Similar presentations:
Nucleic Acid Templated Chemistry (NATC) for the treatment of cancer
1.
Nucleic Acid TemplatedChemistry (NATC) for the
treatment of cancer
developed
by Cancevir AG
By Pavel Sergeev, PhD, email: p.sergeev@cancevir.com
Phone: +41 78 630 40 70
Non-confidential presentation
2.
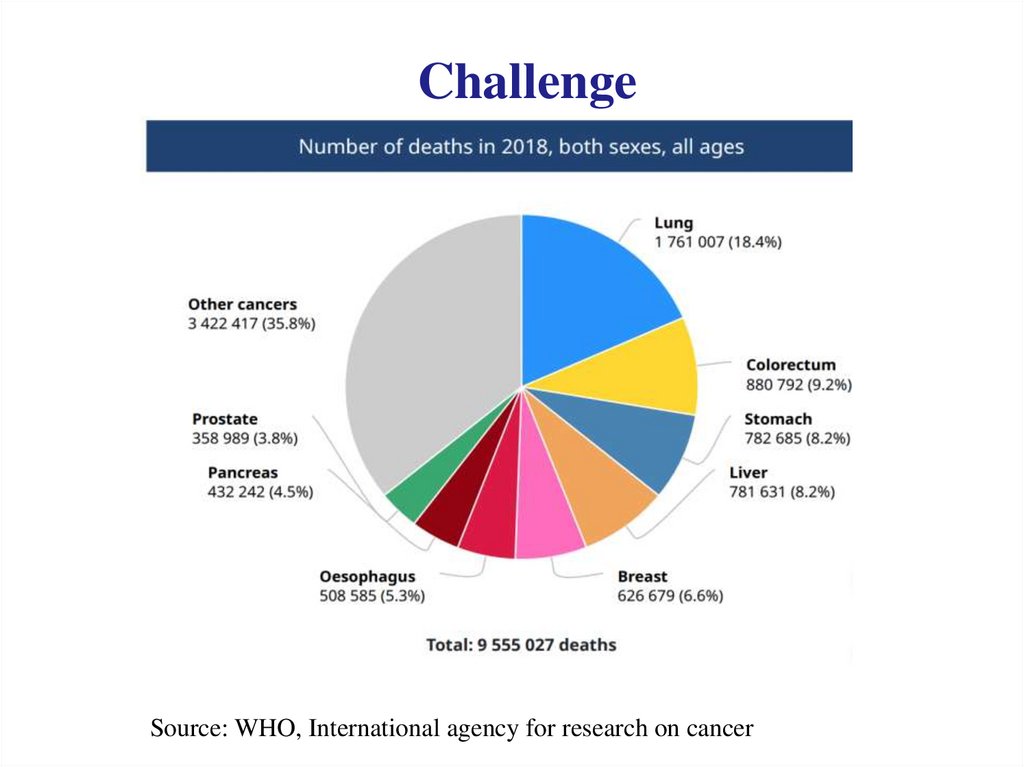
ChallengeSource: WHO, International agency for research on cancer
3.
Vision of CANCEVIR AG• We strive to develop an absolute new medical technology
against cancer diseases at metastatic stage
• We are dedicated to develop a highly specific and efficient
treatment to transform the perspective on approaches of
curing cancer
• Our goal is to have a cancer treatment without side effects
on the heart, liver and other organs of patients for every
type of cancer
4.
NATC technology selective tumor cellkilling
Inactive precursor of medication 1
linked to Oligonucleotide (stable)
Tumor cell
Inactive precursor of medication 2
linked to Oligonucleotide (stable)
tumor specific RNA
(mutated or overexpressed)
active drug is formed =>tumor killing
Tumor cell
5.
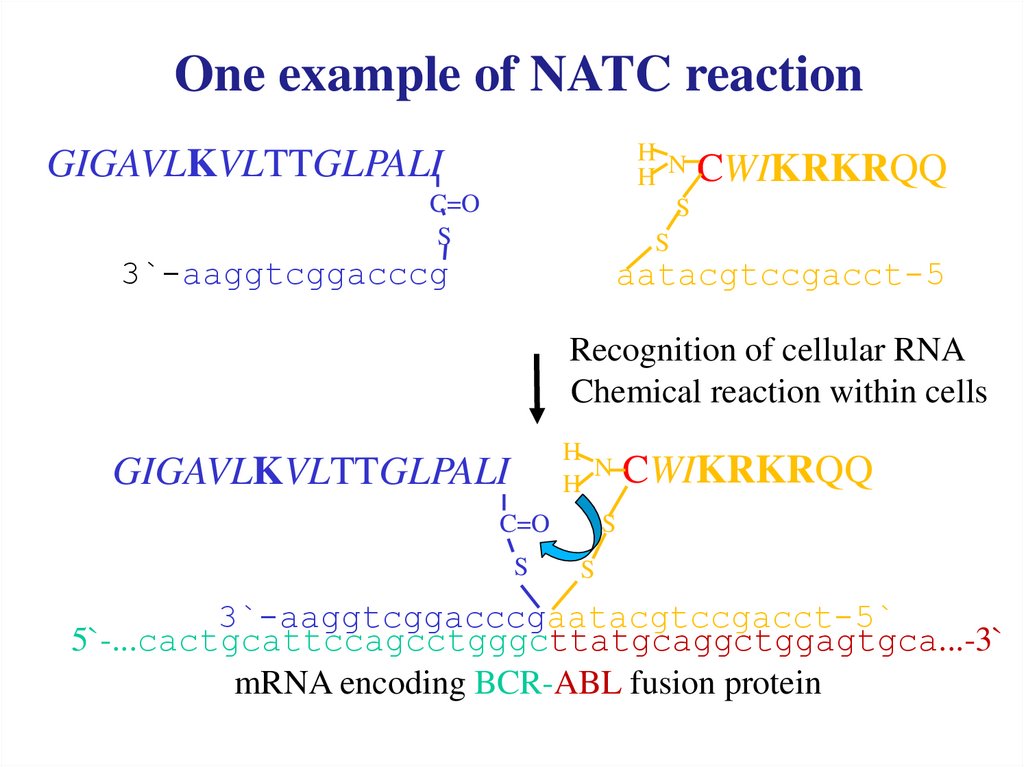
One example of NATC reactionH N
CWIKRKRQQ
H
S
S
GIGAVLKVLTTGLPALI
C=O
S
3`-aaggtcggacccg
aatacgtccgacct-5
Recognition of cellular RNA
Chemical reaction within cells
H
N CWIKRKRQQ
H
GIGAVLKVLTTGLPALI
C=O
S
S
S
3`-aaggtcggacccgaatacgtccgacct-5`
5`-...cactgcattccagcctgggcttatgcaggctggagtgca...-3`
mRNA encoding BCR-ABL fusion protein
6.
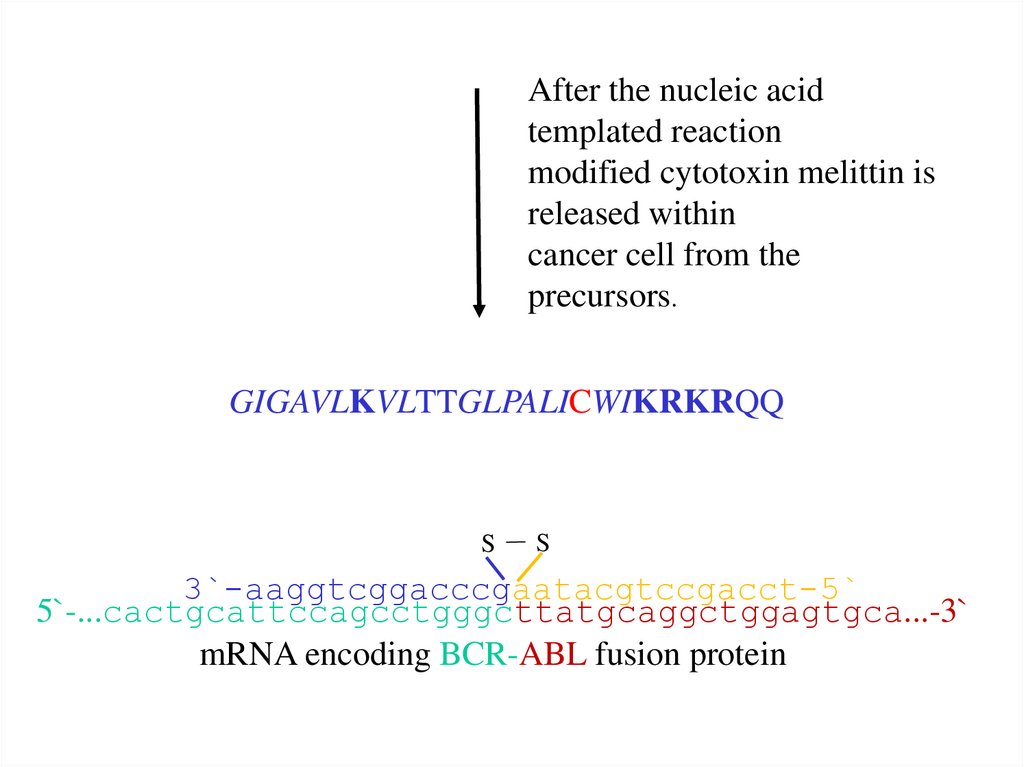
After the nucleic acidtemplated reaction
modified cytotoxin melittin is
released within
cancer cell from the
precursors.
GIGAVLKVLTTGLPALICWIKRKRQQ
S
S
3`-aaggtcggacccgaatacgtccgacct-5`
5`-...cactgcattccagcctgggcttatgcaggctggagtgca...-3`
mRNA encoding BCR-ABL fusion protein
7.
NATC technology offers manyadvantages
• The active drug is only formed in tumor cells => specific killing
• Healthy cells are spared because they don’t have mutated RNA
• Somatic mutations leading to cancer are the target of medications
• Cancer of different origin can be targeted by the technology
• The NATC technology is able to recognize even the change of only
one nucleotide from 3 bln. nucleotides in Human genome
8.
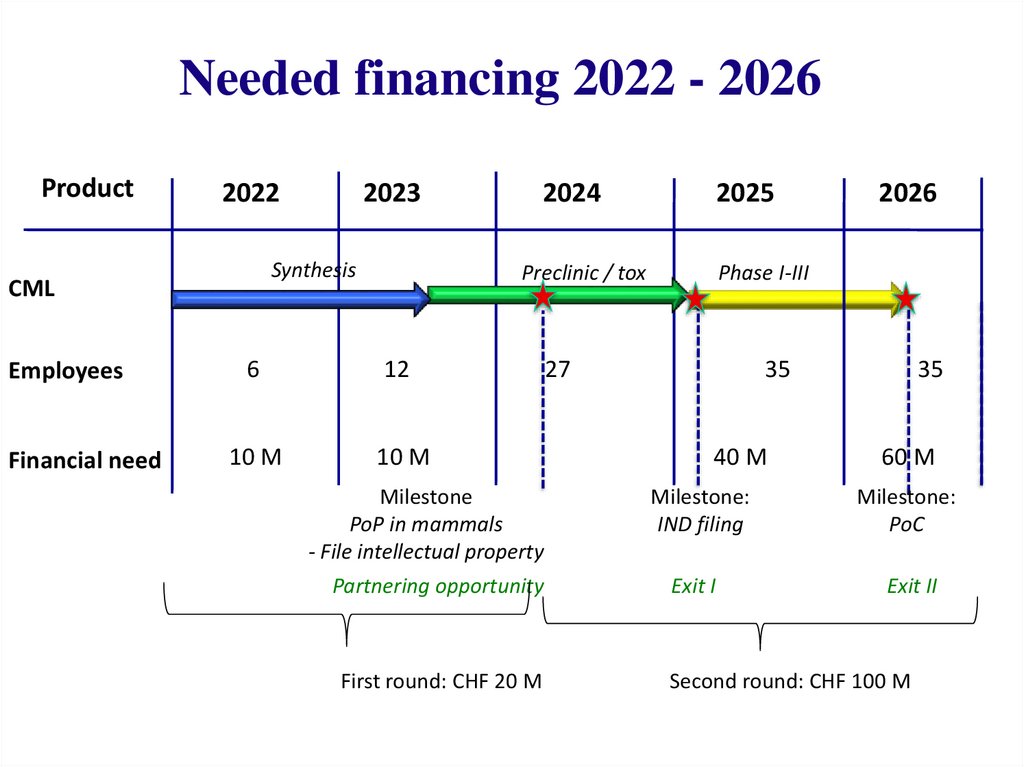
Needed financing 2022 - 2026Product
2022
Synthesis
CML
Employees
Financial need
2023
2024
2025
Preclinic / tox
6
12
10 M
10 M
2026
Phase I-III
27
35
40 M
35
60 M
Milestone
PoP in mammals
- File intellectual property
Milestone:
IND filing
Milestone:
PoC
Partnering opportunity
Exit I
Exit II
First round: CHF 20 M
Second round: CHF 100 M
9.
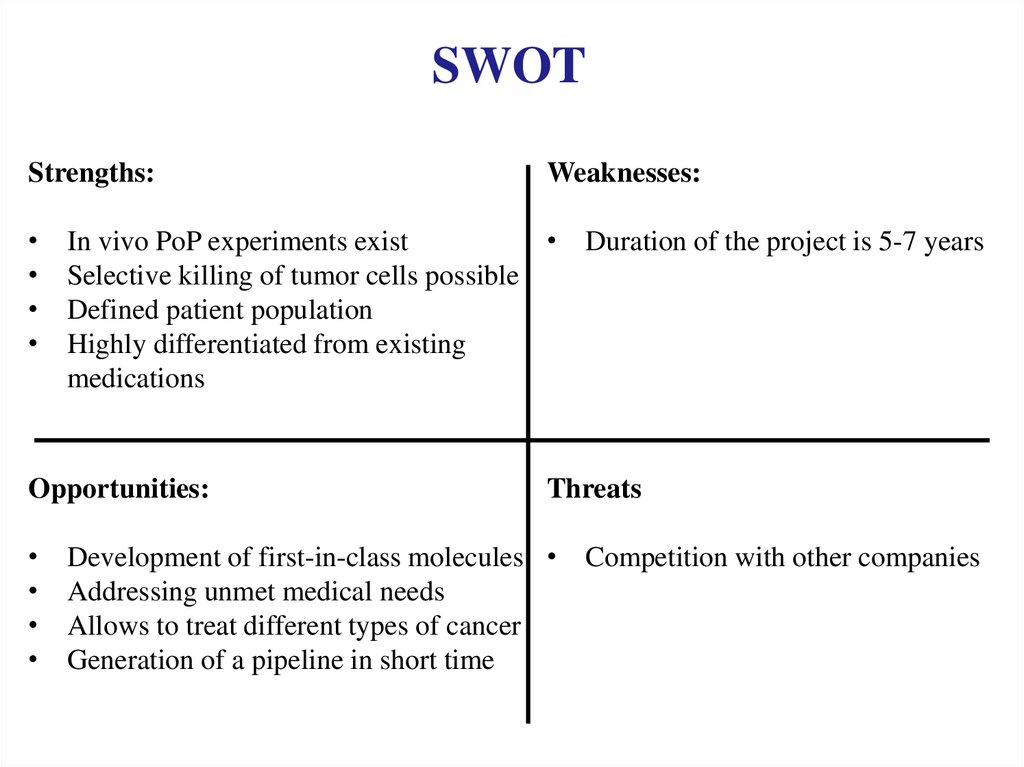
SWOTStrengths:
In vivo PoP experiments exist
• Duration of the project is 5-7 years
Selective killing of tumor cells possible
Defined patient population
Highly differentiated from existing
medications
Opportunities:
Weaknesses:
Threats
Development of first-in-class molecules • Competition with other companies
Addressing unmet medical needs
Allows to treat different types of cancer
Generation of a pipeline in short time
10.
Thank you very muchfor your attention





 medicine
medicine chemistry
chemistry