Similar presentations:
Bladder cancer
1. Bladder cancer
Maya Kolin, MDDepartment Of Oncology
Hillel Yaffe Medical Center
29.11.2021
2.
Bladder cancer represents the fifth most common cancerdiagnosis
• Annually in the United States were diagnosed >76,000 new cases of bladder cancer and
16,000 deaths
• While cancers of the renal pelvis are less frequent than bladder cancer, an additional
20,000 new cases and 5000 deaths are estimated every year
• Bladder cancer typically affects older patients with a median age at diagnosis of 73
years
• Males are four times more frequently affected than females
• More common in Caucasians than in Asian patients
3.
Risk factors for bladder cancer• Smoking of tobacco products (cigarettes, cigars, pipes, etc.) remains
the overwhelming leading risk factor for development of bladder cancer
Among new bladder cancer diagnoses, 90% of cases occur in current
or former smokers
• Toxicologists have estimated that over 70 confirmed carcinogenic
toxins are present within tobacco smoke
• One-third of bladder cancer cases could be prevented through simple
modification of lifestyle choices, in particular cessation of smoking
4.
Other risk factors for bladder cancer• Exposures to hair dyes and hair sprays in workers in the hairstyling
• Much concern has been raised regarding use of the antidiabetic medication,
pioglitazone, and bladder cancer risk
• Chronic inflammatory states and the development of squamous bladder cancer in
patients chronically infected with schistosomiasis and in paraplegic patients with
chronic indwelling catheters
5.
Other risk factors• Aromatic amines benzidine and beta-naphthylamine that can present in industrial
dyes
• Arsenic that can be found in some drinking water supplies in underdeveloped
countries
• Chemicals in the leather, paint, rubber, textiles, and printing industries have been
associated with bladder cancer
6.
Genetic risk factors• Patients with defects in
mismatch repair genes leading
to microsatellite instability
(MLH1, MSH2, MSH6, etc.) as
part of the familial cancer Lynch
syndrome (also known as
hereditary non-polyposis
colorectal cancer, or HNPCC)
• People with Lynch syndrome
have an increased risk of colon,
endometrial cancers… and
might also have an increased
risk of bladder cancer (as well as
other cancers of the urinary
tract)
7.
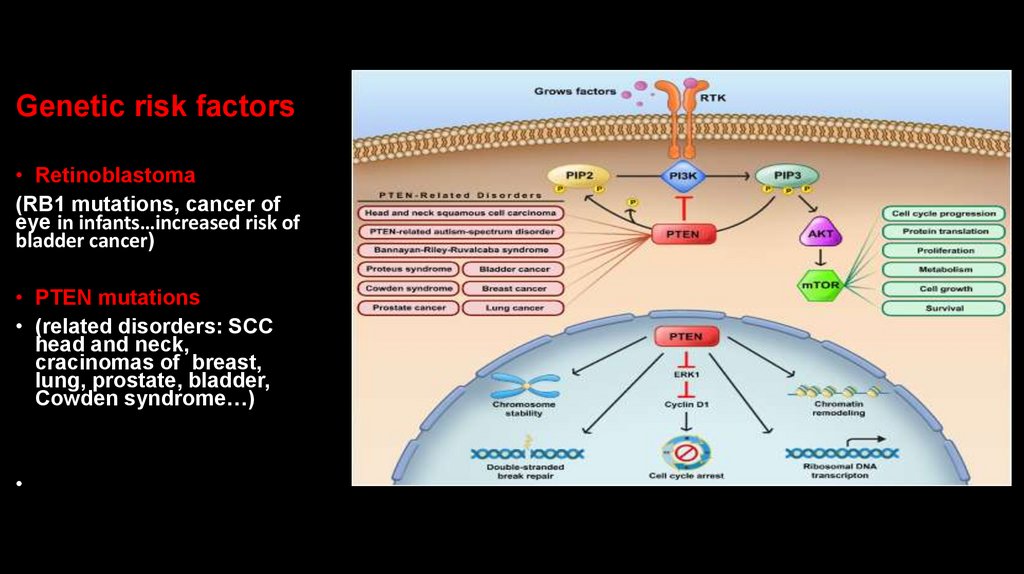
Genetic risk factors• Retinoblastoma
(RB1 mutations, cancer of
eye in infants…increased risk of
bladder cancer)
• PTEN mutations
• (related disorders: SCC
head and neck,
cracinomas of breast,
lung, prostate, bladder,
Cowden syndrome…)
8.
Genetic risk factors• Germline pathogenic variants in
the phosphatase and tensin
homolog (PTEN) gene are
described in a variety of rare
syndromes that are collectively
known as PTEN hamartoma tumor
syndromes (PHTS)
PTEN hamartoma tumor
syndromes (PHTS) are inherited in
an autosomal dominant fashion,
and have an increased risk of both
benign and malignant tumors
• The defining clinical feature of
PTEN hamartoma tumor
syndromes (PHTS) is the presence
of hamartomatous tumors, which
are disorganized growth of native
cells in native tissues
9.
Genetic risk factors• Cowden syndrome is
associated with mutations
in PTEN tumor
suppressor gene, that results in
dysregulation of
the mTOR pathway leading to
errors in cell proliferation, cell
cycling, and apoptosis
• Cowden syndrome is
an autosomal
dominant inherited condition
characterized by benign
overgrowths
called hamartomas as well as
an increased lifetime risk
different cancers
• The most common malignancies associated
with Cowden syndrome, are
adenocarcinoma of the breast (20%),
followed by adenocarcinoma of the thyroid
(7%), squamous cell carcinomas of the
skin (4%), and the remaining from the colon,
uterus…
• 99% of patients report mucocutaneous
symptoms by age 20-29
10.
Clinical presentation of bladder cancer• Painless hematuria (either gross or microscopic) is the initial most often
manifestation of urinary tract cancer
• Flank pain in association with cancer of renal pelvis, or cancer of urteter,
or due to hydro nephrosis caused by bladder tumor obstructing the
orifice of the ureter within the bladder
• Significant cachexia and widespread metastatic disease in rare cases
11.
Clinical presentation of bladder cancer• In females, hematuria due to malignancy can often be mistaken
for a urinary tract infection or menstrual bleeding
• While treatment with antibiotics is warranted if a concurrent
urinary tract infection is suspected, persistent hematuria
requires further workup
• Painless hematuria in males is almost always abnormal and
should be worked up
12.
Work up• Urine cytology ( is positive in only 50% of patients with high-grade bladder cancers)
• Visual examination of the bladder by cystoscopy
• Ureteroscopy or retrograde pyelography for patients in whom upper tract tumors are
suspected
• Because of the increased sensitivity and reduced IV contrast loads, CT scan uro gram
have replaced IV pyelogram for upper urinary tract imaging
• A magnetic resonance MR urogram in patients with poor renal function
13.
WorkupIn all patients with abnormalities noted in the bladder or upper
urinary tracts, complete endoscopic resection for histologic
diagnosis and staging should be performed via either
transurethral resection of bladder tumor (TURBT) or
endoscopic resection of upper tract tumors
14.
HistologyUrothelial carcinoma is
the most common
cancer histology (~90%
of cases)
Bladder and urinary tract cancer histologies. A. Urothelial carcinoma; B. squamous cell
carcinoma; C. small-cell carcinoma; D. plasmacytoid variant. (Courtesy of Alex Baras,
MD, PhD, Johns Hopkins University Department of Pathology.)
15.
HistologySome variants of urothelial
histology including
micropapillary and plasmacytoid
are associated with worse
surgical outcomes compared to
urothelial carcinoma
Bladder and urinary tract cancer histologies. A. Urothelial carcinoma; B.
squamous cell carcinoma; C. small-cell carcinoma; D. plasmacytoid variant.
(Courtesy of Alex Baras, MD, PhD, Johns Hopkins University Department of
Pathology.)
16.
HistologyNon urothelial variant
histology including squamous
cell carcinoma,
adenocarcinoma, small-cell
carcinoma, and
carcinosarcoma account for
≤10% of urinary tract tumors
Bladder and urinary tract cancer histologies. A. Urothelial carcinoma; B. squamous
cell carcinoma; C. small-cell carcinoma; D. plasmacytoid variant. (Courtesy of Alex
Baras, MD, PhD, Johns Hopkins University Department of Pathology.)
17.
Low grade and high grade tumorsBiology
• Low grade papillary tumors of bladder are frequently recur but
rarely invade or metastasize
• High grade tumors are sometimes flat tumors that invade early,
leading to lethal metastatic disease
• In both of these phenotypes, loss of portions of chromosomes 9q
and 9p is an early molecular event
18.
Molecular biologyLow-grade tumors are
characterized by alterations in the
RAS/RAF signaling pathway with
activating FGFR3 mutations or
gene fusions present in 60–80% of
patients
19.
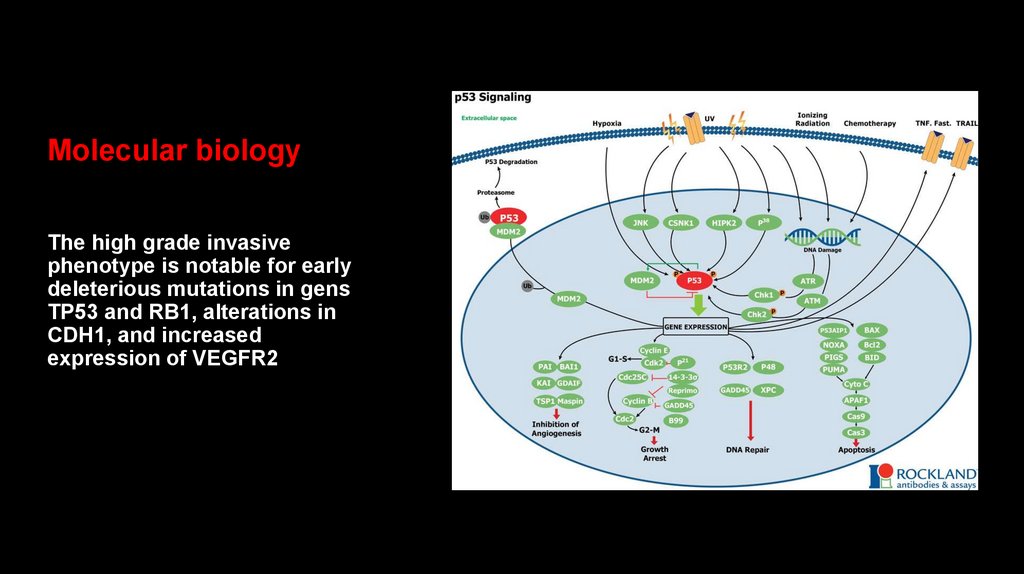
Molecular biologyThe high grade invasive
phenotype is notable for early
deleterious mutations in gens
TP53 and RB1, alterations in
CDH1, and increased
expression of VEGFR2
20.
Molecular biologyTesting for germline
mutations in these genes
is recommended:
10–20% of cases of urothelial
carcinoma of the renal pelvis and ureter
may be associated with Lynch
syndrome (hereditary defects in the
MLH1, MSH2, or MSH6 mismatch repair
genes leading to microsatellite
instability and frequent DNA mutations)
• In patients with upper urinary tract
urothelial carcinoma under the age
of 60 at diagnosis
• With a first-degree relative with a
Lynch syndrome–associated
cancer diagnosed under the age of
50
• Or with two first-degree relatives
with a Lynch syndrome–associated
cancer regardless of the age at
diagnosis
21.
Presentation of bladder cancerApproximately 75% of bladder cancers present with
non–muscle invasive bladder cancer (NMIBC)
• 18% of bladder cancers present with disease invading
into or through the muscular wall of the bladder
(muscle invasive bladder cancer (MIBC))
• 3% of bladder cancers present with metastatic
spread to distant organs
22.
The staging of bladder cancer is dependent:• On the depth of invasion within the bladder wall
• Involvement of lymph nodes
• Spread to surrounding and/or distant organs
23.
Non- muscle invasivebladder cancer (NMIBC)
• NMIBC involves only urothelial
mucosa (flat tumor-carcinoma
in situ [CIS] and Ta) or
penetrates into the connective
tissue below the urothelium (to
lamina propria-T1), but not into
the muscular layer -muscularis
propria
24.
Muscle-invasivebladder cancer (MIBC)
• MIBC invades into the
muscularis propria (T2),
through the muscularis
propria, to perivesical soft
tissue (T3), or into adjacent
pelvic organs such as the
rectum, prostate, vagina, or
cervix (T4)
25.
Bladder cancer TNM stagingUsed with permission of the American College of Surgeons, Chicago,
Illinois. The original source for this information is the AJCC Cancer
Staging Manual, Eighth Edition (2017) published by Springer
International Publishing. Corrected at 4th printing, 2018.
Graphic 110763 Version 8.0
26.
Bladder cancer staging• Stage IVa: T4b/ Any T/ Any N/ M0/M1a
• Stage IVB: Any T/ Any N/ M1b
27.
5-year overall survival rates• 80% for disease confined to the bladder
(stage I–II)
35–50% for disease that penetrates
through the bladder wall (stage III)
10–20% for disease extending to
surrounding organs, lymph nodes, or
metastatic sites (stage IV)
28.
Treatment of non- muscle invasive bladder cancer• Removal of all visible tumors by
TURBT is considered the
mainstay of surgical treatment
• Risk of recurrence can be
classified as low, intermediate,
or high
29.
Treatment of non- muscle invasive bladder cancerLow risk
Primary, solitary, low-grade Ta tumor,
<3 cm in diameter, no carcinoma in
situ (CIS)
Intermediate risk
All tumors not defined in the two adjacent
categories (between the category of low and
high risk)
• Any of the following: CIS, high-grade
disease, or T1 lesion
High risk
• Low-grade Ta tumors having all of the
following: multiple, recurrent, and
large (>3 cm)
30.
Treatment of non- muscle invasive bladder cancerFor patients with intermediate- or high-risk tumors,
weekly intravesical instillations for 6 consecutive weeks of
the attenuated mycobacterium strain known as BacilleCalmette Guerin (BCG) reduce the risk of recurrence at 12
months from 56 to 29%
• BCG treatment has been shown to decrease the rate of
progression to MIBC by 27%
31.
Treatment of non- muscle invasive bladder cancer• Intravesical BCG is generally well tolerated
• Side effects can include dysuria, urinary frequency,
bladder spasms, hematuria, and, in rare cases (<5%), a
systemic inflammatory response that can mimic
disseminated BCG infection
• Following a 6-week induction BCG schedule, additional
maintenance BCG treatments further reduce the risk of
recurrent NMIBC compared to induction BCG alone
32.
Treatment of non- muscle invasive bladder cancer• In patients with NMIBC that recurs long after initial BCG treatment,
a repeat course of BCG can be considered
• For patients with recurrence after a second induction course of
BCG or with relapsed NMIBC within 6 months of initial BCG
exposure, surgical removal of the entire bladder by cystectomy is
recommended due to the high risk of progression to muscle
invasive bladder cancer and potentially metastatic disease
• For patients who are not fit enough for or who refuse cystectomy,
non-BCG alternative intravesical agents (mithomycin C,
gemcitabine, docetaxel) can achieve temporary tumor responses
33.
Treatment of upper tract tumors• In patients with urothelial carcinoma of the renal pelvis or ureter,
endoscopic tissue acquisition and staging are challenging than
primary tumors located in the bladder
• Low risk tumors (processing all of the following: solitary tumor, low
grade, size <1 cm, no invasive component on imaging) can
successfully be treated by laser ureteroscopic ablation or surgical
resection and re anastomosis of the remaining ureter ends
34.
Muscle-invasive disease• In patients with urothelial carcinoma of the bladder that invades into
or through the muscularis propria but with no evidence of metastatic
spread, aggressive therapy is required to achieve cure (radical
cystectomy or bladder-sparing combined modality)
• In patients with no evidence of CIS or hydronephrosis, bladdersparing combined modality therapy (concurrent chemotherapy and
radiation) can achieve cure in ~65% of patients
• Various chemotherapy regimens have been utilized in combination
with radiation including cisplatin, 5-fluorouracil, mithomycin C,
paclitaxel, gemcitabine
35.
Bladder-sparing combined modality therapy• A maximal debulking of all visible tumor by TURBT is
required prior to initiation of chemoradiation of urinary
bladder
• In patients who achieve a complete response to
chemoradiation, regular cystoscopic monitoring of the
bladder is required, with salvage cystectomy offered to
patients who develop muscle -invasive bladder during
follow-up
36.
Partial cystectomy• Bladder-sparing partial cystectomy can be performed
in a very small subset of patients with muscle invasive
bladder cancer
• The ideal patient for partial cystectomy is the patient
with a solitary, clinical T2 urothelial carcinoma in the
dome of the bladder
37.
Resection of the entire bladder• In males a cystoprostatectomy with removal of the
bladder, prostate, and pelvic lymph nodes is performed
• in females an anterior exenteration with removal of the
bladder, uterus, ovaries, cervix, and pelvic lymph nodes
is performed
38.
With the bladder removed,three options exist to re-route
the urine outflow:
• In an ileostomy, the bilateral ureters are
connected to a portion of ileum that is
brought through an incision in the
abdominal wall to create a stoma that
drains urine into a bag outside of the
body
39.
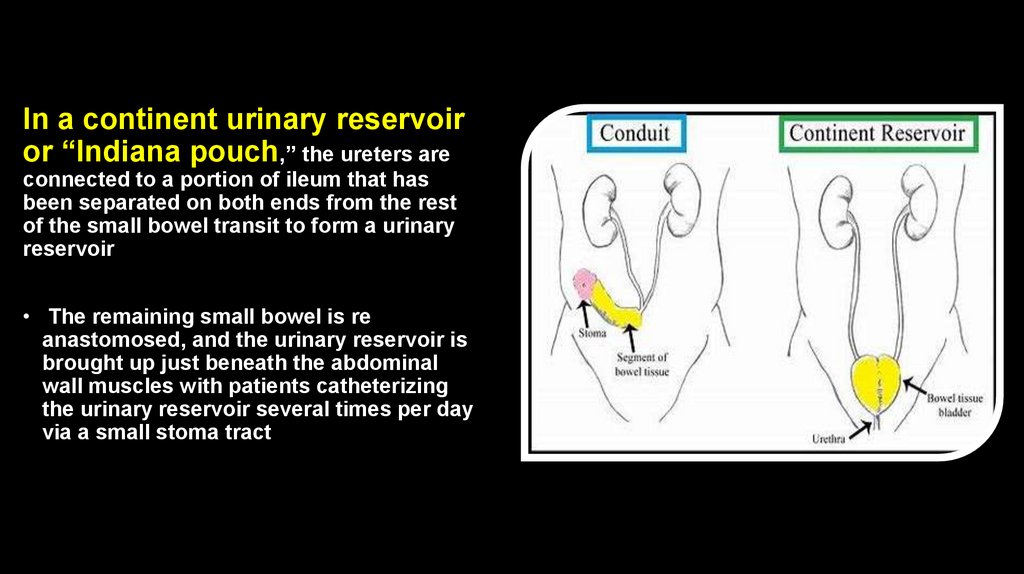
In a continent urinary reservoiror “Indiana pouch,” the ureters are
connected to a portion of ileum that has
been separated on both ends from the rest
of the small bowel transit to form a urinary
reservoir
• The remaining small bowel is re
anastomosed, and the urinary reservoir is
brought up just beneath the abdominal
wall muscles with patients catheterizing
the urinary reservoir several times per day
via a small stoma tract
40.
In a neo bladder, the sameurinary reservoir is brought down into
the pelvis and is anastomosed to the
remaining urethra to provide the
opportunity to void urine through the
urethra
41.
Muscle-invasive disease• The choice of which urinary reconstruction to perform is affected
by patient choice, anatomic tumor considerations and urologist
experience
• Regardless of the type of surgery performed, all patients undergo a
significant catabolic changes in their metabolism following
removal of the bladder:
o Weight loss in the first month postoperatively
o Long-term nutritional changes such as low B12 levels due to
alterations in small bowel physiology caused by all of the urinary
diversion options
42.
Neo adjuvant therapy• Despite aggressive surgery, only half of patients undergoing
cystectomy are cured by surgery alone
• Many clinical trials have investigated the role of systemic
chemotherapy before (neoadjuvant) or after (adjuvant) surgery
• Meta-analyses have shown a 5–10% absolute overall survival
advantage when combination chemotherapy regimens utilizing
cisplatin have been used before surgery
• Non–cisplatin-containing chemotherapy regimens have proven inferior
to cisplatin-containing regimens
• If patients are not suitable candidates for cisplatin administration due
to poor functional status or comorbidities (e.g., poor renal function),
they should proceed directly to surgery
43.
Adjuvant therapyWhat about benefit with cisplatin-based combination
chemotherapy given after surgery?
• The data in the adjuvant setting are based on smaller, older
trials
Furthermore, in the postoperative setting, some patients may
not recover sufficiently from their surgery within a time frame
optimal for chemotherapy administration
44.
Adjuvant therapyOther options?
Immunotherapy!
• Nivolumab for patients with high risk muscle invasive
urothelial carcinoma who had undergone radical
cystectomy!
• Disease free survival was longer among patients with
adjuvant nivolumab than in patients received placebo
(20.8 months versus 10.8 months) after radical
cystectomy
45.
Treatment of muscle -invasive disease non metastatic diseaseTreatment
Patient Selection
Clinical Outcomes
Bladder-sparing chemoradiation
No CIS, no hydronephrosis, maximal TURBT required
65% cure, 55% bladder intact, highly dependent on patient selection
Bladder-sparing partial cystectomy
Solitary tumors in dome of bladder are ideal
Variable, highly dependent on patient selection
Cystectomy
Any MIBC patient
50% cure with surgery alone, highly dependent on pathologic stage
Neoadjuvant cisplatin-based chemotherapy
Cisplatin-eligible MIBC patients
5–10% improvement in overall survival compared to cystectomy alone
Adjuvant cisplatin-based chemotherapy
Cisplatin-eligible high-risk postcystectomy MIBC patients Similar improvement as neoadjuvant treatment, data less robust, many
(pT3-4, N+)
patients not suitable for adjuvant treatment
Adjuvant nivolumab for patients with high risk muscle invasive
urothelial carcinoma who had undergone radical cystectomy
46.
Urothelial carcinoma of the upper urinary tract• For patients with high risk urothelial carcinoma of the upper
urinary tract, resection of the kidney and ureter (including the
ureter bladder cuff) by nephroureterectomy is preferred
• Segmental ureterectomy may be appropriate in patients with
decreased renal function in which nephron-sparing outcomes
are critical to prevent the need for dialysis
• In CIS patients, administration of BCG therapy via a
nephrostomy tube can be considered to preserve intact renal
function
47.
Urothelial carcinoma of the upper urinary tract• In retrospective series, the use of cisplatin-based
neoadjuvant chemotherapy has been associated with a
pathologic complete response at surgery of 14% in upper
tract urothelial carcinoma patients
• Adjuvant chemotherapy is considered in patients with
locally advanced stages (T3, T4, or node positive)
48.
Patients with metastatic urothelialcarcinoma need systemic therapy!
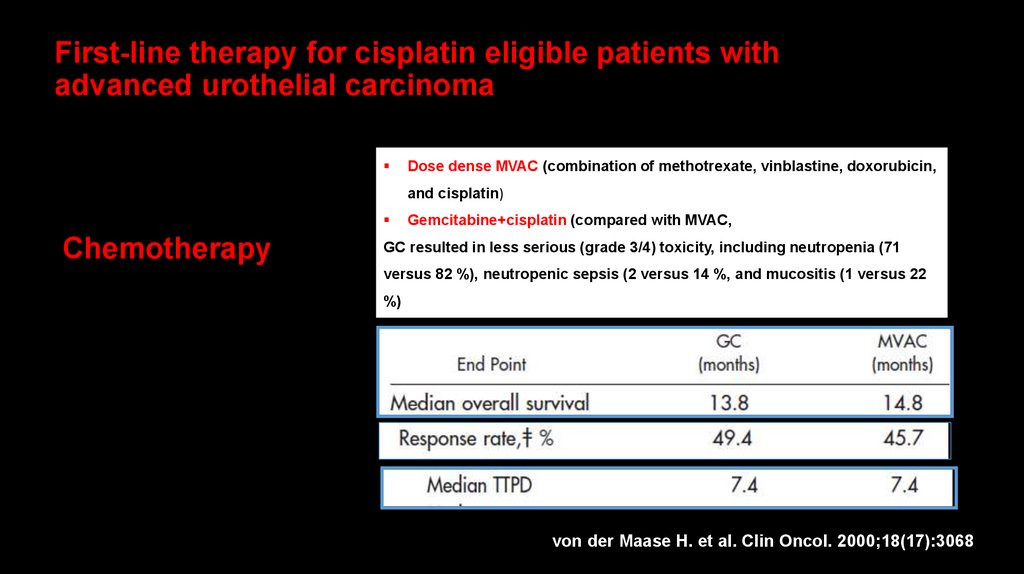
49. First-line therapy for cisplatin eligible patients with advanced urothelial carcinoma
Dose dense MVAC (combination of methotrexate, vinblastine, doxorubicin,and cisplatin)
Chemotherapy
Gemcitabine+cisplatin (compared with MVAC,
GC resulted in less serious (grade 3/4) toxicity, including neutropenia (71
versus 82 %), neutropenic sepsis (2 versus 14 %, and mucositis (1 versus 22
%)
von der Maase H. et al. Clin Oncol. 2000;18(17):3068
50.
Metastatic urothelial carcinoma• Treatment with either MVAC or CG has
remained the standard for first-line
treatment of patients with metastatic
urothelial carcinoma with adequate
renal function and functional status
suitable for cisplatin therapy
51.
Metastatic disease• For patients with lymph node only metastases and good
functional status, cure is achieved in 15–20% of patients
(unfortunately, only ~5% of metastatic patients fulfill both these
criteria)
• For most patients, chemotherapy may prolong survival, but
disease resistance proving lethal eventually develops
52.
Metastatic disease• Approximately half of patients with urothelial
carcinoma have renal insufficiency, comorbidities, or
frail functional status, and are not candidates for
cisplatin treatment
• In cisplatin-ineligible patients, carboplatin-based
chemotherapy regimens are most often used with
median overall survival rates decreased to 9.3 months
• Following front-line chemotherapy treatment, secondline chemotherapy regimens have shown modest 10–
20% response rates, but no overall survival benefit
53.
Metastatic disease• Platinum-based chemotherapy
is standard of care first line
treatment for advanced
urothelial carcinoma, however,
progression free survival and
overall survival are limited by
chemotherapy resistance
• Maintenance avelumab (an anti PDlL1
antibody) plus best supportive care as
compared with best supportive care
alone, significantly prolonged overall
survival (21.4 months in avelumab
group vs 14.3 months in control group)
among patients with urothelial
carcinoma who had disease that not
progressed with first line chemotherapy
54.
Immunotherapy• In recent years, exponential development of novel immunotherapy
approaches has occurred for patients with urothelial carcinoma
• The immune checkpoint inhibitors target programmed cell death
protein 1 (PD-1) and programmed death ligand 1 (PD-L1) have
demonstrated the most encouraging clinical benefits
o
In cancers, including urothelial carcinoma, PD-1/PD-L1 are often
upregulated on the tumor surface or immune cells in the tumor
microenvironment
o
Upregulated PD-1/PD-L1 in this situation serve as a mechanism of
immune escape that facilitates tumor growth
55.
Immunotherapy• Atezolizumab (an anti–PD-L1 antibody) was the first drug approved in the United
States for metastatic urothelial carcinoma in over two decades based on a
response rate of 15% in post platinum patients
• Pembrolizumab (an anti–PD-1 antibody) demonstrated an improvement in overall
survival from 7.4 to 10.3 months compared to standard second-line chemotherapy
options
• Pembrolizumab is recommended too for first -line therapy for patients not suitable
for platinum based chemotherapy
• Multiple other PD-1/PD-L1 agents have demonstrated clinical responses in
urothelial carcinoma
• Clinical trials investigating immunotherapy, chemotherapy combinations are
ongoing
56.
Targeted therapy-erdafitinib• Recently effectiveness of molecularly
targeted therapies in patients with
metastatic urothelial carcinoma, harboring
specific genetic alterations (e.g.,
activating FGFR3 mutations or gene
fusions) was demonstrated in clinical
trials
• The rate of confirmed response to
erdafitinib therapy in patients previously
treated with chemotherapy and/or
immunotherapy was 40% (3% complete
responses)!
• Erdafitinib was approved for patients with
metastatic bladder carcinoma and FGFR
alterations
57.
Targeted chemotherapy• Recently success of enfortumab vedotin,
which is an ADC (antibody-drug
conjugate), was demonstrated (ORR was
44% in previously treated patients)
• Enfortumab vedotin is a monoclonal
antibody that targets nectin-4 (a
transmembrane protein that regulates a
number of cellular functions including
angiogenesis and overexpressed in
multiple tumors including bladder cancer)
and is linked to vedotin (monomethyl
auristatin E) that inhibits tubulin
polymerization
58.
Targeted chemotherapy• It’s a way of getting the
chemotherapy to the cancer
cells!
• It’s also a way to try to reduce
toxicity!
59.
60.
תודה רבה!61. Molecular biology
• In 2014, the initial bladder cancer results of The Cancer Genome Atlas(TCGA) project were published (This effort comprehensively analyzed gene
mutations, fusions, expression, copy number variations, methylation, and
microRNA across the genome of patients with bladder urothelial carcinoma
treated with surgery)
• The initial findings include :
Molecular biology
o genomic alterations in genes (e.g., FGFR3, EGFR, ERBB2, ERBB3, PIK3CA,
TSC1, etc.) targetable by currently approved drugs or drugs in development
in 69% of patients
o
o
genomic alterations in chromatin modifying genes (KDM6A, MLL2,
CREBBP, EP300, etc.) in 89% of patients
hypermethylation of tumor suppressor genes in 34% of patients
o the identification by RNA sequencing of four distinct intrinsic molecular
subtypes (luminal 1, luminal 2, basal 3, and basal 4) closely resembling
luminal and basal sub classifications of breast cancer























































 medicine
medicine