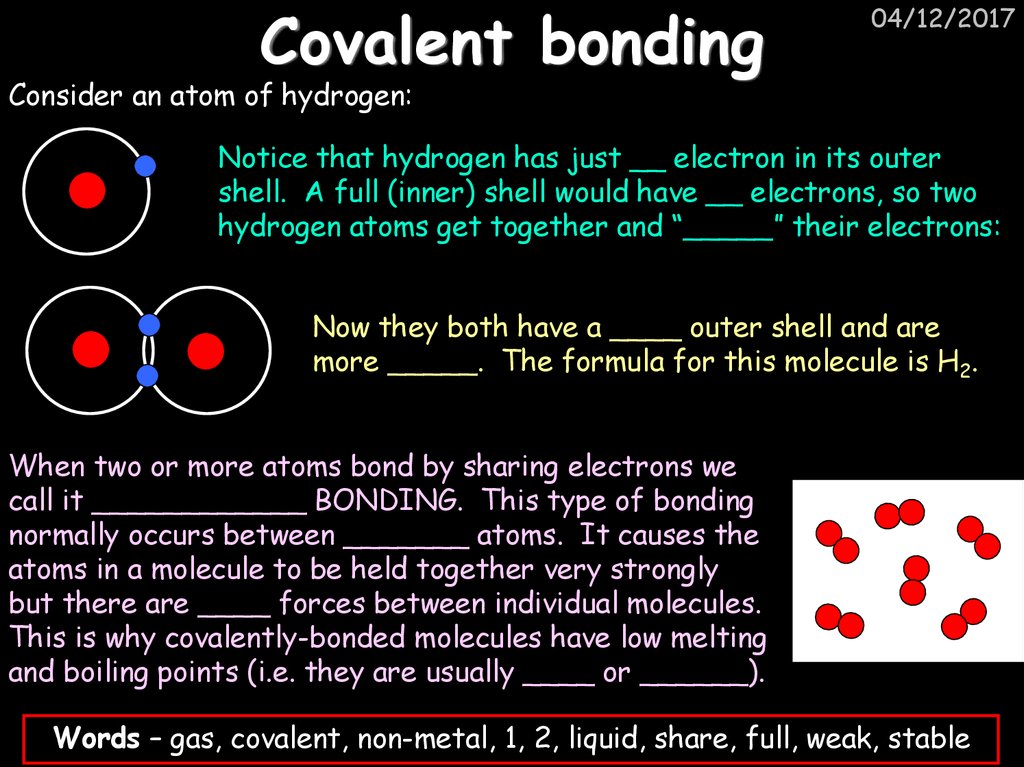
04/12/2017Consider an atom of hydrogen:
Notice that hydrogen has just __ electron in its outer
shell. A full (inner) shell would have __ electrons, so two
hydrogen atoms get together and “_____” their electrons:
Now they both have a ____ outer shell and are
more _____. The formula for this molecule is H2.
When two or more atoms bond by sharing electrons we
call it ____________ BONDING. This type of bonding
normally occurs between _______ atoms. It causes the
atoms in a molecule to be held together very strongly
but there are ____ forces between individual molecules.
This is why covalently-bonded molecules have low melting
and boiling points (i.e. they are usually ____ or ______).
Words – gas, covalent, non-metal, 1, 2, liquid, share, full, weak, stable
43. Dot and Cross Diagrams
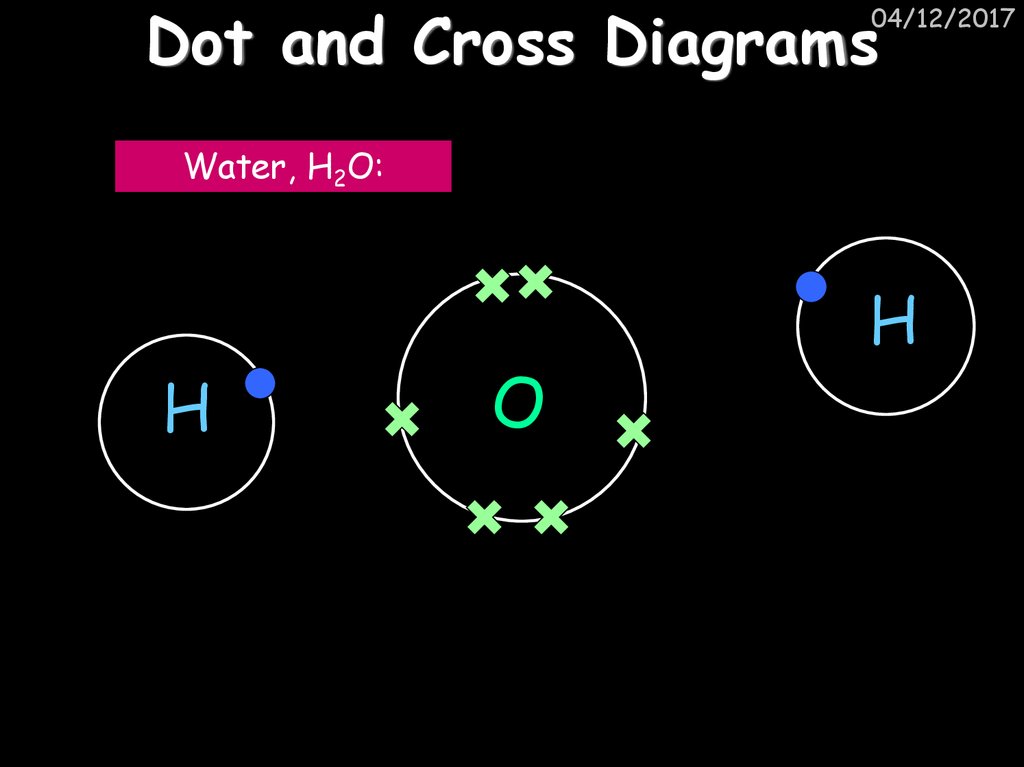
04/12/2017Water, H2O:
H
O
H
44. Dot and Cross Diagrams
04/12/2017Oxygen, O2:
O
O
45. Dot and cross diagrams
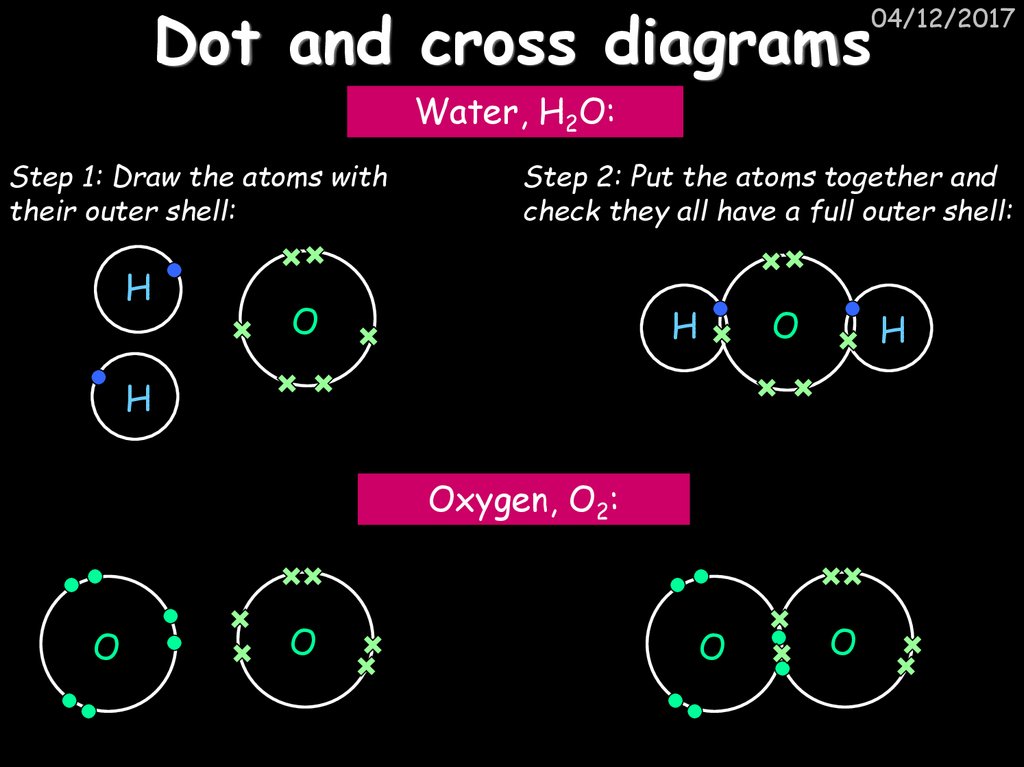
04/12/2017Water, H2O:
Step 1: Draw the atoms with
their outer shell:
H
Step 2: Put the atoms together and
check they all have a full outer shell:
O
H
O
H
H
Oxygen, O2:
O
O
O
O
46. Dot and cross diagrams
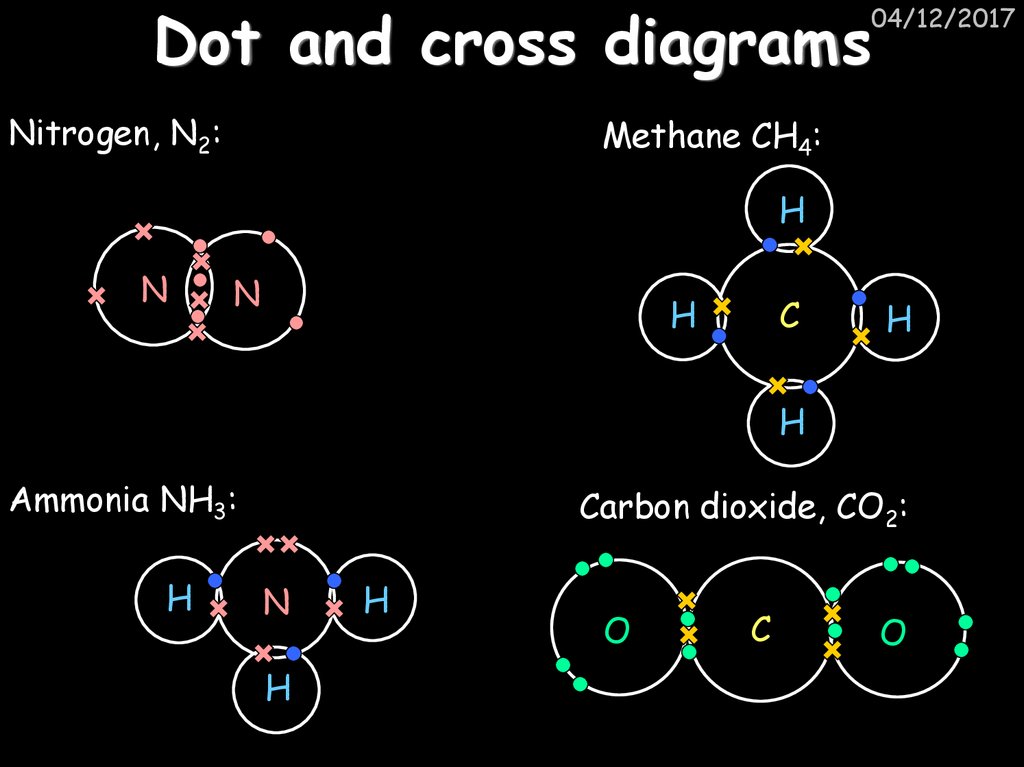
Nitrogen, N2:04/12/2017
Methane CH4:
H
N
N
H
C
H
H
Ammonia NH3:
H
Carbon dioxide, CO2:
N
H
H
O
C
O
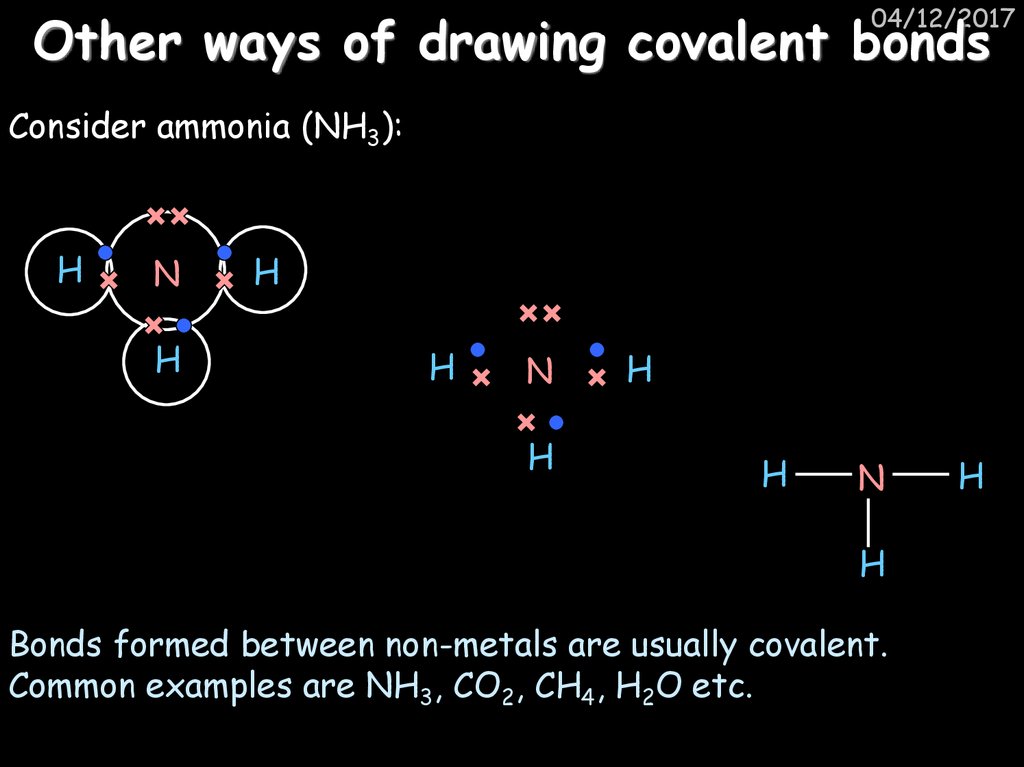
47. Other ways of drawing covalent bonds
04/12/2017Other ways of drawing covalent bonds
Consider ammonia (NH3):
H
N
H
H
H
N
H
H
H
N
H
Bonds formed between non-metals are usually covalent.
Common examples are NH3, CO2, CH4, H2O etc.
H
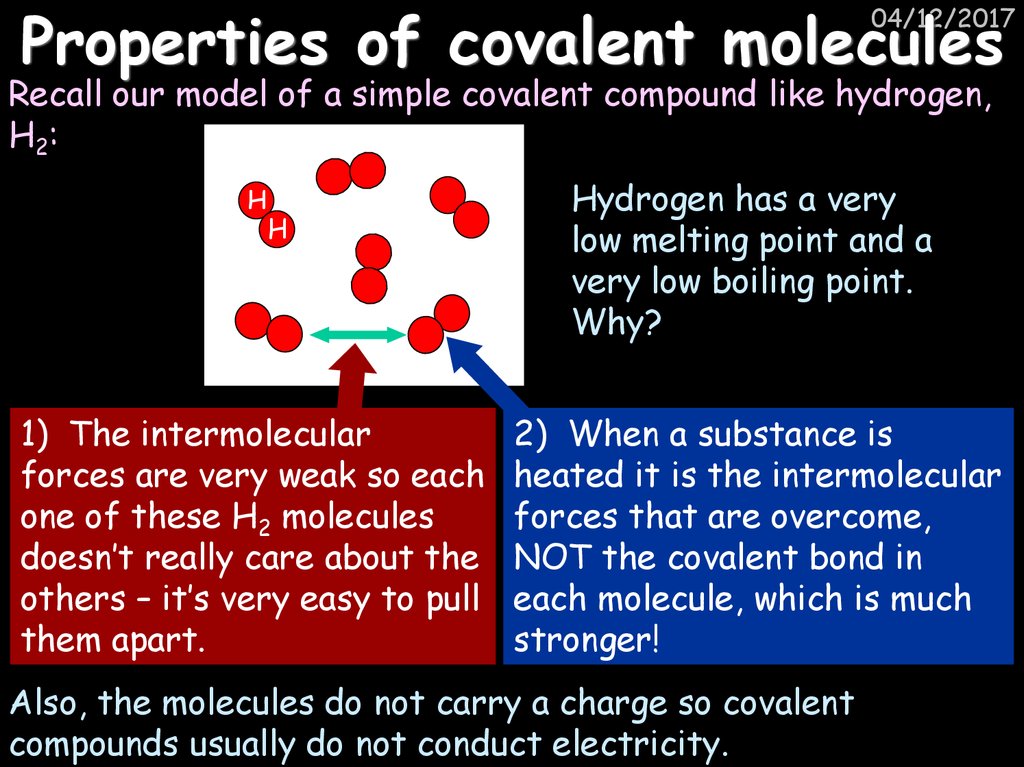
48. Properties of covalent molecules
04/12/2017Recall our model of a simple covalent compound like hydrogen,
H2:
H
H
1) The intermolecular
forces are very weak so each
one of these H2 molecules
doesn’t really care about the
others – it’s very easy to pull
them apart.
Hydrogen has a very
low melting point and a
very low boiling point.
Why?
2) When a substance is
heated it is the intermolecular
forces that are overcome,
NOT the covalent bond in
each molecule, which is much
stronger!
Also, the molecules do not carry a charge so covalent
compounds usually do not conduct electricity.
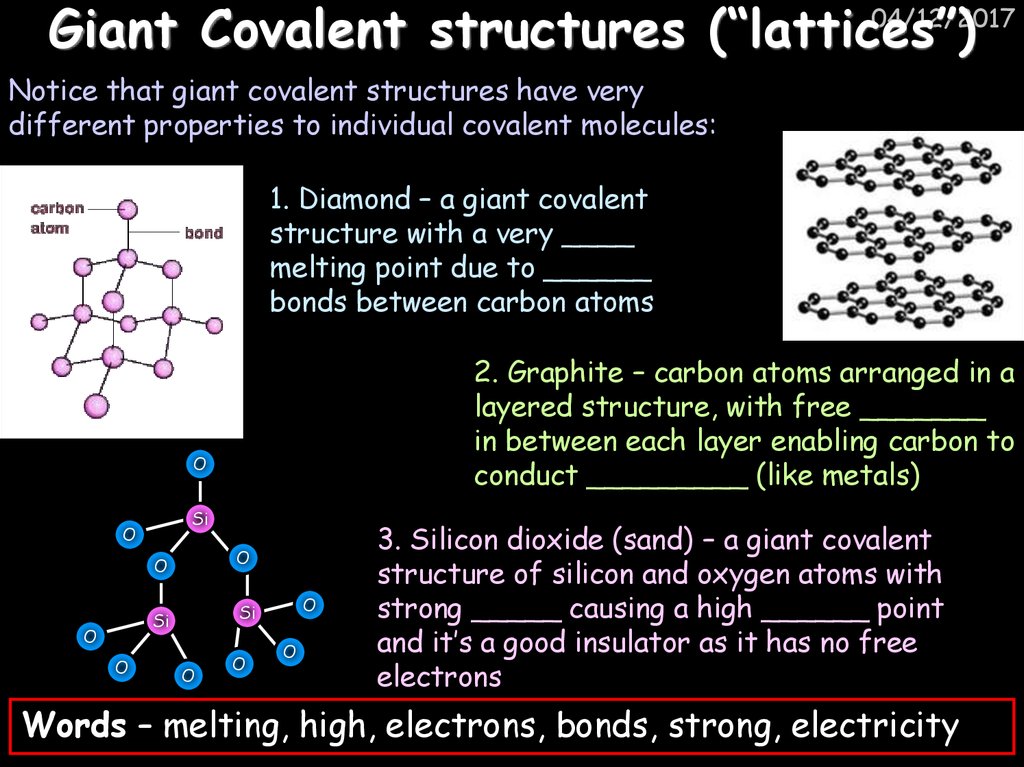
49. Giant Covalent structures (“lattices”)
04/12/2017Giant Covalent structures (“lattices”)
Notice that giant covalent structures have very
different properties to individual covalent molecules:
1. Diamond – a giant covalent
structure with a very ____
melting point due to ______
bonds between carbon atoms
2. Graphite – carbon atoms arranged in a
layered structure, with free _______
in between each layer enabling carbon to
conduct _________ (like metals)
O
Si
O
O
O
O
O
Si
Si
O
O
O
O
3. Silicon dioxide (sand) – a giant covalent
structure of silicon and oxygen atoms with
strong _____ causing a high ______ point
and it’s a good insulator as it has no free
electrons
Words – melting, high, electrons, bonds, strong, electricity










 chemistry
chemistry








