Similar presentations:
Plutonium. The use of plutonium
1.
Kazan National Research Technological UniversityPLUTONIUM
2023
Student: Salimov Airat
4121-13
2.
■ Plutonium is a radioactive chemicalelement atomic number 94. It is an
metal of silvery-gray appearance that
tarnishes when exposed to air, and
forms a dull coating when oxidized.It
is radioactive and can accumulate in
bones, which makes the handling of
plutonium dangerous. Plutonium was
first synthetically produced and
isolated in late 1940 at the University
of California, Berkeley.
3.
■ The use of plutoniumIt is a heat source in thermoelectric generators, which are used to power some
spacecraft. Plutonium isotopes are expensive and inconvenient to separate, so
particular isotopes are usually manufactured in specialized reactors. Plutonium is also
used in the production of nuclear weapons, as in some nuclear power plants. It used
to be used in medicine, namely in pacemakers.
4.
Plutonium-239There are also isotopes of
plutonium, there are about 6
of them. The most famous of
them is Plutonium-239. The
nuclear properties of
plutonium-239 were also
studied; researchers found
that when it is hit by a
neutron it fissions by
releasing more neutrons and
energy.
5.
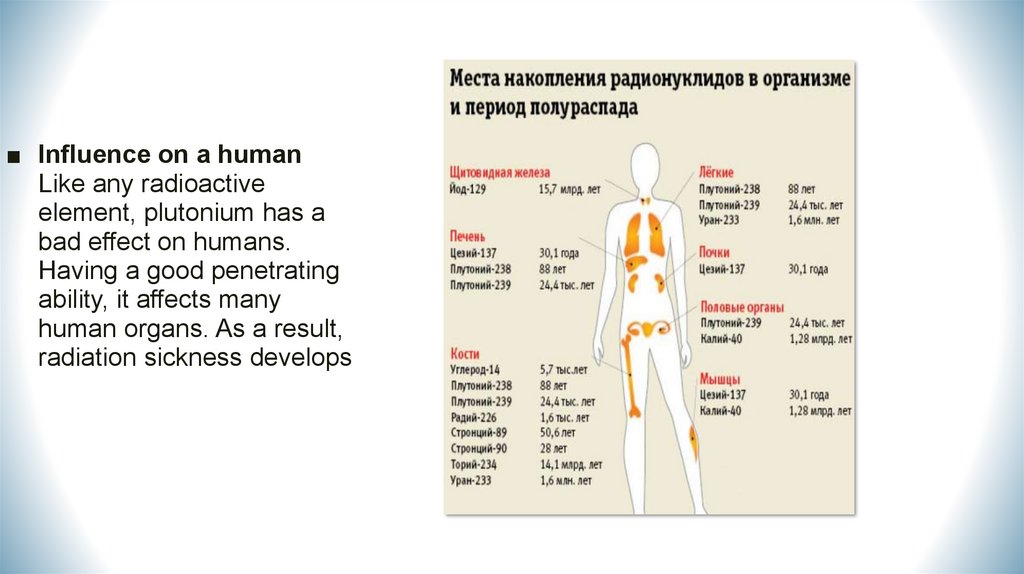
■ Influence on a humanLike any radioactive
element, plutonium has a
bad effect on humans.
Having a good penetrating
ability, it affects many
human organs. As a result,
radiation sickness develops
6.
■ Sad day of NagasakiOn August 9, 1945, a huge plane appeared in the sky in Nagasaki. And he dropped
an atomic bomb on Earth. Inside it was a ball no bigger than a fist, made of plutonium.
A few seconds later, a huge "mushroom" grew right out of the Ground. Which
eventually claimed 40,000 human lives.
Currently, many weapons containing plutonium have been dismantled. However, the
plutonium contained in them does not disappear anywhere.
Вefore
After





 biology
biology chemistry
chemistry








