Similar presentations:
Nuclear magnetic resonance
1.
Nuclear Magnetic Resonance (NMR)2.
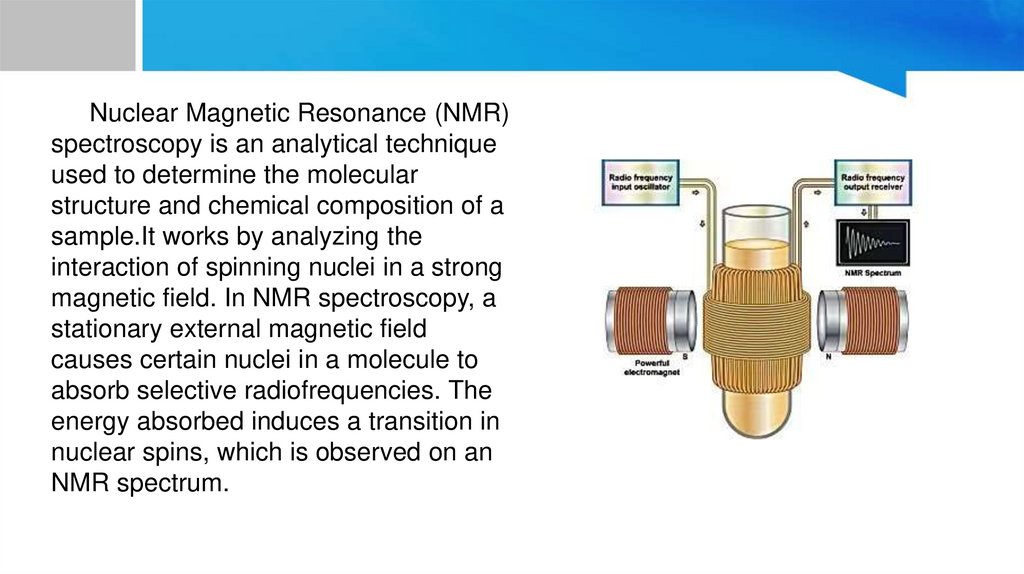
Nuclear Magnetic Resonance (NMR)spectroscopy is an analytical technique
used to determine the molecular
structure and chemical composition of a
sample.It works by analyzing the
interaction of spinning nuclei in a strong
magnetic field. In NMR spectroscopy, a
stationary external magnetic field
causes certain nuclei in a molecule to
absorb selective radiofrequencies. The
energy absorbed induces a transition in
nuclear spins, which is observed on an
NMR spectrum.
3.
Applications of NMR Spectroscopy• NMR spectroscopy is a non-destructive and non-invasive technique that is used to determine
molecular structure and dynamics. The applications of NMR are diverse and include the
following research areas and industries:
• In biology, NMR is applied to study macromolecules, such as proteins, lipids and nucleic
acids. In chemistry, it’s widely used for both qualitative and quantitative analysis to monitor
reactions, identify structures and assess purity.
• In polymer science, to analyze monomer ratio, molecular weight, tacticity, sequencing, chain
length and branching, and to determine end groups.
• In the pharma industry, to determine the purity and quantity of active ingredients, excipients
and impurities in pharmaceutical products
• In the petroleum industry, to assess hydrocarbons of raw petroleum and its products.
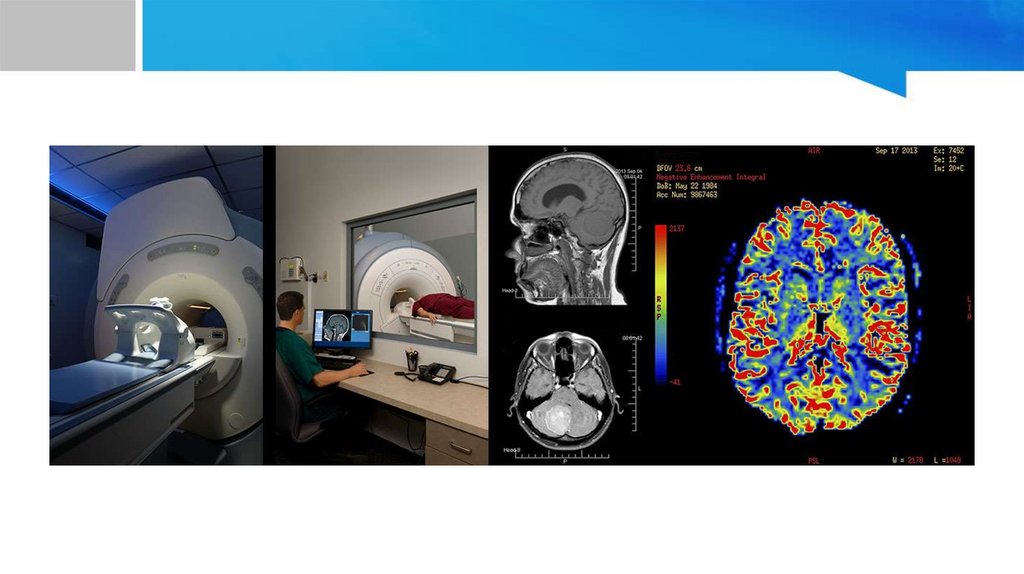
• In medicine, magnetic resonance imaging (MRI) is an application of NMR used for soft tissue
analysis to identify the damaged or diseased tissues.
4.
5.
Principles of NMR SpectroscopyA NMR instrument measures the interaction of nuclear spin
states under the influence of a powerful magnetic field. The
magnetic field causes nuclei to precess (rotate) like a spinning top.
A precessing nucleus selectively absorbs energy from
radiofrequency waves when the frequency of the precessing nuclei
matches the low external frequency of radiofrequency waves
interacting with it. When this absorption occurs, the precessing
nucleus and radiofrequency waves are said to be in ‘resonance’,
hence the term nuclear magnetic resonance. Resonance can be
produced by tuning the frequency of the nuclei to the fixed
frequency of radio waves, or by tuning the frequency of radio waves
to that of the nuclei.
6.
7.
Characteristics of a NMR Spectrum• A NMR spectrum is a plot of the applied radiofrequency against
absorption. The position on the plot at which the nuclei absorbs is
called the chemical shift. Chemical shift is affected by the electron
density around the nucleus. If a nucleus is surrounded by a high
electron density, the nucleus is shielded from the external
magnetic field, which shifts the signals upfield on the NMR
spectrum. If a nucleus is surrounded by an electronegative atom,
it removes electron density around the nucleus and causes a
‘deshielding’ effect. This shifts the signal ‘downfield’ on an NMR
spectrum. The spin of neighboring nuclei also affects the signals
seen on an NMR spectrum and may cause splitting of the NMR
signal, known as ‘spin-spin coupling’.

 physics
physics








