Similar presentations:
Atom overview and subatomic particles
1.
ATOM OVERVIEWand
SUBATOMIC
PARTICLES
• Elements are made up of atoms.
• Atoms are made up of protons and
neutrons located within the nucleus, with
electrons in orbitals surrounding the
nucleus.
2.
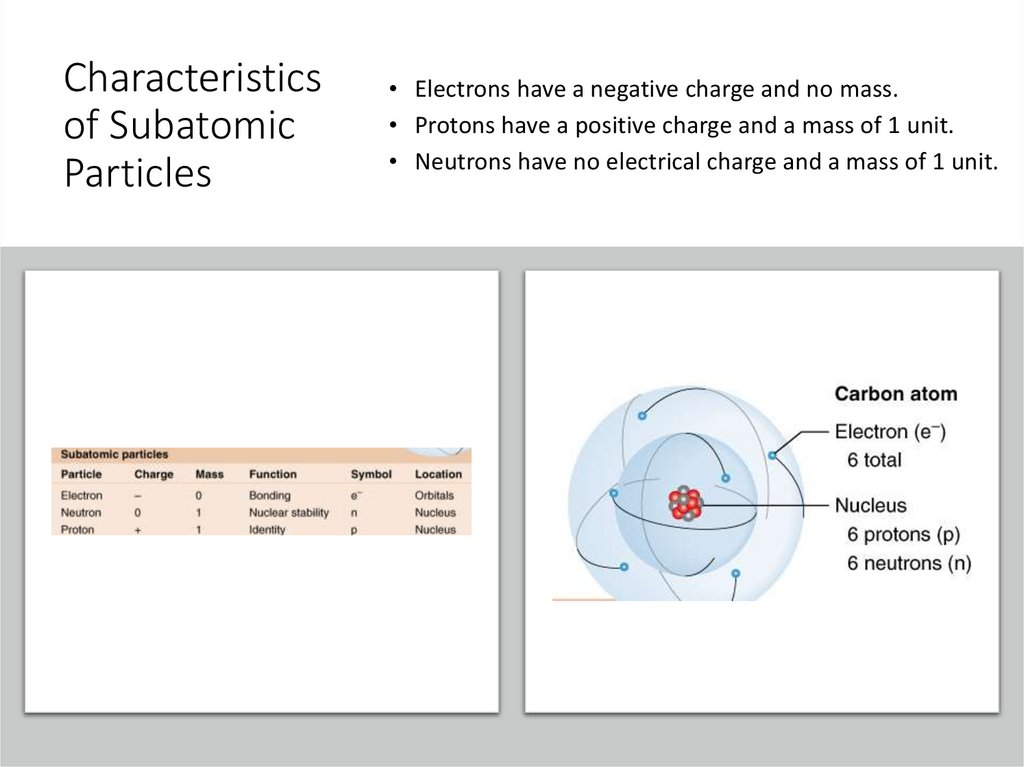
DEFINITIONS• Proton: positively charged particle in
the nucleus of all atoms
• Neutron: uncharged subatomic
particle in the nucleus
• Electron: negatively charged
subatomic particle that occupies
orbitals around the atomic nucleus
• Nucleus: core of atoms, occupied by
neutrons and protons
• Atomic number: number of protons in
the atomic nucleus, determines the
element
• Atomic Mass: total number of protons
and neutrons in the nucleus of an
element’s atom
• Isotope: element that differ in the
number of neutrons
• Radioisotope: isotope with instable
nucleus
3.
Characteristicsof Subatomic
Particles
• Electrons have a negative charge and no mass.
• Protons have a positive charge and a mass of 1 unit.
• Neutrons have no electrical charge and a mass of 1 unit.
4.
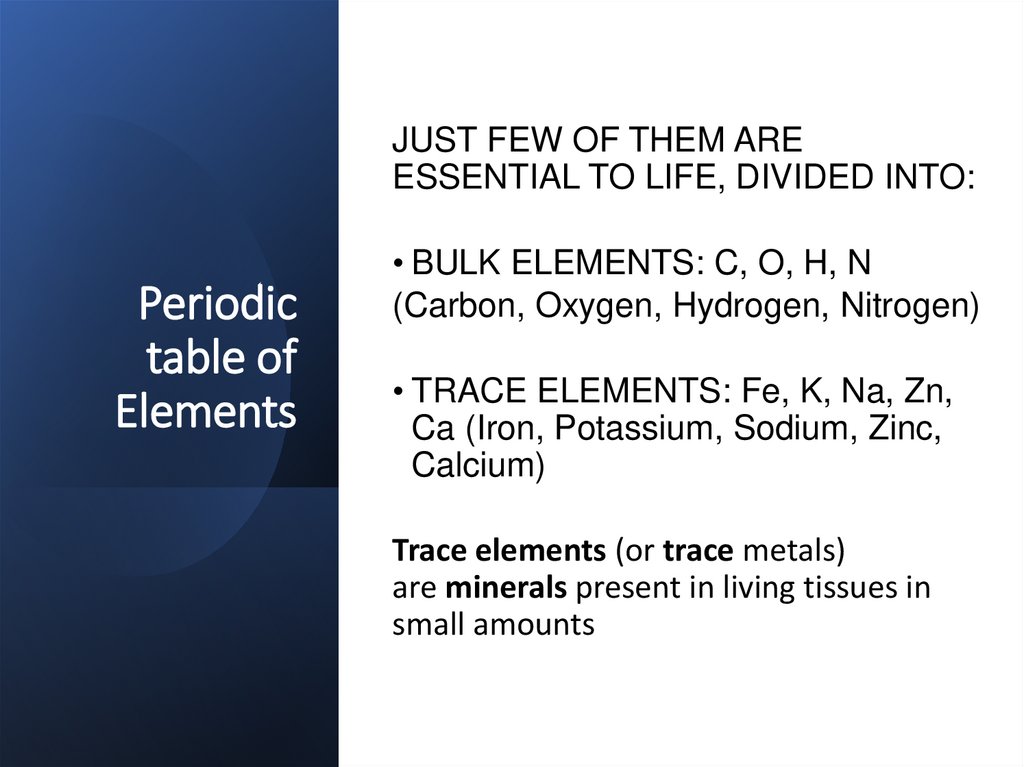
JUST FEW OF THEM AREESSENTIAL TO LIFE, DIVIDED INTO:
Periodic
table of
Elements
• BULK ELEMENTS: C, O, H, N
(Carbon, Oxygen, Hydrogen, Nitrogen)
• TRACE ELEMENTS: Fe, K, Na, Zn,
Ca (Iron, Potassium, Sodium, Zinc,
Calcium)
Trace elements (or trace metals)
are minerals present in living tissues in
small amounts
5.
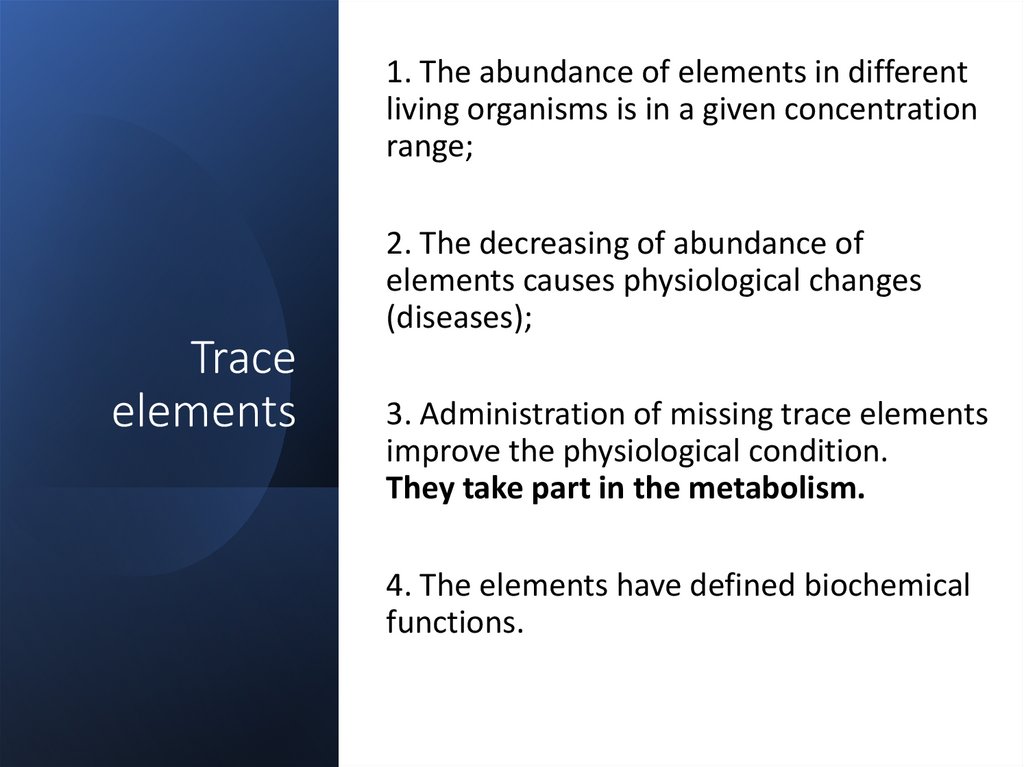
1. The abundance of elements in differentliving organisms is in a given concentration
range;
Trace
elements
2. The decreasing of abundance of
elements causes physiological changes
(diseases);
3. Administration of missing trace elements
improve the physiological condition.
They take part in the metabolism.
4. The elements have defined biochemical
functions.
6.
1. Transport of biological small moleculespl. O2-transport: hemoglobin (Fe), hemocianin (Cu)
O2-storage: mioglobin (Fe)
Roles of
trace
elements
2. Activation of molecules: metalloenzymes, enzymes activated
by metal ions:
a) catalysing of redox processes (Fe, Cu, Mn, Co, Mo, Ni)
biological oxidation, reduction of substrate
b) catalysing of acid-base processes (Zn)
3. Seconder conformation of macromolecules
– determination of conformation of enzymes
– determination of conformation of proteins, nucleic acids
4. Metabolism of microelements
– uptaking, transport, storage of trace elements
7.
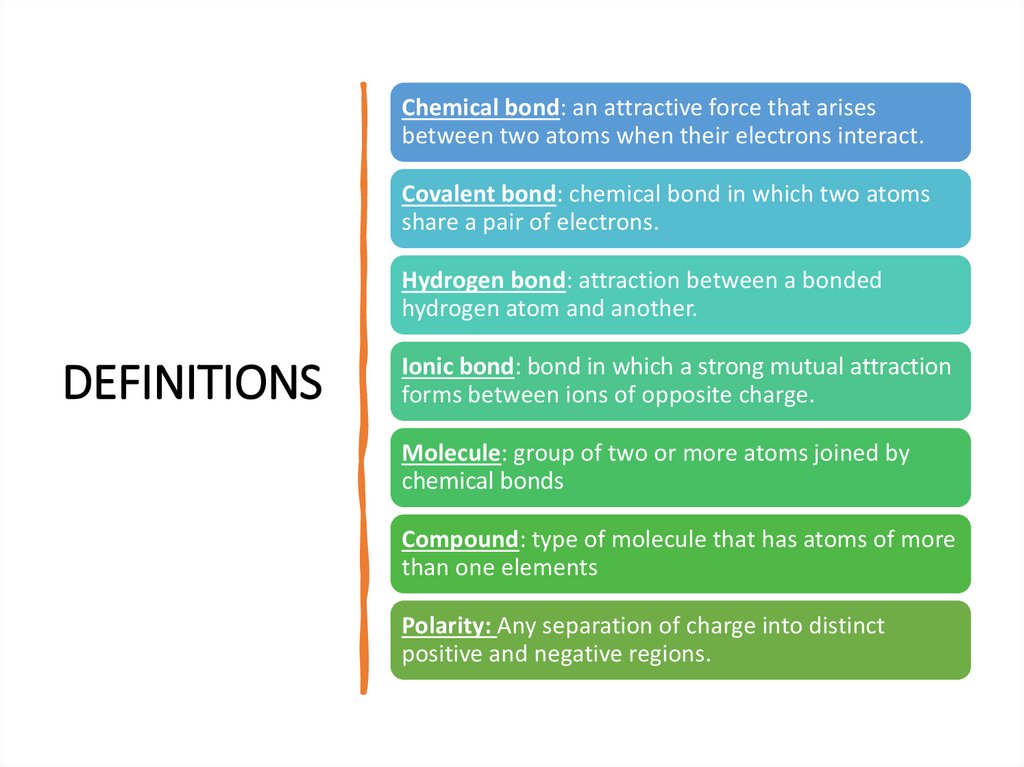
Chemical bond: an attractive force that arisesbetween two atoms when their electrons interact.
Covalent bond: chemical bond in which two atoms
share a pair of electrons.
Hydrogen bond: attraction between a bonded
hydrogen atom and another.
DEFINITIONS
Ionic bond: bond in which a strong mutual attraction
forms between ions of opposite charge.
Molecule: group of two or more atoms joined by
chemical bonds
Compound: type of molecule that has atoms of more
than one elements
Polarity: Any separation of charge into distinct
positive and negative regions.
8.
What is a Chemicalbond?
Atoms accept, share, and donate
electrons; Whether an atom will
interact with other atoms depends
on how many electrons it has. A
chemical bond is the union of the
electron structures of atoms.
Atoms with an unfilled outer shell
tend to interact with other atoms in
ways that fill the shell, such as
forming chemical bonds.
Atoms with a filled outer shell are
inert- they do not form bonds.
9.
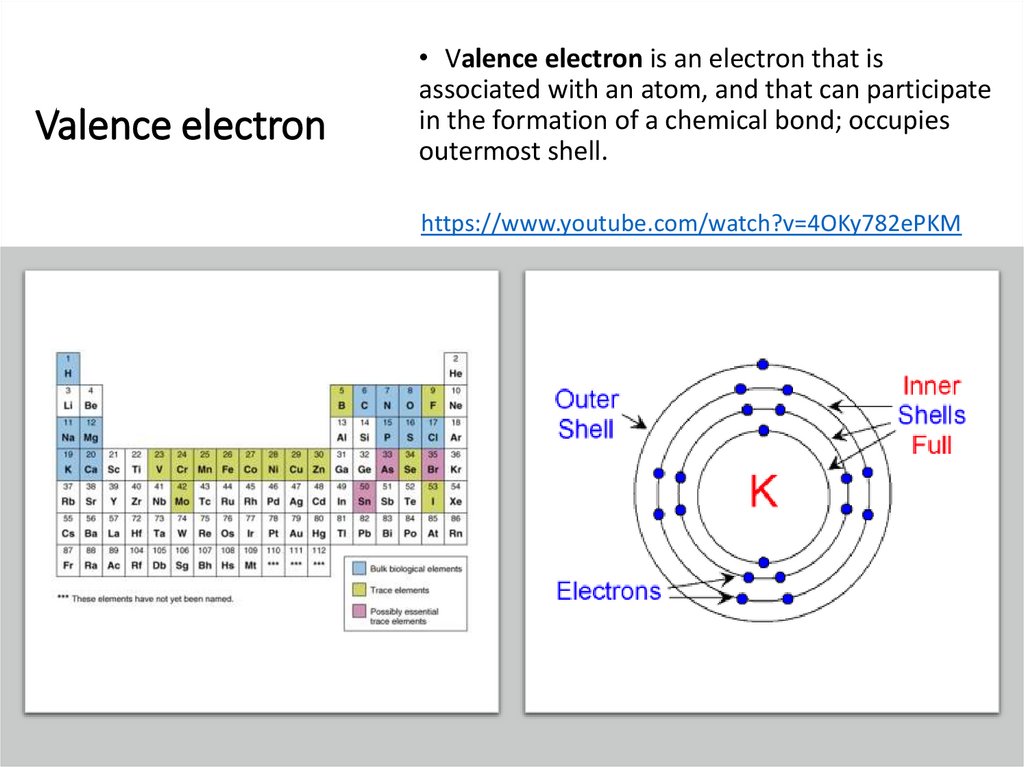
Valence electron• Valence electron is an electron that is
associated with an atom, and that can participate
in the formation of a chemical bond; occupies
outermost shell.
https://www.youtube.com/watch?v=4OKy782ePKM
10.
Types ofbonds:
Three types of bonds:
1. IONIC (giving up of e-); STRONG
2. COVALENT (non- polar and polar)
sharing of e-; STRONG
3. HYDROGEN between (hydrogen atom
which usually has a partial + charge, is
attracted to an atom w/ a partial - charge)
WEAK
11.
IONIC BOND• when oppositely charged ions are
attracted to each other
• weaker than covalent bonds
• Na+Cl=NaCl=SODIUM CHLORIDE=
SALT
12.
Ions• Ion
An atom with a positive or negative charge
due to loss or gain of electrons in its outer
shell.
Examples: Na+, ClIonic bonding takes place when the
difference in electronegativity between the
two atoms is more than 1.7
13.
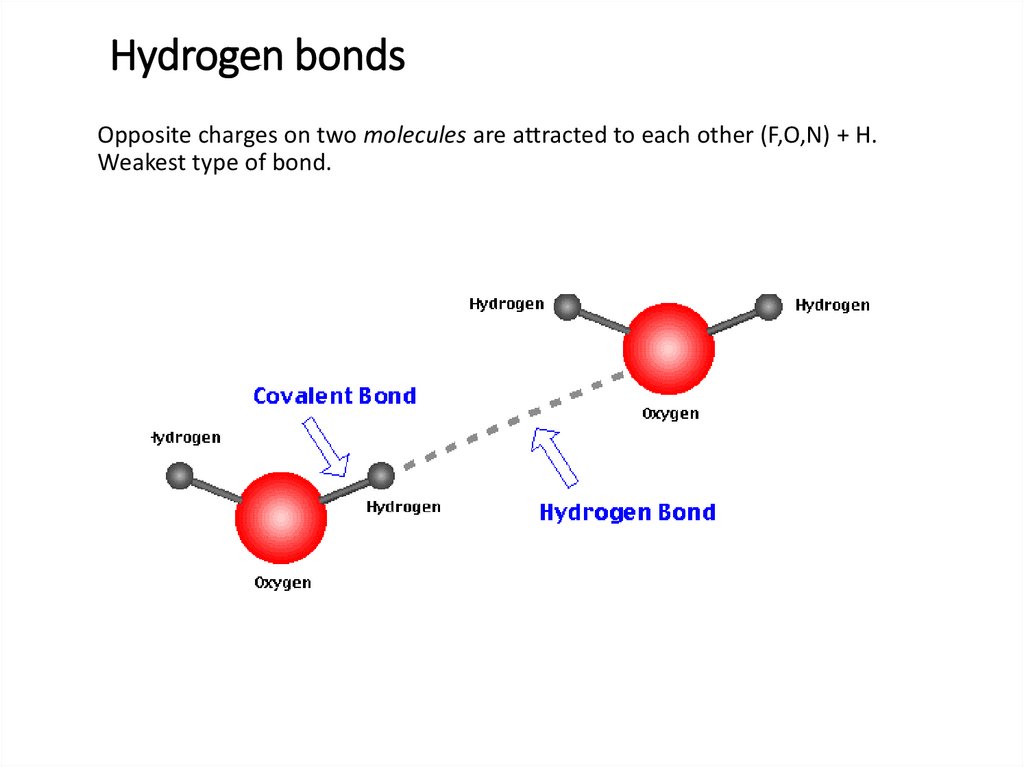
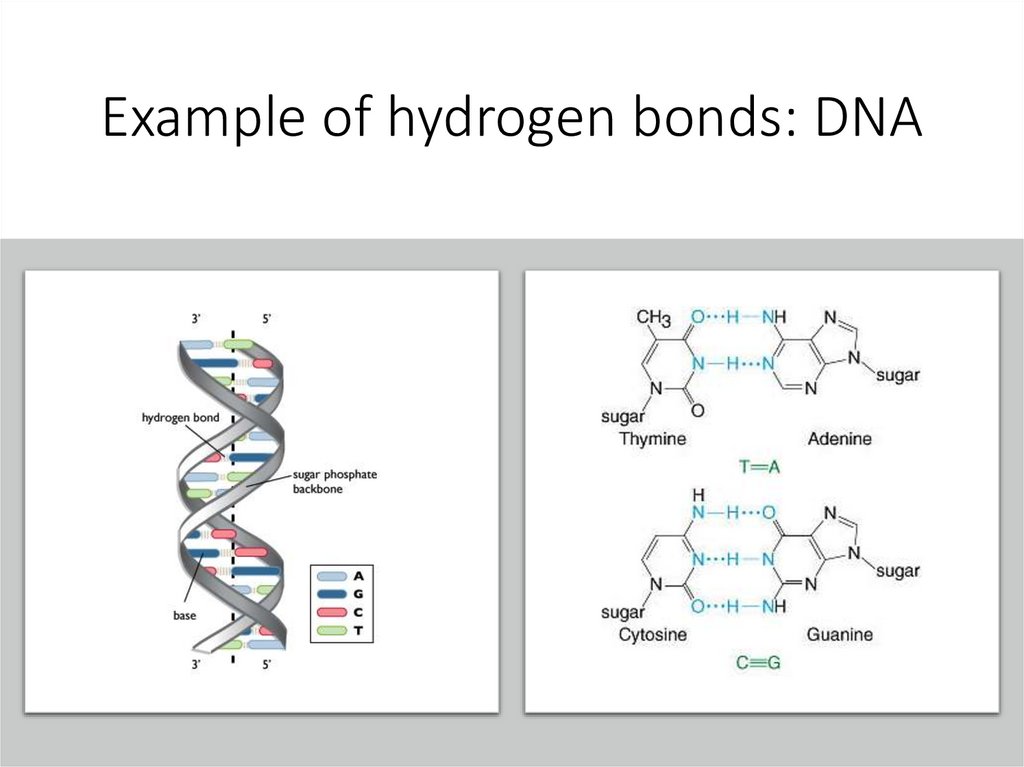
Hydrogen bondsOpposite charges on two molecules are attracted to each other (F,O,N) + H.
Weakest type of bond.
14.
Chemical reactions arerequired to make or break
these bonds.
Properties of Water:
- HYDROGEN bonds make it all
possible








 chemistry
chemistry








