Similar presentations:
Chronic obstructive pulmonary disease
1.
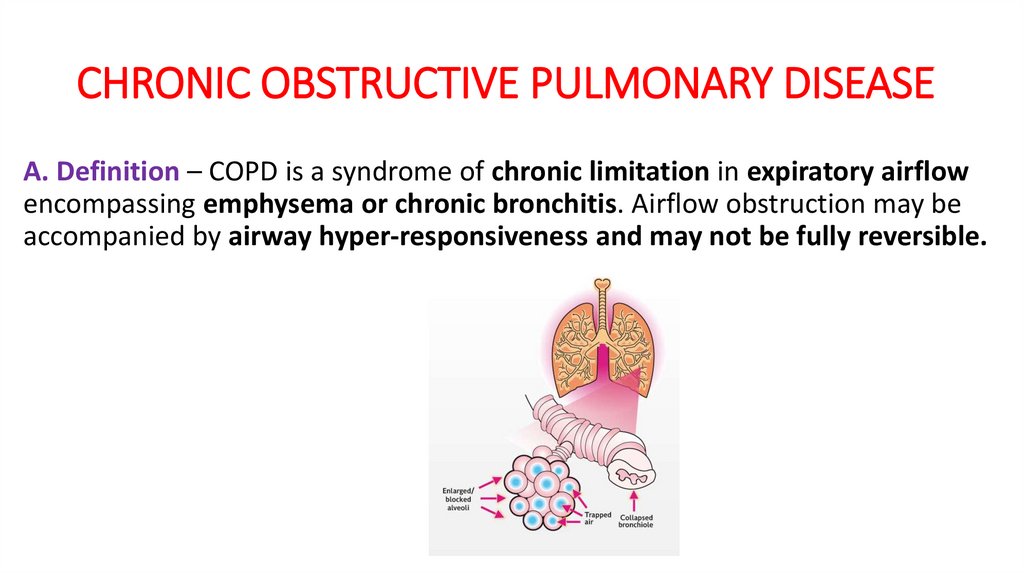
CHRONIC OBSTRUCTIVE PULMONARY DISEASEA. Definition – COPD is a syndrome of chronic limitation in expiratory airflow
encompassing emphysema or chronic bronchitis. Airflow obstruction may be
accompanied by airway hyper-responsiveness and may not be fully reversible.
2.
3.
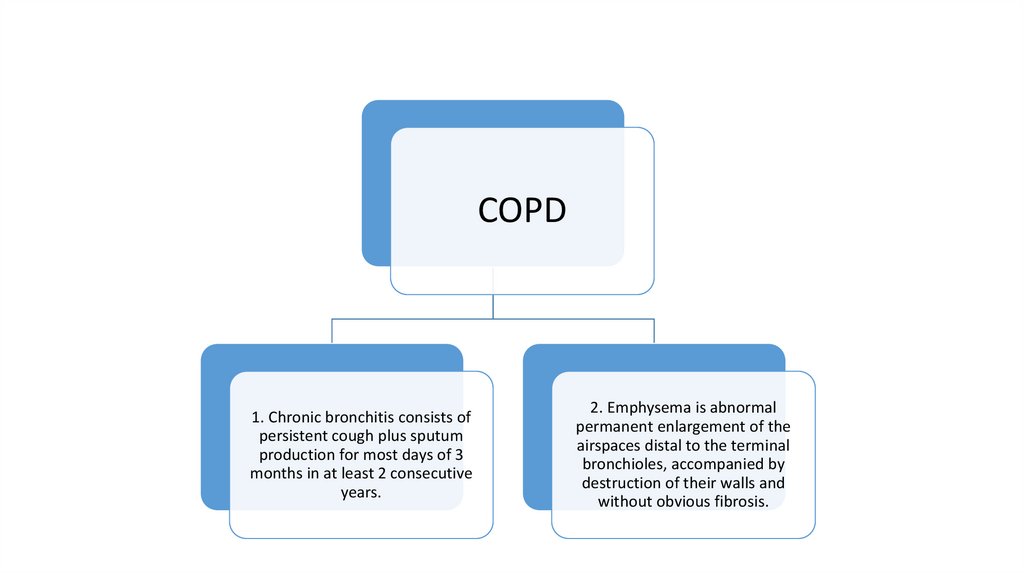
COPD1. Chronic bronchitis consists of
persistent cough plus sputum
production for most days of 3
months in at least 2 consecutive
years.
2. Emphysema is abnormal
permanent enlargement of the
airspaces distal to the terminal
bronchioles, accompanied by
destruction of their walls and
without obvious fibrosis.
4.
B. Diagnosis and Assessment1) The diagnosis of COPD is based on 1) a history of exposure to risk factors and 2) the
presence of airflow limitation that is not fully reversible, with or without the presence of
symptoms.
2) Symptoms: Dyspnea (described by patients as “increased effort to breathe,” “heaviness,”
“air hunger,” or “gasping”), poor exercise tolerance, chronic cough, sputum production,
wheezing
3) consider COPD if an individual is older than 40 years and has any of the following:
i. Dyspnea that is progressive (worsens over time), persistent (present every day), and
worse on exercise
ii. Chronic cough that is present intermittently or every day; often present throughout the
day; seldom only nocturnal. May be nonproductive
iii. Chronic sputum production in any pattern
iv. History of exposure to risk factors, especially tobacco smoke (most common risk factor),
occupational dusts and chemicals, and smoke from home cooking and heating fuels
5.
Notes:The best predictor of airflow obstruction is the
presence of all three of the following:
i. Smoking history of more than 55 pack-years
ii. Wheezing on auscultation
iii. Patient self-reported wheezing
6.
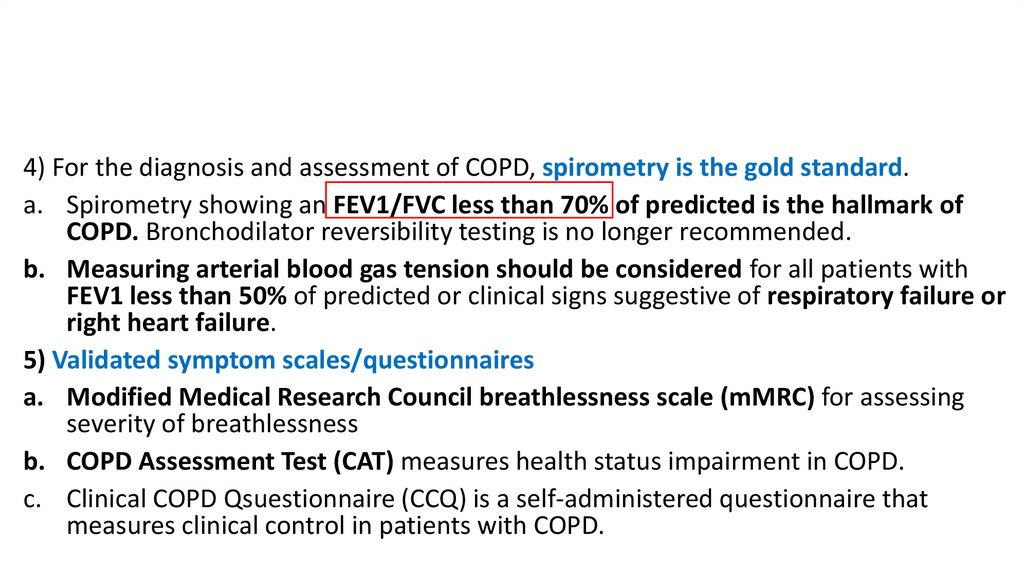
4) For the diagnosis and assessment of COPD, spirometry is the gold standard.a. Spirometry showing an FEV1/FVC less than 70% of predicted is the hallmark of
COPD. Bronchodilator reversibility testing is no longer recommended.
b. Measuring arterial blood gas tension should be considered for all patients with
FEV1 less than 50% of predicted or clinical signs suggestive of respiratory failure or
right heart failure.
5) Validated symptom scales/questionnaires
a. Modified Medical Research Council breathlessness scale (mMRC) for assessing
severity of breathlessness
b. COPD Assessment Test (CAT) measures health status impairment in COPD.
c. Clinical COPD Qsuestionnaire (CCQ) is a self-administered questionnaire that
measures clinical control in patients with COPD.
7.
8.
mMRCGrade
Description of Breathlessness (dyspnea)
0
1
I only get breathless with strenuous exercise.
I get short of breath when hurrying on level ground or walking up a slight
hill
(slight limitation) On level ground, I walk slower than people of the same
age because of breathlessness, or have to stop for breath when walking at
my own pace.
2
3
4
(marked limitation) I stop for breath after walking about 100 yards or after
a few minutes on level ground
Symptoms at rest
9.
Management of Stable COPD1. Existing medications for COPD have not been shown to
modify the long-term decline in lung function, the
hallmark of this disease (Evidence A). Therefore,
pharmacotherapy for COPD is used to decrease
symptoms, complications, or both.
2. Smoking cessation is a critical component of COPD
management.
10.
3. Bronchodilator medications are central to the symptomatic management of COPD(Evidence A).
a. They are given on an as-needed basis (SABA, or short acting anticholinergic, ipratropium)
or on a regular basis (LABA, or long acting anticholenergic, tiotropium) to prevent or
reduce symptoms.
b. The principal bronchodilator treatments are β2-agonists, anticholinergics, or a
combination of these drugs (Evidence A). Theophylline is also a bronchodilator but is not
recommended unless other long-term bronchodilators are unavailable or unaffordable.
c. Inhaled therapy is preferred.
d. Combining bronchodilators from different pharmacologic classes may improve efficacy
with the same or fewer adverse effects compared with increasing the dose of a single
bronchodilator (Evidence A).
e. Adding tiotropium to a LABA-ICS combination (triple therapy) improves lung function
and health-related quality of life and reduces the number of exacerbations (Evidence B),
and retrospective data show decreased mortality, fewer hospital admissions, and fewer
OCS bursts. All bronchodilators improve symptoms and exercise capacity.
11.
12.
Q. tiotropium vs. LABA?A. Tiotropium is more effective than a LABA as initial LA
bronchodilator therapy in moderate to very severe
COPD regarding time to first exacerbation and annual
number of exacerbations.
Q. why we don’t use ICSs in stable COPD?
A. to avoid pneumonia
13.
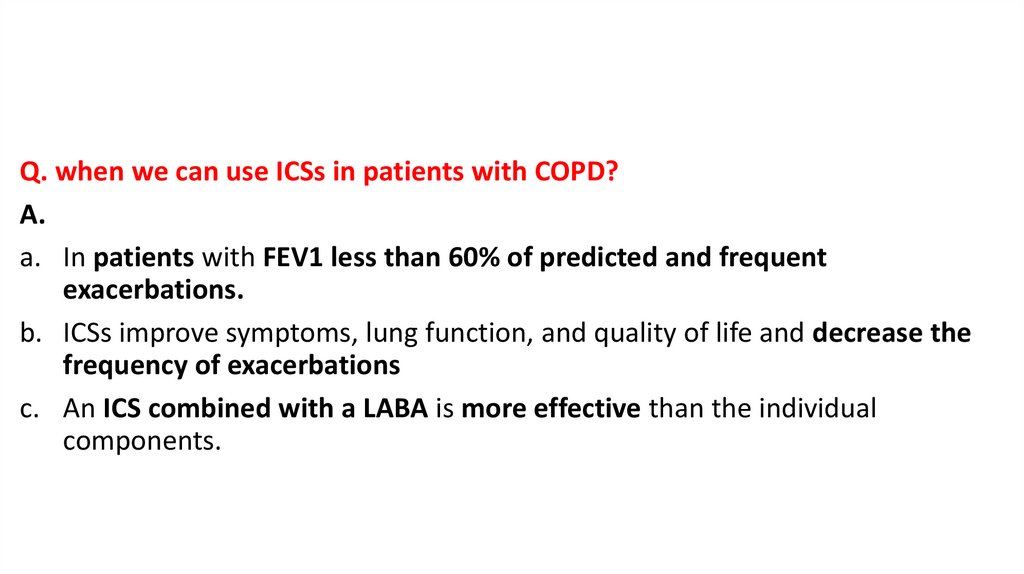
Q. when we can use ICSs in patients with COPD?A.
a. In patients with FEV1 less than 60% of predicted and frequent
exacerbations.
b. ICSs improve symptoms, lung function, and quality of life and decrease the
frequency of exacerbations
c. An ICS combined with a LABA is more effective than the individual
components.
14.
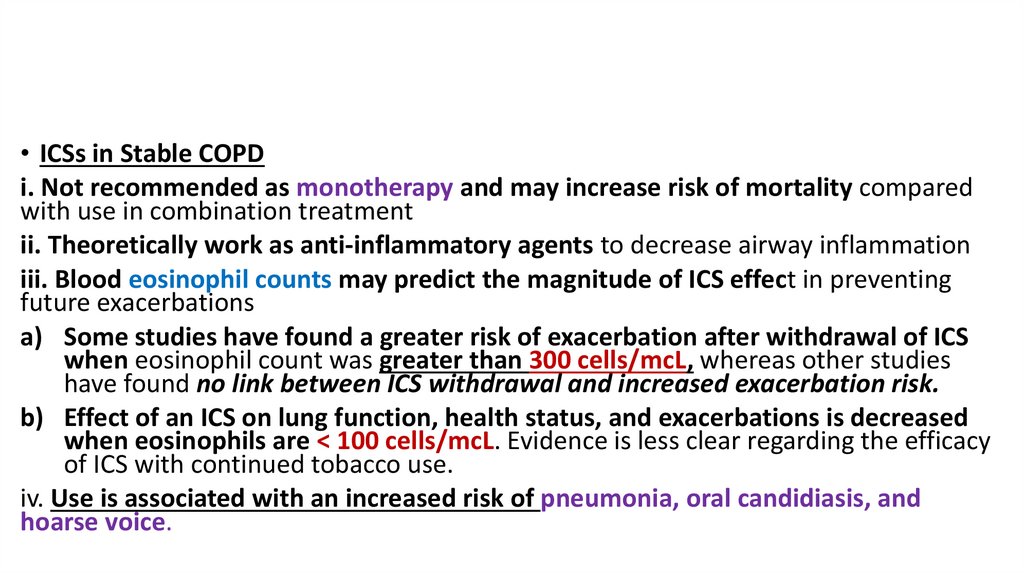
• ICSs in Stable COPDi. Not recommended as monotherapy and may increase risk of mortality compared
with use in combination treatment
ii. Theoretically work as anti-inflammatory agents to decrease airway inflammation
iii. Blood eosinophil counts may predict the magnitude of ICS effect in preventing
future exacerbations
a) Some studies have found a greater risk of exacerbation after withdrawal of ICS
when eosinophil count was greater than 300 cells/mcL, whereas other studies
have found no link between ICS withdrawal and increased exacerbation risk.
b) Effect of an ICS on lung function, health status, and exacerbations is decreased
when eosinophils are < 100 cells/mcL. Evidence is less clear regarding the efficacy
of ICS with continued tobacco use.
iv. Use is associated with an increased risk of pneumonia, oral candidiasis, and
hoarse voice.
15.
v. Use of LABA/ICS in COPD is not encouraged. Tripletherapy (LABA + LAMA + ICS) is preferred when ICS is
indicated based on improved outcomes compared with
LABA/ICS.
vi. Group E: Consider LABA + LAMA + ICS if blood
eosinophil count is ≥ 300 cells/mcL
16.
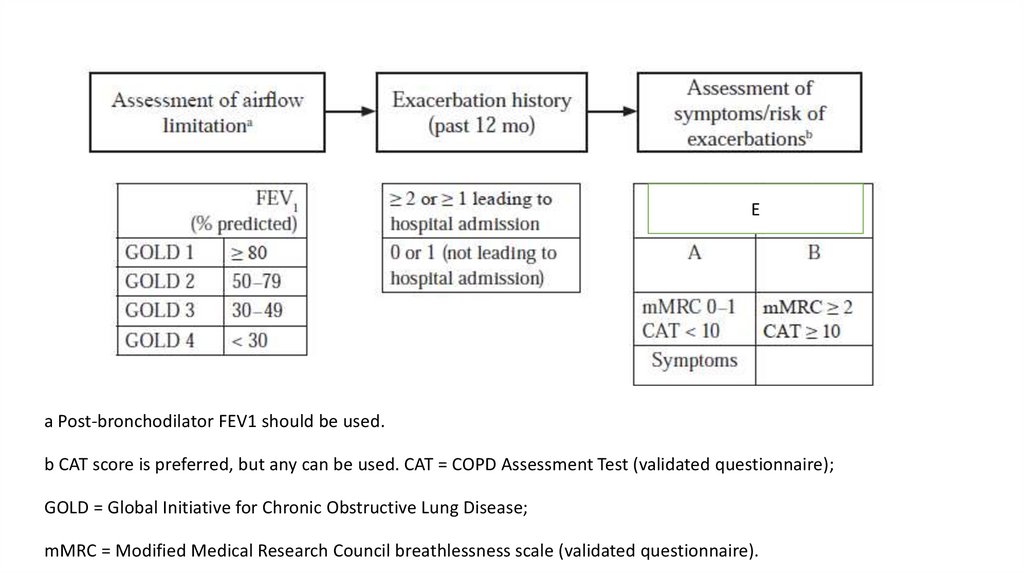
4. Patient assessment and selection of therapya. GOLD guidelines combine symptoms (on the basis of
symptom scores), airflow limitation (on the basis of postbronchodilator FEV1), and frequency of exacerbations to
determine patient risk group and recommended treatment .
b. ACP/ACCP/ATS/ERS guidelines simplify treatment even
further on the basis of FEV1 in patients with COPD with
symptoms. They do not provide detailed treatment guidelines
17.
Ea Post-bronchodilator FEV1 should be used.
b CAT score is preferred, but any can be used. CAT = COPD Assessment Test (validated questionnaire);
GOLD = Global Initiative for Chronic Obstructive Lung Disease;
mMRC = Modified Medical Research Council breathlessness scale (validated questionnaire).
18.
5. GOLD Guidelines: Initial Pharmacologic TreatmentPatient group
A
B
E
Symptoms and Exacerbations
Few symptoms (CAT score < 10) No hospitalizations ≤
1 exacerbation in the past year
Many symptoms (CAT score ≥ 10) No hospitalizations
≤ 1 exacerbations in the past year
Few or many symptoms ≥ 1 COPD-related
hospitalization or ≥ 2 exacerbations in the past year
Recommended Initial
Treatment
Bronchodilator (short- or
long-acting)
LABA + LAMAa
LABA + LAMAa
LABA + LAMA +ICSa,b
Single inhaler may be more convenient and effective than multiple inhalers.
bConsider if eosinophils > 300 cells/mcL.
CAT = COPD Assessment Test (validated questionnaire); COPD = chronic obstructive pulmonary disease;
GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled corticosteroid; LABA = longacting b2-agonist; LAMA = long-acting anticholinergic/muscarinic antagonist.
a
19.
6. Follow-up Treatment for DyspneaCurrent
Recommended Treatment Change(s)
Treatment
LABA or
LABA + LAMA If ineffective: consider changing inhaler devices
LAMA
or molecules, and investigate other causes of dyspnea
Consider changing inhaler devices or molecules, implement or
escalate non-pharmacologic treatments or investigate and
treat other causes of dyspnea
LABA +
LAMAa
Single inhaler may be more convenient and effective than multiple inhalers.
LABA = long-acting β2-agonist; LAMA = long-acting anticholinergic/muscarinic antagonist.
a
20.
7. Follow-up Treatment for ExacerbationsCurrent Treatment
Recommended Treatment Change(s)
LABA or LAMA
LABA + LAMAa (if blood eosinophils < 300 cells/mcL)
OR
LABA + LAMA + ICSa (if blood eosinophils ≥ 300 cells/mcL)
LABA + LAMA + ICSa (if blood eosinophils ≥ 100 cells/mcL)
OR
If blood eosinophils < 100 cells/mcL, consider adding roflumilast if FEV1 < 50% and chronic
bronchitis
AND/OR azithromycin daily if former smoker
LABA + LAMA
LABA + LAMA
+ ICS
Consider de-escalation of ICS and change to LABA + LAMAb
OR
Consider adding roflumilast if FEV1 < 50% and chronic bronchitis
AND/OR azithromycin daily if former smoker
Single inhaler may be more convenient and effective than multiple inhalers.
b Consider if pneumonia, or other considerable adverse effects. De-escalation is more likely to be associated with
future exacerbations if blood eosinophil count is ≥ 300 cells/mcL.
a
21.
8. Other pharmacologic treatmentsa. Phosphodiesterase-4 inhibitor: Roflumilast (Daliresp)
i. Indication: As a daily treatment to reduce the risk of COPD exacerbations in
patients with severe COPD (FEV1 less than 50% of predicted) associated
with chronic bronchitis and a history of frequent exacerbations
ii. Mechanism: Reduces inflammation through inhibition of the breakdown of
intracellular cyclic adenosine monophosphate; no direct bronchodilator
activity
iii. Dose: 500 mcg orally once daily
22.
b. Influenza vaccine annually (essential for all patients)c. Pneumococcal vaccine (essential for all patients)
d. Tdap vaccination should be provided to patients with COPD to protect against
tetanus, diphtheria, and pertussis if not vaccinated during adolescence.
e. COVID-19 vaccination according to CDC guidelines
f. Shingles vaccination according to CDC guidelines
23.
g. α1-Antitrypsin augmentation therapy:i. For young patients with severe hereditary α1-antitrypsin
deficiency and established emphysema, but an expensive
treatment
ii. Patients with α1-antitrypsin deficiency usually are white, usually
develop COPD at a young age (younger than 45 years), and have a
strong family history of it. It may be worthwhile screening such
patients.
24.
9. Non-pharmacologic therapya. Home oxygen therapy
i. Recommended in patients who have a Pao2 of 55 mm Hg or Sao2 of 88% or
less.
ii. Long-term (more than 15 hours/day) use in patients with chronic respiratory
failure improves survival.
b. Pulmonary rehabilitation
i. Essential for patient groups B–D
ii. Includes exercise training, nutrition counseling, and education
iii. Improves many outcomes in COPD, including quality of life and survival
25.
10. New data in COPDa. Antibiotic (azithromycine) use in COPD
i. Actions are anti-inflammatory and antibacterial
ii. Daily azithromycin at 250 mg orally daily for 1 year found to lengthen time to first
exacerbation, decrease overall exacerbation rate, and improve quality of life
iii. Azithromycin 500 mg orally 3 times per week and erythromycin 500 mg orally
twice daily have reduced exacerbations
iv. Potential adverse effects include hearing loss (contradictory evidence),
pneumonia, GI disturbances, and QTc prolongation
v. Recommended as add-on to treatment intensification with LABA + LAMA +/- ICS if
eosinophils < 100 cells/mcL and former smoker
26.
b. β-Blockersi. long-term treatment with β-blockers may reduce risk of exacerbations and
improves survival, even in patients without overt cardiovascular disease.
ii. It is too early to recommend β-blockers for the treatment of COPD, but βblockers should not be withheld in patients with COPD who also have
heart disease, chronic heart failure (CHF), or other cardiovascular
conditions in which β-blockers are beneficial
iii. Mechanism for benefit in COPD is unknown, but β-blockers can upregulate β2-receptors in the lungs, which may improve the effectiveness of
inhaled β-agonists.
27.
Management of Acute Exacerbations of Chronic COPD1. A COPD exacerbation is an acute worsening of a patient’s baseline respiratory
symptoms (dyspnea and/ or cough and/or an increase in quantity or purulence of
sputum) that is worse than normal day-to-day variation and results in a change in
medication. Diagnosis is based purely on clinical presentation.
2. Common precipitating factors include upper respiratory tract infections (most
common) and air pollution, but the cause of one-third of exacerbations cannot be
determined.
3. Spirometry is not accurate during an exacerbation and is not recommended.
4. Pulse oximetry can be used to determine the need for supplemental oxygen,
which should be given in severe exacerbations.
28.
Pulse oximetry29.
5. Classification of acute exacerbation:a. Mild: SA bronchodilators only
b. Moderate: SA bronchodilators plus antibiotics and/or oral corticosteroids
c. Severe: hospitalization or ED visits
i. No respiratory failure: respiratory rate of 20 to 30 breaths/minute; no accessory
muscles; no mental status changes; hypoxemia improved with supplemental oxygen;
no increase in PaCO2
ii. Acute respiratory failure, non–life-threatening: more than 30 breaths/min;
accessory respiratory muscles in use; no change in mental status; hypoxemia
improved with supplemental oxygen; PaCO2.increased compared to baseline
iii. Acute respiratory failure, life-threatening: more than 30 breaths/minute
accessory respiratory muscles in use; acute changes in mental status; hypoxemia not
improved with supplemental oxygen; PaCO2 increased compared to baseline or pH of
7.25 or greater
30.
Management of Acute Exacerbations of Chronic COPD6. Systemic corticosteroids are effective, and they shorten
recovery time, improve FEV1, and improve hypoxemia. They
may also lower the length of hospital stay.
7. Systemic corticosteroids should be used in most
exacerbations (very important);
i. According to one study: a shorter course of systemic
corticosteroids (5 days) was non-inferior to a longer (14
days) course with respect to re-exacerbation within 6
months.
31.
9. Antibiotic treatmenta. should be initiated for exacerbations if the criteria below are met.
i. The three cardinal symptoms in COPD exacerbations are 1) increased
dyspnea, 2) increased sputum volume, and 3) increased sputum purulence.
ii. Antibiotics should be given if all three cardinal symptoms are present.
iii. Antibiotics should be given if two of the three cardinal symptoms are
present AND if increased sputum purulence is one of the symptoms.
iv. Antibiotics should be given to patients with a severe exacerbation requiring
mechanical ventilation.
v. Recommended duration of antibiotic treatment is usually 5–10 days.
32.
b. The most common pathogens in COPD exacerbations: Streptococcus pneumoniae,Haemophilus influenzae, and Moraxella catarrhalis. In patients with GOLD 3 and 4
severity, Pseudomonas aeruginosa should be considered a potential pathogen..
c. Recommended antibiotics:
i. Usual initial antibiotics for uncomplicated COPD include azithromycin,
clarithromycin, doxycycline, trimethoprim/sulfamethoxazole, and amoxicillin,
with or without clavulanate.
ii. In complicated COPD with risk factors: Amoxicillin/clavulanate, levofloxacin,
moxifloxacin.
iii. Risk factors for complicated COPD: Comorbid diseases, severe COPD (FEV1 less
than 50% of predicted), greater than 3 exacerbations/year, antibiotic use in past
3 months
33.
iv. If at risk of Pseudomonas infection: High-dose levofloxacin(750 mg) or ciprofloxacin; obtain sputum culture.
v. Risk factors for Pseudomonas infections are: 1) Four or
more courses of antibiotics in past year, 2) recent
hospitalization (past 90 days), 3) isolation of Pseudomonas
during past hospitalization,
4) severe COPD (FEV1 less than 50% of predicted)
vi. If exacerbation does not respond to initial antibiotic, then
sputum culture and sensitivity should be performed.






















 medicine
medicine