Similar presentations:
Жалпы химия (Керімбаева К.З.)
1.
ԔȺɁȺԔɋɌȺɇ ɊȿɋɉɍȻɅɂɄȺɋɕɇɕԘ ȻȱɅȱɆ ɀԤɇȿ ԐɕɅɕɆɆɂɇɂɋɌɊɅȱȽȱ
ɈԘɌԚɋɌȱɄ ԔȺɁȺԔɋɌȺɇ ɆȿɆɅȿɄȿɌɌȱɄ ɉȿȾȺȽɈȽɂɄȺɅɕԔ
ɂɇɋɌɂɌɍɌɕ
ɄȿɊȱɆȻȺȿȼȺ Ʉ Ɂ
ɀȺɅɉɕ ɏɂɆɂə
ɥɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɚɪԑɚ ɚɪɧɚɥԑɚɧ
ɨԕɭ-ԥɞɿɫɬɟɦɟɥɿɤ ɧԝɫԕɚɭ
ɒɕɆɄȿɇɌ
2014
2.
ɍȾɄ 6 - 038 (6)Ɉԙɬԛɫɬɿɤ Ԕɚɡɚԕɫɬɚɧ ɦɟɦɥɟɤɟɬɬɿɤ ɩɟɞɚɝɨɝɢɤɚɥɵԕ ɢɧɫɬɢɬɭɬɵ
Ɉԕɭ ԥɞɿɫɬɟɦɟɥɿɤ ɤɟԙɟɫ ɲɟɲɿɦɿ ʋ ɯɚɬɬɚɦɚ ԕɚԙɬɚɪ ɠ
Ʉɟɪɿɦɛɚɟɜɚ Ʉ Ɂ
ɀɚɥɩɵ ɯɢɦɢɹ Ɉԕɭ-ԥɞɿɫɬɟɦɟɥɿɤ ɧԝɫԕɚɭ
-ɒɵɦɤɟɧɬ
ɉɿɤɿɪ ɠɚɡԑɚɧɞɚɪ
Ȼɟɝɠɿɝɿɬɨɜɚ Ʉ Ⱥ ± ɬ ԑ ɤ ɞɨɰɟɧɬ Ɇ Ԥɭɟɡɨɜ ɚɬɵɧɞɚԑɵ ɈԔɆɍ
ɒɚԑɪɚɟɜɚ Ȼ Ȼ ± ɯ ԑ ɤ ɞɨɰɟɧɬ ɈԔɆɉɂ
Ɉԕɭ-ԥɞɿɫɬɟɦɟɥɿɤ ɧԝɫԕɚɭɞɚ ɛɢɨɥɨɝɢɹ ɦɚɦɚɧɞɵԑɵɧɵԙ ɫɬɭɞɟɧɬɬɟɪɿ ԛɲɿɧ
ɠɚɥɩɵ ɯɢɦɢɹ ɩԥɧɿɧɟɧ ɥɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɚɪ ԛɣ ɬɚɩɫɵɪɦɚɥɚɪɵɧɵԙ
ɫԝɪɚԕɬɚɪɵ ɥɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɚɪɞɵԙ ɨɪɵɧɞɚɥɭ ԥɞɿɫɬɟɦɟɥɟɪɿ
ԕɚɠɟɬɬɿ ԕɨɧɞɵɪԑɵɥɚɪ ɦɟɧ ԕԝɪɚɥ-ɠɚɛɞɵԕɬɚɪ ɯɢɦɢɹɥɵԕ ɪɟɚɤɬɢɜɬɟɪ ɠԥɧɟ
ɵɞɵɫɬɚɪ ɟɪɿɬɿɧɿɞɿɥɟɪ ɤɟɥɬɿɪɿɥɝɟɧ Ɉԕɭ-ԥɞɿɫɬɟɦɟɥɿɤ ɧԝɫԕɚɭ ɠɨԑɚɪɵ ɨԕɭ
ɨɪɵɧɞɚɪɵɧɵԙ ȼ -Ȼɢɨɥɨɝɢɹ ɦɚɦɚɧɞɵԑɵɧɵԙ ɫɬɭɞɟɧɬɬɟɪɿɧɟ
ɚɪɧɚɥԑɚɧ ɩԥɧɧɿԙ ɬɢɩɬɿɤ ɛɚԑɞɚɪɥɚɦɚɫɵɧɚ ɫԥɣɤɟɫ ɠɚɡɵɥԑɚɧ
2
3.
Ɇ Ⱥ Ɂ Ɇ Ԝ ɇ ɕ1. Ɍɟɯɧɢɤɚɥɵԕ ԕɚɭɿɩɫɿɡɞɿɤ ɟɪɟɠɟɥɟɪɿɦɟɧ ɬɚɧɵɫɭ
ɋԛɡɭ ɬԝɧɛɚɧɵ ɠɭɭ Ɍɢɬɪɥɟɭ ............................................4
2. Ƚɚɡ ɬԥɪɿɡɞɿ ɡɚɬɬɚɪɞɵԙ ɦɨɥɟɤɭɥɚɥɵԕ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚɭ ........................15
3. ɀɚɣ ɠԥɧɟ ɤԛɪɞɟɥɿ ɡɚɬɬɚɪɞɵԙ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ ɦɨɥɹɪɥɵԕ
ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚɭ ...............................18
Ȼɟɣɨɪɝɚɧɢɤɚɥɵԕ ԕɨɫɵɥɵɫɬɚɪɞɵԙ ɧɟɝɿɡɝɿ ɯɢɦɢɹɥɵԕ
ԕɚɫɢɟɬɬɟɪɿ ....................................21
ɏɢɦɢɹɥɵԕ ɪɟɚɤɰɢɹɥɚɪɞɵԙ ɠɵɥɞɚɦɞɵԑɵ ...................................23
6. ȿɪɿɬɿɧɞɿɥɟɪ .........................................27
7 ɗɥɟɤɬɪɨɥɢɬɬɿɤ ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɭ ɬɟɨɪɢɹɫɵ ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ
ɪɇ ɚɧɵԕɬɚɭ ɬԥɫɿɥɞɟɪɿ ..............................................................30
ɋɭɞɵԙ ɢɨɧɞɵԕ ɤԧɛɟɣɬɿɧɞɿɫɿ ...33
Ɍԝɡɞɚɪ ɝɢɞɪɨɥɢɡɿ .................35
10. Ɍɨɬɵԑɭ-ɬɨɬɵԕɫɵɡɞɚɧɭ ɪɟɚɤɰɢɹɥɚɪɵ ...36
11. ɋɭɬɟɤ Ɉɬɬɟɤ ɋɭɬɟɤ ɩɟɪɨɤɫɢɞɿ ..............................................................39
12. 9ȱȱ ɬɨɩɬɵԙ ɪ-ɷɥɟɦɟɧɬɬɟɪɿ Ƚɚɥɨɝɟɧɞɟɪ.................................................43
9ȱ ɬɨɩɬɵԙ ɪ-ɷɥɟɦɟɧɬɬɟɪɿ .......................46
14. 9 ɬɨɩɬɵԙ ɪ-ɷɥɟɦɟɧɬɬɟɪɿ ....................50
15 ȱ9 ɬɨɩɬɵԙ ɪ-ɷɥɟɦɟɧɬɬɟɪɿ ...57
Ԥɞɟɛɢɟɬɬɟɪ ɬɿɡɿɦɿ
3
4.
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ 1Ɍɚԕɵɪɵɛɵ Ɍɟɯɧɢɤɚɥɵԕ ԕɚɭɿɩɫɿɡɞɟɧɞɿɪɭ ɟɪɟɠɟɥɟɪɿɦɟɧ ɬɚɧɵɫɭ
1. ɍɥɵ ɡɚɬɬɚɪɦɟɧ ɠԥɧɟ ɢɿɫɿ ɠɚɦɚɧ ɡɚɬɬɚɪɦɟɧ ɛɚɪɥɵԕ ɬԥɠɿɪɢɛɟɥɟɪɞɿ
ɥɚɫ ɚɭɚ ɫɨɪԑɵɲ ɲɤɚɮɬɵԙ ɿɲɿɧɞɟ ɠɚɫɚɣɞɵ
2. ɕɞɵɫԕɚ ɠɚԕɵɧ ԛԙɿɥɿɩ ɛԧɥɿɧɝɟɧ ɝɚɡɞɚɪɞɵ ɢɿɫɤɟɦɟɭ ɤɟɪɟɤ Ɍԥɠɿɪɢɛɟ
ɠɚɫɚɭ ɛɚɪɵɫɵɧɞɚ ɝɚɡɞɵԙ ɢɿɫɿɧ ɛɚɣԕɚɫɚԙɵɡ ɧɟɦɟɫɟ ɝɚɡɞɵԙ ɫԝɣɵԕɬɵԑɵɧ
ɤԧɪɫɟԙɿɡ ɚԕɵɪɵɧ ɢɿɫɤɟɝɟɧ ɚɭɚɧɵ ɤɟɪɿ ɲɵԑɚɪɵԙɵɡ
3. Ʉɨɧɰɟɧɬɪɥɟɧɝɟɧ ԕɵɲԕɵɥɞɚɪɞɵ ɫԝɣɵɥɬԕɚɧɞɚ ԥɫɿɪɟɫɟ ɤԛɤɿɪɬ
ԕɵɲԕɵɥɵɧ ԕɵɲԕɵɥɞɵ ɫɭԑɚ ԕԝɣɵԙɵɡɞɚɪ
4. Ɋɟɚɤɬɢɜɬɟɪɞɿ ɵɞɵɫɬɚɪԑɚ ԕԝɣԑɚɧɞɚ ԧɡɿԙɿɡɞɿ ɵɞɵɫԕɚ ɠɚԕɵɧ
ԝɫɬɚɦɚԙɵɡ ɠԥɧɟ ɠɚԕɵɧ ɟԙɤɟɣɿɩ ԕɚɪɚɦɚԙɵɡ Ɋɟɚɤɬɢɜ ɛɟɬɿԙɿɡɝɟ ɧɟɦɟɫɟ
ɤɢɿɦɿԙɿɡɝɟ ɲɚɲɵɪɚɭɵ ɦԛɦɤɿɧ
5. Ԕɵɡɞɵɪɵɥԑɚɧ ɫԝɣɵԑɵ ɛɚɪ ɵɞɵɫԕɚ ɠɚԕɵɧ ɟԙɤɟɣɿɩ ԕɚɪɚɦɚԙɵɡ
Ʉɟɣɛɿɪ ɠɚԑɞɚɣɥɚɪɞɚ ɫԝɣɵԕ ɵɞɵɫɬɚɧ ɚɬԕɵɥɚɭɵ ɦԛɦɤɿɧ
6. ɉɪɨɛɢɪɤɚɧɵ ԕɵɡɞɵɪԑɚɧɞɚ ԝɫɬɚԑɵɲɩɟɧ ԕɵɫԕɵɲɩɟɧ ɩɪɨɛɢɪɤɚɧɵԙ
ɚɭɡɵɧ ԧɡɿԙɿɡɞɟɧ ɛɚɫԕɚ ɠɚԕԕɚ ɛɚԑɵɬɬɚԙɵɡ
7. Ȼɚɥԕɭ ɬɟɦɩɟɪɚɬɭɪɚɫɵ ɬԧɦɟɧ ɠԥɧɟ ԝɲԕɵɲ ɡɚɬɬɚɪɦɟɧ ɠԝɦɵɫ
ɿɫɬɟɝɟɧɞɟ ɨɬɬɚɧ ԕɚɲɵԕ ԝɫɬɚɭ ɤɟɪɟɤ ɠԥɧɟ ɥɚɫ ɚɭɚ ɫɨɪԑɵɲ ɲɤɚɮɬɚ ɠԝɦɵɫ
ɿɫɬɟɭ ɤɟɪɟɤ
8. ɀɚɧԑɚɧ ɛɟɧɡɢɧɞɿ ɫɩɢɪɬɬɿ ɷɮɢɪɞɿ ԧɲɿɪɝɟɧɞɟ ԧɪɬ ɫԧɧɞɿɪɝɿɲ ԕԝɦɞɵ
ɨɥɚɪɞɵԙ ɠɚɥɵɧɵɧɚ ɬԧɝɿԙɿɡɞɟɪ
9. Ԧɡɿԙɿɡɞɿԙ ɠԝɦɵɫ ɨɪɧɵԙɵɡɞɵ ɬԥɠɿɪɢɛɟɝɟ ɤɟɪɟɤ ɟɦɟɫ ɡɚɬɬɚɪɦɟɧ
ɬɨɥɬɵɪɦɚԙɵɡ
10. Ɂɚɬɬɵ ԕɨɥɵԙɵɡɛɟɧ ԝɫɬɚɦɚԙɵɡ ɠԥɧɟ ɚɭɡɵԙɵɡԑɚ ɫɚɥɦɚԙɵɡ
ɏɢɦɢɹ ɩԥɧɿɧɿԙ ɥɚɛɨɪɚɬɨɪɢɹɫɵɧɞɚ ɠɚɥɩɵ ɠԝɦɵɫ ɿɫɬɟɭ ɬԥɪɬɿɛɿ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵ ɨɪɵɧɞɚɭ ɠԥɧɟ ɬɿɪɤɟɭ ɬԥɪɬɿɛɿ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵ ɠɚɫɚɭ ɚɥɞɵɧɞɚ ɢɧɫɬɪɭɤɰɢɹɫɵɦɟɧ
ɬɟɯɧɢɤɚɥɵԕ ԕɚɭɿɩɫɿɡɞɟɧɞɿɪɭ ɟɪɟɠɟɥɟɪɿɦɟɧ ɬɚɧɵɫɵɩ ɚɥɭ ɤɟɪɟɤ ɀԝɦɵɫ
ɨɪɧɵɧɞɚ ɬɚɡɚɥɵԕ ɫɚԕɬɚɥɵɩ ɩɚɣɞɚɥɚɧɚɬɵɧ ԕԝɪɚɥɞɚɪ ɪɟɬɬɟɥɿɩ ɬԝɪɭɵ
ɤɟɪɟɤ Ⱥɬɚɥԑɚɧ ɬԥɠɿɪɢɛɟ ɛɨɣɵɧɲɚ ԕɨɥɞɚɧɵɥɚɬɵɧ ɪɟɚɤɬɢɜɬɟɪɞɿԙ ɦԧɥɲɟɪɿ
ɤԧɪɫɟɬɿɥɦɟɝɟɧ ɛɨɥɫɚ ɨɧɞɚ ɚɡ ɦԧɥɲɟɪɞɟ ɚɥɵɩ ɩɚɣɞɚɥɚɧɭ ɤɟɪɟɤ Ȼɚɪɥɵԕ
ɬԥɠɿɪɢɛɟɥɟɪɞɟ ɬɟɤ ԕɚɧɚ ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭɦɟɧ ɠԝɦɵɫ ɿɫɬɟɭ ɤɟɪɟɤ
ɋɤɥɹɧɤɚɥɚɪɞɵԙ
ɬɵԑɵɧɞɚɪɵɧ
ɛɿɪ-ɛɿɪɿɦɟɧ
ɚɥɦɚɫɬɵɪɦɚԙɵɡɞɚɪ
Ɍԥɠɿɪɢɛɟɞɟɧ ɚɪɬɵɥԑɚɧ ɡɚɬɬɚɪɞɵ ԕɚɣɬɚ ɫɤɥɹɧɤɚԑɚ ԕԝɣɦɚԙɵɡɞɚɪ Ԕԝɪԑɚԕ
ɡɚɬɬɚɪɞɵ ԕɚɣɬɚ ɵɞɵɫɵɧɚ ɫɚɥɦɚԙɵɡɞɚɪ ɛɚɫɬɚɩԕɵ ɚɥԑɚɧ ɵɞɵɫԕɚ
ɗɬɢɤɟɬɤɚɫɵ ɠɨԕ ɧɟɦɟɫɟ ɤԛɞɿɤ ɬɭԑɵɡɚɬɵɧ ɪɟɚɤɬɢɜɬɟɪɦɟɧ ɠԝɦɵɫ ɿɫɬɟɭɝɟ
ԕɚɬɚԙ ɬɵɣɵɦ ɫɚɥɵɧɚɞɵ Ԥɪɛɿɪ ɫɬɭɞɟɧɬ ɥɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠɭɪɧɚɥ
ɠԛɪɝɿɡɟɞɿ ɀɭɪɧɚɥɞɵԙ ɞԥɩɬɟɪɞɿԙ ɫɵɪɬԕɵ ɛɟɬɿɧɞɟ ɮɚɦɢɥɢɹɫɵɧ ɚɬɵɧ
ɬɨɩ ɧԧɦɿɪɿɧ ɤԧɪɫɟɬɭɿ ɤɟɪɟɤ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵ ɠԥɧɟ ɠɭɪɧɚɥɞɵ ɦɵɧɚ ɮɨɪɦɚɞɚ ɠԛɪɝɿɡɭ
ɤɟɪɟɤ:
4
5.
Ɉɪɵɧɞɚɥԑɚɧ ɤԛɧɿ ɚɣɵ ɠɚɡɵɥɚɞɵ;2) Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵԙ ɚɬɚɥɭɵ ɠԥɧɟ ɧԧɦɿɪɿ
Ɍԥɠɿɪɢɛɟɧɿԙ ɚɬɚɥɭɵ;
4) Ɋɟɚɤɰɢɹ ɬɟԙɞɟɭɿ ɛɚɣԕɚɥԑɚɧɞɚɪ ԕԝɪɚɥɞɵԙ ɫɯɟɦɚɫɵ ɟɫɟɩɬɟɭɥɟɪɿ
ɤɟɫɬɟɥɟɪɿ ɝɪɚɮɢɤɬɟɪɿ
Ԕɨɪɵɬɵɧɞɵ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠɭɪɧɚɥɞɵ ɠԝɦɵɫɬɵ ɨɪɵɧɞɚɭ ɛɚɪɵɫɵɧɞɚ
ɬɨɥɬɵɪɚɞɵ
ɀԝɦɵɫɬɵԙ
ɫɨԙɵɧɞɚ
ɠɭɪɧɚɥɞɵ
ɨԕɵɬɭɲɵ
ɬɟɤɫɟɪɟɞɿ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɫɚɛɚԕ ɚɹԕɬɚɥɵɫɵɦɟɧ ԕɨɥɞɚɧԑɚɧ ɵɞɵɫɬɚɪɞɵ ɠɭɵɩ ԧɡ
ɠԝɦɵɫ ɨɪɧɵԙɵɡɞɵ ɠɢɧɚɫɬɵɪɵԙɵɡ Ʉԧɩɬɟɝɟɧ ɥɚɛɨɪɚɬɨɪɢɹɥɵԕ
ɠԝɦɵɫɬɚɪɞɚ ɦɿɧɞɟɬɬɿ ɬԛɪɞɟ ɟԙ ɚɥɞɵɦɟɧ ɟɫɟɩɬɟɭ ɠԝɦɵɫɬɚɪɵ ɠԛɪɝɿɡɿɥɟɞɿ
Ԥɪɛɿɪ ɠԝɦɵɫɬɵԙ ɧɟɝɿɡɝɿ ɛԧɥɿɝɿ ԕɚɬɟɥɿɤɬɿ ɟɫɟɩɬɟɭ ɛɨɥɭɵ ɤɟɪɟɤ
Ʌɚɛɨɪɚɬɨɪɢɹɞɚԑɵ ɯɢɦɢɹɥɵԕ ɵɞɵɫɬɚɪɦɟɧ ɬɚɧɵɫɭ
1. ɀɚɥɩɵԑɚ ɚɪɧɚɥԑɚɧ ɵɞɵɫɬɚɪ
2. Ⱥɪɧɚɣɵ ɵɞɵɫɬɚɪ
3. Ԧɥɲɟɭɿɲ ɵɞɵɫɬɚɪ
4. Ɏɚɪɮɨɪ ɠԥɧɟ ɠɚɥɵɧԑɚ ɬԧɡɿɦɞɿ ɵɞɵɫɬɚɪ
ɚ
ɛ
ɜ
1-ɫɭɪɟɬ ɋɵɧɚɭɵԕɬɚɪ: ɚ-ԧɥɲɟɭɿɲ ɫɵɧɚɭɵԕ ɛ-ԕɚɪɚɩɚɣɵɦ
ɫɵɧɚɭɵԕɬɚɪ
ɜ-ɬԛɬɿɤɲɟɫɿ ɛɚɪ ɫɵɧɚɭɵԕ
5
6.
2-ɫɭɪɟɬ ɋɬɚɤɚɧɞɚɪɚ
ɛ
ɜ
ɝ
3-ɫɭɪɟɬ Ʉɨɥɛɚɥɚɪ ɚ-ɤɨɧɭɫɬɵԕ ɤɨɥɛɚ ɛ-ɞԧԙɝɟɥɟɤ ɬԛɛɬɿ ɤɨɥɛɚ
ɜ-ȼɸɪɰ ɤɨɥɛɚɫɵ ɝ-ԛɲ ɬԛɬɿɤɬɿ ɤɨɥɛɚ
ɚ
ɛ
4-ɫɭɪɟɬ. Ԕԝɣԑɵɲɬɚɪ.
6
ɜ
7.
5-ɫɭɪɟɬ ɗɤɫɢɤɚɬɨɪɥɚɪ.ɚ
ɛ
ɜ
6-ɫɭɪɟɬ ɀɭԑɵɲ ɫɤɥɹɧɤɚɥɚɪ ɚ-Ⱦɪɟɤɫɟɥɶ ɫɤɥɹɧɤɚɫɵ ɛ-ȼɭɥɶɮ
ɫɤɥɹɧɤɚɫɵ ɜ-Ɍɢɳɟɧɤɨ ɫɤɥɹɧɤɚɫɵ
7
8.
ɚɛ
ɜ
7-ɫɭɪɟɬ ͞ɥɲɟɭɿɲ ɰɢɥɢɧɞɪɥɟɪ
8-ɫɭɪɟɬ ͞ɥɲɟɭɿɲ ɩɢɩɟɬɤɚ
9-ɫɭɪɟɬ ͞ɥɲɟɭɿɲ ɛɸɪɟɬɤɚ
8
9.
ɚɛ
ɜ
ɝ
10-ɫɭɪɟɬ Ɏɚɪɮɨɪ ɵɞɵɫɬɚɪ ɚ- ɤɟɩɬɿɪɟɬɿɧ ɬɚɛɚԕɲɚ ɛ-ɬɢɝɟɥɶ
ɜ-Ȼɸɯɧɟɪ ԕԝɣԑɵɲɵ ɝ-ԝɧɬɚԕɬɚԑɵɲ
Ɍԝɧɛɚɧɵ ɚɥɭ ɠɭɭ ɫԛɡɭ
ɋԚɁɍ ± ԕɚɬɬɵ ɠԥɧɟ ɫԝɣɵԕ ɤɨɦɩɨɧɟɧɬɬɟɪɞɿ ɦɟɯɚɧɢɤɚɥɵԕ ɛԧɥɭ
ɩɪɨɰɟɫɿ ɋԛɡɭɞɿԙ ɦԥɧɿ ɦɵɧɚɞɚ ɿɲɿɧɞɟ ԕɚɬɬɵ ɛԧɥɲɟɝɿ ɛɚɪ ɫԝɣɵԕɬɵ ɫԛɡɝɿɲ
ԕɚԑɚɡԑɚ ԕԝɹɞɵ ɋԛɡɝɿɲ ԕɚԑɚɡɞɚɧ ɫԝɣɵԕ ɡɚɬ ԧɬɿɩ ԕɚɬɬɵ ɛԧɥɲɟɤ ɫԛɡɝɿɲ
ԕɚԑɚɡɞɚ ԕɚɥɚɞɵ Ȼԝɥ ԥɞɿɫɬɿԙ ɟԙ ɤԧɩ ԕɨɥɞɚɧɵɥɚɬɵɧɵ ԕɚԑɚɡɞɚ ɫԛɡɭ ɠԥɧɟ ԥɪ
ɬԛɪɥɿ ɬɵԑɵɡɞɵԕɬɚԑɵ ԕԝɦɞɚ ɚɫɛɟɫɬɟ ɲɵɧɵ ɜɚɬɚɞɚ ɫԛɡɭ ɋԛɡɝɿɲ
ɦɚɬɟɪɢɚɥɞɚɪɞɵ ɬɚԙɞɚɭ ɫԛɡɟɬɿɧ ɫԝɣɵԕɬɵԙ ԕɚɫɢɟɬɿɧɟ ɠԥɧɟ ԕɚɬɬɵ
ɛԧɥɲɟɤɬɿԙ ɤԧɥɟɦɿɧɟ ɧɟɝɿɡɞɟɥɝɟɧ Ԕɚԑɚɡ ɫԛɡɝɿɲɬɟɪɞɿ ԕɚԑɚɡɞɚɪɞɵԙ
ɬɵԑɵɡɞɵԑɵɧɚ ԕɚɪɚɣ ɬɚԙɞɚɣɞɵ Ȼԝɥ ɬɚԙɞɚɭ ԕɚԑɚɡ ɥɟɧɬɚɫɵɧɵԙ ɬԛɫɿɦɟɧ
ɚɧɵԕɬɚɥɚɞɵ Ɉɥɚɪ ɞɚɣɵɧ ɠɢɧɚԕɬɚɥԑɚɧ ɬԛɪɞɟ ɛɨɥɚɞɵ Ɇɵɧɚ ɬԧɦɟɧɞɟɝɿ
ɛɟɥɝɿɥɟɧɝɟɧ ɬԛɪɥɟɪɿ ԕɨɥɞɚɧɵɥɚɞɵ
Ɋɨɡɨɜɚɹ ɧɟɦɟɫɟ ԕɵɡԑɵɲ ɥɟɧɬɚ-ɬɟɡ ɫԛɡɝɿɲɬɟɪ ɞɢɚɦɟɬɪɿ- ɆɆɄ
Ⱥԕ ɥɟɧɬɚ-ɨɪɬɚɲɚ ɫɿԙɿɪɝɿɲ ɞɢɚɦɟɬɪɿ- ɆɆɄ
Ʉԧɤ ɥɟɧɬɚ - ɬɵԑɵɡ ɫԛɡɝɿɲ ɞɢɚɦɟɬɪɿ- ɆɆɄ
Ȼԝɥɚɪ ԧɬɟ ɤɿɲɿ ɛԧɥɲɟɤɬɟɪɞɿ ɫԛɡɭɝɟ ɚɪɧɚɥԑɚɧ
ɋɚɪɵ ɥɟɧɬɚ - ɦɚɣɥɵ ɡɚɬɬɚɪɞɵ ɫԛɡɭ ԛɲɿɧ ɩɚɣɞɚɥɚɧɵɥɚɞɵ
Ԕɚɪɚɩɚɣɵɦ ɫԛɡɝɿɲ ɬԝɧɛɚɧɵ ɛԧɥɿɩ ɚɥɭ ԛɲɿɧ ɩɚɣɞɚɥɚɧɵɥɚɞɵ Ԕɚɪɚɩɚɣɵɦ
ɫԛɡɝɿɲɬɿ ɤɜɚɞɪɚɬ ԕɚԑɚɡɞɚɧ ɠɚɫɚɣɞɵ Ʉɜɚɞɪɚɬ ԕɚԑɚɡ ԕԝɣԑɵɲɬɵԙ ɤԧɥɟɦɿɧɟ
ɫԥɣɤɟɫ ɤɟɥɭɿ ɤɟɪɟɤ
ɋԛɡɝɿɲ ԕɚԑɚɡɞɵ -ɫɭɪɟɬɬɟɝɿ ɬԥɪɬɿɩɩɟɧ ԕԝɣԑɵɲԕɚ ɨɪɧɚɥɚɫɬɵɪɚɞɵ
11-ɫɭɪɟɬ
9
10.
Ԕɚԑɚɡɞɵԙ ɫɵɪɬԕɵ ɛԝɪɵɲɬɚɪɵɧ ɫɵɪɬԕɵ ԕԝɣԑɵɲ ɞɟԙɝɟɣɿɧɟɧ ɬԧɦɟɧɛɨɥɚɬɵɧɞɚɣ ɟɬɿɩ ɤɟɫɟɞɿ Ԕԝɣԑɵɲɬɵԙ ɞɟԙɝɟɣɿɧɟɧ - ɫɦ ɬԧɦɟɧ ɛɨɥɭɵ
ɤɟɪɟɤ ɀɢɧɚԕɬɚɥԑɚɧ ԕɚԑɚɡɞɵԙ ɛɿɪ ɛԧɥɿɝɿɧ ɚɲɵɩ ԕԝɣԑɵɲɬɵԙ ɿɲɿɧɟ
ɟɧɝɿɡɟɞɿ Ԕɚԑɚɡɞɵ ԕԝɣԑɵɲɬɵԙ ɿɲɿɧɟ ɧɟɦɟɫɟ ԕɚɛɵɪԑɚɫɵɧɚ ɫɚɭɫɚԕɬɚɪɵɦɟɧ
ɧɵԕɬɚɩ ɤɢɝɿɡɟɞɿ ɋԛɡɟɬɿɧ ɟɪɿɬɿɧɞɿɧɿ ԕԝɹɪ ɚɥɞɵɧɞɚ ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭ ԕԝɹɞɵ
Ȼԝɥ ɤɟɡɞɟ ԕɚԑɚɡ ԕԝɣԑɵɲ ԕɚɛɵɪԑɚɫɵɧɚ ɠɚԕɫɵ ɠɚɛɵɫɵɩ ɨɬɵɪɚɞɵ
Ԕɚɛɚɬɬɚɥԑɚɧ ɫԛɡɝɿɲ Ԕɚɛɚɬɬɚɥԑɚɧ ɫԛɡɝɿɲɬɿ ɞɚɣɵɧɞɚɭ ԥɞɿɫɿɦɟɧ ɫɭɪɟɬɬɿ ɩɚɣɞɚɥɚɧɵɩ ɬɚɧɵɫɵԙɞɚɪ
Ԕɚɛɚɬɬɚɥԑɚɧ ɫԛɡɝɿɲɬɿ ɞɚɣɵɧɞɚɩ ɨԕɵɬɭɲɵԑɚ ɤԧɪɫɟɬɿԙɞɟɪ ɀɟԙɿɥ
ɫԛɡɿɥɟɬɿɧ ɫԝɣɵԕɬɚɪɞɵ ɤԥɞɿɦɝɿ ԕɵɫɵɦɞɚ ԧɬɤɿɡɟɞɿ ɀɚɛɵɫԕɵɲ ɫԝɣɵԕɬɚɪɞɵ
ɠԥɧɟ ԕɚɧɵԕԕɚɧ ɟɪɿɬɿɧɞɿɥɟɪɞɿ ɵɫɬɵԕɬɚ ɫԛɡɟɞɿ
12-ɫɭɪɟɬ
Ʉԥɞɿɦɝɿ ԕɵɫɵɦɞɚԑɵ ɫԛɡɭ
Ȼԝɥ ɫԛɡɭɞɿ ɠԛɪɝɿɡɭ ԛɲɿɧ -ɫɭɪɟɬɬɟɝɿ ɫɯɟɦɚɧɵ ɠɢɧɚԕɬɚɣɞɵ ɋԛɡɝɿɞɟ
ɚɡɞɚԑɚɧ ɫԝɣɵԕ ԕɚɥɫɚ ɬԝɧɛɚɧɵ ɲɚɣԕɚɩ ɫԛɡɝɿ ԕɚԑɚɡԑɚ ԕԝɹɞɵ ɋԛɡɝɿɞɟɧ ԧɬɤɟɧ
ɫԝɣɵԕ ɮɢɥɶɬɪɚɬ ɞɟɩ ɚɬɚɥɚɞɵ Ɍԝɧɛɚɧɵԙ ɵɞɵɫɬɚԑɵ ԕɚɥɞɵԕɬɚɪɵɧ
ɠɭԑɵɲɬɚԑɵ ɞɢɫɬɢɥɶɞɟɧɝɟɧ ɫɭ ɧɟɦɟɫɟ ɚɪɧɚɣɵ ɟɪɿɬɤɿɲɬɟɪ ԕɨɥɞɚɧɵɥɚɞɵ
Ȼԝɥ ɠɭɚɬɵɧ ɡɚɬɬɚɪɞɵ ɩɚɣɞɚɥɚɧԑɚɧɞɚ ɚɡ ɦԧɥɲɟɪɞɟ ԕԝɹɞɵ Ʉɟɥɟɫɿ ɛԧɥɿɝɿɧ
ɚɥԑɚɲԕɵ ԕԝɣɵɥԑɚɧ ɛԧɥɿɝɿ ɬɨɥɵԕ ɫԛɡɝɿɞɟɧ ԧɬɤɟɧɧɟɧ ɤɟɣɿɧ ԕԝɹɞɵ
Ɍԝɧɛɚɧɵ - ɪɟɬ ɠɭԑɚɧɧɚɧ ɤɟɣɿɧ ɫɚɩɚɥɵԑɵɧ ɚɥԑɚɲԕɵ ԕɨɫɩɚɥɚɪԑɚ
ɫɚɥɵɫɬɵɪɭ ɛɚɪɵɫɵɧɞɚ ɬɟɤɫɟɪɟɞɿ Ȼԝɥ ԛɲɿɧ ɬɚɡɚ ɩɪɨɛɢɪɤɚԑɚ ɬɚɦɲɵɥɚɩ
ɬԝɪԑɚɧ ɫԝɣɵԕɬɚɧ ԕԝɹɞɵ ɠԥɧɟ ɠɭɵɥԑɚɧ ɢɨɧԑɚ ɪɟɚɤɰɢɹ ɛɟɪɟɬɿɧ ɟɪɿɬɿɧɞɿɦɟɧ
ԥɫɟɪ ɟɬɟɞɿ ɦɵɫɚɥɵ ɢɨɧ Cl-- AgNO3 ɢɨɧ SO4)2- - BaCl2 ȿɝɟɪ ɟɪɿɬɿɧɞɿ
ɥɚɣɥɚɧɫɚ ɨɧɞɚ ɬԝɧɛɚɧɵ ԥɪɿ ԕɚɪɚɣ ɠɭɭɞɵ ɠɚɥԑɚɫɬɵɪɚɞɵ Ɍԝɧɛɚɧɵ ɠɭԑɚɧ
ɫԝɣɵԕɬɵ ɮɢɥɶɬɪɚɬɬɚɧ ɛԧɥɟɤ ɵɞɵɫԕɚ ɠɢɧɚԕɬɚɣɞɵ (ɇɟɝɟ?)
10
11.
13-ɫɭɪɟɬ Ʉԥɞɿɦɝɿ ɫԛɡɭȺɡ ɟɪɢɬɿɧ ɠɚɣ ɫԛɡɿɥɟɬɿɧ ɬԝɧɛɚɥɚɪɞɵ ɠɭɭ ԛɲɿɧ ɞɟɤɚɧɬɚɰɢɹ ԥɞɿɫɿ
ԕɨɥɞɚɧɵɥɚɞɵ ɋԛɡɭɝɟ ɞɟɣɿɧ ɬԛɡɿɥɟɬɿɧ ɬԝɧɛɚɧɵ ɵɞɵɫɬɵԙ ɬԛɛɿɧɟ ɬɨɥɵԕ
ɨɬɵɪɭɵɧ ɤԛɬɟɞɿ ȿɪɿɬɿɧɞɿ ɬԝɧԑɚɧɧɚɧ ɤɟɣɿɧ ɛɟɬɿɧɞɟɝɿ ɟɪɿɬɿɧɞɿɧɿ ɬԝɧɛɚɞɚɧ
ɛԧɥɿɩ ɫԛɡɝɿɝɟ ԕԝɹɞɵ Ⱥɥ ɬԝɧɛɚԑɚ ɬɚԑɵ ɞɚ ɟɪɿɬɤɿɲ ԕԝɹɞɵ ɠɚԕɫɵɥɚɩ
ɚɪɚɥɚɫɬɵɪɵɩ ɟɪɿɬɿɧɞɿɧɿ ɬɚԑɵ ɛɿɪɚɡ ɬԝɧɞɵɪɚɞɵ ɋԝɣɵԕɬɵ ɬɚԑɵ ɞɚ ɬԧɝɿɩ
ɬɚɫɬɚɩ ɬԝɧɛɚԑɚ ɟɪɿɬɤɿɲɬɿ ԕԝɹɞɵ ɨɫɵɥɚɣ ɛɿɪɧɟɲɟ ɪɟɬ ԕɚɣɬɚɥɚɧɚɞɵ
ɋɨԙɵɧɚɧ ɬԝɧɛɚɧɵ ɫԛɡɝɿɲɤɟ ԕԝɣɵɩ ɬԝɧɛɚɧɵ ɠɭɭɞɵ ɠɚɥԑɚɫɬɵɪɚɞɵ
ɋԛɡɭɞɿ ɨԕɵɬɭɲɵɧɵԙ ɬɚɩɫɵɪɦɚɫɵɦɟɧ ɠԛɪɝɿɡɟɞɿ ɦɥ-ɞɿ ԕԝɦ-ɫɭɞɚ
ɧɟɦɟɫɟ ɥɚɣ ɫɭɞɚ ɨԕɵɬɭɲɵɧɵԙ ɧԝɫԕɚɭɵɦɟɧ ɨɪɵɧɞɚɥɚɞɵ Ɍԝɧɛɚɧɵ
ɫɚɧɞɵԕ ԥɞɿɫɩɟɧ ɚɧɵԕɬɚɭɞɵ ɛɟɤɿɬɭ ԛɲɿɧ ɲɵɧɵ ɬɚɹԕɲɚ ɠԥɧɟ ɠɭԑɵɲ
ԕɨɥɞɚɧɵɥɚɞɵ
ȼɚɤɭɭɦɞɚ ɫԛɡɭ
Ԕɚɬɬɵ ɡɚɬɬɚɪɞɵ ɬɟɡ ɛԧɥɭ ԛɲɿɧ ɜɚɤɭɭɦɞɚԑɵ ɫԛɡɭ ԕɨɥɞɚɧɵɥɚɞɵ ɋԛɡɭ
ԕɵɫɵɦɞɵ ɬԧɦɟɧɞɟɬɭ ԥɞɿɫɿɦɟɧ ɨɪɵɧɞɚɥɚɞɵ Ȼԝɥ ԕԝɪɚɥ -ɫɭɪɟɬɬɟ
ɤԧɪɫɟɬɿɥɝɟɧ Ɉɥ ԕɚɥɵԙ ԕɚɛɵɪԑɚɥɵ ɤɨɥɛɚ Ȼɭɧɡɟɧɧɟɧ ɬԝɪɚɞɵ Ɋɟɡɢɧɚ
ɬɵԑɵɧɧɵԙ ɤԧɦɟɝɿɦɟɧ ɤɨɥɛɚԑɚ ɮɚɪɮɨɪ Ȼɸɯɧɟɪ ԕԝɣԑɵɲɵ
ɨɬɵɪԑɵɡɵɥԑɚɧ Ԕԝɣԑɵɲɬɵԙ ɬɟɫɿɤɬɟɪɿ ɬɨɪ ɪɟɲɟɬɤɚ ɬԥɪɿɡɞɿԕԝɣԑɵɲɬɵԙ
ɬɟɫɿɝɿɧɟ ɟɤɿ ԕɚɛɚɬ ɮɢɥɶɬɪ ɬԧɫɟɥɟɞɿ ɬɟɫɿɝɿɧɿԙ ɞɢɚɦɟɬɪɿ ɫɦ ɋԛɡɝɿɲ
ԕɚԑɚɡɞɵ ԕԝɣԑɵɲɬɵԙ ɞɢɚɦɟɬɪɿɦɟɧ ɫԥɣɤɟɫɬɟɧɞɿɪɿɩ ɤɟɫɿɩ ɫɚɥɚɞɵ Ⱦɢɚɦɟɬɪɿ
ɤɿɲɿɥɟɭ ɫԛɡɝɿɲɬɿ ɫɭɦɟɧ ɞɵɦԕɵɥɞɚɩ ɞɢɚɦɟɬɪɿ ԛɥɤɟɧ ɫԛɡɝɿɲɬɿԙ ɛɟɬɿɧɟ
ɬɵԑɵɡ ɟɬɿɩ ɨɪɧɚɥɚɫɬɵɪɚɞɵ ɋԛɡɝɿɲɬɿ ԕɨɸɞɵԙ ɤɟɥɟɫɿ ɬԥɫɿɥɿ ɛɨɣɵɧɲɚ
ɫԛɡɝɿɲ ԕɚԑɚɡ ԕԝɣԑɵɲɬɵԙ ԕɚɛɵɪԑɚɥɚɪɵɧɚ ɧɵԕɬɚɥɵɩ ԕɨɣɵɥԑɚɧ ɫɨԙ ɬɨɥɵԕ
11
12.
ɨɪɧɵԕɬɵɪɭ ԛɲɿɧ ɫɨɪԑɵɲ ɧɚɫɨɫ ԕɨɥɞɚɧɵɥɚɞɵ ɇɚɫɨɫɬɵԙ ɤԧɦɟɝɿɦɟɧɫԛɡɝɿɲ ԕԝɣԑɵɲԕɚ ɬɵԑɵɡ ɨɪɧɚɥɚɫɬɵɪɚɞɵ ɫԛɡɝɿɲɬɿԙ ɨɪɧɚɥɚɫԕɚɧɵɧ
ɚɧɵԕɬɚɩ ɩɪɢɛɨɪɞɵ ԧɲɿɪɟɞɿ Ȼɸɯɧɟɪ ԕԝɣԑɵɲɵɧɚ ɲɵɧɵ ɬɚɹԕɲɚɧɵԙ
ɤԧɦɟɝɿɦɟɧ ɬԝɧɛɚɫɵ ɛɚɪ ɟɪɿɬɿɧɞɿ ԕԝɹɞɵ ȿɪɿɬɿɧɞɿɧɿԙ ɛɚɪɥɵԑɵɧ ԕԝɣɵɩ
ɛɨɥԑɚɧɧɚɧ ɫɨԙ ɧɚɫɨɫԕɚ ԕɨɫɚɞɵ Ɍԝɧɛɚɧɵ ɫԛɡɝɿɞɟ ɫɵԑɚɞɵ ɋԛɡɭ ɠԝɦɵɫɵ
ɛɿɬɤɟɧ ɫɨԙ ɫԛɡɝɿɧɿ ɬԝɧɛɚɫɵɦɟɧ ԕԝɣԑɵɲɬɚɧ ɚɥɵɩ ɤɟɩɬɿɪɭɝɟ ԕɨɹɞɵ
14-cɭɪɟɬ ȼɚɤɭɭɦɞɟ ɫԛɡɭ: 1-Ȼɸɯɧɟɪ ԕԝɣԑɵɲɵ - ɫɚԕɬɚɭɲɵ ԕɚɩɬɚɦɚ
3-Ȼɭɧɡɟɪ ɤɨɥɛɚɫɵ -ɫɚԕɬɚɭɲɵ ɲɵɧɵ ɵɞɵɫɵ -ɫɭ ɚԑɵɧɞɵ ɧɚɫɨɫ
ɌɂɌɊɅȿɍ
Ȼԝɥ ԥɞɿɫ ɡɚɬɬɵԙ ɪɟɚɤɰɢɹԑɚ ɬԛɫɟɬɿɧ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵɧ ɟɪɿɬɿɧɞɿɧɿԙ
ɤԧɥɟɦɿɧ ԧɥɲɟɭɝɟ ɧɟɝɿɡɞɟɥɝɟɧ Ȼɿɪ ɡɚɬɬɵԙ ɟɪɿɬɿɧɞɿɞɟɝɿ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵɧ
ɠԥɧɟ ɠԝɦɫɚɥԑɚɧ ɤԧɥɟɦɿɧ ɛɿɥɟ ɨɬɵɪɵɩ ɛɚɫԕɚ ɡɚɬɬɵԙ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵɧ
ɟɫɟɩɬɟɩ ɚɧɵԕɬɚɣɞɵ
Ɍɢɬɪɥɟɭ ԛɲɿɧ ɛɸɪɟɬɤɚԑɚ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵ ɛɟɥɝɿɥɿ ɟɪɿɬɿɧɞɿɧɿ ԕԝɣɵɩ
ɛɸɪɟɬɤɚɧɵ ɠԝɦɵɫɲɵ ԕɚɥɵɩԕɚ ɤɟɥɬɿɪɟɞɿ
ɉɢɩɟɬɤɚɦɟɧ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵ ɛɟɥɝɿɫɿɡ ɡɚɬɬɵԙ ɟɪɿɬɿɧɞɿɫɿɧ ԧɥɲɟɩ
ɚɥɵɩ ɤɨɧɭɫ ɤɨɥɛɚԑɚ ԕԝɹɞɵ ȿɝɟɪ ɢɧɞɢɤɚɬɨɪɞɵԙ ɤԧɦɟɝɿɦɟɧ ɚɧɵԕɬɚɭ ɤɟɪɟɤ
ɛɨɥɫɚ ɨɧɞɚ ɤɨɥɛɚԑɚ - ɬɚɦɲɵ ɢɧɞɢɤɚɬɨɪɞɵ ɥɚɤɦɭɫ ɮɟɧɨɥɮɬɚɥɟɢɧ
ɬɚɦɵɡɚɞɵ -ɫɭɪɟɬ
Ɍɢɬɪɥɟɭɞɿ ɚԕ ԕɚԑɚɡɞɵԙ ɛɟɬɿɧɞɟ ɠԛɪɝɿɡɟɞɿ Ɍɢɬɪɥɟɭ ɤɟɡɿɧɞɟ
ɤɨɧɰɟɧɬɪɚɰɢɹɫɵ ɛɟɥɝɿɥɿ ɟɪɿɬɿɧɞɿɧɿ ɛɸɪɟɬɤɚɞɚɧ ɬɚɦɲɵɥɚɬɵɩ ɬɢɬɪɥɟɣɞɿ
ɠԥɧɟ ɚɧɵԕɬɚɥɚɬɵɧ ɟɪɿɬɿɧɞɿɧɿ ɠɢɿ-ɠɢɿ ɲɚɣԕɚɩ ɨɬɵɪɚɞɵ Ɍɢɬɪɥɟɭɞɿ
ɟɪɿɬɿɧɞɿɞɟɝɿ ɢɧɞɢɤɚɬɨɪɞɵԙ ɬԛɫɿ ԧɡɝɟɪɝɟɧɞɟ ɬɨԕɬɚɬɚɞɵ ȿɝɟɪ ɟɤɿ ɪɟɬ
ɬɢɬɪɥɟɭɞɿԙ ɧԥɬɢɠɟɫɿɧɞɟɝɿ ɦԥɧɞɟɪɿɧɿԙ ɚɣɵɪɦɚɲɵɥɵԑɵ ɦɥ ɛɨɥɫɚ
ɬɢɬɪɥɟɭɞɿ ɠɚɥԑɚɫɬɵɪɚɞɵ
12
13.
15-ɫɭɪɟɬ Ɍɢɬɪɥɟɭ ԕɨɧɞɵɪԑɵɫɵɌɚɪɚɡɵ ɠԥɧɟ ɨɧɵɦɟɧ ԧɥɲɟɭ
Ԧɥɲɟɭ- ɞɟɝɟɧɿɦɿɡ ɛɟɪɿɥɝɟɧ ɦɚɫɫɚɧɵ ɝɢɪɶ ɦɚɫɫɚɫɵɦɟɧ ɫɚɥɵɫɬɵɪɭ
Ԧɥɲɟɭ ɞԥɥɞɿɝɿɧɟ ԕɚɪɚɣ ɬɚɪɚɡɵɥɚɪɞɵ ɤɟɥɟɫɿ ɬɨɩɬɚɪԑɚ ɛԧɥɟɞɿ
1. Ɍɟɯɧɢɤɚɥɵԕ ɬɚɪɚɡɵ ԛɥɤɟɧ ɞԥɥɞɿɤɬɟ ɞԥɥɞɿɝɿ ɝ ɞɟɣɿɧ
2. Ɍɟɯɧɢɤɚɥɵԕ ɬɚɪɚɡɵ ɤɿɲɿ ɞԥɥɞɿɤɬɟ ɞԥɥɞɿɝɿ ɝ ɞɟɣɿɧ
Ⱥɧɚɥɢɬɢɤɚɥɵԕ ɬɚɪɚɡɵ ɤɿɲɿ ɞԥɥɞɿɤɬɟ ɞԥɥɞɿɝɿ -410-6ɝ ɞɟɣɿɧ
4. Ⱥɪɧɚɣɵ ɬɚɪɚɡɵ ɩɪɨɛɢɪɥɿ ɬɨɪɡɢɨɧɞɵ
Ɍɟɯɧɢɤɚɥɵԕ ɬɚɪɚɡɵ ɞɟɩ ɚɬɚɥɚɬɵɧ ɬɟɯɧɢɤɚɥɵԕ ԧɬɟ ɤɿɲɿ ɞԥɥɞɿɤɬɟ
ԧɥɲɟɣɬɿɧ ɬɚɪɚɡɵɧɵԙ ԕԝɪɵɥɵɫɵ -ɫɭɪɟɬ
13
14.
ɚɛ
16-ɫɭɪɟɬ ɚ Ɍɚɪɚɡɵ -ɛɟɤɿɬɿɥɟɬɿɧ ɬɚɛɚɧ -ɤɨɥɨɧɤɚ -ɤɨɪɨɦɵɫɥɨ
4-ɬɚɛɚԕɲɚɥɚɪ -ɚɪɪɟɬɢɪ -ɬɚɪɚɡɵ ɛɚԑɞɚɪɵ -ɲɤɚɥɚ -ɬɿɪɟɭɲɿ ɜɢɧɬɬɟɪ
9-ɛɨɫ ɬɚɛɚԕɲɚɥɚɪɞɵ ɬɟԙɟɫɬɿɪɟɬɿɧ ɜɢɧɬɬɟɪ -ɬɿɤɬɟɭɲɿ -ɬɿɤɬɟɭɲɿɧɿԙ
ɠɵɥɠɵɦɚɣɬɵɧ ɤɨɧɭɫɵ -ɨɪɬɚɥɵԕ ɩɪɢɡɦɚ 3-ɛԛɣɿɪɥɟɪɞɟɝɿɩɪɢɡɦɚɥɚɪ
14-ɿɥɝɿɲ -ɫɚɯɢɧɚ ɛ ɗɥɟɤɬɪɨɧɞɵԕ ɬɚɪɚɡɵɥɚɪ
Ɍɟɯɧɢɤɚɥɵԕ ɬɚɪɚɡɵɥɚɪɞɚ ɡɚɬɬɚɪɞɵԙ ɦɚɫɫɚɫɵɧ ԧɥɲɟɭ ɠԛɡ ɝɪɚɦɞɵԕ
ɬɚɫɬɚɪɞɵ ɠԥɧɟ ɦɚɫɫɚɥɚɪɵ ԥɪ ɬԛɪɥɿ ɝɪɚɦɞɚɪɦɟɧ ɦɢɥɢɝɪɚɦɞɚɪɞɚɧ
ɬԝɪɚɬɵɧ ɝɢɪɶɥɟɪɞɿԙ ɠɢɧɚԕɬɚɪɵɧ ɩɚɣɞɚɥɚɧɚɞɵ Ƚɢɪɶɥɟɪ ɚɪɧɚɣɵ
ɠԥɲɿɤɬɟɪɞɟ ɫɚԕɬɚɥɚɞɵ
17-ɫɭɪɟɬ Ɍɚɪɚɡɵ ɬɚɫɬɚɪɵ: 1-ɫɚɥɦɚԕ ɬɚɫɬɚɪ ɠԥɲɿɝɿ -ɝɪɚɦɞɵԕ
ɬɚɫɬɚɪ -ɦɢɥɥɢɝɪɚɦɞɵԕ ɬɚɫɬɚɪ -ɩɢɧɰɟɬ
14
15.
Ԧɥɲɟɭɝɟ ɤɿɪɿɫɟɪ ɚɥɞɵɧɞɚ ɬɚɪɚɡɵɧɵԙ ɞԝɪɵɫ ɠԝɦɵɫ ɿɫɬɟɣɬɿɧɞɿɝɿɧɚɧɵԕɬɚɩ ɚɥɚɦɵɡ Ȼԝɥ ԛɲɿɧ ɚɪɪɟɬɢɪɞɿԙ ԝɫɬɚԑɵɲɵɧ ɠɚɣɥɚɩ ɨԙԑɚ ɛԝɪɵɩ
ɫɬɪɟɥɤɚɧɵԙ ɬɟɪɛɟɥɿɫɿɧ ɛɚɣԕɚɣɦɵɡ ȿɝɟɪ ɬɚɪɚɡɵ ɞԝɪɵɫ ɠԝɦɵɫ ɿɫɬɟɩ
ɬԝɪԑɚɧ ɛɨɥɫɚ ɫɬɪɟɥɤɚɫɵ ɛԧɥɿɧɝɟɧ ɲɤɚɥɚɥɚɪɞɵԙ ɛɨɣɵɦɟɧ ɬɟɪɛɟɥɝɟɧɞɟ
ɨԙԑɚ ɧɟɦɟɫɟ ɫɨɥԑɚ ɬɟɪɛɟɥɝɟɧɞɟ ɧԧɥɞɟɧ ɤԧɩ ɚɭɵɬԕɭ ɤԧɪɫɟɬɫɟ ɨɧɞɚ ɬɚɪɚɡɵ
ɬɭɪɚ ɠԝɦɵɫ ɿɫɬɟɦɟɣɞɿ Ȼԝɥ ɠɚԑɞɚɣɞɚ ɥɚɛɨɪɚɧɬɬɵԙ ɬɚɪɚɡɵɧɵ ɠԧɧɝɟ
ɤɟɥɬɿɪɿɩ ɛɟɪɭɿɧ ԧɬɿɧɭ ɤɟɪɟɤ Ɂɚɬɬɵ ԧɥɲɟɝɟɧɞɟ ɛԧɥɦɟ ɬɟɦɩɟɪɚɬɭɪɚɫɵɧɚ
ɞɟɣɿɧ ɫɚɥԕɵɧɞɚɬɵɩ ԧɥɲɟɣɞɿ Ɍɚɪɚɡɵ ɬɚɛɚԕɲɚɫɵɧ ԧɥɲɟɧɟɬɿɧ ɡɚɬɬɵ ɠԥɧɟ
ɝɢɪɶɥɟɪɞɿ ɚɥɭ ɠԥɧɟ ɫɚɥɭ ɬɟɤ ԕɚɧɚ ɚɪɪɟɬɢɪɥɟɧɝɟɧ ɬɚɪɚɡɵɥɚɪɞɚ
ɠԛɪɝɿɡɿɥɟɞɿ Ԧɥɲɟɭ ɤɟɡɿɧɞɟ ɚɪɧɚɣɵ ɵɞɵɫɬɚɪɞɵ ԕɚɬɚԙ ɩɚɣɞɚɥɚɧɭ ɤɟɪɟɤ
Ɉɥɚɪ ɫɚԑɚɬ ɲɵɧɵɫɵ ɬɢɝɟɥɶ ɛɸɤɫɬɟɪ Ԧɥɲɟɭ ɠԝɦɵɫɵ ɛɿɬɤɟɧɧɟɧ ɤɟɣɿɧ
ɞɟɧɟɧɿԙ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚɣɬɵɧ ɝɢɪɶɥɟɪɞɿ ɟɫɟɩɬɟɩ ɚɥɦɚɣ ɨɥɚɪɞɵ
ɬɚɛɚԕɲɚɞɚɧ ɚɥɵɩ ɬɚɫɬɚɦɚɭ ɤɟɪɟɤ Ɍɚɪɚɡɵ ɬɚɫɬɚɪɵɧ ɠԥɲɿɤɤɟ ɫɚɥɚɪɞɚ ɬɚԑɵ
ɞɚ ɦɚɫɫɚɧɵ ɬɟɤɫɟɪɭ ɤɟɪɟɤ ȿɝɟɪ ɛɿɪ ɠԝɦɵɫɬɚ ɛɿɪɧɟɲɟ ԧɥɲɟɭ
ɠԝɦɵɫɬɚɪɵɧ ɠԛɪɝɿɡɭ ɤɟɪɟɤ ɛɨɥɫɚ ɨɧɞɚ ɬɟɤ ԕɚɧɚ ɫɨɥ ɩɚɣɞɚɥɚɧԑɚɧ ɬɚɪɚɡɵ
ɬɚɫɬɚɪɵɦɟɧ ɠԝɦɵɫɬɵԙ ɚɹԑɵɧɚ ɞɟɣɿɧ ɠԝɦɵɫ ɿɫɬɟɭ ɤɟɪɟɤ Ԧɥɲɟɭ ɠԝɦɵɫɵ
ɛɿɬɤɟɧ ɫɨԙ ɬɚɪɚɡɵ ԛɫɬɿɧɞɟ ɟɲɬɟԙɟ ԕɚɥɞɵɪɦɚɣ ɠɢɧɚɩ ԕɨɸ ɤɟɪɟɤ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ 1
Ɍɚԕɵɪɵɛɵ Ƚɚɡ ɬԥɪɿɡɞɿ ɡɚɬɬɚɪɞɵԙ ɦɨɥɟɤɭɥɚɥɵԕ ɦɚɫɫɚɫɵɧ
ɆɋɈ Ʉɢɩɩ ɚɩɩɚɪɚɬɵɧɞɚ ɚɧɵԕɬɚɭ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵԙ ɦɚԕɫɚɬɵ. Ʉԧɦɿɪɬɟɤ ȱ9 ԕɨɫ ɨɤɫɢɞɿɧɿԙ
ɦɨɥɟɤɭɥɚɥɵԕ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚɭ
Ȼɚԕɵɥɚɭ ɠԝɦɵɫɵɧɵԙ ɫԝɪɚԕɬɚɪɵ ɦɟɧ ɟɫɟɩɬɟɪɿ
1. ɏɢɦɢɹɧɵԙ ɧɟɝɿɡɝɿ ɡɚԙɞɚɪɵ ɡɚɬ ɦɚɫɫɚɫɵɧɵԙ ɫɚԕɬɚɥɭ ɡɚԙɵ ԕԝɪɚɦ
ɬԝɪɚԕɬɵɥɵԕ ɡɚԙɵ ɟɫɟɥɿɤ ԕɚɬɵɧɚɫ ɡɚԙɵ ɤԧɥɟɦɞɿɤ ԕɚɬɵɧɚɫ ɡɚԙɵ
2. Ⱥɬɨɦ ɠԥɧɟ ɫɚɥɵɫɬɵɪɦɚɥɵ ɚɬɨɦɞɵԕ ɦɚɫɫɚ ɦɨɥɟɤɭɥɚ ɠԥɧɟ
ɫɚɥɵɫɬɵɪɦɚɥɵ ɦɨɥɟɤɭɥɚɥɵԕ ɦɚɫɫɚ ɬɭɪɚɥɵ ԝԑɵɦ Ԧɥɲɟɦ ɛɿɪɥɿɤɬɟɪɿ
Ɇɨɥɶ Ⱥɜɨɝɚɞɪɨ ɡɚԙɵ Ɇɨɥɶɞɿɤ ɤԧɥɟɦ Ƚɚɡɞɚɪɞɵԙ ɦɨɥɟɤɭɥɚɥɵԕ
ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚɭ ԥɞɿɫɬɟɪɿ
3. ȿɫɟɩ ɝ NH3 ɠԥɧɟ ɝ N2 ɦɨɥɟɤɭɥɚ ɫɚɧɵɧ ɫɚɥɵɫɬɵɪɵԙɞɚɪ Ԕɚɧɞɚɣ
ɦɨɥɟɤɭɥɚɞɚ ɠԥɧɟ ɧɟɲɟ ɪɟɬ ɦɨɥɟɤɭɥɚ ɫɚɧɵ ɚɪɬɵԕ ɛɨɥɚɞɵ"
4. ȿɫɟɩ ɗɥɟɦɟɧɬɬɿԙ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵ ɝ ɦɨɥɶ
ɨɧɵԙ ɦɟɧɲɿɤɬɿ ɠɵɥɭ ɫɵɣɵɦɞɵɥɵԑɵ Ⱦɠ ɦɨɥɶ Ʉ ɗɥɟɦɟɧɬɬɿԙ
ɚɧɵԕ ɚɬɨɦɞɵԕ ɦɚɫɫɚɫɵɧ ɟɫɟɩɬɟԙɞɟɪ
5. ȿɫɟɩ ɋɭɪɶɦɚɧɵԙ (Sb ɫɭɬɟɤ ɛɨɣɵɧɲɚ ɬɵԑɵɡɞɵԑɵ
2000ɋ ɝɪɚɞɭɫɬɚ ɫɭɪɶɦɚɧɵԙ ɦɨɥɟɤɭɥɚɫɵ ɧɟɲɟ ɚɬɨɦɧɚɧ ɬԝɪɚɞɵ"
6. ȿɫɟɩ ȿɝɟɪ ɞɟ ɋɈ2 ɦɚɫɫɚɥɵԕ ԛɥɟɫɿ ԕ ɠ ɬɟԙ ɛɨɥɫɚ, ɥ ɚɭɚɞɚ
ɤԧɦɿɪ ԕɵɲԕɵɥ ɝɚɡɞɵԙ ԕɚɧɲɚ ɦɨɥɟɤɭɥɚɫɵ ɛɚɪ"
15
16.
7. ȿɫɟɩ 0ɋ ɦɟɧ ɦɦ ɫ ɛ ɝ ɤɚɥɶɰɢɣ ɤɚɪɛɨɧɚɬɵ ɵɞɵɪɚԑɚɧɞɚɛԧɥɿɧɿɩ ɲɵԕԕɚɧ ɤԧɦɿɪ ԕɵɲԕɵɥ ɝɚɡɞɵԙ ɤԧɥɟɦɿɧ ɬɚɛɵԙɞɚɪ
8. ȿɫɟɩ 0ɋ ɦɟɧ ɤɉȺ ɦɦ ɫ ɛ ɛɨɥԑɚɧɞɚ ɦɥ ɝɚɡɞɵԙ ɦɚɫɫɚɫɵ
ɝ ɬɟԙ Ƚɚɡɞɵԙ ɦɨɥɟɤɭɥɚɥɵԕ ɦɚɫɫɚɫɵɧ ɟɫɟɩɬɟԙɞɟɪ
9. ȿɫɟɩ Ʉɨɥɛɚɧɵԙ ɤԧɥɟɦɿ ɦɥ 0ɋ ɤɨɥɛɚɧɵ ɨɬɬɟɤɩɟɧ ɬɨɥɬɵɪԑɚɧɞɚ
ɦɚɫɫɚɫɵ ɝ ɬɟԙ ɛɨɥɚɞɵ 0ɋ ɤɨɥɛɚɧɵԙ ɫɚɥɦɚԑɵ ɝ Ɉɬɬɟɤɬɿԙ
ԕɵɫɵɦɵɧ ɟɫɟɩɬɟԙɞɟɪ
10. ɦ3 ɝɚɡɞɵԙ ɦɚɫɫɚɫɵ ԕ ɠ ɤɝ Ƚɚɡɞɵԙ ɦɨɥɟɤɭɥɚɥɵԕ
ɦɚɫɫɚɫɵɧ ɚɭɚ ɚɪԕɵɥɵ ɬɵԑɵɡɞɵԑɵɧ ɠԥɧɟ ɛɿɪ ɦɨɥɟɤɭɥɚɧɵԙ ɦɚɫɫɚɫɵɧ
ɬɚɛɵԙɞɚɪ
11.ȿɫɟɩ 0ɋ ɠԥɧɟ ɤɉȺ ɦɦ ɫ ɛ ԕɵɫɵɦɞɚԑɵ ɦ3 ɚɭɚɧɵԙ
ɦɚɫɫɚɫɵɧ ɟɫɟɩɬɟԙɞɟɪ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɬԥɠɿɪɢɛɟ
Ԕɚɠɟɬɬɿ ԕɨɧɞɵɪԑɵ ɠԥɧɟ ԕԝɪɚɥ-ɠɚɛɞɵԕɬɚɪ ɯɢɦɢɹɥɵԕ ɵɞɵɫɬɚɪ.
Ʉɢɩɩ ɚɩɩɚɪɚɬɵ ɰɢɥɢɧɞɪ ɫɬɚɤɚɧ ɤɨɥɛɚ ɦɥ ɛɚɪɨɦɟɬɪ ɬɟɯɧɢɤɚɥɵԕ
ɬɚɪɚɡɵ ɬɵԑɵɧ ɤɚɪɚɧɞɚɲ
Ɋɟɚɤɬɢɜɬɟɪ ɋɚɋɈ3 ɦɪɚɦɨɪ ɢɡɜɟɫɬɶ ɦɟɥ ɇɋȱ ɤɨɧɰ 36,23%. U=1,18
1-ɬԥɠɿɪɢɛɟ ɋɈ2 ɦɨɥɟɤɭɥɚɥɵԕ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚɭ
Ɉɥ ԛɲɿɧ ɤԧɥɟɦɿ - ɦɥ ԕԝɪԑɚԕ ɤɨɥɛɚ ɚɥɵɩ ɬɵԑɵɧɦɟɧ ɚɭɡɵɧ
ɦɵԕɬɚɩ ɠɚɭɵɩ ɤɚɪɚɧɞɚɲɩɟɧ ɬɵԑɵɧ ɠɟɬɤɟɧ ɠɟɪɞɿ ɛɟɥɝɿɥɟԙɞɟɪ
Ʉɨɥɛɚɧɵ ɿɲɿɧɞɟɝɿ ɚɭɚɫɵ ɦɟɧ ɠԥɧɟ ɬɵԑɵɧɵɦɟɧ ɝ ɞԥɥɞɿɤɤɟ ɞɟɣɿɧ
ԧɥɲɟɭ ȼ1 ɤɟɪɟɤ Ʉɨɥɛɚɧɵ Ʉɢɩɩ ɚɩɩɚɪɚɬɵɧɚɧ ɚɥɵɧԑɚɧ ɤԧɦɿɪɬɟɤɬɿԙ ԕɨɫ
ɨɤɫɢɞɿɦɟɧ ɬɨɥɬɵɪɵԙɞɚɪ
18-ɫɭɪɟɬ Ʉɢɩɩ ɚɩɩɚɪɚɬɵ ɠԥɧɟ ɠɭԑɵɲ ɫɤɥɹɧɤɚɥɚɪ
16
17.
Ƚɚɡ ɬɚɡɚ ɠԥɧɟ ԕԝɪԑɚԕ ɛɨɥɭ ԛɲɿɧ NaHCO3 ɦɟɧ H2SO4 ɤɨɧɰ ɛɚɪɵɞɵɫɬɚɪɞɚɧ ԧɬɤɿɡɭ ԕɚɠɟɬ Ƚɚɡ ԧɬɤɿɡɝɿɲ ɬԛɬɿɤɲɟɧɿ ɤɨɥɛɚɧɵԙ ɬԛɛɿɧɟ ɞɟɣɿɧ
ɬԛɫɿɪɿɩ - ɦɢɧɭɬ ɋɈ2 ɦɟɧ ɬɨɥɬɵɪɭ ɤɟɪɟɤ ɀɚɧԑɚɧ ɲɵɪɩɵɧɵԙ
ɤԧɦɟɝɿɦɟɧ ɤɨɥɛɚɧɵԙ ɝɚɡԑɚ ɬɨɥԑɚɧɵɧ ɬɟɤɫɟɪɿԙɞɟɪ Ƚɚɡ ԧɬɤɿɡɝɿɲ ɬԛɬɿɤɲɟɧɿ
ɲɵԑɚɪɵɩ ɤɨɥɛɚɧɵ ɬɵԑɵɧɦɵɧ ɛɟɥɝɿɝɟ ɞɟɣɿɧ ɬɵԑɵɧɞɚɩ ɠɚɭɵɩ ԕɚɣɬɚɞɚɧ
ȼ2 ԧɥɲɟԙɞɟɪ Ԧɥɲɟɭ ɧԥɬɢɠɟɫɿ ɝ ɚɣɵɪɦɚɫɵ ɛɨɥԑɚɧԑɚ ɞɟɣɿɧ ɤɨɥɛɚɧɵ
ɝɚɡɛɟɧ ɬɨɥɬɵɪɵɩ - ɪɟɬ ԕɚɣɬɚɥɚɭ ȼ3 ɤɟɪɟɤ Ʉɨɥɛɚɞɚԑɵ ɝɚɡɞɵԙ ɤԧɥɟɦɿ
ɨԑɚɧ ɛɟɥɝɿɫɿɧɟ ɞɟɣɿɧ ԕԝɣɵɥԑɚɧ ɫɭɞɵԙ ɤԧɥɟɦɿɦɟɧ ԧɥɲɟɧɟɞɿ
Ʌɚɛɨɪɚɬɨɪɢɹɞɚԑɵ ɬɟɦɩɟɪɚɬɭɪɚ ɦɟɧ ɚɬɦɨɫɮɟɪɚɥɵԕ ԕɵɫɵɦɞɵ ɛɚɣԕɚԙɞɚɪ
Ⱥɥɵɧԑɚɧ ɦԥɧɞɟɪ ɛɨɣɵɧɲɚ ɤɨɥɛɚɞɚԑɵ ɚɭɚɧɵԙ ɦɚɫɫɚɫɵ ɦɟɧ ɤԧɦɿɪɬɟɤɬɿԙ
ԕɨɫ ɨɤɫɢɞɿɧɿԙ ɦɚɫɫɚɫɵ ɠԥɧɟ ɦɨɥɟɤɭɥɚɥɵԕ ɦɚɫɫɚɫɵɧ ɟɫɟɩɬɟԙɞɟɪ
Ȼɚԕɵɥɚɧԑɚɧ ɬԥɠɿɪɢɛɟ ɧԥɬɢɠɟɫɿ
Ʉɨɥɛɚɧɵԙ
Ʉɨɥɛɚɧɵԙ ɦɚɫɫɚɫɵ Ʉɨɥɛɚ
ɦɚɫɫɚɫɵ ɚɭɚɦɟɧ ɋɈ2-ɦɟɧ ɠԥɧɟ ɤԧɥɟɦɿ
ɠԥɧɟ ɬɵԑɵɧɦɟɧ ɝ ɬɵԑɵɧɦɟɧ ɝ
ɦɥ
ȼ1=
B2=
B3=
Ʉɟɫɬɟ
Ⱥɭɚ ɬɟɦ- Ԕɵɫɵɦ
ɩɟɪɚɬɭɪɚɫɵ ɦɦ ɫ ɛ
0
ɋ ɛԧɥɦɟ ɛԧɥɦɟ
Ɍԥɠɿɪɢɛɟɧɿԙ ɧԥɬɢɠɟɫɿɧ ԧԙɞɟɭ
V-ɤɨɥɛɚɞɚԑɵ ɚɭɚɧɵԙ ɤԧɥɟɦɿ Ʉɨɥɛɚԑɚ ɛɟɥɝɿɝɟ ɞɟɣɿɧ ɫɭ ԕԝɣɵɩ
ɰɢɥɢɧɞɪɦɟɧ ԧɥɲɟɭ
1. Ʉɨɥɛɚɞɚԑɵ ɚɭɚɧɵԙ ɤԧɥɟɦɿɧ ԕɚɥɵɩɬɵ ɠɚԑɞɚɣԑɚ ɤɟɥɬɿɪɭ ԛɲɿɧ ɦɵɧɚ
ɮɨɪɦɭɥɚɧɵ ɩɚɣɞɚɥɚɧɚɞɵ
V0=P.V.T0/P0 .T
Ɇԝɧɞɚԑɵ Ɋ-ɚɬɦɨɫɮɟɪɚɥɵԕ ԕɵɫɵɦ ɛԧɥɦɟ
Ɍ0-ԕɚɥɵɩɬɵ ɠɚԑɞɚɣɞɚԑɵ Ʉɟɥɶɜɢɧ ɛɨɣɵɧɲɚ ɬɟɦɩɟɪɚɬɭɪɚ 0)
Ɋ0- ԕɚɥɵɩɬɵ ɠɚԑɞɚɣɞɚԑɵ ԕɵɫɵɦ ɦɦ ɫ ɛ ɧɟɦɟɫɟ ɤɉȺ
Ɍ T0+t0C-ɬԥɠɿɪɢɛɟɧɿԙ ɛԧɥɦɟɧɿԙ ɬɟɦɩɟɪɚɬɭɪɚɫɵ
2. V0-ɛɨɣɵɧɲɚ ɤɨɥɛɚɞɚԑɵ ɚɭɚɧɵԙ ɦɚɫɫɚɫɵɧ ɟɫɟɩɬɟԙɞɟɪ ɨɥ ԛɲɿɧ ɬɟԙɞɟɭ
ɠɚɡɭ ɤɟɪɟɤ ɟɫɤɟ ɫɚԕɬɚɩ ɥ ɚɭɚɧɵԙ ԕ ɠ ɦɚɫɫɚɫɵ ɝ ȼ4).
ȼ1-ȼ4 ɚɣɵɪɦɚɥɚɪɵɧɚɧ ɛɨɫ ɤɨɥɛɚɧɵԙ ɬɵԑɵɧɦɟɧ ɟɫɟɩɬɟɝɟɧɞɟɝɿ
ɦɚɫɫɚɫɵɧ ɟɫɟɩɬɟԙɞɟɪ ȼ5).
ȼ3-ȼ5 ɚɣɵɪɦɚɥɚɪɵɧɚɧ ɤԧɦɿɪɬɟɤɬɿԙ ԕɨɫ ɨɤɫɢɞɿɧɿԙ ɤԧɦɿɪ ԕɵɲԕɵɥ
ɝɚɡɵɧɵԙ ɤɨɥɛɚɞɚԑɵ ȼ6 ɦɚɫɫɚɫɵɧ ɟɫɟɩɬɟԙɞɟɪ
5. ɋɈ2 ±ɧɿԙ ɚɭɚ ɛɨɣɵɧɲɚ ɬɵԑɵɡɞɵԑɵɧ ɟɫɟɩɬɟԙɞɟɪ
Ⱦɚɭɚ= mCO2/mɚɭɚ=B6/B4
6. Ʉԧɦɿɪɬɟɤɬɿԙ ԕɨɫ ɨɤɫɢɞɿɧɿԙ (ɤԧɦɿɪ ԕɵɲԕɵɥ ɝɚɡɵɧɵԙ) ɦɨɥɟɤɭɥɚɥɵԕ
ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚԙɞɚɪ:
Ɇɬԥɠɿɪ. =29 .Ⱦɚɭɚ (ɦ.ɚ.ɛ)
7. Ɇɬɟɨɪ - ɦ ɚ ɛ ɝ ɦɨɥɶ
8. Ɍԥɠɿɪɢɛɟɧɿԙ ɚɛɫɨɥɸɬɬɿɤ ԕɚɬɟɫɿɧ ɟɫɟɩɬɟԙɞɟɪ
17
18.
Ⱥɛɫɨɥɸɬɬɿɤ ԕɚɬɟ =Mɬɟɨɪ - Ɇɬԥɠɿɪɋɚɥɵɫɬɵɪɦɚɥɵ ԕɚɬɟ Ɇɬɟɨɪ Ɇɬԥɠɿɪ /Ɇɬɟɨɪ .100%.
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ N 3
Ɍɚԕɵɪɵɛɵ ɀɚɣ ɠԥɧɟ ɤԛɪɞɟɥɿ ɡɚɬɬɚɪɞɵԙ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ
ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚɭ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵԙ ɦɚԕɫɚɬɵ Ɇɟɬɚɥɞɵԙ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ
ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚɭ
Ȼɚԕɵɥɚɭ ɠԝɦɵɫɵɧɵԙ ɫԝɪɚԕɬɚɪɵ ɦɟɧ ɠɚɬɬɵԑɭɥɚɪɵ
ɀɚɣ ɠԥɧɟ ɤԛɪɞɟɥɿ ɡɚɬɬɚɪɞɵԙ ɷɤɜɢɜɚɥɟɧɬɿ ɬɭɪɚɥɵ ɬԛɫɿɧɿɤ
2. ɗɤɜɢɜɚɥɟɧɬɬɿɤ ɮɚɤɬɨɪɵ ɬɭɪɚɥɵ ɬԛɫɿɧɿɤ ɗɤɜɢɜɚɥɟɧɬɬɿԙ ɫɚɧɵ
ɦԧɥɲɟɪɿ
3. ɗɤɜɢɜɚɥɟɧɬɬɿԙ ɠԥɧɟ ɷɤɜɢɜɚɥɟɧɬɬɿԙ ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧɵԙ ԧɥɲɟɦɿ
ɗɥɟɦɟɧɬɬɟɪɞɿԙ ɷɤɜɢɜɚɥɟɧɬɬɿɧɿԙ ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚɭ
ɗɤɜɢɜɚɥɟɧɬɬɿԙ ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧɵԙ ɤԧɥɟɦɿ
5. Ʉԛɪɞɟɥɿ ɡɚɬɬɚɪɞɵԙ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚɭ
ɨɤɫɢɞɬɟɪɞɿԙ ɝɢɞɪɨɤɫɢɞɬɟɪɞɿԙ ԕɵɲԕɵɥɞɚɪɞɵԙ ɨɪɬɚ ԕɵɲԕɵɥɞɵԕ
ɧɟɝɿɡɞɿɤ ɬԝɡɞɚɪɞɵԙ Ɇɵɫɚɥ ɤɟɥɬɿɪɿԙɞɟɪ
6. ɗɤɜɢɜɚɥɟɧɬ ɡɚԙɵ ɦɚɬɟɦɚɬɢɤɚɥɵԕ ԧɪɧɟɝɿ
7. Ȼɟɪɿɥɝɟɧ ɤԛɪɞɟɥɿ ɡɚɬɬɚɪɞɵԙ ɷɤɜɢɜɚɥɟɧɬɿɧ ɠԥɧɟ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ
ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚԙɞɚɪ ɇȼr ɇ2S ɇ3ɊɈ4 Ɇg3 ɊɈ4)2, ɄɇSO4,
ɋɚ ɇɋɈ3)2 ԐɟɈɇɋȱ2, ɋr Ɉɇ 2NO3, Ⱥȱ2O3, SO2, N2Ɉ5, ɄȺȱ SO4)2.
8. Ⱥɥɸɦɢɧɢɣɞɿԙ ɟɤɿ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ ɤԛɤɿɪɬɬɿԙ ȱV ԛɲ ɷɤɜɢɜɚɥɟɧɬɬɿɧɿԙ,
ɧɢɤɟɥɶɞɿԙ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ ɦɚɫɫɚɫɵɧ ɬɚɛɵԙɞɚɪ
9. Ɍԧɦɟɧɞɟɝɿ ɛɟɪɿɥɝɟɧ ɪɟɚɤɰɢɹɥɚɪɞɚԑɵ:
Ԑɟ Ɉɇ 2ɋɇ3ɋɈɈ ɠԥɧɟ Ʉɇ2ɊɈ4 ɡɚɬɬɚɪɵɧɵԙ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ
ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚԙɞɚɪ
ȿɫɟɩ ɝ ɦɟɬɚɥ ɤɚɪɛɨɧɚɬɵ ɚɡɨɬ ԕɵɲԕɵɥɵɦɟɧ ԥɪɟɤɟɬɬɟɫɤɟɧɞɟ ɝ
ɦɟɬɚɥ ɧɢɬɪɚɬɵ ɬԛɡɿɥɟɞɿ Ɇɟɬɚɥɞɵԙ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ
ɚɧɵԕɬɚԙɞɚɪ
11. ȿɫɟɩ ɝ ɮɨɫɮɨɪɥɵ ԕɵɲԕɵɥɵɧ ɇ3ɊɈ3 ɧɟɣɬɪɚɥɞɚɭ ԛɲɿɧ ɝ
ɄɈɇ ԕɚɠɟɬ ɠԝɦɫɚɥɚɞɵ Ɏɨɫɮɨɪɥɵ ԕɵɲԕɵɥɞɵԙ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ
ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ ɧɟɝɿɡɞɿɥɿɝɿɧ ɚɧɵԕɬɚԙɞɚɪ Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ
ɠɚɡɵԙɞɚɪ
12. ȿɫɟɩ Ɉɬɬɟɤɬɿԙ ԕ ɠ ɷɤɜɢɜɚɥɟɧɬɬɿɤ ɦɚɫɫɚɫɵɧɵԙ ɤԧɥɟɦɿ ԕɚɧɲɚԑɚ ɬɟԙ?
ɝ ɦɟɬɚɥɞɵ ɠɚɧɞɵɪɭ ԛɲɿɧ ɥ ɨɬɬɟɤ ԕ ɠ ԕɚɠɟɬ Ɇɟɬɚɥɞɵԙ
ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚԙɞɚɪ Ԕɚɧɞɚɣ ɦɟɬɚɥ ɟɝɟɪɞɟ
ɜɚɥɟɧɬɬɿɥɿɝɿ ɬɟԙ ɛɨɥɫɚ"
18
19.
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɬԥɠɿɪɢɛɟɁɚɬɬɚɪɞɵԙ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚɭ
Ԕɚɠɟɬɬɿ ԕԝɪɚɥ ɠɚɛɞɵԕɬɚɪ Ȼɸɪɟɬɤɚ ɲɬɚɬɢɜ Ɉɫɬɜɚɥɶɞ ɩɪɨɛɢɪɤɚɫɵ
ɞԥɥɞɿɤɤɟ ɞɟɣɿɧ ԧɥɲɟɧɝɟɧ ɛɿɪɧɟɲɟ ɦɟɬɚɥɞɚɪ ɜɨɪɨɧɤɚ ɛɚɪɨɦɟɬɪ
Ɋɟɚɤɬɢɜɬɟɪ ɇɋȱ
ɀԝɦɵɫɬɵԙ ɨɪɵɧɞɚɥɭɵ. Ɂɚɬɬɵԙ ɷɤɜɢɜɚɥɟɧɬɬɿɤ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚɭ
ԛɲɿɧ ɦɵɧɚɞɚɣ ɩɪɢɛɨɪ ԕɚɠɟɬ -ɫɭɪɟɬ ɉɪɢɛɨɪ ɛɸɪɟɬɤɚɞɚɧ
Ɉɫɬɜɚɥɶɞ ɩɪɨɛɢɪɤɚɫɵɧɚɧ ɬɟԙɟɫɬɿɪɝɿɲ ɬԛɬɿɤɲɟɞɟɧ ɬԝɪɚɞɵ
Ⱥɧɚɥɢɬɢɤɚɥɵԕ ɬɚɪɚɡɵɞɚ ɝ ɞԥɥɞɿɤɤɟ ɞɟɣɿɧ ԧɥɲɟɧɿɩ ɚɥɵɧԑɚɧ
ɦɟɬɚɥɞɵ Ɉɫɬɜɚɥɶɞ ɩɪɨɛɢɪɤɚɫɵɧɵԙ ɛɿɪ ɠɚԑɵɧɚ ɫɚɥɚɞɵ ȿɤɿɧɲɿ ɠɚԑɵɧɚ
ɜɨɪɨɧɤɚ ɚɪԕɵɥɵ ɬԝɡ ԕɵɲԕɵɥɵ ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɹɞɵ Ɍԥɠɿɪɢɛɟ ɚɥɞɵɧɞɚ
ɩɪɢɛɨɪɞɵԙ ɞԝɪɵɫɬɵԑɵɧ ɬɟɤɫɟɪɿɩ ɚɥɭ ԕɚɠɟɬ Ɉɥ ԛɲɿɧ ɚɲɵԕ ɬԛɬɿɤɲɟɧɿ
ɠɨԑɚɪɵ-ɬԧɦɟɧ ɬԛɫɿɪɭ ɤɟɪɟɤ Ȼԝɥ ɤɟɡɞɟ ɬԛɬɿɤɲɟ ɿɲɿɧɞɟɝɿ ɫɭ ɬɟɡ ɬԧɦɟɧɠɨԑɚɪɵ ɬԛɫɩɟɣ ɠɚɣ ԑɚɧɚ ɚɭɵɬԕɭɵ ɬɢɿɫ Ȼԝɥɚɣ ɛɨɥɦɚԑɚɧ ɠɚԑɞɚɣɞɚ
ɬԛɬɿɤɲɟɝɟ ɩɪɨɛɢɪɤɚԑɚ ɬɵԑɵɧɵɧ ɠɚԕɫɵɥɚɩ ɬɵԑɵɡɞɚɭ ԕɚɠɟɬ
19-ɫɭɪɟɬ Ɂɚɬɬɚɪɞɵԙ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚɭ
ԕԝɪɚɥɵ
1-ɛɸɪɟɬɤɚ 2-Ɉɫɬɜɚɥɶɞ ɩɪɨɛɢɪɤɚɫɵ -ɬɟԙɟɫɬɿɪɝɿɲ ɬԛɬɿɤɲɟ
19
20.
ȿɝɟɪ ɞɟ ɩɪɢɛɨɪ -ɫɭɪɟɬ Ɂɚɬɬɚɪɞɵԙ ɷɤɜɢɜɚɥɟɧɬɬɿɤ ɦɚɫɫɚɫɵɧɚɧɵԕɬɚɭ ԕԝɪɚɥɵ ɝɟɪɦɟɬɢɤɚɥɵԕ ɛɨɥɫɚ ɬԥɠɿɪɢɛɟɧɿ ɛɚɫɬɚɭԑɚ ɛɨɥɚɞɵ
Ɇɟɬɚɥɥ ɠԥɧɟ ԕɵɲԕɵɥ ԕԝɣɵɥԑɚɧ ɩɪɨɛɢɪɤɚɧɵ ɛɸɪɟɬɤɚԑɚ ԕɨɫɵɩ
ɩɪɢɛɨɪɞɵԙ ɞԝɪɵɫɬɵԑɵɧ ɬɟɤɫɟɪɭ ԕɚɠɟɬ Ɍԛɬɿɤɲɟɥɟɪɞɟɝɿ ɫɭɞɵԙ ɞɟԙɝɟɣɿɧ
ɛɿɪɞɟɣ ɟɬɿɩ ɬԧɦɟɧɝɿ ɫɵɡɵԑɵ ɛɨɣɵɧɲɚ ɤԧɪɫɟɬɭɿɧ ɠɚɡɵɩ K1 ɚɥɭ ԕɚɠɟɬ
Ȼԛɞɚɧ ɫɨԙ ԕɵɲԕɵɥɞɵ ɦɟɬɚɥԑɚ ԕԝɣɵԙɞɚɪ Ȼɟɪɿɥɝɟɧ ɪɟɚɤɰɢɹ ɧԥɬɢɠɟɝɿ
ɫɭɬɟɤ ɛɸɪɟɬɤɚɞɚԑɵ ɫɭɞɵ ɵԑɵɫɬɵɪɚ ɛɚɫɬɚɣɞɵ Ɋɟɚɤɰɢɹ ɚɹԕɬɚɥԑɚɧɚɧ
ɤɟɣɿɧ - ɦɢɧɭɬ ԧɬɤɟɫɿɧ ɛɸɪɟɬɤɚɞɚԑɵ ɫɭɞɵ ɚɲɵԕ ɬԛɬɿɤɲɟ ɚɪԕɵɥɵ ɛɿɪ
ɞɟԙɝɟɣɝɟ ɤɟɥɬɿɪɭ ɤɟɪɟɤ ɠɚԙɚ ɩɚɣɞɚ ɛɨɥԑɚɧ ɫɭɞɵԙ ɤԧɪɫɟɬɭɿɧ h2 ɠɚɡɵɩ
ɚɥɵԙɞɚɪ Ȼɚɪɨɦɟɬɪ ɦɟɧ ɬɟɪɦɨɦɟɬɪɞɿԙ ɤԧɪɫɟɬɭɥɟɪɿɧ ɠɚɡɵɩ ɚɥɵԙɞɚɪ
Ɍԥɠɿɪɢɛɟɧɿԙ ɧԥɬɢɠɟɫɿɧ ɤɟɫɬɟɝɟ ɠɚɡɵԙɞɚɪ
Ȼԧɥɿɧɿɩ ɲɵԕԕɚɧ ɫɭɬɟɤɬɿԙ ԕԧɥɟɦɿɧ ԕɚɥɵɩɬɵ ɠɚԑɞɚɣԑɚ ɤɟɥɬɿɪɭ ԛɲɿɧ
ɦɵɧɚ ɮɨɪɦɭɥɚɧɵ ɩɚɣɞɚɥɚɧɭ ɤɚɠɟɬ
V0=V.(P-PH2O) .T0 Ɋ0 Ɍ
Ɇԝɧɞɚԑɵ V-ɛԧɥɿɧɿɩ ɲɵԕԕɚɧ ɫɭɬɟɤɬɿԙ ɤԧɥɟɦɿ ɦɥ
Ɋɇ Ɉ- ɛɸɪɟɬɤɚɞɚԑɵ ɫɭɞɵԙ ԕɵɫɵɦɵ ɚɧɵԕɬɚɦɚɧɵ ɩɚɣɞɚɥɚɧɭ ɤɟɪɟɤ
Ɋ- ɬԥɠɿɪɢɛɟɧɿԙ ԕɵɫɵɦɵ ɛԧɥɦɟ
Ɋ0-ԕɚɥɵɩɬɵ ɠɚԑɞɚɣɞɚԑɵ ɚɬɦɨɫɮɟɪɚɥɵԕ ԕɵɫɵɦ ɦɦ ɫ ɛ ɧɟɦɟɫɟ
ɤɉȺ
Ɍ-ɬԥɠɿɪɢɛɟɧɿԙ ɛԧɥɦɟɧɿԙ Ʉɟɥɶɜɢɧ ɛɨɣɵɧɲɚ ɬɟɦɩɟɪɚɬɭɪɚɫɵ Ɍ0+t0ɋ
Ɍ0 ±ԕɚɥɵɩɬɵ ɠɚԑɞɚɣɞɚԑɵ Ʉɟɥɶɜɢɧ ɬɟɦɩɟɪɚɬɭɪɚɫɵ 0 Ʉ
Ɇɟɬɚɥɞɵԙ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ Ɇ z Ɇɟ
ɚɧɵԕɬɚɭ ԛɲɿɧ ɚɥɞɵɦɟɧ ɫɭɬɟɤɬɿԙ ɦɚɫɫɚɫɵɧ mH2 ɟɫɟɩɬɟɭ ɤɟɪɟɤ Ɉɥ ԛɲɿɧ
ɬɟԙɞɟɭ ɠɚɡɭ ɤɟɪɟɤ ɟɫɤɟ ɫɚԕɬɚɩ ɥ ɫɭɬɟɤɬɿԙ ԕɚɥɵɩɬɵ ɠɚԑɞɚɣɞɚ ɦɚɫɫɚɫɵ
ɝ
Ɇɟɬɚɥɞɵԙ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚɭ ԛɲɿɧ
ɷɤɜɢɜɚɥɟɧɬ ɡɚԙɵ ɩɚɣɞɚɥɚɧɚɞɵ
m Ɇɟ /m ɇ2 Ɇ /zɆɟ /Ɇ /zɇ2)
Ɇɟɬɚɥɞɵԙ ɬɟɨɪɢɹ ɛɨɣɵɧɲɚ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ
ɬɚɛɭ ԛɲɿɧ ɨɧɵԙ Ⱦ ɂ Ɇɟɧɞɟɥɟɟɜ ɤɟɫɬɟɫɿɧɞɟɝɿ ɨɪɧɵ ɠԥɧɟ ɜɚɥɟɧɬɬɿɥɿɝɿɧ
ɬɚɛɭ ɤɟɪɟɤ
Ɇ zɆɟ Ⱥ Ɇɟ ȼ
Ⱥ Ɇɟ ± ɦɟɬɚɥɞɵԙ ɚɬɨɦɞɵԕ ɦɚɫɫɚɫɵ
ȼ - ɦɟɬɚɥɞɵԙ ɜɚɥɟɧɬɬɿɥɿɝɿ
20
21.
5) Ɍԥɠɿɪɢɛɟɧɿԙ ԕɚɬɟɫɿɧ ɟɫɟɩɬɟԙɞɟɪ ɦɵɧɚɞɚɣ ɮɨɪɦɭɥɚ ɚɪԕɵɥɵɅɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ 1
Ɍɚԕɵɪɵɛɵ Ȼɟɣɨɪɝɚɧɢɤɚɥɵԕ ԕɨɫɵɥɵɫɬɚɪɞɵԙ ɧɟɝɿɡɝɿ
ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵԙ ɦɚԕɫɚɬɵ Ȼɟɣɨɪɝɚɧɢɤɚɥɵԕ
ԕɨɫɵɥɵɫɬɚɪɞɵԙ ɧɟɝɿɡɝɿ ɤɥɚɫɬɚɪɵɧ ɠԥɧɟ ɨɥɚɪɞɵԙ ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿɧ
ɨԕɵɩ ԛɣɪɟɧɭ
Ȼɚԕɵɥɚɭ ɠԝɦɵɫɵɧɵԙ ɫԝɪɚԕɬɚɪɵ ɦɟɧ ɠɚɬɬɵԑɭɥɚɪɵ
1. Ɍԧɦɟɧɝɿ ɛɟɪɿɥɝɟɧ ԕɨɫɵɥɵɫɬɚɪɞɵԙ ԕɚɣɫɵɥɚɪɵ ԕɵɲԕɵɥɞɵԕ ɧɟɝɿɡɞɿɤ
ɠԥɧɟ ɚɦɮɨɬɟɪɥɿɤ ɨɤɫɢɞɬɟɪɝɟ ɠɚɬɚɞɵ" %H2 %U23 =Q2 6ɿ22, CO2,
CuSO4, Ԑe2O3, ԐeO, Pb(OH)2, SnO, CdO, Al(NO3)3, H2CrO4, NaOH, SO3,
Mg3(PO4)2, FeOHSO4 Ɉɤɫɢɞɬɟɪɞɿԙ ɚɥɵɧɭ ɠɨɥɞɚɪɵ
2. Ɍԧɦɟɧɝɿ ɛɟɪɿɥɝɟɧ ԕɨɫɵɥɵɫɬɚɪɞɵԙ ԕɚɣɫɵɥɚɪɵ ԕɵɲԕɵɥɞɚɪԑɚ
ɝɢɞɪɨɤɫɢɞɬɟɪɝɟ ɠԥɧɟ ɬԝɡɞɚɪԑɚ ɨɪɬɚ ԕɵɲԕɵɥɞɵԕ ɧɟɝɿɡɞɿɤ ɬԝɡɞɚɪԑɚ
ɠɚɬɚɞɵ" +3PO3, NaHCO3, FeHSO4, Al(NO3)3, +&ȱ23, HClO4, Cu(OH)2,
MgBr2, Na2CO3, AlPO4, Ca(HCO3)2, CaHPO4, Ca(H2PO4)2, CuOHCl,
Cr(OH)2NO3, Mg(H2PO4)2, 1ɿ 2+ 2, K2MnO4, H2Cr2O7.
3. Ԕɵɲԕɵɥɞɚɪɞɵԙ ɝɢɞɪɨɤɫɢɞɬɟɪɞɿԙ ɚɥɵɧɭ ɠɨɥɞɚɪɵ ɏɢɦɢɹɥɵԕ
ԕɚɫɢɟɬɬɟɪɿ
4. Ʉɟɥɟɫɿ ɡɚɬɬɚɪɞɵԙ ɮɨɪɦɭɥɚɫɵɧ ɠɚɡɵԙɞɚɪ Ʌɢɬɢɣɞɿԙ ȱ ɝɢɞɪɨɤɫɢɞɿ
ɯɪɨɦɧɵԙ ȱȱȱ ɝɢɞɪɨɤɫɢɞɿ ɦɚɪɝɚɧɟɰɬɿԙ ȱ9 ɝɢɞɪɨɤɫɢɞɿ ɨɪɬɨɛɨɪ
ԕɵɲԕɵɥɵ ɯɪɨɦ ԕɵɲԕɵɥɵ ɤɚɥɶɰɢɣɞɿԙ ɞɢɝɢɞɪɨɮɨɫɮɚɬɵ ɦɚɝɧɢɣɞɿԙ
ɝɢɞɪɨɤɚɪɛɨɧɚɬɵ ɤɚɥɶɰɢɣɞɿԙ ɮɨɫɮɚɬɵ ɯɪɨɦɧɵԙ ȱȱȱ ɝɢɞɪɨɫɭɥɶɮɚɬɵ
ɜɚɧɚɞɢɣɞɿԙ 9 ɨɤɫɢɞɿ ɋɬɪɭɤɬɭɪɚɥɵԕ ɮɨɪɦɭɥɚɫɵɧ ɠɚɡɵԙɞɚɪ
5. Ɉɪɬɚ ԕɵɲԕɵɥɞɵԕ ɠԥɧɟ ɧɟɝɿɡɞɿɤ ɬԝɡɞɚɪɞɵԙ ɚɥɵɧɭɵɧ ɠɚɡɵɩ ɨɪɬɚ
ɬԝɡɞɚɪɞɵԙ ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿɧ ɤԧɪɫɟɬɿԙɞɟɪ ɪɟɚɤɰɢɹ ɬɟԙɞɟɭɥɟɪɿɧ
ɠɚɡɵԙɞɚɪ
6. Ʉԧɩ ɧɟɝɿɡɞɿ ԕɵɲԕɵɥɞɚɪɞɵԙ ɤԧɩ ԕɵɲԕɵɥɞɵ ɧɟɝɿɡɞɟɪɞɿԙ ɨɪɬɚ
ԕɵɲԕɵɥɞɵԕ ɠԥɧɟ ɧɟɝɿɡɞɿɤ ɬԝɡɞɚɪɞɵԙ ɞɢɫɫɨɰɢɚɰɢɹɫɵɧ ɠɚɡɵԙɞɚɪ ɛɿɪɛɿɪ ɦɵɫɚɥ ɤɟɥɬɿɪɿԙɞɟɪ
7. ȿɫɟɩ Ɍɟԙɿɡɞɿԙ ɫɭɵɧɞɚ ɬԝɡɞɚɪɞɵԙ ɦɚɫɫɚɥɵԕ ԛɥɟɫɿ ɬɟԙ ɤɝ ɬɟԙɿɡ
ɫɭɵɧ ɛɭɥɚɧɞɵɪԑɚɧɞɚ ԕɚɧɲɚ ɬԝɡ ԕɚɥɚɞɵ"
8. ȿɫɟɩ Ɍɚɡɚɪɬԕɚɧ ɲɚɪɚɩ ɫɩɢɪɬ ԕԝɪɚɦɵɧɞɚ ɫɭɞɵԙ ɦɚɫɫɚɥɵԕ ԛɥɟɫɿ
ɬɟԙ ɥ ɫɩɢɪɬɬɟ p ɝ cɦ3 ԕɚɧɲɚ ɬԝɡ ɛɨɥɭɵ ɦԛɦɤɿɧ"
21
22.
9. ȿɫɟɩ ɝ ɚɥɸɦɢɧɢɣɦɟɧ ɠԥɧɟ ɚɥɸɦɢɧɢɣ ɨɤɫɢɞɿɧɟɧ ɬԝɪɚɬɵɧ ԕɨɫɩɚԑɚɬԝɡ ԕɵɲԕɵɥɵɧ ԕɨɫԕɚɧɞɚ ɦɥ ɝɚɡ ԕ ɠ ɛԧɥɿɧɝɟɧ Ԕɨɫɩɚɞɚԑɵ
ɚɥɸɦɢɧɢɣɞɿԙ ɦɚɫɫɚɥɵԕ ԛɥɟɫɿɧ ɟɫɟɩɬɟԙɞɟɪ
10.ȿɫɟɩ ɝ ɦɚɝɧɢɣ ɨɤɫɢɞɿɧɟ ɝ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵ ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɹɞɵ
Ԕɚɧɲɚ ɝɪɚɦɦ ɬԝɡ ɬԛɡɿɥɟɞɿ"
11.ȿɫɟɩ ɤɝ ɪɭɞɚɞɚɧ ԕԝɪɚɦɵɧɞɚ PbS ɛɚɪ ɛɨɥɫɚ ԕɚɧɲɚ ɥɢɬɪ SO2
ԕ ɠ ɚɥɭԑɚ ɛɨɥɚɞɵ"
12.ȿɫɟɩ ɝ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɧ ɛɟɣɬɚɪɚɩɬɚɭ ԛɲɿɧ ɝ ɧɚɬɪɢɣ ɝɢɞɪɨɤɫɢɞɿ
ɠԝɦɫɚɥɞɵ Ȼɚɫɬɚɩԕɵ ɟɪɿɬɿɧɞɿɞɟɝɿ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɧɵԙ ɦɚɫɫɚɥɵԕ
ԛɥɟɫɿɧ ɬɚɛɵԙɞɚɪ
13.Ɇɵɧɚ ԧɡɝɟɪɿɫɬɟɪ ɠԛɡɟɝɟ ɚɫɚɬɵɧ ɪɟɚɤɰɢɹ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
a) NaoNaOHoNa2SO4oNaNO3oNaNO2
b) AloAl2O3oAl2(SO4)3oAl(OH)3oKAlO2oAl(OH)3
oAlOHSO4oAl2(SO4)3 o Al oAl(NO3)3;
c) N2 oNH3oNOoHNO3oZn(NO3)2 oZnO oZnCl2oZn(OH)2 ;
d) FeS oSoSO2oSO3oH2SO4 ;
H2S
e) Mg oMgCl2oMg(OH)2o Mg(NO3)2 oMgOoMgSO4.
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɬԥɠɿɪɢɛɟɥɟɪ
Ԕɚɠɟɬɬɿ ԕɨɧɞɵɪԑɵ ɦɟɧ ɪɟɚɤɬɢɜɬɟɪ Ʉɢɩɩ ɚɩɩɚɪɚɬɵ ɤɟɩɬɿɪɝɿɲ ɲɤɚɮ
ɷɤɫɢɤɚɬɨɪ ɮɟɧɨɥɮɬɚɥɟɢɧ ɢɧɞɢɤɚɬɨɪ ɮɚɪɮɨɪ ɵɞɵɫɵ ɩɪɨɛɢɪɤɚɥɚɪ 1ɚɦɟɬɚɥɵ ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭ ԥɤ ɫɭɵ ɥɚɤɦɭɫ ɋɢ624 ȼɟɈ =Q2 $ȱ2O3, SnO,
PbO, 2% NaOH, NaOH ɤɨɧɰ Ԑɟɋȱ3 ɇ2SO4 +&ȱ ɤɨɧɰ
1-ɬԥɠɿɪɢɛɟ Ԕɵɲԕɵɥɞɵԕ ɚɦɮɨɬɟɪɥɿɤ ɧɟɝɿɡɞɿɤ ɨɤɫɢɞɬɟɪɞɿԙ ɚɥɵɧɭ
ɠɨɥɞɚɪɵ ɠԥɧɟ ɨɥɚɪɞɵԙ ԕɚɫɢɟɬɬɟɪɿ
ɚ Ʉɢɩɩ ɚɩɩɚɪɚɬɵɧɵԙ ɤԧɦɿɪɬɟɤɬɿԙ ԕɨɫ ɨɤɫɢɞɿɧ ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭɵ ɛɚɪ
ɩɪɨɛɢɪɤɚԑɚ ɠɿɛɟɪɭ ɤɟɪɟɤ Ʌɚɤɦɭɫ ɟɪɿɬɿɧɞɿɫɿɧ ԕɨɫɵԙɞɚɪ
ɇɟ ɛɚɣԕɚԑɚɧɞɚɪɵԙɞɵ ɬԛɫɿɧɞɿɪɿɩ ɪɟɚɤɰɢɹ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
ɛ Ʉԧɦɿɪ ԕɵɲԕɵɥ ɝɚɡɵɧ ɤԧɦɿɪɬɟɤɬɿԙ ԕɨɫ ɨɤɫɢɞɿɧ &D 2+ 2 ɟɪɿɬɿɧɞɿɫɿɧɟ
ɠɿɛɟɪɿԙɞɟɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ɋɟɚɤɰɢɹ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
ɜ ɇɚɬɪɢɣɞɵԙ ɤɿɲɤɟɧɬɚɣ ɛԧɥɲɟɝɿɧ ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭɵ ɛɚɪ ɮɚɪɮɨɪ
ɵɞɵɫԕɚ ɫɚɥɵԙɞɚɪ ɮɟɧɨɥɮɬɚɥɟɢɧ ɢɧɞɢɤɚɬɨɪɵɧ ԕɨɫɵԙɞɚɪ Ԕɚɧɞɚɣ
ԧɡɝɟɪɿɫɬɟɪ ɛɚɣԕɚɥɚɞɵ" Ɋɟɚɤɰɢɹ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
ɝ Ɍԝɧɛɚ ɩɚɣɞɚ ɛɨɥԑɚɧɲɚ ɦɵɫ ɫɭɥɶɮɚɬɵ ɟɪɿɬɿɧɞɿɫɿɧɟ ɬɚɦɲɵɥɚɬɵɩ
ɧɚɬɪɢɣ ɝɢɞɪɨɤɫɢɞɿɧɿԙ ɟɪɿɬɿɧɞɿɫɿɧ ԕɨɫɵԙɞɚ Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ
ɠɚɡɵԙɞɚɪ ɬԛɡɿɥɝɟɧ ɬԝɧɛɚɧɵԙ ɬԛɫɿɧ ɛɟɥɝɿɥɟԙɞɟɪ
ɞ Ȼɟɪɿɥɝɟɧ ɚɦɮɨɬɟɪɥɿɤ ɨɤɫɢɞɬɟɪɞɟɧ ȼɟɈ =Q2 $O2O3 6Q2 3E2 ɛɟɥɝɿɥɿ
ɛɿɪ ɨɤɫɢɞɬɿ ɚɥɵɩ ɨɧɵԙ ԕɵɲԕɵɥԑɚ ɠԥɧɟ ɫɿɥɬɿɝɟ ԕɚɬɵɫɵɧ ɛɚɣԕɚԙɞɚɪ
22
23.
ɪɟɚɤɰɢɹ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ Ⱥɦɮɨɬɟɪɥɿɤ ԕɚɫɢɟɬ ɞɟɝɟɧɞɿ ԕɚɥɚɣɬԛɫɿɧɞɿɪɭɝɟ ɛɨɥɚɞɵ"
2-ɬԥɠɿɪɢɛɟ ɇɟɝɿɡɞɿɤ ɚɦɮɨɬɟɪɥɿɤ ɝɢɞɪɨɤɫɢɞɬɟɪɞɿԙ ɚɥɵɧɭɵ ɠԥɧɟ
ɨɥɚɪɞɵԙ ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿ
ɚ ɦɥ -ɬɿɤ ԐH&O3 ɟɪɿɬɿɧɞɿɫɿɧɟ ɦɥ -ɬɿɤ 1D2+ ɟɪɿɬɿɧɞɿɫɿɧ
ԕɨɫɵԙɞɚɪ Ȼɚɫɬɚɩԕɵ ɟɪɿɬɿɧɞɿ ɦɟɧ ɬԛɡɿɥɝɟɧ ɬԝɧɛɚɧɵԙ ɬԛɫɿɧ ɛɟɥɝɿɥɟԙɞɟɪ
Ⱥɥɵɧԑɚɧ ɬɟɦɿɪ ɝɢɞɪɨɤɫɢɞɿɧɿԙ ȱȱȱ ɬԝɧɛɚɫɵɧ ɫԛɡɿɩ ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭɦɟɧ
ɠɭɵɩ 0ɋ ɬɟɦɩɟɪɚɬɭɪɚɞɚ ɤɟɩɬɿɪɝɿɲ ɲɤɚɮɬɚ ɤɟɩɬɿɪɭ ɤɟɪɟɤ
ɗɤɫɢɤɚɬɨɪɞɚ ɫɭɵɬɵɩ ԧɥɲɟɭ ɤɟɪɟɤ Ɍɟɨɪɢɹɥɵԕ ɠԥɧɟ ɿɫ ɠԛɡɿɧɞɟɝɿ
ɲɵԑɵɦɵɧ ɫɚɥɵɫɬɵɪɵɩ ԕɚɬɟɫɿɧ ɟɫɟɩɬɟԙɞɟɪ
ɛ Ⱥɥɵɧԑɚɧ )H 2+ 3 ɬԝɧɛɚɧɵԙ ɛɿɪ ɛԧɥɿɝɿɧ ɛԧɥɟɤ-ɛԧɥɟɤ ɟɤɿ ɩɪɨɛɢɪɤɚԑɚ
ԕԝɣɵԙɞɚɪ ɛɿɪɟɭɿɧɟ ɬԝɡ ԕɵɲԕɵɥɵɧ ɟɤɿɧɲɿɫɿɧɟ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ 1D2+
ԕԝɣɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ɋɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
Ⱥɦɮɨɬɟɪɥɿɤ ԕɚɫɢɟɬ ɬɭɪɚɥɵ ɬԛɫɿɧɿɤɬɟɦɟ Ԕɚɧɞɚɣ ԕɨɪɵɬɵɧɞɵ ɠɚɫɚɭԑɚ
ɛɨɥɚɞɵ"
3-ɬԥɠɿɪɢɛɟ ɋɿɥɬɿɥɟɪɞɿԙ ԕɚɫɢɟɬɬɟɪɿ.
ɚ 1D2+ ɟɪɿɬɿɧɞɿɫɿɧɟ ɢɧɞɢɤɚɬɨɪɥɚɪɞɵԙ -ɥɚɤɦɭɫ ɮɟɧɨɥɮɬɚɥɟɢɧ
ɦɟɬɢɥɨɪɚɧɠ ԥɫɟɪɿɧ ɚɧɵԕɬɚɩ ԕɨɪɵɬɵɧɞɵ ɠɚɫɚԙɞɚɪ
ɛ ɦɥ 1D2+ ɟɪɿɬɿɧɞɿɫɿɧɟ ɚɡɞɚԑɚɧ ɮɟɧɨɥɮɬɚɥɟɢɧ ɠԥɧɟ ɢɧɞɢɤɚɬɨɪɞɵԙ ɬԛɫɿ
ɠɨɣɵɥԑɚɧɲɚ ɚɡɞɚԑɚɧ ɬɚɦɲɵɞɚɧ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵ ɟɪɿɬɿɧɞɿɫɿɧ ԕɨɫɵԙɞɚɪ
Ɋɟɚɤɰɢɹɧɵԙ ɦɨɥɟɤɭɥɚɥɵԕ ɠԥɧɟ ɢɨɧɞɵ±ɦɨɥɟɤɭɥɚɥɵԕ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ 1
Ɍɚԕɵɪɵɛɵ ɏɢɦɢɹɥɵԕ ɪɟɚɤɰɢɹɥɚɪɞɵԙ ɠɵɥɞɚɦɞɵԑɵ
ɏɢɦɢɹɥɵԕ ɬɟɩɟ-ɬɟԙɞɿɤ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵԙ ɦɚԕɫɚɬɵ ɏɢɦɢɹɥɵԕ ɪɟɚɤɰɢɹɥɚɪɞɵԙ
ɠɵɥɞɚɦɞɵԑɵɧɚ ԥɪɟɤɟɬɬɟɫɭɲɿ ɡɚɬɬɚɪɞɵԙ ɤɨɧɰɟɧɬɪɚɰɢɹɥɚɪɵɧɵԙ ԥɫɟɪɿɧ
ɚɧɵԕɬɚɭ
Ȼɚԕɵɥɚɭ ɠԝɦɵɫɵɧɵԙ ɫԝɪɚԕɬɚɪɵ ɦɟɧ ɠɚɬɬɵԑɭɥɚɪɵ:
1. ɏɢɦɢɹɥɵԕ ɪɟɚɤɰɢɹ ɠɵɥɞɚɦɞɵԑɵ ɞɟɝɟɧ ɧɟ" ɏɢɦɢɹɥɵԕ ɪɟɚɤɰɢɹ
ɠɵɥɞɚɦɞɵԑɵ ɨɥ ԕɚɧɞɚɣ ɮɚɤɬɨɪɥɚɪԑɚ ɛɚɣɥɚɧɵɫɬɵ ɬԥɭɟɥɞɿ "
2. Ԥɪɟɤɟɬɬɟɫɭɲɿ ɦɚɫɫɚɥɚɪ ɡɚԙɵɧɵԙ ɚɧɵԕɬɚɦɚɫɵ ɨɧɵԙ ɦɚɬɟɦɚɬɢɤɚɥɵԕ
ԧɪɧɟɝɿ ɪɟɚɤɰɢɹɧɵԙ ɠɵɥɞɚɦɞɵԕ ɤɨɧɫɬɚɧɬɚɫɵ ɨɥ ԕɚɧɞɚɣ ɮɚɤɬɨɪɥɚɪԑɚ
ɛɚɣɥɚɧɵɫɬɵ ɬԥɭɟɥɞɿ "
Ɍɟɦɩɟɪɚɬɭɪɚ ԧɡɝɟɪɝɟɧɞɟ ɯɢɦɢɹɥɵԕ ɪɟɚɤɰɢɹ ɠɵɥɞɚɦɞɵԑɵ ԕɚɥɚɣ ɠԥɧɟ
ɧɟɥɿɤɬɟɧ ԧɡɝɟɪɟɞɿ" ȼɚɧɬ-Ƚɨɮɮ ɡɚԙɵɧɵԙ ɦɚɬɟɦɚɬɢɤɚɥɵԕ ԧɪɧɟɝɿ
Ⱥɤɬɢɜɬɟɧɭ ɷɧɟɪɝɢɹɫɵ ɞɟɝɟɧ ɧɟ"
4. Ʉɚɬɚɥɢɡɚɬɨɪ ɞɟɝɟɧɿɦɿɡ ɧɟ" Ƚɨɦɨɝɟɧɞɿɤ ɠԥɧɟ ɝɟɬɟɪɨɝɟɧɞɿɤ ɤɚɬɚɥɢɡ
ɨɥɚɪɞɵԙ ɦɟɯɚɧɢɡɦɿ Ɇɵɫɚɥ ɤɟɥɬɿɪɿԙɞɟɪ
5. Ԕɚɣɬɵɦɞɵ ɠԥɧɟ ԕɚɣɬɵɦɫɵɡ ɪɟɚɤɰɢɹɥɚɪ Ɇɵɫɚɥɞɚɪ
23
24.
6. ɏɢɦɢɹɥɵԕ ɬɟɩɟ-ɬɟԙɞɿɤ Ɍɟɩɟ-ɬɟԙɞɿɤɬɿԙ ɤɨɧɫɬɚɧɬɚɫɵ ɏɢɦɢɹɥɵԕ ɬɟɩɟɬɟԙɞɿɤɬɿԙ ɵԑɵɫɭɵɧɚ ԕɚɧɞɚɣ ɮɚɤɬɨɪɥɚɪ ԥɫɟɪ ɟɬɟɞɿ" Ʌɟ-ɒɚɬɟɥɶɟ ɩɪɢɧɰɢɩɿȿɫɟɩ Ɇɵɧɚɞɚɣ ɪɟɚɤɰɢɹ ɬɟԙɞɟɭɿɧɞɟ N2 Ɉ2=2NO ԥɪɟɤɟɬɬɟɫɭɲɿ
ɡɚɬɬɚɪɞɵԙ ɛɚɫɬɚɩԕɵ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵ ɦɨɥɶ ɥ >N2@ ɦɨɥɶ ɥ
>Ɉ2@ ɦɨɥɶ ɥ >NɈ@ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵ ɦɨɥɶ ɥ ɬɟԙ ɛɨɥԑɚɧɞɚ ɚɡɨɬ
ɩɟɧ ɨɬɬɟɤɬɿԙ ɬɟɩɟ-ɬɟԙɞɿɤ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵɧ ɟɫɟɩɬɟԙɞɟɪ
8. ȿɫɟɩ Ɇɵɧɚ ɝɟɬɟɨɪɨɝɟɧɞɿɤ ɪɟɚɤɰɢɹɧɵԙ ɬɟɩɟ-ɬɟԙɞɿɤ ɤɨɧɫɬɚɧɬɚɫɵɧɵԙ
ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
Ʉɨɧɰɟɧɬɪɚɰɢɹɫɵɧ ɬԧɪɬ ɟɫɟ ɚɡɚɣɬԕɚɧɞɚ ɬɭɪɚ ɪɟɚɤɰɢɹɧɵԙ
ɠɵɥɞɚɦɞɵԑɵ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ" ɋɈ ɲɵԑɵɦɵɧ ɤԧɛɟɣɬɭ ԛɲɿɧ ԕɵɫɵɦɞɵ
ԕɚɧɞɚɣ ԧɡɝɟɪɬɭ ɤɟɪɟɤ"
9. ȿɫɟɩ Ʉԛɤɿɪɬɬɿԙ ɠԥɧɟ ɤԛɤɿɪɬ ɨɤɫɢɞɿɧɿԙ ȱV ɬɨɬɵԑɭɵ ɦɵɧɚ ɬɟԙɞɟɭɥɟɪ
ɚɪԕɵɥɵ ɠԛɪɟɞɿ
Ɋɟɚɤɰɢɹɥɚɪԑɚ ԕɚɬɵɫԕɚɧ ɠԥɧɟ ɬԛɡɿɥɝɟɧ ɡɚɬɬɚɪɞɵԙ ɤԧɥɟɦɞɟɪɿ ɬԧɪɬ ɟɫɟ
ɬԧɦɟɧɞɟɬɤɟɧɞɟ ɨɫɵ ɪɟɚɤɰɢɹɥɚɪɞɵԙ ɠɵɥɞɚɦɞɵԕɬɚɪɵ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ"
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɬԥɠɿɪɢɛɟ
Ԕɚɠɟɬɬɿ ԕԝɪɚɥ ɠɚɛɞɵԕɬɚɪ ɠԥɧɟ ɪɟɚɤɬɢɜɬɟɪ Ȼɸɪɟɬɤɚ ɲɬɚɬɢɜ
ɩɪɨɛɢɪɤɚ ɫɟɤɭɧɞɨɦɟɪ ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭ ɧ ɇ2SO4 ɧ 1D2S2O3.
1-ɬԥɠɿɪɢɛɟ Ԥɪɟɤɟɬɬɟɫɭɲɿ ɡɚɬɬɚɪ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵɧɵԙ ɪɟɚɤɰɢɹ
ɠɵɥɞɚɦɞɵԑɵɧɚ ԥɫɟɪɿ
Ԛɲ ɛɸɪɟɬɤɚɧɵ ɬɨɥɬɵɪɵԙɞɚɪ ɛɿɪɿɧɲɿɫɿɧɟ ± ɧ +2SO4ɟɪɿɬɿɧɞɿɫɿɧ
ɟɤɿɧɲɿɫɿɧɟ ± ɧ Na2S2O3 ɟɪɿɬɿɧɞɿɫɿɧ ԛɲɿɧɲɿɫɿɧɟ- ɫɭ ԕԝɣɵԙɞɚɪ ɩɪɨɛɢɪɤɚԑɚ ɛɸɪɟɬɤɚɞɚɧ ɦɥ ɧ +2SO4 ԕԝɣɵԙɞɚɪ Ȼɚɫԕɚ ɩɪɨɛɢɪɤɚԑɚ
ɛɿɪɿɧɲɿɫɿɧɟ ɦɥ ɧ 1D2S2O3 ɟɪɿɬɿɧɞɿɫɿ ɦɟɧ ɦɥ ɫɭ ɟɤɿɧɲɿɫɿɧɟ ± ɦɥ ɧ
Na2S2O3 ɟɪɿɬɿɧɞɿɫɿ ɦɟɧ ɦɥ ɫɭ ԛɲɿɧɲɿɫɿɧɟ ± ɦɥ ɧ 1D2S2O3 ɩɟɧ ɦɥ ɫɭ
ɬԧɪɬɿɧɲɿɫɿɧɟ- ɦɥ ɧ 1D2S2O3 ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ ȿɤɿ-ɟɤɿɞɟɧ ɩɚɪɥɚɩ
ɩɪɨɛɢɪɤɚɥɚɪɞɵ ɛɿɪ-ɛɿɪɿɧɟ ԕԝɣɵԙɞɚɪ ɫɟɤɭɧɞɨɦɟɬɪɦɟɧ ԕɵɲԕɵɥɞɵ
ԕԝɣԑɚɧɧɚɧ ɛɚɫɬɚɩ ɥɚɣɥɚɧԑɚɧԑɚ ɞɟɣɿɧ ɭɚԕɵɬɬɵ W ԧɥɲɟԙɞɟɪ ɇɚɬɪɢɣ
ɬɢɨɫɭɥɶɮɚɬɵ ɦɟɧ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɧɵԙ ɚɪɚɫɵɧɞɚ ɦɵɧɚɞɚɣ ɪɟɚɤɰɢɹ
ɠԛɪɟɞɿ
24
25.
Ɋɟɚɤɰɢɹ ɧԥɬɢɠɟɫɿɧɞɟ ɚɥɵɧԑɚɧ ɦԥɧɞɟɪɞɿ ɤɟɫɬɟɝɟ ɠɚɡɵԙɞɚɪɊɟɚɤɰɢɹ ɠɵɥɞɚɦɞɵԑɵɧ ɥɚɣɥɚɧԑɚɧ ɭɚԕɵɬ t ɛɨɣɵɧɲɚ ɟɫɟɩɬɟɭ ԕɚɠɟɬ
Ɇɢɥɥɢɦɟɬɪɥɿɤ ԕɚԑɚɡԑɚ ɪɟɚɤɰɢɹ ɠɵɥɞɚɦɞɵԑɵɧɵԙ X ɨɫɶ ɨɪɞɢɧɚɬɵ
ɤɨɧɰɟɧɬɪɚɰɢɹԑɚ ɋ ɨɫɶ ɚɛɫɰɢɫɫɚ ɛɚɣɥɚɧɵɫɬɵԑɵɧɵԙ u=f ɋ ɝɪɚɮɢɝɿɧ
ɫɵɡɵԙɞɚɪ Ɇɚɫɲɬɚɛɬɵ ɚɥɭ ԛɲɿɧ ɟԙ ɠɨԑɚɪɵ ɦԥɧɞɟɪɿ ɨɫɶ ɨɪɞɢɧɚɬ ɩɟɧ ɨɫɶ
ɚɛɫɰɢɫɬɚ ɲɚɦɚɞɚ ɫɦ ɬɟԙ ɛɨɥɭɵ ɦԛɦɤɿɧ Ԥɪɟɤɟɬɬɟɫɭɲɿ ɦɚɫɫɚɥɚɪ ɡɚԙɵɧɚ
ɛɚɣɥɚɧɵɫɬɵ ɝɪɚɮɢɤɬɿԙ ɫɵɡɵԑɵ ɤɨɨɪɞɢɧɚɬɬɵԙ ɛɚɫɵɧɚɧ ԧɬɭɿ ɤɟɪɟɤ
Ȼɟɪɿɥɝɟɧ ɠɚԑɞɚɣԑɚ ɪɟɚɤɰɢɹ ɠɵɥɞɚɦɞɵԑɵɧɵԙ
ɤɨɧɰɟɧɬɪɚɰɢɹԑɚ
ɛɚɣɥɚɧɵɫɬɵԑɵɧɚ ԕɨɪɵɬɵɧɞɵ ɠɚɫɚԙɞɚɪ
2-ɬԥɠɿɪɢɛɟ Ɍɟɦɩɟɪɚɬɭɪɚɧɵԙ ɪɟɚɤɰɢɹ ɠɵɥɞɚɦɞɵԑɵɧɚ ԥɫɟɪɿ
Na2S2O3, H2SO4 ɟɪɿɬɿɧɞɿɥɟɪɿ ɛɚɪ ɛɸɪɟɬɤɚɥɚɪɞɵ ɠԝɦɵɫ ɿɫɬɟɭ
ɠɚԑɞɚɣɵɧɚ ɤɟɥɬɿɪɭ ɤɟɪɟɤ Ɍԧɪɬ ɧԧɦɿɪɥɟɧɝɟɧ ɩɪɨɛɢɪɤɚԑɚ
ɛɸɪɟɬɤɚɞɚɧ ɦɥ-ɞɟɧ ɧ 1D2S2O3 ɟɪɿɬɿɧɞɿɫɿɧ ɛɚɫԕɚ ɩɪɨɛɢɪɤɚԑɚ
ɚ ɚ ɚ ɚ ɦɥ-ɞɟɧ ɧ +2SO4 ԕԝɣɵԙɞɚɪ Ʌɚɛɨɪɚɬɨɪɢɹɞɚԑɵ ɛԧɥɦɟɞɟɝɿ
ɬɟɦɩɟɪɚɬɭɪɚɧɵ ɛɚɣԕɚԙɞɚɪ 1 ɠԥɧɟ 1 D ɩɪɨɛɢɪɤɚɥɚɪɞɵ ɛɿɪ-ɛɿɪɿɧɟ
ԕԝɣɵԙɞɚɪ Ԕɵɲԕɵɥɞɵ ԕԝɣԑɚɧɧɚɧ ɛɚɫɬɚɩ ɥɚɣɥɚɧԑɚɧԑɚ ɞɟɣɿɧɝɿ ɭɚԕɵɬɬɵ
W ԧɥɲɟԙɞɟɪ ȿɤɿɧɲɿ ɩɚɪɥɚɧԑɚɧ ɩɪɨɛɢɪɤɚɥɚɪɞɵ ɫɭ ԕԝɣɵɥԑɚɧ ɯɢɦɢɹɥɵԕ
ɫɬɚɤɚɧԑɚ ɨɪɧɚɥɚɫɬɵɪɵԙɞɚɪ ԕɵɡɞɵɪɵԙɞɚɪ ɋɬɚɤɚɧɞɚԑɵ ɫɭɞɵԙ
ɬɟɦɩɟɪɚɬɭɪɚɫɵɧ 0ɋ ɲɚɦɚɫɵɧɚ ɛԧɥɦɟ ɥɚɛɨɪɚɬɨɪɢɹ ɬɟɦɩɟɪɚɬɭɪɚɫɵɧɚɧ
ɠɨԑɚɪɵ ɛɨɥɭ ɤɟɪɟɤ Ɍɟɦɩɟɪɚɬɭɪɚɧɵԙ ԧɡɝɟɪɭɿɧ ɬɟɪɦɨɦɟɬɪ ɚɪԕɵɥɵ
ɛɚɣԕɚԙɞɚɪ
ɉɪɨɛɢɪɤɚɥɚɪɞɵ ɛɿɪ-ɛɿɪɿɧɟ ԕԝɣɵԙɞɚɪ ɛɟɥɝɿɥɿ ɛɿɪ ɬɟɦɩɟɪɚɬɭɪɚԑɚ
ɞɟɣɿɧ ԕɵɡԑɚɧɲɚ Ȼɿɪɿɧɲɿ ԥɞɿɫɬɟɝɿɞɟɣ ɟɪɿɬɿɧɞɿɧɿԙ ɥɚɣɥɚɧɭ ɭɚԕɵɬɵɧ
ɠɚɡɵԙɞɚɪ Ԕɚɥԑɚɧ ɩɪɨɛɢɪɤɚɥɚɪɦɟɧ ԥɪ ɞɚɣɵɦ ɬɟɦɩɟɪɚɬɭɪɚ 0ɋ
ɠɨԑɚɪɵɥɚɬɵԙɞɚɪ Ⱥɥɵɧԑɚɧ ɦԥɧɞɟɪɞɿ ɤɟɫɬɟɝɟ ɠɚɡɵԙɞɚɪ
25
26.
Ʌɚɣɥɚɧԑɚɧ ɭɚԕɵɬ W ɛɨɣɵɧɲɚ ɪɟɚɤɰɢɹɧɵԙ ɠɵɥɞɚɦɞɵԑɵɧ ɟɫɟɩɬɟɭ ԕɚɠɟɬɆɢɥɥɢɦɟɬɪɥɿɤ ԕɚԑɚɡԑɚ ɠɵɥɞɚɦɞɵԕɬɵԙ ɬɟɦɩɟɪɚɬɭɪɚԑɚ
ɛɚɣɥɚɧɵɫɬɵɥɵԑɵɧ ɝɪɚɮɢɝɿɧ ɫɵɡɵԙɞɚɪ Ɉɫɶ ɚɛɫɰɢɫɬɚ ɬɟɦɩɟɪɚɬɭɪɚɧɵԙ
ɦԥɧɞɟɪɿ ɨɫɶ ɨɪɞɢɧɚɬɬɚ ɪɟɚɤɰɢɹ ɠɵɥɞɚɦɞɵԑɵ u W ɦԥɧɞɟɪɿɧ ԕɨɣɵԙɞɚɪ
ɠԥɧɟ ԕɨɪɵɬɵɧɞɵ ɠɚɫɚԙɞɚɪ X f(t ɝɪɚɮɢɝɿɧ ɫɵɡɵԙɞɚɪ
3-ɬԥɠɿɪɢɛɟ Ƚɟɬɟɪɨɝɟɧɞɿ ɠԛɣɟɞɟɝɿ ԥɪɟɤɟɬɬɟɫɭɲɿ ɡɚɬɬɚɪɞɵԙ ɛԧɥɿɧɭ
ɛɟɬɿɧɿԙ ɪɟɚɤɰɢɹ ɠɵɥɞɚɦɞɵԑɵɧɚ ԥɫɟɪɿ
ɒɚɦɚɦɟɧ ɛɿɪɞɟɣ ɝ ɛɨɪɞɵԙ ɟɤɿ ɛԧɥɿɝɿɧ ɚɥɵԙɞɚɪ Ȼɿɪɟɭɿɧ ԛɝɿɬɿɩ
ɟɤɿɧɲɿɫɿɧ ɛԧɥɿɤ ɬԛɪɿɧɞɟ ɟɤɿ ɩɪɨɛɢɪɤɚԑɚ ɫɚɥɵԙɞɚɪ ȿɤɟɭɿɧɟ ɞɟ ɛɿɪ ɦɟɡɝɿɥɞɟ
ɦɥ ɫԝɣɵɬɵɥԑɚɧ ɬԝɡ ԕɵɲԕɵɥɵɧ ԕԝɣɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" ɇɟɥɿɤɬɟɧ ɟɤɿ
ɠɚԑɞɚɣɞɚ ɛɨɪɞɵԙ ɟɪɭɿ ɟɤɿ ɬԛɪɥɿ" Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ ɠԥɧɟ
ԕɨɪɵɬɵɧɞɵ ɠɚɫɚԙɞɚɪ
4-ɬԥɠɿɪɢɛɟ ɏɢɦɢɹɥɵԕ ɬɟɩɟ-ɬɟԙɞɿɤ Ԥɪɟɤɟɬɬɟɫɭɲɿ ɡɚɬɬɚɪ
ɤɨɧɰɟɧɬɪɚɰɢɹ-ɫɵɧɵԙ ɯɢɦɢɹɥɵԕ ɬɟɩɟ-ɬɟԙɞɿɤɤɟ ԥɫɟɪɿ
Ɋɟɚɤɰɢɹ ɦɵɧɚ ɬɟԙɞɟɭ ɚɪԕɵɥɵ ɠԛɪɟɞɿ
Ȼɚɫɬɚɩԕɵ ɠԥɧɟ ɬԛɡɿɥɝɟɧ ɡɚɬɬɚɪɞɵԙ ɬԛɫɿ ԥɪɬԛɪɥɿ ɛɨɥԑɚɧɞɵԕɬɚɧ
ԥɪɟɤɟɬɬɟɫɭɲɿ ɡɚɬɬɚɪ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵɧɵԙ ɯɢɦɢɹɥɵԕ ɬɟɩɟ-ɬɟԙɞɿɤɬɿԙ
ɵԑɵɫɭɵɧɚ ԥɫɟɪɿɧ ɛɚɣԕɚɭԑɚ ɛɨɥɚɞɵ Ɋɟɚɤɰɢɹ ɧԥɬɢɠɟɫɿɧɞɟ ɬԛɡɿɥɝɟɧ
Fe(SCN)3-ɬԛɫɿ ԕɵɩ-ԕɵɡɵɥ ԕɚɧ ɬԥɪɿɡɞɿ ɛɨɥɚɞɵ ȿɪɿɬɿɧɞɿɧɿԙ ɬԛɫɿɧɿԙ
ԧɡɝɟɪɭɿ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵɧɚ ɛɚɣɥɚɧɵɫɬɵ ȿɝɟɪ ɞɟ ɟɪɿɬɿɧɞɿɧɿԙ ɬԛɫɿ ԕɵɩԕɵɡɵɥ ɛɨɥɫɚ ɨɧɞɚ ɬɟɩɟ-ɬɟԙɞɿɤ ɨԙԑɚ ɵԑɵɫɚɞɵ ɟɪɿɬɿɧɞɿɧɿԙ ɬԛɫɿ ɲɚɦɚɥɵ
ԕɵɡԑɵɥɬ ɛɨɥɫɚ ɬɟɩɟ-ɬɟԙɞɿɤ ɫɨɥԑɚ ɵԑɵɫɚɞɵ
ɉɪɨɛɢɪɤɚԑɚ ɛԧɥɿɝɿɧɟ ɫԝɣɵɬɵɥԑɚɧ FeCl3 ɟɪɿɬɿɧɞɿɫɿɧɟ ɬɟԙ ɤԧɥɟɦ
ɫԝɣɵɬɵɥԑɚɧ NH4SCN ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ Ȼɚɫɬɚɩԕɵ ɚɥɵɧԑɚɧ
ɟɪɿɬɿɧɞɿɥɟɪ ɬԛɫɫɿɡ ɛɨɥɭɵ ɤɟɪɟɤ ɪɟɚɤɰɢɹ ɧԥɬɢɠɟɫɿɧɞɟ ɩɚɣɞɚ ɛɨɥԑɚɧ
ɟɪɿɬɿɧɞɿɧɿԙ ɬԛɫɿ ± ɲɚɣ ɬԥɪɿɡɞɿ Ɍԛɡɿɥɝɟɧ ɟɪɿɬɿɧɞɿɧɿ ɩɪɨɛɢɪɤɚԑɚ ɛԧɥɿɩ
ԕԝɣɵԙɞɚɪ Ȼɿɪɿɧɲɿ ɩɪɨɛɢɪɤɚԑɚ- 3- ɬɚɦɲɵ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ FeCl3
ɟɪɿɬɿɧɞɿɫɿɧ ɟɤɿɧɲɿɫɿɧɟ- 3- ɬɚɦɲɵ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ NH4SCN ɟɪɿɬɿɧɞɿɫɿɧ
ԕԝɣɵԙɞɚɪ ԝɲɿɧɲɿɫɿɧɟ- NH4Cl ɤɪɢɫɬɚɥɞɚɪɵɧ ԕɨɫɵԙɞɚɪ Ɍԧɪɬɿɧɲɿ
ɩɪɨɛɢɪɤɚ ɫɚɥɵɫɬɵɪɭ ԛɲɿɧ ɇɟ ɛɚɣԕɚɥɚɞɵ" ɉɪɨɛɢɪɤɚɥɚɪɞɚԑɵ
26
27.
ɟɪɿɬɿɧɞɿɥɟɪɞɿԙ ɬԛɫɬɟɪɿ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ" Ɍԥɠɿɪɢɛɟɞɟɧ ɚɥɵɧԑɚɧ ɦԥɧɞɟɪɞɿɤɟɫɬɟɝɟ ɠɚɡɵԙɞɚɪ
Ʌɟ-ɒɚɬɟɥɶɟ ɩɪɢɧɰɢɩɿɧ ɩɚɣɞɚɥɚɧɵɩ ɪɟɚɤɰɢɹɧɵԙ ɵԑɵɫɭɵɧ
ɬԛɫɿɧɞɿɪɿԙɞɟɪ Ɋɟɚɤɰɢɹɧɵԙ ɬɟɩɟ-ɬɟԙɞɿɤ ɤɨɧɫɬɚɧɬɚɫɵɧɵԙ ɬɟԙɞɟɭɿɧ
ɠɚɡɵԙɞɚɪ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ N6
ȿɪɿɬɿɧɞɿɥɟɪ ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ ɤɨɧɰɟɧɬɪɚɰɢɹɥɚɪɵɧ ԧɪɧɟɤɬɟɭ
Ɇɚɫɫɚɥɵԕ ԛɥɟɫ ɋɦ ɋɧ ɋm Ɍ ɦɨɥɶɞɿɤ ԛɥɟɫɿ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵԙ ɦɚԕɫɚɬɵ ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ
ɤɨɧɰɟɧɬɪɚɰɢɹɥɚɪɵɧ ɟɫɟɩɬɟɭ ɠԥɧɟ ɟɪɿɬɿɧɞɿɥɟɪ ɞɚɣɵɧɞɚɭ
Ȼɚԕɵɥɚɭ ɠԝɦɵɫɵɧɵԙ ɫԝɪɚԕɬɚɪɵ ɦɟɧ ɠɚɬɬɵԑɭɥɚɪɵ
1. ȿɪɿɬɿɧɞɿɥɟɪ ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ ɬɚɛɢԑɚɬɵɧɚ ɮɢɡɢɤɚɥɵԕ ɠԥɧɟ
ɯɢɦɢɹɥɵԕ ɬԝɪԑɵɞɚɧ ɤԧɡԕɚɪɚɫ ȿɪɿɬɿɧɞɿɥɟɪ ɬԛɡɿɥɝɟɧɞɟ ɛɚɣԕɚɥɚɬɵɧ
ɩɪɨɰɟɫɬɟɪ
2. ȿɪɿɝɟɧ ɡɚɬɬɚɪɞɵԙ ɟɪɿɝɿɲɬɿɝɿɧɿԙ ɬɟɦɩɟɪɚɬɭɪɚԑɚ ɛɚɣɥɚɧɵɫɬɵɥɵԑɵ
3. Ʉɨɧɰɟɧɬɪɚɰɢɹ ɞɟɝɟɧɿɦɿɡ ɧɟ" ɋԝɣɵɬɵɥԑɚɧ ɠԥɧɟ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ
ԕɚɧɵԕɩɚԑɚɧ ɠԥɧɟ ԕɚɧɵԕԕɚɧ ɚɫɚ ԕɚɧɵԕԕɚɧ ɟɪɿɬɿɧɞɿɥɟɪ
4. ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ ɤɨɧɰɟɧɬɪɚɰɢɹɥɚɪɵɧ ԧɪɧɟɤɬɟɭ ԥɞɿɫɬɟɪɿ ɦɚɫɫɚɥɵԕ
ԛɥɟɫ ɦɨɥɹɪɥɵԕ ɧɨɪɦɚɥɶɞɵԕ ɷɤɜɢɜɚɥɟɧɬɬɿɤ ɦɨɥɹɥɶɞɵԕ ɤɨɧɰɟɧɬɪɚɰɢɹ
ɠԥɧɟ ɬɢɬɪ
5. ȿɫɟɩ ɝ ɫɭɞɚ ɝ ɬɟɦɿɪ ɤɪɢɫɬɚɥɥɝɢɞɪɚɬɵɧ FeSO4ā H2O ɟɪɿɬɤɟɧ
Ʉɪɢɫɬɚɥɥɝɢɞɪɚɬɬɵɧ ɠԥɧɟ ɫɭɫɵɡ ɬɟɦɿɪ ɫɭɥɶɮɚɬɵɧɵԙ ɟɪɿɬɿɧɞɿɞɟɝɿ
ɦɚɫɫɚɥɵԕ ԛɥɟɫɬɟɪɿɧ ɟɫɟɩɬɟԙɞɟɪ
6. ȿɫɟɩ -ɬɿɤ Al(NO3)3(p ɝ ɫɦ3 ɟɪɿɬɿɧɞɿɫɿɧɿԙ ɦɨɥɹɪɥɵԕ
ɧɨɪɦɚɥɶɞɵԕ ɠԥɧɟ ɦɨɥɹɥɶɞɵԕ ɤɨɧɰɟɧɬɪɚɰɢɹɥɚɪɵɧ ɟɫɟɩɬɟԙɞɟɪ
7. ȿɫɟɩ ɥ ɧɨɪɦɚɥɶɞɵԕ H2SO4 ɟɪɿɬɿɧɞɿɫɿɧ ɞɚɣɵɧɞɚɭ ԛɲɿɧ ɬɿɤ p ɝ ɫɦ3) H2SO4 ɟɪɿɬɿɧɞɿɫɿɧɟɧ ɧɟɲɟ ɤԧɥɟɦ ԕɚɠɟɬ"
27
28.
8. ȿɫɟɩ ɇ2SO4 ɟɪɿɬɿɧɞɿ ɚɥɭ ԛɲɿɧ ɦɥ -ɬɿɤ H2SO4(p ɝ ɫɦ3 ɟɪɿɬɿɧɞɿɫɿɧɟ ԕɚɧɲɚ ɤԧɥɟɦ ɫɭ ԕɨɫɭ ԕɚɠɟɬ"
9. ȿɫɟɩ ɦɥ -ɬɿɤ HNO3 (p ɝ ɫɦ3 ɟɪɿɬɿɧɞɿɫɿɧɟ ɥ ɫɭ
ԕԝɣɞɵ ɀɚԙɚ ɩɚɣɞɚ ɛɨɥԑɚɧ ɟɪɿɬɿɧɞɿɞɟ HNO3 ɦɚɫɫɚɥɵԕ ԛɥɟɫɿ ԕɚɧɲɚԑɚ ɬɟԙ?
ȿɫɟɩ ɦɥ ɫɿɥɬɿɧɿ ɧɟɣɬɪɚɥɞɚɭ ԛɲɿɧ ɦɥ ɧ H2SO4 ɟɪɿɬɿɧɞɿɫɿ
ɠԝɦɫɚɥԑɚɧ ɋɿɥɬɿɧɿԙ ɧɨɪɦɚɥɶɞɵԑɵ ɧɟɝɟ ɬɟԙ" Ɉɫɵɧɞɚɣ ɫɿɥɬɿ ԛɲɿɧ ɧ
ɇɋl ɟɪɿɬɿɧɞɿɫɿɧɿԙ ԕɚɧɞɚɣ ɤԧɥɟɦɿ ԕɚɠɟɬ ɛɨɥɚɪ ɟɞɿ"
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɬԥɠɿɪɢɛɟ
Ԕɚɠɟɬɬɿ ԕԝɪɚɥ-ɠɚɛɵԕɬɚɪ ɠԥɧɟ ɪɟɚɤɬɢɜɬɟɪ ɋɬɚɤɚɧɞɚɪ ɰɢɥɢɧɞɪ
ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭ ɚɪɟɨɦɟɬɪ ɬɟɪɦɨɦɟɬɪ 1D2CO ɤɪɢɫ ā ɇ2Ɉ
1-ɬԥɠɿɪɢɛɟ. Ԕɚɬɬɵ ɡɚɬ ɩɟɧ ɫɭɞɚɧ ɩɪɨɰɟɧɬɬɿɤ ɛɟɪɿɥɝɟɧ ɦɚɫɫɚɥɵԕ
ԛɥɟɫ ɤɨɧɰɟɧɬɪɚɰɢɹ ɟɪɿɬɿɧɞɿɥɟɪɿɧ ɞɚɣɵɧɞɚɭ
ɚ Ʉɪɢɫɬɚɥɞɵԕ ɫɨɞɚɞɚɧ 1D2CO3.10H22 ɠԥɧɟ ɫɭɞɚɧ ɝ - ɬɿɤ
ɟɪɿɬɿɧɞɿ ɞɚɣɵɧɞɚԙɞɚɪ ɝ - ɬɿɤ ɟɪɿɬɿɧɞɿ ɞɚɣɵɧɞɚɭ ԛɲɿɧ ɤɪɢɫɬɚɥɞɵԕ
ɫɨɞɚɧɵԙ Na2CO3.10H2O ԕɚɠɟɬ ɦɚɫɫɚɫɵɧ ɟɫɟɩɬɟԙɞɟɪ
ɝ ɞԥɥɞɿɤɤɟ ɞɟɣɿɧ ɟɫɟɩɬɟɥɝɟɧ ɤɪɢɫɬɚɥɞɵԕ ɫɨɞɚɧɵԙ ɦɚɫɫɚɫɵɧ ɬɚɪɚɡɵɞɚ
ԧɥɲɟԙɞɟɪ ɨɫɵ ɬԝɡɞɵԙ ɦɚɫɫɚɫɵɧɚ ԕɨɫɚɬɵɧ ɫɭɞɵԙ ԕɚɧɲɚ ɤԧɥɟɦɿ ԕɚɠɟɬ
ɟɤɟɧɿɧ ɟɫɟɩɬɟɩ
ɰɢɥɢɧɞɪɦɟɧ ԧɥɲɟɩ ɫɬɚɤɚɧԑɚ ԕԝɣɵԙɞɚɪ ȿɫɟɩɬɟɥɝɟɧ ɠԥɧɟ ԧɥɲɟɧɝɟɧ
ɬԝɡɞɵԙ ɦɚɫɫɚɫɵɧ ɫɬɚɤɚɧɞɚԑɵ ɫɭɞɵԙ ԕɚɠɟɬɬɿ ԕԧɥɟɦɿɧɞɟ ɟɪɿɬɿԙɞɟɪ
Ⱦɚɣɵɧɞɚԑɚɧ ɟɪɿɬɿɧɞɿɧɿԙ ɬɟɦɩɟɪɚɬɭɪɚɫɵɧ ԧɥɲɟԙɞɟɪ ɨɧɵ 0ɋ
ɠɟɬɤɿɡɿԙɞɟɪ ԕɵɡɞɵɪɭ ɧɟɦɟɫɟ ɫɚɥԕɵɧɞɚɬɭ ɚɪԕɵɥɵ Ⱦɚɣɵɧ ɛɨɥԑɚɧ
ɟɪɿɬɿɧɞɿɧɿ ɰɢɥɢɧɞɪɝɟ ԕԝɣɵɩ ɚɥɵɩ ɚɪɟɨɦɟɬɪɦɟɧ ɨɧɵԙ ɬɵԑɵɡɞɵԑɵɧ
ɚɧɵԕɬɚɭ ԕɚɠɟɬ Ⱦɚɣɵɧ ɤɟɫɬɟɧɿ ɩɚɣɞɚɥɚɧɵɩ ɬɵԑɵɡɞɵԕ ɚɪԕɵɥɵ
ɞɚɣɵɧɞɚɥԑɚɧ Na2CO3 ɇ2Ɉ ɟɪɿɬɿɧɞɿɫɿɧɿԙ ɩɪɨɰɟɧɬɬɿɤ ԕԝɪɚɦɵɧ
ɚɧɵԕɬɚԙɞɚɪ ȿɪɿɬɿɧɞɿɧɿԙ ɦɨɥɹɪɥɵԑɵɧ ɧɨɪɦɚɥɶɞɵԑɵɧ ɟɫɟɩɬɟԙɞɟɪ
ɋɭɫɵɡ ɬԝɡɞɵԙ ɦɚɫɫɚɥɵԕ ԛɥɟɫɿɧ ɫɭɫɵɡ ɫɨɞɚɧɵԙ ɦɚɫɫɚɫɵɧ ɟɫɟɩɬɟɭ
ɚɪԕɵɥɵ ɚɧɵԕɬɚɣɦɵɡ
28
29.
2-ɬԥɠɿɪɢɛɟ Ʉɨɧɰɟɧɬɪɥɟɧɝɟɧ ɟɪɿɬɿɧɞɿɞɟɧ ɫԝɣɵɥɬɭ ɚɪԕɵɥɵɟɪɿɬɿɧɞɿɥɟɪ ɞɚɣɵɧɞɚɭ
Ȼɚɫɬɚɩԕɵ ɛɟɪɿɥɝɟɧ ɟɪɿɬɿɧɞɿɞɟɧ ɇ2SO4 ɤɨɧɰɟɧɬɪ ɝ ɇ2SO4
ɟɪɿɬɿɧɞɿɫɿɧ ɞɚɣɵɧɞɚɭ ԕɚɠɟɬ
1. Ȼԝɥ ԛɲɿɧ ɛɚɫɬɚɩԕɵ ɛɟɪɿɥɝɟɧ ɇ2SO4ɤɨɧɰ ɟɪɿɬɿɧɞɿɧɿԙ ɰɢɥɢɧɞɪɞɟ
ɚɪɟɨɦɟɬɪ ɚɪԕɵɥɵ ɬɵԑɵɡɞɵԑɵɧ Uɛɚɫɬ ɚɧɵԕɬɚԙɞɚɪ ɤɟɫɬɟɞɟɧ ɫɨԑɚɧ ɫԥɣɤɟɫ
ɩɪɨɰɟɧɬɬɿɤ ԕԝɪɚɦɵɧ Zɛɚɫɬ ɬɚɛɵԙɞɚɪ
2. 25 ɝ -ɬɿɤ ɇ2SO4) ɞɚɣɵɧɞɚɥɚɬɵɧ ɟɪɿɬɿɧɞɿ ԛɲɿɧ ɟɪɿɝɟɧ
ɡɚɬɬɵԙ ɦɚɫɫɚɫɵɧ ɟɫɟɩɬɟԙɞɟɪ
3. Ⱦɚɣɵɧɞɚɥɚɬɵɧ ɟɪɿɬɿɧɞɿɞɟ ɟɪɿɝɟɧ ɡɚɬɬɵԙ
ɛɚɫɬɚɩԕɵ ɛɟɪɿɥɝɟɧ ɟɪɿɬɿɧɞɿɧɿԙ ɤԧɥɟɦɿɧ ɟɫɟɩɬɟԙɞɟɪ
ȼ ɦɚɫɫɚɫɵ ɛɚɪ
4. ɋԝɣɵɥɬɭԑɚ ԕɚɠɟɬɬɿ ɫɭɞɵԙ ɤԧɥɟɦɿɧ ɟɫɟɩɬɟԙɞɟɪ
ȿɫɟɩɬɟɥɝɟɧ ɛɚɫɬɚɩԕɵ ɛɟɪɿɥɝɟɧ ɟɪɿɬɿɧɞɿɧɿԙ ɤԧɥɟɦɿɧ ɰɢɥɢɧɞɪɦɟɧ
ɛɸɪɟɬɤɚɦɟɧ ɧɟɦɟɫɟ ɩɢɩɟɬɤɚɦɟɧ ԧɥɲɟɧɝɟɧ ɤɨɥɛɚԑɚ ɫɬɚɤɚɧԑɚ ԕԝɣɵԙɞɚɪ
ɨԑɚɧ ɟɫɟɩɬɟɥɝɟɧ ɫɭɞɵԙ ɤԧɥɟɦɿɧ ԕɨɫɵԙɞɚɪ ɠԥɧɟ ɚɪɚɥɚɫɬɵɪɵԙɞɚɪ
Ⱦɚɣɵɧɞɚɥԑɚɧ ɟɪɿɬɿɧɞɿɧɿԙ ɰɢɥɢɧɞɪɞɟ ɚɪɟɨɦɟɬɪ ɚɪԕɵɥɵ ɬɵԑɵɡɞɵԑɵɧ
(rɞɚɣɵɧ ɚɧɵԕɬɚԙɞɚɪ ɤɟɫɬɟɞɟɧ ɫɨԑɚɧ ɫԥɣɤɟɫ ɩɪɨɰɟɧɬɬɿɤ ԕԝɪɚɦɵɧ Zɞɚɣɵɧ
10%) ɬɚɛɵԙɞɚɪ
ȿɪɿɬɿɧɞɿɧɿԙ ɦɨɥɹɪɥɵԑɵɧ ɠԥɧɟ ɧɨɪɦɚɥɶɞɵԑɵɧ ɟɫɟɩɬɟԙɞɟɪ
3-ɬԥɠɿɪɢɛɟ ɦɥ ɦ ɇɋȱ ɟɪɿɬɿɧɞɿɫɿɧ ɞɚɣɵɧɞɚɭ ԕɚɠɟɬ
Ȼԝɥ ԛɲɿɧ ɛɚɫɬɚɩԕɵ ɛɟɪɿɥɝɟɧ ɇɋȱɤɨɧɰ ɟɪɿɬɿɧɞɿɧɿԙ ɰɢɥɢɧɞɪɞɟ
ɚɪɟɨɦɟɬɪ ɚɪԕɵɥɵ ɬɵԑɵɡɞɵԑɵɧ rɛɚɫɬ ɚɧɵԕɬɚԙɞɚɪ ɤɟɫɬɟɞɟɧ ɫɨԑɚɧ ɫԥɣɤɟɫ
ɩɪɨɰɟɧɬɬɿɤ ԕԝɪɚɦɵɧ wɛɚɫɬ ɬɚɛɵԙɞɚɪ
29
30.
2. ɦɥ Ɇ ɬԝɡ ԕɵɲԕɵɥɵɧɵԙ ɞɚɣɵɧɞɚɥɚɬɵɧ ɟɪɿɬɿɧɞɿɫɿ ԛɲɿɧɟɪɿɝɟɧ ɡɚɬɬɵԙ ɦɚɫɫɚɫɵɧ ɟɫɟɩɬɟԙɞɟɪ
3. Ⱦɚɣɵɧɞɚɥɚɬɵɧ ɟɪɿɬɿɧɞɿɞɟ ɟɪɿɝɟɧ ɡɚɬɬɵԙ ȼ ɦɚɫɫɚɫɵ ɛɚɪ
ɛɚɫɬɚɩԕɵ ɛɟɪɿɥɝɟɧ ɟɪɿɬɿɧ-ɞɿɧɿԙ ɤԧɥɟɦɿɧ ɟɫɟɩɬɟԙɞɟɪ
ȿɫɟɩɬɟɥɝɟɧ ɛɚɫɬɚɩԕɵ ԕɵɲԕɵɥɞɵԙ ɤԧɥɟɦɿɧ ԧɥɲɟɩ ɩɢɩɟɬɤɚɦɟɧ
ɛɟɥɝɿɫɿ ɛɚɪ ɦɥ ԧɥɲɟɭɿɲ ɤɨɥɛɚԑɚ ԕԝɣɵɩ ɨԑɚɧ ɠɚɪɬɵ ɦԧɥɲɟɪɞɟ ɫɭ
ԕɨɫɵԙɞɚɪ
ɚɪɚɥɚɫɬɵɪɵԙɞɚɪ
ɛԧɥɦɟ
ɬɟɦɩɟɪɚɬɭɪɚɫɵɧɚ
ɞɟɣɿɧ
ɫɚɥԕɵɧɞɚɬɵԙɞɚɪ Ɉɧɚɧ ɫɨԙ ɛɟɥɝɿɫɿɧɟ ɞɟɣɿɧ ɫɭ ԕԝɣɵɩ ɚɭɡɵɧ ɬɵԑɵɧɞɚɩ
ɚɪɚɥɚɫɬɵɪɵԙɞɚɪ Ⱦɚɣɵɧɞɚɥԑɚɧ ɟɪɿɬɿɧɞɿɧɿԙ ɰɢɥɢɧɞɪɞɟ ɬɵԑɵɡɞɵԑɵɧ
ɚɪɟɨɦɟɬɪɦɟɧ ԧɥɲɟԙɞɟɪ ɤɟɫɬɟ ɚɪԕɵɥɵ ɦɚɫɫɚɥɵԕ ԛɥɟɫɿɧ Zɞɚɣɵɧ ɠԥɧɟ
ɦɨɥɹɪɥɵԑɵɧ ɦ ɚɧɵԕɬɚԙɞɚɪ Ⱦɚɣɵɧɞɚɥɚɬɵɧ ɠԥɧɟ ɞɚɣɵɧɞɚɥԑɚɧ
ɟɪɿɬɿɧɞɿɥɟɪɞɿԙ ɦɨɥɹɪɥɵԕ ɤɨɧɰɟɧɬɪɚɰɢɹɥɚɪɵɧ ɫɚɥɵɫɬɵɪɵԙɞɚɪ Ɉɫɵ
ɟɪɿɬɿɧɞɿɧɿԙ ɧɨɪɦɚɥɶɞɵԕ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵɧ ɟɫɟɩɬɟԙɞɟɪ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ N7
Ɍɚԕɵɪɵɛɵ ɗɥɟɤɬɪɨɥɢɬɬɿɤ ɞɢɫɫɨɰɢɚɰɢɹ ɬɟɨɪɢɹɫɵ
ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ ɪɇ-ɵɧ ɚɧɵԕɬɚɭ ɬԥɫɿɥɞɟɪɿ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵԙ
ԧɬɤɿɡɝɿɲɬɿɝɿɧ ɨԕɵɩ ԛɣɪɟɧɭ
1.
2.
3.
4.
5.
ɦɚԕɫɚɬɵ
ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ
ɷɥɟɤɬɪ
Ȼɚԕɵɥɚɭ ɠԝɦɵɫɵɧɵԙ ɫԝɪɚԕɬɚɪɵ ɦɟɧ ɠɚɬɬɵԑɭɥɚɪɵ
ɗɥɟɤɬɪɨɥɢɬɬɿɤ ɞɢɫɫɨɰɢɚɰɢɹ ɬɟɨɪɢɹɫɵɧɵԙ ɧɟɝɿɡɝɿ ԕɚԑɢɞɚɥɚɪɵ
Ⱦɢɫɫɨɰɢɚɰɢɹɧɵԙ ɦɟɯɚɧɢɡɦɿ ɂɨɧ-ɞɢɩɨɥɶɞɿ ɞɢɩɨɥɶ-ɞɢɩɨɥɶɞɿ
Ԕɵɲԕɵɥɞɚɪ ɧɟɝɿɡɞɟɪ ɠԥɧɟ ɬԝɡɞɚɪ-ɷɥɟɤɬɪɨɥɢɬɬɿɤ ɞɢɫɫɨɰɢɚɰɢɹ
ɬɟɨɪɢɹɫɵ ɬԝɪԑɵɫɵɧɚɧ ɚɧɵԕɬɚɦɚ Ʉԧɩɧɟɝɿɡɞɿɤ ԕɵɲԕɵɥɞɚɪɞɵԙ ɠԥɧɟ
ɤԧɩԕɵɲԕɵɥɞɵ ɧɟɝɿɡɞɟɪɞɿԙ ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɭɵ
ɗɥɟɤɬɪɨɥɢɬɬɟɪ ɟɪɿɬɿɧɞɿɥɟɪɿ Ʉԛɲɬɿ ɠԥɧɟ ԥɥɫɿɡ ɷɥɟɤɬɪɨɥɢɬɬɟɪ
Ⱦɢɫɫɨɰɢɚɰɢɹɥɚɧɭ ɞԥɪɟɠɟɫɿ ɨɧɵԙ ɚɧɵԕɬɚɦɚɫɵ Ⱦɢɫɫɨɰɢɚɰɢɹɥɚɧɭ
ɞԥɪɟɠɟɫɿ ԕɚɧɞɚɣ ɮɚɤɬɨɪɥɚɪԑɚ ɬԥɭɟɥɞɿ "
Ⱦɢɫɫɨɰɢɚɰɢɹɥɚɧɭ ɤɨɧɫɬɚɧɬɚɫɵ ɨɧɵԙ ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɭ ɞԥɪɟɠɟɫɿɦɟɧ
ɛɚɣɥɚɧɵɫɵ Ɉɫɬɜɚɥɶɞɬɵԙ ɫԝɣɵɥɬɭ ɡɚԙɵ
30
31.
6. ɋɭɞɵԙ ɷɥɟɤɬɪɨɥɢɬɬɿɤ ɞɢɫɫɨɰɢɚɰɢɹɫɵ ɋɭɬɟɤɬɿɤ ɤԧɪɫɟɬɤɿɲ ȿɫɟɩɆ ɋɇ3ɋɈɈɇ ɟɪɿɬɿɧɞɿɫɿɧɞɟɝɿ Ʉg=1,810-5 ɪɇ ɠԥɧɟ >ɇ+@ ɟɫɟɩɬɟԙɞɟɪ
Ʉԧɪɫɟɬɿɥɝɟɧ ɟɪɿɬɿɧɞɿɧɿԙ ɥ ɧɟɲɟ ɝ ɋɇ3ɋɈɈ- ɢɨɧ ɬԛɪɿɧɞɟ ɛɨɥɚɞɵ
7. ȿɫɟɩ Ɇ ɇɋN ɟɪɿɬɿɧɞɿɫɿɧɞɟɝɿ ɫɭɬɟɤ ɤɨɧɰɟɧɬɪɚɰɢɚɰɢɹɫɵ
>ɇ+@|6,910-4 ɦɨɥɶ ɥ ȿɪɿɬɿɧɞɿɧɿԙ ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɭ ɤɨɧɫɬɚɧɬɚɫɵɧ
ɟɫɟɩɬɟԙɞɟɪ
8. ȿɫɟɩ Ɍԧɦɟɧɞɟɝɿ ɛɟɪɿɥɝɟɧ
ɚ Ɇ ɇԐ ɟɪɿɬɿɧɞɿɫɿɧɿԙ Ʉg=6,8 10-4);
ɛ Ɇ ɇԐ ɟɪɿɬɿɧɞɿɫɿɧɿԙ D ɠԥɧɟ >ɇ+@ ɟɫɟɩɬɟԙɞɟɪ
9. ȿɫɟɩ ɧ ɤԧɦɿɪ ԕɵɲԕɵɥɵɧɵԙ ɛɿɪɿɧɲɿ ɫɚɬɵɫɵ ɛɨɣɵɧɲɚ
ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɭ ɞԥɪɟɠɟɫɿ 10-3 ɬɟԙ ɛɨɥɫɚ ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɭ
ɤɨɧɫɬɚɧɬɚɫɵ ɧɟɝɟ ɬɟԙ?
10. ȿɫɟɩ Ԕɚɧɞɚɣ ɤɨɧɰɟɧɬɪɚɰɢɹɞɚ ɚɡɨɬɬɵ ԕɵɲԕɵɥɞɵԙ ɇNɈ2
ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɭ ɞԥɪɟɠɟɫɿ -ɟ ɬɟԙ Ʉg HNO =4 10-4)?
11. ȿɫɟɩ ɧ Nɇ4Ɉɇ ɟɪɿɬɿɧɞɿɫɿɧɞɟɝɿ ɪɇ ɪɈɇ ɠԥɧɟ >Ɉɇ-@ ɦԥɧɞɟɪɿɧ
ɟɫɟɩɬɟԙɞɟɪ Ʉg NH OH =1,76 10-5
2
4
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɬԥɠɿɪɢɛɟ
Ԕɚɠɟɬɬɿ ԕԝɪɚɥ ɠɚɛɞɵԕɬɚɪ ɦɟɧ ɪɟɚɤɬɢɜɬɟɪ Ⱦɢɫɬɢɥɞɟɧɝɟɧ ɫɭ ɦɥ
ɫɬɚɤɚɧ ɤԧɦɿɪ ɷɥɟɤɬɪɨɞɵ ɥɚɦɩɚ Ɇ ɋɇ3ɋɈɈɇ NH4OH, HNO3, HCȱ
NaOH, KOH, KNO3 ɮɟɧɨɥɮɬɚɥɟɢɧ ɦɟɬɢɥɨɪɚɧɠ ɥɚɤɦɭɫ NH4Cȱ ɤɪɢɫ ,
ɮɢɥɶɬɪ ԕɚԑɚɡɵ
1-ɬԥɠɿɪɢɛɟ ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ ɷɥɟɤɬɪ ԧɬɤɿɡɝɿɲɬɿɝɿɧ ɚɧɵԕɬɚɭ
ɋɯɟɦɚɫɵ -ɫɭɪɟɬɬɟ ɤԧɪɫɟɬɿɥɝɟɧɞɟɣ ԕԝɪɚɥɞɵ ɩɚɣɞɚɥɚɧɵɩ
ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭɞɵԙ ɷɥɟɤɬɪ ԧɬɤɿɡɝɿɲɬɿɝɿɧ ɛɚɣԕɚԙɞɚɪ Ɉɥ ԛɲɿɧ ɦɥ-ɥɿɤ
ɫɬɚɤɚɧ ɚɥɵɩ - ɦɥ ɫɭ ԕԝɣɵɩ ɤԧɦɿɪ ɷɥɟɤɬɪɨɞɬɚɪɞɵ ɛɚɬɵɪɵԙɵɡ Ɍɨɤԕɚ
ԕɨɫԕɚɧɧɚɧ ɤɟɣɿɧ ɥɚɦɩɚɧɵԙ ԕɵɡɭɵɧ ɛɚɣԕɚԙɞɚɪ
Ʌɚɦɩɚ
20-ɫɭɪɟɬ
ɫɯɟɦɚɫɵ
ɗɥɟɤɬɪɨɥɢɬɬɟɪɞɿʃ
ɷɥɟɤɬɪԧɬɤɿɡɝɿɲɬɿɝɿɧ
31
ɚɧɵԕɬɚɭ
32.
2-ɬԥɠɿɪɢɛɟ Ɍԧɦɟɧɞɟɝɿ ɛɟɪɿɥɝɟɧ Ɇ ɡɚɬɬɚɪɞɵԙ ɟɪɿɬɿɧɞɿɥɟɪɿɧɿԙ:ɋɇ3ɋɈɈɇ NH4OH, HNO3, HCȱ NaOH, HNO3, KOH, KNO3 ɷɥɟɤɬɪ
ԧɬɤɿɡɝɿɲɬɿɝɿɧ ɬɟɤɫɟɪɿԙɞɟɪ ȿɪɿɬɿɧɞɿɞɟɝɿ ɷɥɟɤɬɪɨɞɬɚɪɞɵ ɛɚɬɵɪɦɚɫ ɛԝɪɵɧ
ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭɦɟɧ ɠɭɵɩ ɚɥɭ ɤɟɪɟɤ ɇɟ ɛɚɣԕɚɥɚɞɵ" ɗɥɟɤɬɪ
ɥɚɦɩɚɫɵɧɵԙ ԕɵɡɭɵɧ ɛɚɣԕɚԙɞɚɪ Ԕɚɧɞɚɣ ɷɥɟɤɬɪɨɥɢɬɬɟɪɝɟ ɠɚɬɚɞɵ"
3-ɬԥɠɿɪɢɛɟ Ȼɿɪɞɟɣ ɤԧɥɟɦɞɟ ɚɥɵɧԑɚɧ ɟɪɿɬɿɧɞɿɥɟɪɞɿ ɚɪɚɥɚɫɬɵɪɵԙɞɚɪ
1) Ʉԛɲɬɿ ԕɵɲԕɵɥ ɦɟɧ ɤԛɲɬɿ ɧɟɝɿɡɞɿ
2) Ԥɥɫɿɡ ԕɵɲԕɵɥ ɦɟɧ ԥɥɫɿɡ ɧɟɝɿɡɞɿ
ɉɚɣɞɚ ɛɨɥԑɚɧ ɟɪɿɬɿɧɞɿɥɟɪɞɿԙ ɷɥɟɤɬɪ ԧɬɤɿɡɝɿɲɬɿɝɿɧ ɬɟɤɫɟɪɿԙɞɟɪ Ԕɚɧɞɚɣ
ԕɨɪɵɬɵɧɞɵ ɠɚɫɚɭԑɚ ɛɨɥɚɞɵ" Ɋɟɚɤɰɢɹ ɬɟԙɞɟɭɥɟɪɿɧ ɦɨɥɟɤɭɥɚɥɵԕ ɠԥɧɟ
ɢɨɧɞɵԕ ɬԛɪɞɟ ɠɚɡɵԙɞɚɪ
4-ɬԥɠɿɪɢɛɟ ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ ɷɥɟɤɬɪ ԧɬɤɿɡɝɿɲɬɿɝɿɧɟ ɷɥɟɤɬɪɨɥɢɬɬɿԙ
ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɭ ɞԥɪɟɠɟɫɿɧɿԙ ԥɫɟɪɿ
ɋɬɚɤɚɧԑɚ ɚɡ ɲɚɦɚɫɵɧɞɚ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ ɫɿɪɤɟ ԕɵɲԕɵɥɞɵԙ ɟɪɿɬɿɧɞɿɫɿɧ
ԕԝɣɵԙɞɚɪ ɬɨɤԕɚ ԕɨɫɵԙɞɚɪ ɗɥɟɤɬɪ ɥɚɦɩɚɧɵԙ ԕɵɡɭɵɧ ɛɚɣԕɚԙɞɚɪ
Ԕɵɲԕɵɥԑɚ ɛɿɪɬɿɧɞɟɩ ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭ ԕԝɣɵԙɞɚɪ Ʌɚɦɩɚɧɵԙ ԕɵɡɭɵ ԕɚɥɚɣ
ԧɡɝɟɪɟɞɿ" Ȼɚɣԕɚɥԑɚɧ ԧɡɝɟɪɿɫɬɟɪɞɿ ɬԛɫɿɧɞɿɪɿԙɞɟɪ Ɇ
Ɇ Ɇ ɋɇ3ɋɈɈɇ ɟɪɿɬɿɧɞɿɫɿɧɿԙ ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɭ ɞԥɪɟɠɟɫɿɧ
ɟɫɟɩɬɟԙɞɟɪ ɉɪɢɛɨɪ ɦɟɧ ɷɥɟɤɬɪɨɞɬɚɪɞɵ ɫɭɦɟɧ ɠɭɵɩ ɲɚɣɵɩ ɬԥɠɿɪɢɛɟɧɿ
ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ NH4OH ɟɪɿɬɿɧɞɿɫɿɦɟɧ ԕɚɣɬɚɥɚԙɞɚɪ D=f(C).ɝɪɚɮɢɝɿɧ
ɫɵɡɵԙɞɚɪ.
Kg=D2C. D= Kg / C . KgCH3COOH=1.8.10-5. KgNH4OH=1.76.10-5.
5-ɬԥɠɿɪɢɛɟ Ԥɥɫɿɡ ɷɥɟɤɬɪɨɥɢɬɬɿԙ ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɭ ɞԥɪɟɠɟɫɿɧɟ ɚɬɬɚɫ
ɢɨɧɧɵԙ ԥɫɟɪɿ
ɉɪɨɛɢɪɤɚԑɚ ɫԝɣɵɬɵɥԑɚɧ 1+42+ ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ - ɬɚɦɲɵ
ɮɟɧɨɥɮɬɚɥɟɢɧ ԕɨɫɵԙɞɚɪ ȿɪɿɬɿɧɞɿɧɿ ɟɤɿ ɩɪɨɛɢɪɤɚԑɚ ɛԧɥɿԙɞɟɪ Ȼɿɪ
ɩɪɨɛɢɪɤɚɧɵ ɟɪɿɬɿɧɞɿɫɿɦɟɧ ɫɚɥɵɫɬɵɪɭ ԛɲɿɧ ԕɨɣɵԙɞɚɪ ɟɤɿɧɲɿɫɿɧɟ 1+4&ȱ
ɤɪɢɫɬɚɥɞɚɪɵɧ ԕɨɫɵԙɞɚɪ, ɠɚԕɫɵɥɚɩ ɚɪɚɥɚɫɬɵɪɵԙɞɚɪ. ȿɪɿɬɿɧɞɿɧɿԙ ɬԛɫɿ
ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ? Ʌɟ-ɒɚɬɟɥɶɟ ɩɪɢɧɰɢɩɿɧɟ ɫԛɣɟɧɿɩ, ԕɚɧɞɚɣ ԕɨɪɵɬɵɧɞɵ
ɠɚɫɚɭԑɚ ɛɨɥɚɞɵ?
6-ɬԥɠɿɪɢɛɟ. ɪɇ-ɬɵ ɚɧɵԕɬɚɭ, ɢɧɞɢɤɚɬɨɪɞɵԙ ɬԛɫɿɧɿԙ ɪɇ-ԕɚ
ɛɚɣɥɚɧɵɫɬɵɥɵԑɵ. ɪɇ-ɦɟɬɪɦɟɧ ɠԝɦɵɫ ɿɫɬɟɭ ɟɪɟɠɟɥɟɪɿ.
ɚ) ɪɇ-ɦɟɬɪɞɿԙ ɤԧɦɟɝɿɦɟɧ ɧɟɣɬɪɚɥɞɵ, ԕɵɲԕɵɥɞɵԕ ɠԥɧɟ ɧɟɝɿɡɞɿɤ
ɟɪɿɬɿɧɞɿɥɟɪɿɧɿԙ ɪɇ-ɵɧɵԙ ɦԥɧɿɧ ɚɧɵԕɬɚԙɞɚɪ. Ɉɥ ԛɲɿɧ 3 ɫɬɚɤɚɧԑɚ
ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭ, 0,1ɦ ɬԝɡ ԕɵɲԕɵɥɵ, 0,1ɦ ɫɿɥɬɿ ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵɩ,
ɨɪɬɚɧɵԙ ɪɇ-ɧ ɚɧɵԕɬɚԙɞɚɪ.
Ԥɪ ɫɬɚɤɚɧɞɚԑɵɧɵ 3 ɩɪɨɛɢɪɤɚԑɚ ɛԧɥɿɩ ԕԝɣɵԙɞɚɪ, ɢɧɞɢɤɚɬɨɪɞɵԙ
ɬԛɫɿɧ ɚɧɵԕɬɚԙɞɚɪ. Ɉɥ ԛɲɿɧ ɩɪɨɛɢɪɤɚԑɚ ɛɿɪ ɟɤɿ ɬɚɦɲɵɞɚɧ ɦɟɬɢɥɨɪɚɧɠ,
ɮɟɧɨɥɮɬɚɥɟɢɧ, ɥɚɤɦɭɫ ɬɚɦɵɡɵԙɞɚɪ. Ȼɚɣԕɚԑɚɧɞɚɪɵԙɞɵ ɤɟɫɬɟɝɟ
ɠɚɡɵԙɞɚɪ
32
33.
Ʉɟɫɬɟ 9ɂɧɞɢɤɚɬɨɪ
Ʌɚɤɦɭɫ
Ɇɟɬɢɥɨɪɚɧɠ
Ɏɟɧɨɥɮɬɚɥɟɢɧ
ɂɧɞɢɤɚɬɨɪɞɵԙ
ԧɡɝɟɪɭ ɬԛɫɿ
ɇɟɣɬɪɚɥɶɞɵ
Ɉɪɬɚ
Ԕɵɲԕɵɥɞɵԕ
ɇɟɝɿɡɞɿɤ
ɪɇ
ɬԛɫɿ
ɪɇ
ɬԛɫɿ
ɪɇ
ɬԛɫɿ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ N 8
Ɍɚԕɵɪɵɛɵ ɋɭɞɵԙ ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɭɵ ɋɭɞɵԙ ɢɨɧɞɵԕ ɤԧɛɟɣɬɿɧɞɿɫɿ
ɂɨɧɞɵԕ ɪɟɚɤɰɢɹɥɚɪ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵԙ ɦɚԕɫɚɬɵ
ɂɨɧ ɚɥɦɚɫɭ ɪɟɚɤɰɢɹɥɚɪɵɧ ɚɧɵԕɬɚɭ
ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ ɪɇ ɚɧɵԕɬɚɭ
Ȼɚԕɵɥɚɭ ɠԝɦɵɫɵɧɵԙ ɫԝɪɚԕɬɚɪɵ ɦɟɧ ɠɚɬɬɵԑɭɥɚɪɵ
1. ɋɭɞɵԙ ɢɨɧɞɵԕ ɤԧɛɟɣɬɿɧɞɿɫɿ ɞɟɩ ɧɟɧɿ ɚɣɬɚɦɵɡ"
2. ɋɭɬɟɤɬɿɤ ɠԥɧɟ ɝɢɞɪɨɤɫɢɥɞɿɤ ɤԧɪɫɟɬɤɿɲ ɞɟɝɟɧ ɧɟ"
3. Ԕɚɧɞɚɣ ɪɟɚɤɰɢɹɥɚɪ ɢɨɧɞɵԕ ɞɟɩ ɚɬɚɥɚɞɵ ɠԥɧɟ ԕɚɧɞɚɣ ɠɚԑɞɚɣɞɚ
ɠԛɪɟɞɿ"
4. ɚ ɬԝɧɛɚ ɛ Ƚɚɡ ɬԛɪɿɧɞɟɝɿ ԕɨɫɵɥɵɫɬɚɪ ɜ ɇɚɲɚɪ ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɚɬɵɧ
ɡɚɬɬɚɪ ɬԛɡɿɥɟ ɠԛɪɟɬɿɧ ɪɟɚɤɰɢɹɥɚɪԑɚ ɦɵɫɚɥɞɚɪ ɤɟɥɬɿɪɿԙɞɟɪ
5. ȿɫɟɩ ɦɨɥɹɪɥɵԕ 1+4&O ɟɪɿɬɿɧɞɿɫɿɧɿԙ ɪɇ-ɵɧ ɟɫɟɩɬɟԙɞɟɪ
6. ȿɫɟɩ ɪɇ ɛɨɥԑɚɧɞɚԑɵ >OH-@ɤɨɧɰɟɧɬɪɚɰɢɹɫɵɧ ɟɫɟɩɬɟԙɞɟɪ
7. Ʉɟɥɟɫɿ ɡɚɬɬɚɪ ɚɪɚɫɵɧɞɚ ɠԛɪɟɬɿɧ ɪɟɚɤɰɢɹɧɵԙ ɢɨɧɞɵԕ ɬɟԙɞɟɭɿɧ
ԕԝɪɚɫɬɵɪɵԙɞɚɪ
ɚ) BaCl2(`ɟɪ)+Na2SO4(ɟɪ)o
ɛ ɄɈɇ Cɟɪ ɇ2SO ɟɪ o
ɜ NaCl Cɟɪ +KNO ɟɪ o
ɝ CuSO ɟɪ +NaOH ɟɪ)o
ɞ &D&23(`k)+HCl(ɟɪ)o
Ɉɫɵ ɪɟɚɤɰɢɹɥɚɪɞɵԙ ԕɚɥɵɩɬɵ ɠɚԑɞɚɣɞɚԑɵ Ƚɢɛɛɫ ɷɧɟɪɝɢɹɫɵɧɵԙ
ԧɡɝɟɪɭɿɧ ɟɫɟɩɬɟԙɞɟɪ. Ԕɚɣɫɵ ɪɟɚɤɰɢɹɥɚɪ ԧɡɞɿɝɿɧɟɧ ɠԛɪɟɞɿ"
8. Ʉɟɥɟɫɿ ɪɟɚɤɰɢɹ ɬɟԙɞɟɭɥɟɪɿɧ ɦɨɥɟɤɭɥɚɥɵԕ ɬԛɪɞɟ ɠɚɡɵԙɞɚɪ
ɚ) Mg2++CO32-o
ɛ &D2++PO43-o
ɜ) Pb2+ ««oPb(NO3)2 «
ɝ SɿO3 2- « oH2SɿO3«
33
34.
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɬԥɠɿɪɢɛɟԔɚɠɟɬɬɿ ԕԝɪɚɥ ɠɚɛɞɵԕɬɚɪ ɦɟɧ ɪɟɚɤɬɢɜɬɟɪ ɇ2Ɉ M H2SO4, KOH,
NH4OH, ZnCl2, Na2CO3, CH3COONa ɢɧɞɢɤɚɬɨɪɥɚɪ- ɮɟɧɨɥɮɬɚɥɟɢɧ
ɦɟɬɢɥɨɪɚɧɠ ɥɚɤɦɭɫ ɪɇ ɦɟɬɪ ȺȱCȱ3 , Na2CO3 ɇɋȱ Ԑɟɋȱ3 Ʉ3ɊɈ4,
ɋɢSO4, Na2SɿɈ3 ԐeSO4, (NH4)2SO4
1-ɬԥɠɿɪɢɛɟ ɋɭɞɵԙ ԕɵɲԕɵɥɞɵԙ ɫɿɥɬɿ-ɧɟɝɿɡɞɟɪɞɿԙ ɬԝɡɞɚɪɞɵԙ ɪɇɵɧ
ɚɧɵԕɬɚɭ ɫɚɥɵɫɬɵɪɭ
ɂɧɞɢɤɚɬɨɪ ɦɟɧ ɪɇ-ɦɟɬɪɞɿԙ ɤԧɦɟɝɿɦɟɧ H2O, 0,1M H2SO4, KOH,
NH4OH, ZnCl2, Na2CO3, CH3COONa ɟɪɿɬɿɧɞɿɥɟɪɿɧɿԙ ɪɇ ɫɭɬɟɤɬɿɤ
ɤԧɪɫɟɬɤɿɲɿɧ ɚɧɵԕɬɚɩ ɚɥɵɧԑɚɧ ɦԥɧɞɟɪɞɿ ɤɟɫɬɟɝɟ ɠɚɡɵԙɞɚɪ
Ʉɟɫɬɟ 10
ȿɪɿɬɿɧɞɿ
ɂɧɞɢɤɚɬɨɪ
ɬԛɫɿ
Ɉɪɬɚɧɵԙ
ɪɇ
Ɉɪɬɚ
ɫɢɩɚɬɵ
Ɋɟɚɤɰɢɹ
ɬɟԙɞɟɭɿ
H2O
H2SO4
KOH
NH4OH
ZnCl2
Na2CO3
CH3COONa
2-ɬԥɠɿɪɢɛɟ ɋɨԙɵɧɚ ɞɟɣɿɧ ԕɚɣɬɵɦɫɵɡ ɠԛɪɟɬɿɧ ɢɨɧɞɵԕ ɪɟɚɤɰɢɹɥɚɪ
ɬԝɧɛɚ ɝɚɡ ԥɥɫɿɡ ɷɥɟɤɬɪɨɥɢɬ ɬԛɡɭ
ɚ Ȼɚɪ ɪɟɚɤɬɢɜɬɟɪɞɟɧ ɬɟɦɿɪ ȱȱȱ ɝɢɞɪɨɤɫɢɞɿ ɤɚɥɶɰɢɣ ɮɨɫɮɚɬɵ ɠԥɧɟ ɦɵɫ
ɫɭɥɶɮɢɞɿ ȱȱ ɚɥɭԑɚ ɛɨɥɚɬɵɧɞɚɪɵɧ ɬɚԙɞɚɩ ɚɥɵԙɞɚɪ 7ԝɧɛɚɧɵԙ ɬԛɫɿɧ
ɤԧɪɫɟɬɿԙɞɟɪ Ɇɨɥɟɤɭɥɚɥɵԕ ɢɨɧɞɵԕ ɠԥɧɟ ɢɨɧɞɵԕ ɬԛɪɞɟɝɿ ɬɟԙɞɟɭɿɧ
ɠɚɡɵԙɞɚɪ
ɛ Ʉɨɧɰɟɧɬɪɥɟɧɝɟɧ ɧɚɬɪɢɣ ɫɢɥɢɤɚɬɵ ɟɪɿɬɿɧɞɿɫɿɧɟ ɬԝɡ ԕɵɲԕɵɥɵɧ
ɬɚɦɵɡɵԙɞɚɪ Ɋɟɚɤɰɢɹɧɵԙ ɦɨɥɟɤɭɥɚɥɵԕ ɠԥɧɟ ɢɨɧɞɵԕ ɬԛɪɞɟɝɿ ɬɟԙɞɟɭɿɧ
ɠɚɡɵԙɞɚɪ
3-ɬԥɠɿɪɢɛɟ Ɍԝɧɛɚɧɵԙ ɬԛɡɿɥɭ ɠɚԑɞɚɣɵ
ȿɤɿ ɩɪɨɛɢɪɤɚԑɚ - ɬɚɦɲɵ ɬɟɦɿɪ ɫɭɥɶɮɚɬɵ ȱȱ ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ
Ȼɿɪɟɭɿɧɟ ɨɫɵɧɞɚɣ ɦԧɥɲɟɪɞɟ ɤԛɤɿɪɬɬɿ ɫɭɬɟɤ ԕɵɲԕɵɥɵɧ ɚɥ ɟɤɿɧɲɿɫɿɧɟ
ɚɦɦɨɧɢɣ ɫɭɥɶɮɢɞɿ ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ Ԕɚɣ ɠɚԑɞɚɣɞɚ ɬԝɧɛɚ ɬԛɫɟɞɿ"
Ɋɟɚɤɰɢɹɧɵԙ ɢɨɧɞɵԕ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ ȿɪɿɝɿɲɬɿɤ ɤԧɛɟɣɬɿɧɞɿɫɿɧ
ɩɚɣɞɚɥɚɧɵɩ ɛɿɪɿɧɲɿ ɠɚԑɞɚɣɞɚ ɬɟɦɿɪ ɫɭɥɶɮɢɞɿɧɿԙ ɬԝɧɛɚԑɚ ɬԛɫɭɿɧ ɚɥ
ɟɤɿɧɲɿ ɠɚԑɞɚɣɞɚ ɬԛɫɩɟɭɿɧ ɬԛɫɿɧɞɿɪɿԙɞɟɪ
34
35.
4-ɬԥɠɿɪɢɛɟ Ⱥɡ ɟɪɢɬɿɧ ɷɥɟɤɬɪɨɥɢɬ ɬԝɧɛɚɥɚɪɵɧɵԙ ɟɪɭ ɠɚԑɞɚɣɵȿɤɿ ɩɪɨɛɢɪɤɚ ɞɚɣɵɧɞɚԙɞɚɪ Ȼɿɪɟɭɿɧɟ - ɦɥ ɬɟɦɿɪ ɫɭɥɶɮɚɬɵ ȱȱ
ɟɪɿɬɿɧɞɿɫɿɧ ɟɤɿɧɲɿɫɿɧɟ - ɦɥ ɦɵɫ ɫɭɥɶɮɚɬɵɧ ȱȱ ԕԝɣɵԙɞɚɪ Ԥɪɛɿɪ
ɩɪɨɛɢɪɤɚԑɚ - ɦɥ ɚɦɦɨɧɢɣ ɫɭɥɶɮɢɞɿɧ ԕɨɫɵԙɞɚɪ Ⱥɥɵɧԑɚɧ ɬԝɧɛɚɥɚɪԑɚ
ɬԝɡ ԕɵɲԕɵɥɵ ɟɪɿɬɿɧɞɿɫɿɧ ԕɨɫɵԙɞɚɪ ɇɟ ɛɚɣԕɚԑɚɧɞɚɪɵԙɞɵ ɠɚɡɵɩ
ɪɟɚɤɰɢɹɧɵԙ ɢɨɧɞɵԕ ɬɟԙɞɟɭɿɧ ԕԝɪɚɫɬɵɪɵԙɞɚɪ ȿɪɿɝɿɲɬɿɤ ɤԧɛɟɣɬɿɧɞɿɫɿɧɿԙ
ɤɟɫɬɟɥɿɤ ɦԥɧɿɧ ɩɚɣɞɚɥɚɧɵɩ ɇɋȱ ԕɨɫԕɚɧɞɚ ɛɿɪ ɫɭɥɶɮɢɞɿ ɟɪɿɩ ɟɤɿɧɲɿɫɿ
ɧɟɝɟ ɟɪɿɦɟɝɟɧɿɧ ɬԛɫɿɧɞɿɪɿԙɞɟɪ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ N 9
Ɍɚԕɵɪɵɛɵ Ɍԝɡɞɚɪɞɵԙ ɝɢɞɪɨɥɢɡɿ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵԙ ɦɚԕɫɚɬɵ
ɚɧɵԕɬɚɭ
Ɍԝɡɞɚɪ ɝɢɞɪɨɥɢɡɿɧɿԙ ɪɇ-ɧ
Ȼɚԕɵɥɚɭ ɠԝɦɵɫɵɧɵԙ ɫԝɪɚԕɬɚɪɵ ɦɟɧ ɠɚɬɬɵԑɭɥɚɪɵ
1. Ɍԝɡɞɚɪ ɝɢɞɪɨɥɢɡɿ ɞɟɩ ɧɟɧɿ ɚɣɬɚɦɵɡ " Ƚɢɞɪɨɥɢɡɞɟɧɭ ɞԥɪɟɠɟɫɿ ɦɟɧ
ɤɨɧɫɬɚɧɬɚɫɵ ɠԥɧɟ ɨԑɚɧ ԥɫɟɪ ɟɬɟɬɿɧ ɮɚɤɬɨɪɥɚɪ
2. Ƚɢɞɪɨɥɢɡɞɿԙ ԥɪ ɬԛɪɥɿ ɠɚԑɞɚɣɥɚɪɵ Ɇɵɫɚɥ ɤɟɥɬɿɪɿԙɞɟɪ
3. ȿɫɟɩ Ƚɢɞɪɨɥɢɡɞɿԙ ɛɿɪɿɧɲɿ ɫɚɬɵɫɵɧ ɟɫɤɟɪɟ ɨɬɵɪɵɩ Ɇ ɋɢɋȱ2
ɟɪɿɬɿɧɞɿɫɿɧɿԙ ɪɇ-ɧ ɟɫɟɩɬɟԙɞɟɪ
4. ȿɫɟɩ Ƚɢɞɪɨɥɢɡɞɿԙ ɛɿɪɿɧɲɿ ɫɚɬɵɫɵɧ ɟɫɤɟɪɟ ɨɬɵɪɵɩ 0Ʉ Ɇ
1ɚ2ɋɈ3 ɟɪɿɬɿɧɞɿɫɿɧɿԙ ɝɢɞɪɨɥɢɡɞɟɧɭ ɤɨɧɫɬɚɧɬɚɫɵ ɦɟɧ ɞԥɪɟɠɟɫɿɧ
ɟɫɟɩɬɟԙɞɟɪ
5. ȿɫɟɩ Ⱥɦɦɨɧɢɣ ɯɥɨɪɢɞɿ ɟɪɿɬɿɧɞɿɫɿɧɿԙ ɤɨɧɫɬɚɧɬɚɫɵɧ ɟɫɟɩɬɟԙɞɟɪ Ɉɫɵ
ɬԝɡɞɵԙ Ɇ ɟɪɿɬɿɧɞɿɫɿɧɞɟɝɿ ɝɢɞɪɨɥɢɡɞɟɧɭ ɞԥɪɟɠɟɫɿ ɦɟɧ ɪɇ-ɧ
ɚɧɵԕɬɚԙɞɚɪ
6. 1ɚ2ɋɈ3 ɋɢɋȱ2 ɝɢɞɪɨɥɢɡɿɧɿԙ ɤɨɧɫɬɚɧɬɚɫɵɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
ȿɝɟɪ ɛɿɪɿɧɲɿ ɟɪɿɬɿɧɞɿɝɟ ԕɵɲԕɵɥ ɟɤɿɧɲɿɫɿɧɟ ɫɿɥɬɿ ԕԝɹɬɵɧ ɛɨɥɫɚԙ ɬɟɩɟɬɟԙɞɿɤ ԕɚɣ ɛɚԑɵɬԕɚ ɵԑɵɫɚɞɵ "
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɬԥɠɿɪɢɛɟ
Ԕɚɠɟɬɬɿ ԕԝɪɚɥ-ɠɚɛɞɵԕɬɚɪ ɦɟɧ ɪɟɚɤɬɢɜɬɟɪ ɍɧɢɜɟɪɫɚɥɞɵ ɢɧɞɢɤɚɬɨɪ
ɪɇ-ɦɟɬɪ ɩɪɨɛɢɪɤɚ 1ɚ3ɊɈ4 ɧ 1ɚ2ɇɊɈ4 1ɚɇ2ɊɈ4 Ԑɟɋȱ3 Ԑɟɋȱ2 1ɚ2ɋɈ3,
1ɚɇɋɈ3 Ʉ3ɊɈ4 ɧ =Q 123)2 Ⱥȱ&ȱ3 ɆJɋȱ2.
1-Ɍԥɠɿɪɢɛɟ ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ ɪɇ-ɵɧ ɚɧɵԕɬɚɭ ɚɧɢɨɧ ɛɨɣɵɧɲɚ ɝɢɞɪɨɥɢɡ
ɍɧɢɜɟɪɫɚɥɞɵ ɢɧɞɢɤɚɬɨɪɦɟɧ ɪɇ-ɦɟɬɪɞɿԙ ɤԧɦɟɝɿɦɟɧ ɤɚɥɢɣ ɮɨɫɮɚɬɵ ɦɟɧ
ɧɚɬɪɢɣ ɤɚɪɛɨɧɚɬɵ ɟɪɿɬɿɧɞɿɥɟɪɿɧɿԙ ɪɇ-ɧ ɚɧɵԕɬɚԙɞɚɪ
Ƚɢɞɪɨɥɢɡ ɬɟԙɞɟɭɿɧ ɦɨɥɟɤɭɥɚɥɵԕ ɠԥɧɟ ɢɨɧɞɵԕ ɬԛɪɿɧɞɟ ɠɚɡɵԙɞɚɪ
35
36.
2-ɬԥɠɿɪɢɛɟ 1ɚ3ɊɈ4 1ɚ2ɇɊɈ4 1ɚɇ2ɊɈ4 ɝɢɞɪɨɥɢɡɿɧ ɫɚɥɵɫɬɵɪɭ Ԑɟɋȱ3ɩɟɧ Ԑɟɋȱ2 ɧɟɦɟɫɟ 1ɚ2ɋɈ3 1ɚɇɋɈ3.
ɂɧɞɢɤɚɬɨɪ ɠԥɧɟ ɪɇ-ɦɟɬɪɞɿԙ ɤԧɦɟɝɿɦɟɧ ɧ 1ɚɇ2ɊɈ4 1ɚ2ɇɊɈ4 ɠԥɧɟ
1ɚ3ɊɈ4 ɧɟɦɟɫɟ Ԑɟɋȱ3 ɩɟɧ Ԑɟɋȱ2 ɧɟɦɟɫɟ 1ɚ2ɋɈ3 1ɚɇɋɈ3
ɟɪɿɬɿɧɞɿɥɟɪɿɧɿԙ ɪɇ-ɧ ɚɧɵԕɬɚԙɞɚɪ ɇɟ ɛɚɣԕɚԑɚɧɞɚɪɵԙɞɵ ɬԛɫɿɧɞɿɪɿԙɞɟɪ
Ƚɢɞɪɨɥɢɡɞɟɧɭ ɤɨɧɫɬɚɧɬɚɫɵɧɵԙ
ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ Ƚɢɞɪɨɥɢɡ
ɬɟԙɞɟɭɥɟɪɿɧ ɦɨɥɟɤɭɥɚɥɵԕ ɠԥɧɟ ɢɨɧɞɵԕ ɬԛɪɿɧɞɟ ɠɚɡɵԙɞɚɪ
3-ɬԥɠɿɪɢɛɟ Ԕɵɡɞɵɪɭɞɵԙ ɝɢɞɪɨɥɢɡɝɟ ԥɫɟɪɿ
Ⱥȱ&ȱ3 ɧɟɦɟɫɟ 1ɚ2ɋɈ3 ɟɪɿɬɿɧɞɿɥɟɪɿɧɿԙ ɛɿɪɧɟɲɟ ɬɚɦɲɵɫɵɧ ɫɚɥԕɵɧ ɠԥɧɟ
ɵɫɬɵԕ ɫɭɵ ɛɚɪ ɩɪɨɛɢɪɤɚԑɚ ɬɚɦɵɡɵɩ ɟɪɿɬɿɧɞɿɥɟɪɞɿԙ ɬԛɫɿɧɿԙ ԧɡɝɟɪɭɿɧ
ɬԛɫɿɧɞɿɪɿԙɞɟɪ Ɉɫɵ ɟɪɿɬɿɧɞɿɥɟɪɞɿԙ ɪɇ-ɵɧ ԧɥɲɟԙɞɟɪ Ƚɢɞɪɨɥɢɡ
ɬɟԙɞɟɭɥɟɪɿɧ ɦɨɥɟɤɭɥɚɥɵԕ ɠԥɧɟ ɢɨɧɞɵԕ ɬԛɪɿɧɞɟ ɠɚɡɵԙɞɚɪ
4-ɬԥɠɿɪɢɛɟ ȿɪɿɬɿɧɞɿ ɫԝɣɵɬɵɥԑɚɧ ɤɟɡɞɟ ɝɢɞɪɨɥɢɡɞɿԙ ɬɟɩɟ-ɬɟԙɞɿɝɿɧɿԙ
ɵԑɵɫɭɵ
ɧ =Q 123)2 ɧɟɦɟɫɟ ɆJɋȱ2
ɟɪɿɬɿɧɞɿɥɟɪɿɧɿԙ ɪɇ-ɧ ɚɧɵԕɬɚԙɞɚɪ
ȿɪɿɬɿɧɞɿɥɟɪɞɿ ɟɤɿ ɟɫɟ ɫԝɣɵɥɬɵɩ ɨɪɬɚɧɵԙ ɪɇ-ɧ ԧɥɲɟԙɞɟɪ Ɍԥɠɿɪɢɛɟɧɿԙ
ɧԥɬɢɠɟɫɿɧ ɬԛɫɿɧɞɿɪɿɩ ɝɢɞɪɨɥɢɡɞɟɧɭ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ Ƚɢɞɪɨɥɢɡ
ɬɟԙɞɟɭɥɟɪɿɧ ɦɨɥɟɤɭɥɚɥɵԕ ɠԥɧɟ ɢɨɧɞɵԕ ɬԛɪɿɧɞɟ ɠɚɡɵԙɞɚɪ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ 1 0
Ɍɚԕɵɪɵɛɵ Ɍɨɬɵԑɭ-ɬɨɬɵԕɫɵɡɞɚɧɭ ɪɟɚɤɰɢɹɥɚɪɵ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵԙ ɦɚԕɫɚɬɵ ɗɥɟɦɟɧɬɬɟɪɞɿԙ ɠԥɧɟ ɨɥɚɪɞɵԙ
ԕɨɫɵɥɵɫɬɚɪɵɧɵԙ
ɬɨɬɵԕɬɵɪԑɵɲɬɵԕ-ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ
ԕɚɫɢɟɬɬɟɪɿɧ ɨԕɵɩ ԛɣɪɟɧɭ
Ȼɚԕɵɥɚɭ ɠԝɦɵɫɵɧɵԙ ɫԝɪɚԕɬɚɪɵ ɦɟɧ ɠɚɬɬɵԑɭɥɚɪɵ
1. Ԕɚɧɞɚɣ ɪɟɚɤɢɹɥɚɪ ɬɨɬɵԑɭ-ɬɨɬɵԕɫɵɡɞɚɧɭ ɪɟɚɤɢɹɥɚɪɵ ɞɟɩ ɚɬɚɥɚɞɵ"
2. Ɍɨɬɵԑɭ ɞԥɪɟɠɟɫɿ ɜɚɥɟɧɬɬɿɥɿɤ ɬɭɪɚɥɵ ɬԛɫɿɧɿɤɬɟɪ ɗɥɟɦɟɧɬɬɿԙ ɬɨɬɵԑɭ
ɞԥɪɟɠɟɫɿɧɿԙ
Ⱦ ɂ Ɇɟɧɞɟɥɟɟɜɬɿԙ
ɩɟɪɢɨɞɬɵԕ ɠԛɣɟɞɟɝɿ ɨɪɧɵɧɚ
ɛɚɣɥɚɧɵɫɬɵɥɵԑɵ
3. Ɇɵɧɚ ɛɟɪɿɥɝɟɧ ԕɨɫɵɥɵɫɬɚɪɞɵԙ:
ɚ NH4NO3,NH4NO2-ɚɡɨɬɬɵԙ;
ɛ NH4)2Cr2O7-ɚɡɨɬ ɩɟɧ ɯɪɨɦɧɵԙ;
ɜ Al2(SO4)3- ɤԛɤɿɪɬɬɿԙ;
ɝ Cr2O3 ɯɪɨɦɧɵԙ;
ɞ MnO2, K2MnO4, KMnO4-ɦɚɪɝɚɧɟɰɬɿԙ ɬɨɬɵԑɭ ɞԥɪɟɠɟɥɟɪɿɧ ɚɧɵԕɬɚԙɞɚɪ
36
37.
4. Ɍɨɬɵԕɬɵɪԑɵɲ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲ ɬɨɬɵԑɭ ɬɨɬɵԕɫɵɡɞɚɧɭɩɪɨɰɟɫɫɬɟɪɿ ɇɟɝɿɡɝɿ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɚɪ ɦɟɧ ɬɨɬɵԕɬɵɪԑɵɲɬɚɪ
5. Ɇɵɧɚ ԕɨɫɵɥɵɫɬɚɪɞɵԙ:
ɚ NH3, HNO2, HNO3
ɛ K2Cr2O7, K2MnO4, PH3
ԕɚɣɫɵɫɵ ɬɨɬɵԕɬɵɪԑɵɲ, ԕɚɣɫɵɫɵ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲ, ɚɥ ԕɚɣ
ԕɨɫɵɥɵɫɬɚɪ
ɬɨɬɵԕɬɵɪԑɵɲɬɵԕ-ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ
ԕɚɫɢɟɬ
ɤԧɪɫɟɬɟɞɿ?
6. Ɍɨɬɵԑɭ-ɬɨɬɵԕɫɵɡɞɚɧɭ ɪɟɚɤɰɢɹɥɚɪɵɧɵԙ ɬɢɩɬɟɪɿ
7. Ɋɟɚɤɰɢɹɧɵԙ ɬɨɬɵԑɭ-ɬɨɬɵԕɫɵɡɞɚɧɭ ɩɨɬɟɧɰɢɚɥɵ ԕɚɥɚɣ ɚɧɵԕɬɚɥɚɞɵ"
8. ɗԔɄ ɩɟɧ 'G ɪɟɚɤɰɢɹɧɵԙ ɚɪɚɫɵɧɞɚ ԕɚɧɞɚɣ ɛɚɣɥɚɧɵɫ ɛɚɪ"
9. ɂɨɧɞɵ-ɷɥɟɤɬɪɨɧɞɵ ԥɞɿɫɿɧ ɩɚɣɞɚɥɚɧɵɩ ɪɟɚɤɰɢɹ ɬɟԙɞɟɭɿɧ ɚɹԕɬɚԙɞɚɪ
ɤɨɷɮɮɢɰɢɟɧɬɬɟɪɿɧ ԕɨɣɵԙɞɚɪ ɬɨɬɵԕɬɵɪԑɵɲ ɩɟɧ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵ
ɚɧɵԕɬɚԙɞɚɪ
1.
KMnO4toMnO2 ««
2.
Mg+HNO3(ɫԝɣɵɬ)oNH4NO3 ««
3.
As2S3+HNO3 oH3AsO4 12 ««
4.
FeSO4 .ɋO23+H2SO4o««««
5.
MnO2+Br2+KOH o««««
6.
MnSO4+KMnO4+H2Oo««
7.
Fe2O3+NaNO3+NaOH o««
8.
K2Cr2O7+HCloCl2 «««
9.
Zn+KNO2+KOHoNH3 «
10. CrCl3+Br2+KOHo ««
ȿɫɟɩ Ɇɵɧɚ ɪɟɚɤɰɢɹɧɵԙ:
t
o
2(NH4)2Cr2O7(k)
Cr2O3(k)+2NH3 ɝ H2O+N2 ɝ
0
0
'+ 298, 'S 298 ɠԥɧɟ 'G0298 ɟɫɟɩɬɟԙɞɟɪ
ɚ ɨɫɵ ɩɪɨɰɟɫɬɿԙ ɠԛɪɭɿɧɞɟɝɿ ɷɧɬɚɥɶɩɢɹɧɵԙ ɠԥɧɟ ɷɧɬɪɨɩɢɹɧɵԙ ɪɨɥɿ
ԕɚɧɞɚɣ"
ɛ ɪɟɚɤɰɢɹ ԕɚɣɬɵɦɞɵ ɛɨɥɭɵ ɦԛɦɤɿɧ ɛɟ"
ɜ ɛԝɥ ɪɟɚɤɰɢɹɞɚ ԕɚɣ ɷɥɟɦɟɧɬɬɟɪ ɬɨɬɵԑɭ ɞԥɪɟɠɟɫɿɧ ԧɡɝɟɪɬɟɞɿ"
ȿɫɟɩ ɚ Na2SO3 ɤ ԕɵɡɞɵɪԑɚɧɞɚ Na2S ɠԥɧɟ Na2SO4 ɬԛɡɿɥɟɞɿ
Ɋɟɚɤɰɢɹɧɵԙ 'G0298 ɟɫɟɩɬɟɩ ɬɟɪɦɢɹɥɵԕ ɚɣɵɪɵɥɭɞɵԙ ɠԛɪɭ
ɦԛɦɤɿɧɞɿɝɿɧ ɞԥɥɟɥɞɟԙɞɟɪ
ɛ Ȼԝɥ ɩɪɨɰɟɫɫ ɬɨɬɵԑɭ-ɬɨɬɵԕɫɵɡɞɚɧɭ ɪɟɚɤɰɢɹɥɚɪɞɵԙ ԕɚɣ ɬԛɪɿɧɟ
ɠɚɬɚɞɵ"
ȿɫɟɩ Ɍɟɦɿɪɞɿԙ Fe2+ ɢɨɧɵɧ ԕɚɥɚɣɵɧɵԙ Sn4+ ɢɨɧɵɦɟɧ ɬɨɬɵԕɬɵɪɭԑɚ
ɛɨɥɚ ɦɚ" ɀɚɭɚɩ ɬɨɥɵԕ ɛɨɥɭ ԛɲɿɧ ɗԔɄ ɟɫɟɩɬɟԙɞɟɪ
ȿɫɟɩ KMnO4 ԕɵɲԕɵɥɞɵԕ ɧɟɝɿɡɞɿɤ ɠԥɧɟ ɧɟɣɬɪɚɥɶɞɵ ɨɪɬɚɞɚԑɵ
ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ ɦɨɥɹɪɥɵԕ ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚԙɞɚɪ
37
38.
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɬԥɠɿɪɢɛɟԔɚɠɟɬɬɿ ԕԝɪɚɥ-ɠɚɛɞɵԕɬɚɪ ɦɟɧ ɪɟɚɤɬɢɜɬɟɪ Ɏɚɪɮɨɪ ɵɞɵɫɵ
ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭ ɲɬɚɬɢɜ ɫɩɢɪɬ ɲɚɦɵ Na-ɦɟɬɚɥɵ ɮɟɧɨɥɮɬɚɥɟɢɧ ɲɵɧɵ
ɬɚɹԕɲɚ ɋuSO4, ZnSO4, Fe-ɦɟɬɚɥ Ʉ3>Ԑɟ ɋN)6@ ȱ2, Cȱ2, Br2, H2S Ʉ2ɋr2O7,
Na2S; H2SO4; Na2SO3; Cu(NO3)2 ɇ2Ɉ ɄMnɈ4; H2O2; KOH ɤ NaOH.
1-ɬԥɠɿɪɢɛɟ Ɇɟɬɚɥɞɚɪɞɵԙ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɬɟɪɿ
Ɏɚɪɮɨɪ ɵɞɵɫɵɧɚ ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭ ԕԝɣɵɩ ɧɚɬɪɢɣɞɿԙ ɬԛɣɿɪɲɿɝɿɧ
ɫɚɥɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ԕɚɧɞɚɣ ɝɚɡ ɛԧɥɿɧɟɞɿ" - ɬɚɦɲɵ ɮɟɧɨɥɮɬɚɥɟɢɧ
ɬɚɦɲɵɥɚɬɵԙɞɚɪ ȿɪɿɬɿɧɞɿɧɿԙ ɬԛɫɿ ɧɟɝɟ ԧɡɝɟɪɟɞɿ" Ɋɟɚɤɰɢɹ ɬɟԙɞɟɭɿɧ
ԕԝɪɵԙɞɚɪ
2-ɬԥɠɿɪɢɛɟ Ɇɟɬɚɥɞɚɪɞɵԙ ɚɤɬɢɜɬɿɥɿɝɿɧ ɚɧɵԕɬɚɭ
Ȼɿɪ ɩɪɨɛɢɪɤɚԑɚ- 10- ɬɚɦɲɵ ɦɵɫ ɫɭɥɶɮɚɬɵ ɟɪɿɬɿɧɞɿɫɿɧ ɟɤɿɧɲɿɩɪɨɛɢɪɤɚԑɚ ɦɵɪɵɲ ɫɭɥɶɮɚɬɵ ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ ɟɤɟɭɿɧɟ ɞɟ ɬɟɦɿɪɞɿԙ
ɛɿɪ ɬɿɥɿɦɿɧ ɫɚɥɵԙɞɚɪ Ɇɵɫ ɫɭɥɶɮɚɬɵ ɛɚɪ ɩɪɨɛɢɪɤɚɞɚ - ɦɢɧɭɬɬɟ
ɬɟɦɿɪɞɿԙ ɛɟɬɿɧɞɟ ԕɵɡɵɥ ɬԛɫɬɿ ԕɚɬɩɚɪ ɛɚɣԕɚɥɚɞɵ Ɉɫɵ ɩɪɨɛɢɪɤɚԑɚ
ɬɟɦɿɪɞɿԙ K3[Fe(CN)6@ ɬԝɡɵɧ ԕɨɫɵԙɞɚɪ ȿɪɿɬɿɧɞɿ ɬԛɫɿɧ ԧɡɝɟɪɬɟɞɿ ȿɤɿɧɲɿ
ɩɪɨɛɢɪɤɚԑɚ ZnSO4 ɟɪɿɬɿɧɞɿɫɿɧɟ K3[Fe(CN)6@ ԕԝɣɵԙɞɚɪ Ԧɡɝɟɪɿɫ ɛɚɣԕɚɥɚ
ɦɚ" Ɋɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ ɬɟɦɿɪ ԕɚɧɞɚɣ ԕɚɫɢɟɬ
ɤԧɪɫɟɬɟɞɿ"
3-ɬԥɠɿɪɢɛɟ ɪ-ɷɥɟɦɟɧɬɬɟɪɞɿԙ ɬɨɬɵԕɬɵɪԑɵɲɬɵԕ ɠԥɧɟ
ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɬɟɪɿ
2-ɩɪɨɛɢɪɤɚԑɚ - ɬɚɦɲɵɞɚɧ ɢɨɞ ɫɭɵɧ ԕԝɣɵԙɞɚɪ Ȼɿɪɿɧɲɿ ɩɪɨɛɢɪɤɚԑɚ ɯɥɨɪ ɫɭɵɧ ɟɤɿɧɲɿɫɿɧɟ- ɤԛɤɿɪɬɬɿ ɫɭɬɟɤ ɫɭɵɧ ԕɨɫɵԙɞɚɪ ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ
ɬԛɫɬɟɪɿ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ" Ɋɟɚɤɰɢɹɥɚɪ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ ɪɟɚɤɰɢɹɥɚɪ
ɧԥɬɢɠɟɫɿɧɞɟ ɇɋO ɠԥɧɟ ɇJɈ3 ԕɵɲԕɵɥɞɚɪ ɬԛɡɿɥɟɞɿ Ɍɨɬɵԕɬɵɪԑɵɲ ɩɟɧ
ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵ ɚɧɵԕɬɚԙɞɚɪ
4-ɬԥɠɿɪɢɛɟ ɪ-ɠԥɧɟ d-ɷɥɟɦɟɧɬɬɟɪɞɿԙ ɬɨɬɵԕɬɵɪԑɵɲɬɵԕ ɩɟɧ
ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɬɟɪɿ
K2Cr2O7 ɟɪɿɬɿɧɞɿɫɿ ɛɚɪ ɛɿɪ ɩɪɨɛɢɪɤɚԑɚ Na2S ɟɪɿɬɿɧɞɿɫɿ ɛɚɪ ɟɤɿɧɲɿ
ɩɪɨɛɢɪɤɚԑɚ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵ ɦɟɧ Na2SO3 ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ
ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ ɬԛɫɬɟɪɿ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ" Ɋɟɚɤɰɢɹɥɚɪ ɬɟԙɞɟɭɥɟɪɿɧ
ɠɚɡɵԙɞɚɪ
5-ɬԥɠɿɪɢɛɟ Ⱦɢɫɩɪɨɩɨɪɰɢɹɥɚɧɭ ɪɟɚɤɰɢɹɥɚɪɵ ԧɡɿɧ-ԧɡɿ
ɬɨɬɵԕɬɵɪɚɞɵ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪɚɞɵ
ɩɪɨɛɢɪɤɚԑɚ Na2SO3 ɤɪɢɫɬɚɥɞɚɪɵɧ ɫɚɥɵԙɞɚɪ ɛɿɪɟɭɿɧ ԕɵɡɞɵɪɵԙɞɚɪ ɦɢɧ ȿɤɟɭɿɧɟ ɞɟ ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭ ԕԝɣɵԙɞɚɪ ɬԝɡɞɚɪ ɟɪɿɝɟɧɲɟ ɲɵɧɵ
ɬɚɹԕɲɚɦɟɧ ɚɪɚɥɚɫɬɵɪɵԙɞɚɪ - ɩɪɨɛɢɪɤɚԑɚ ɦɵɫ ɫɭɥɶɮɚɬɵ ȱȱ ɟɪɿɬɿɧɞɿɫɿɧ
ԕɨɫɵԙɞɚɪ ɬԛɡɿɥɝɟɧ ɬԝɧɛɚɥɚɪɞɵԙ ɬԛɫɬɟɪɿɧ ɛɚɣԕɚԙɞɚɪ Ɋɟɚɤɰɢɹ
ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
38
39.
6-ɬԥɠɿɪɢɛɟ Ɇɨɥɟɤɭɥɚ ɿɲɿɧɞɟɝɿ ԧɡɿɧ-ԧɡɿ ɬɨɬɵԕɬɵɪɭ ɠԥɧɟɬɨɬɵԕɫɵɡɞɚɧɞɵɪɭ ɪɟɚɤɰɢɹɥɚɪɵ
ɉɪɨɛɢɪɤɚɧɵԙ ɛԧɥɿɝɿɧɟ ɦɵɫ ȱȱ ɧɢɬɪɚɬɵɧ Cu(NO3)2*3H2O
ɤɪɢɫɬɚɥɞɚɪɵɧ ɫɚɥɵɩ ɲɬɚɬɢɜɤɟ ɛɟɤɿɬɿɩ ɛɚɥԕɵԑɚɧԑɚ ɞɟɣɿɧ ԕɵɡɞɵɪɵԙɞɚɪ
Ԕɚɧɞɚɣ ԧɡɝɟɪɿɫɬɟɪ ɛɚɣԕɚɥɚɞɵ" Ԕɚɧɞɚɣ ɝɚɡ ɛԧɥɿɧɟɞɿ" Ɋɟɚɤɰɢɹɧɵԙ
ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
7-ɬԥɠɿɪɢɛɟ Ɉɪɬɚɧɵԙ ɪɇ-ɧɵԙ ɬɨɬɵԑɭ-ɬɨɬɵԕɫɵɡɞɚɧɭ ɪɟɚɤɰɢɹɫɵɧɚ
ԥɫɟɪɿ
3-ɩɪɨɛɢɪɤɚԑɚ - ɦɥ KMnO4 ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ Ȼɿɪɿɧɲɿɫɿɧɟ- 1- ɦɥ
ɤԛɤɿɪɬ ԕɵɲԕɵɥɵ ɟɤɿɧɲɿɫɿɧɟ- 1- ɦɥ ɇ2Ɉ ԛɲɿɧɲɿɫɿɧɟ- 1- ɦɥ
ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ ɄɈɇ ɧɟɦɟɫɟ NaOH ɟɪɿɬɿɧɞɿɫɿɧ ԕɨɫɵԙɞɚɪ Ȼɚɪɥɵԕ
ɩɪɨɛɢɪɤɚԑɚ ɧɚɬɪɢɣ ɫɭɥɶɮɢɬɿɧ ɫɚɥɵԙɞɚɪ ɧɟ ɛɚɣԕɚԑɚɧɞɚɪɵԙɞɵ ɠɚɡɵɩ
ɬɨɬɵԑɭ-ɬɨɬɵԕɫɵɡɞɚɧɭ ɪɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ԕԝɪɚɫɬɵɪɵԙɞɚɪ ɠԥɧɟ
ɬɟԙɟɫɬɿɪɿԙɞɟɪ ɄMnɈ4 ԥɪ ɬԛɪɥɿ ɨɪɬɚɞɚԑɵ ɷɤɜɢɜɚɥɟɧɬɿɧɿԙ ɦɨɥɹɪɥɵԕ
ɦɚɫɫɚɫɵɧ ɚɧɵԕɬɚԙɞɚɪ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ N11
Ɍɚԕɵɪɵɛɵ ɋɭɬɟɤ Ɉɬɬɟɤ ɋɭɬɟɤ ɩɟɪɨɤɫɢɞɿ Ⱥɥɵɧɭɵ ԕɚɫɢɟɬɬɟɪɿ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɫɚɛɚԕɬɵԙ ɦɚԕɫɚɬɵ ɋɭɬɟɤɬɿԙ ɨɬɬɟɤɬɿԙ ɠԥɧɟ
ɨɥɚɪɞɵԙ ԕɨɫɵɥɵɫɬɚɪɵɧɵԙ ɚɥɵɧɭ ɠɨɥɞɚɪɵ ɦɟɧ ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿɧ
ɨԕɵɩ ԛɣɪɟɧɭ
Ȼɚԕɵɥɚɭ ɠԝɦɵɫɵɧɵԙ ɫԝɪɚԕɬɚɪɵ ɦɟɧ ɠɚɬɬɵԑɭɥɚɪɵ
1. ɋɭɬɟɤɬɿԙ ɩɟɧ ɨɬɬɟɤɬɿԙ ɬɚɛɢԑɚɬɬɚԑɵ ԕɨɫɵɥɵɫɬɚɪɵ
2. ɋɭɬɟɤɬɿԙ Ⱦ ɂ Ɇɟɧɞɟɥɟɟɜɬɿԙ ɩɟɪɢɨɞɬɵԕ ɫɢɫɬɟɦɚɫɵɧɞɚԑɵ ȱ ɠԥɧɟ Vȱȱ
ɬɨɩɬɚԑɵ ɨɪɧɵ Ɉɫɵ ɠɚԑɞɚɣɞɵ ԕɚɥɚɣ ɬԛɫɿɧɞɿɪɭɝɟ ɛɨɥɚɞɵ"
3. ɋɭɬɟɤɬɿԙ ɩɟɧ ɨɬɬɟɤɬɿԙ ɚɥɵɧɭ ɠɨɥɞɚɪɵ ɮɢɡɢɤɚɥɵԕ ɠԥɧɟ ɯɢɦɢɹɥɵԕ
ԕɚɫɢɟɬɬɟɪɿ
4. ȿɫɟɩ ɥ ɫɭɬɟɤ ԕ ɠ ɚɥɭ ԛɲɿɧ ɤԛɤɿɪɬ ԕɵɲԕɵɥɦɟɧ ԥɪɟɤɟɬɬɟɫɭ ԛɲɿɧ
ԕɚɧɲɚ ɝɪɚɦɦ ɦɵɪɵɲ ԕɚɠɟɬ"
5. ȿɫɟɩ ɝ ɚɥɸɦɢɧɢɣɦɟɧ ԥɪɟɤɟɬɬɟɫɭ ԛɲɿɧ ԕɚɧɲɚ ɦɥ 1D2+
ɠԝɦɫɚɥɚɞɵ" Ԕɚɧɲɚ ɤԧɥɟɦ ɫɭɬɟɤ ԕ ɠ ɛԧɥɿɧɿɩ ɲɵԑɚɞɵ"
6. ȿɫɟɩ ɦɥ ɫɭɞɚ ɝ ɦɟɬɚɥ ɧɚɬɪɢɣ ɟɪɿɬɬɿ ɉɚɣɞɚ ɛɨɥԑɚɧ ɟɪɿɬɿɧɞɿɞɟɝɿ
ɫɿɥɬɿɧɿԙ ɦɚɫɫɚɥɵԕ ԛɥɟɫɿɧ ɟɫɟɩɬɟԙɞɟɪ
7. ȿɫɟɩ ɝ ɦɵɫ ȱȱ ɝɢɞɪɨɤɫɢɞɿɧ ɬɟɪɦɢɹɥɵԕ ԥɞɿɫ ɚɪԕɵɥɵ
ɵɞɵɪɚɬԕɚɧɞɚ ɬԛɡɿɥɝɟɧ ɦɵɫ ɨɤɫɢɞɿɧ ȱȱ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪɭ ԛɲɿɧ ԕɚɧɲɚ
ɤԧɥɟɦ ԕ ɠ ɫɭɬɟɤ ɤɟɪɟɤ"
39
40.
8. ȿɫɟɩ ȿɝɟɪ ɞɟ ɛԧɥɿɧɝɟɧ ɫɭɬɟɤ ɝ Fe3O4 ɬɟɦɿɪɝɟ ɞɟɣɿɧɬɨɬɵԕɫɵɡɞɚɧɞɵɪca ɦɵɪɵɲɩɟɧ ԥɪɟɤɟɬɬɟɫɭ ԛɲɿɧ -ɬɿɤ p ɝ ɫɦ3)
ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɧɵԙ ԕɚɧɲɚ ɤԧɥɟɦɿ ɠԝɦɫɚɥɚɞɵ"
9. ȿɫɟɩ Ʉɚɥɶɰɢɣ ɝɢɞɪɢɞɿ ɫɭɦɟɧ ԥɪɟɤɟɬɬɟɫɤɟɧɞɟ ɬԛɡɿɥɝɟɧ ɝɢɞɪɨɤɫɢɞɿɧ
ɧɟɣɬɪɚɥɞɚɭ ԛɲɿɧ -ɬɿɤ p ɝ ɫɦ3 ɦɥ ɬԝɡ ԕɵɲԕɵɥɵ
ɠԝɦɫɚɥɚɞɵ Ԕɚɥɵɩɬɵ ɠɚԑɞɚɣɞɚ ɛԧɥɿɧɿɩ ɲɵԕԕɚɧ ɫɭɬɟɤɬɿԙ ɤԧɥɟɦɿɧ
ɟɫɟɩɬɟԙɞɟɪ
ȿɫɟɩ -ɬɿɤ ɤɝ ɇ2Ɉ2 ɟɪɿɬɿɧɞɿɫɿɧ ɤɝ ɇ2Ɉ2 ɟɪɿɬɿɧɞɿɫɿɦɟɧ
ɚɪɚɥɚɫɬɵɪԑɚɧ ɠɚԑɞɚɣɞɚ ɠɚԙɚɞɚɧ ɩɚɣɞɚ ɛɨɥԑɚɧ ɟɪɿɬɿɧɞɿɧɿԙ ɦɚɫɫɚɥɵԕ
ԛɥɟɫɿɧ ɬɚɛɵԙɵɡɞɚɪ
11 ȿɫɟɩ ɦɥ ɦɨɥɹɪɥɵԕ ɟɪɿɬɿɧɞɿ ɞɚɣɵɧɞɚɭ ԛɲɿɧ ԕɚɧɲɚ ɤԧɥɟɦ ɬɿɤ ɇ2Ɉ2 ɟɪɿɬɿɧɞɿɫɿ ɪ ɝ ɫɦ3 ɠԥɧɟ ɫɭ ԕɚɠɟɬ" ȿɪɿɬɿɧɞɿɧɿԙ >ɇ+@ ɠԥɧɟ
ɪɇ ɚɧɵԕɬɚԙɞɚɪ
ȿɫɟɩ KCȱO3 ɵɞɵɪɚԑɚɧɞɚ ɥ ɨɬɬɟɤ ɛԧɥɿɧɿɩ ɲɵԑɚɞɵ ԕ ɠ Ɉɫɵ
ɪɟɚɤɰɢɹ ɠԛɪɝɟɧɞɟ ԕɚɧɞɚɣ ɦԧɥɲɟɪɞɟ ɠɵɥɭ ɛԧɥɿɧɿɩ ɲɵԑɚɞɵ"
ȿɫɟɩ ɤɝ -ɬɿɤ ɇ2Ɉ2 ɟɪɿɬɿɧɞɿɫɿɧ ɞɚɣɵɧɞɚɭ ԛɲɿɧ ɧɟɲɟ ɤɝ ȼɚɈ2
ɠԥɧɟ -ɬɿɤ H2SO4 ɟɪɿɬɿɧɞɿɫɿ ɠԝɦɚɫɚɥɚɞɵ"
ȿɫɟɩ ɝ ɇ2Ɉ2 ɟɪɿɬɿɧɞɿɫɿ H2SO4 ԕɵɲԕɵɥɞɚɧԑɚɧ Kȱ ɟɪɿɬɿɧɞɿɫɿɧɟɧ ɝ
ɢɨɞɬɵ ɛԧɥɿɩ ɲɵԑɚɪɚɞɵ ɇ2Ɉ2±ɧɿԙ ɟɪɿɬɿɧɞɿɞɟɝɿ ɦɚɫɫɚɥɵԕ ԛɥɟɫɿɧ
ɟɫɟɩɬɟԙɞɟɪ
15 Ɍԧɦɟɧɞɟɝɿ ɪɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɚɹԕɬɚɩ ɷɥɟɤɬɪɨɧɞɵԕ-ɢɨɧɞɵԕ
ԥɞɿɫɩɟɧ ɤɨɷɮɮɢɰɢɟɧɬɬɟɪɿɧ ɬɚɛɵԙɞɚɪ ɬɟԙɟɫɬɿɪɿԙɞɟɪ
ɚ +2S+H2O2 o 6 «
ɛ &ȱ2+H2O2 o O2 «
ɜ) Hg(NO3)2+H2O2+NaOH o +J «
ɝ Ʉ2Cr2O7+H2O2+KOH oK3 CrO3 «
ɞ) N2H4+H2O2 o N2 «
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɬԥɠɿɪɢɛɟɥɟɪ
Ⱥɩɩɚɪɚɬɬɚɪ ɠԥɧɟ ɵɞɵɫɬɚɪ Ɉɬɬɟɤɩɟɧ ɬɨɥɬɵɪɵɥԑɚɧ ɝɚɡɨɦɟɬɪ Ʉɢɩɩ
ɚɩɩɚɪɚɬɵ ԕɵɫԕɵɲɵ ɛɚɪ ɲɬɚɬɢɜ ɠɚɧɞɵɪԑɵɲ ɫɵɣɵɦɞɵɥɵԑɵ - ɦɥ
ɲɵɧɵ ɰɢɥɢɧɞɪɥɟɪ ɤɪɢɫɬɚɥɥɢɡɚɬɨɪ ɫɵɣɵɦɞɵɥɵԑɵ - ɦɥ ɫɬɚɤɚɧɞɚɪ
ɲɵɧɵ ɩɥɚɫɬɢɧɤɚɥɚɪ ɩɪɨɛɢɪɤɚɥɚɪ ɫɚɥɵɧԑɚɧ ɲɬɚɬɢɜ
Ɋɟɚɤɬɢɜɬɟɪ ɠԥɧɟ ɦɚɬɟɪɢɚɥɞɚɪ Ɇɵɪɵɲ ɬԛɣɿɪɲɿɤɬɟɪɿ ɚɥɸɦɢɧɢɣ
ɤɟɫɿɧɞɿɥɟɪɿ ɦɵɫ ȱȱ ɨɤɫɢɞɿ &X2 ɫԛɥɝɿ Ʉԛɤɿɪɬ ɦɚɝɧɢɣ ɥɟɧɬɚɫɵ ɚԑɚɲ
ɤԧɦɿɪ ɬԛɣɿɪɿ ԕɵɡɵɥ ɮɨɫɮɨɪ ɬɟɦɿɪ ɫɵɦɵ ɧɚɬɪɢɣ ɩɟɪɨɤɫɢɞɿ ɦɚɪɝɚɧɟɰ
ȱ9 ɨɤɫɢɞɿ ɛɚɪɢɣ ɩɟɪɨɤɫɢɞɿ ɤɚɥɢɣ ɯɥɨɪɚɬɵ ɤɚɥɢɣ ɧɢɬɪɚɬɵ
ɞɢɷɬɢɥɷɮɢɪ ɥɚɤɦɭɫ ԕɚԑɚɡɵ ԕɚɪ ɧɟɦɟɫɟ ɦԝɡ ɫԛɡɝɿ ԕɚԑɚɡɵ ɚԑɚɲ
ɠɚԙԕɚɥɚɪɵ
ȿɪɿɬɿɧɞɿɥɟɪ Ʉԛɤɿɪɬ ԕɵɲԕɵɥɵ +2SO4 ɤԛɣɞɿɪɝɿɲ ɧɚɬɪ 1D2+
ɤɚɥɢɣ ɩɟɪɦɚɧɝɚɧɚɬɵ .0Q24 ɧ ɋɭɬɟɤ ɩɟɪɨɤɫɢɞɿ ɠԥɧɟ
ɤԛɤɿɪɬ ԕɵɲԕɵɥɵ ɧ ɚɦɦɢɚɤ ɧ ɛɚɪɢɣ ɝɢɞɪɨɤɫɢɞɿ ԕɚɧɵԕ ɧɚɬɪɢɣ
ɝɢɞɪɨɤɫɢɞɿ ɧ ɤɚɥɢɣ ɢɨɞɢɞɿ ɧ ɤɚɥɢɣ ɞɢɯɪɨɦɚɬɵ ɧ ɤɚɥɢɣ
ɩɟɪɦɚɧɝɚɧɚɬɵ ɤɨɧɰ ɠԥɧɟ ɧ ɦɚɪɝɚɧɟɰ ɫɭɥɶɮɚɬɵ ɧ ɧɚɬɪɢɣ
40
41.
ɫɭɥɶɮɢɞɿ ɧ ɤԛɦɿɫ ɧɢɬɪɚɬɵ ɧ ԕɨɪԑɚɫɵɧ ɧɢɬɪɚɬɵ ɧ ɯɪɨɦȱȱȱ ɧɢɬɪɚɬɵ ɧ ɯɪɨɦ ȱȱȱ ɫɭɥɶɮɚɬɵ ɧ ɤɪɚɯɦɚɥ ɤɥɟɣɫɬɟɪɿ
ɥɚɤɦɭɫ ɮɟɧɨɥɮɬɚɥɟɢɧ
1-ɬԥɠɿɪɢɛɟ Ɇɟɬɚɥɞɚɪɞɵ ԕɵɲԕɵɥɦɟɧ ԥɪɟɤɟɬɬɟɫɬɿɪɭ ɚɪԕɵɥɵ
ɫɭɬɟɤɬɿԙ ɚɥɵɧɭɵ
Ɍɵԑɵɧɵ ɠԥɧɟ ɬԛɬɿɤɲɟɫɿ ɛɚɪ ɩɪɨɛɢɪɤɚԑɚ - ɦɵɪɵɲɬɵԙ ɛԧɥɿɝɿɧ ɫɚɥɵɩ
ԛɫɬɿɧɟ -ɬɿɤ +2SO4 ԕԝɣɵԙɞɚɪ Ƚɚɡ ɛԧɥɿɧɝɟɧɿɧ ɛɚɣԕɚԙɞɚɪ
Ƚɚɡɞɵԙ ɬɚɡɚɥɵԑɵɧ ɬɟɤɫɟɪɿԙɞɟɪ Ɉɥ ԛɲɿɧ ɩɪɨɛɢɪɤɚɧɵ ɝɚɡɛɟɧ ɬɨɥɬɵɪɵɩ
ɚɭɞɚɪɵԙɞɚɪ ɞɚ ɠɚɧɞɵɪɵԙɞɚɪ Ƚɚɡ ɬɚɡɚ ɛɨɥɫɚ ɠɚɣ ɤԧɤ ɬԛɫɬɿ ɛɨɥɵɩ
ɠɚɧɚɞɵ ԕɨɫɩɚ ɛɨɥɫɚ-ԕɨɩɚɪɵɥɵɫ ɪɟɬɿɧɞɟ ɪɟɚɤɰɢɹ ɠԛɪɟɞɿ ɀɚɧɵɩ ɠɚɬԕɚɧ
ɝɚɡɞɵԙ ԛɫɬɿɧɟ ɫɬɚɤɚɧɞɵ ɧɟɦɟɫɟ ɩɪɨɛɢɪɤɚɧɵ ԝɫɬɚԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ"
Ɋɟɚɤɰɢɹ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
2-ɬԥɠɿɪɢɛɟ ɋɭɬɟɤɬɿԙ ɚɥɵɧɭɵ ɦɚɬɟɪɢɚɥɞɚɪ ɦɟɧ ɫɿɥɬɿɥɟɪɦɟɧ
ԥɪɟɤɟɬɬɟɫɬɿɪɭ ɚɪԕɵɥɵ
Ƚɚɡ ɛԧɥɿɧɟɬɿɧ ɬԛɬɿɤɲɟɫɿ ɛɚɪ ɩɪɨɛɢɪɤɚԑɚ Ⱥȱ ɠɚɧԕɚɫɵɧ ɫɚɥɵɩ ԛɫɬɿɧɟɧ
ɫɿɥɬɿ ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ Ԕɚɧɞɚɣ ɝɚɡ ɛԧɥɿɧɟɞɿ" Ɋɟɚɤɰɢɹ ɬɟԙɞɟɭɿɧ
ɠɚɡɵԙɞɚɪ
3-ɬԥɠɿɪɢɛɟ Ɇɨɥɟɤɭɥɚɥɵԕ ɫɭɬɟɤɬɿԙ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ
ԕɚɫɢɟɬɿ
ɉɪɢɛɨɪ ɲɵɧɵ ɬɪɭɛɤɚɞɚɧ ɛɿɪ ɠɚԑɵ ɬɵԑɵɧɦɟɧ ɠɚɛɵɥɚɞɵ Ɍԛɬɤɿɲ
ɬɪɭɛɤɚɫɵ ɛɚɪ ɿɲɿɧɟ ɲɚɦɚɞɚ ɦɵɫ ɨɤɫɢɞɿɧ ȱȱ ɫɚɥɵԙɞɚɪ ɲɬɚɬɢɜɤɟ
ɛɟɤɿɬɿɩ Ʉɢɩɩ ɚɩɩɚɪɚɬɵɧɚɧ ɫɭɬɟɤ ɠɿɛɟɪɿɩ ɨɧɵ ɠɚɧɞɵɪɵԙɞɚɪ Ɇɵɫ
ɨɤɫɢɞɿɧ ԕɵɡɞɵɪɵԙɞɚɪ Ȼɚɣԕɚɥԑɚɧ ԧɡɝɟɪɿɫɬɟɪɞɿ ɚɧɵԕɬɚԙɞɚɪ ɦɵɫ
ɨɤɫɢɞɿɧɿԙ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪɭ ɦɟɧ ɫɭɞɵԙ ɬԛɡɿɥɭɿɧ
Ԕɵɡɞɵɪԑɚɧɞɵ ɬɨԕɬɚɬɵɩ ɫɭɬɟɤ ɠɿɛɟɪɿԙɞɟɪ ɦɵɫɬɵ ɬɨɬɵԕɬɵɪɭɞɚɧ ɫɚԕɬɚɭ
ԛɲɿɧ ɋɭɬɟɤɬɿԙ ɦɵɫ ɨɤɫɢɞɿɦɟɧ ԥɪɟɤɟɬɬɟɫɭ ɪɟɚɤɰɢɹ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
21-ɫɭɪɟɬ Ɇɨɥɟɤɭɥɚɥɵԕ ɫɭɬɟɤɬɿʃ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɿ
1-ɬԛɬɿɤɲɟ; 2-ɲɬɚɬɢɜ -ɫɩɢɪɬ ɲɚɦɵ - ɲɵɧɵ ɬԛɬɿɤɲɟ
41
42.
4-ɬԥɠɿɪɢɛɟ Ɇɨɥɟɤɭɥɚɥɵԕ ɩԥɧ ɚɬɨɦɞɵԕ ɫɭɬɟɤɬɿԙ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪɭԕɚɫɢɟɬɬɟɪɿ
ɩɪɨɛɢɪɤɚԑɚ ɦɥ ɫԝɣɵɬɵɥԑɚɧ H2SO4 ԕԝɣɵԙɞɚɪ ԛɫɬɿɧɟ ɛɿɪɧɟɲɟ
ɬɚɦɲɵ KMnO4 ɧɟɦɟɫɟ K2Cr2O7 ɟɪɿɬɿɧɞɿɥɟɪɿɧ ԕɨɫɵԙɞɚɪ Ȼɿɪ ɩɪɨɛɢɪɤɚԑɚ
Zn ɫɚɥɵԙɞɚɪ ɟɤɿɧɲɿɫɿɧɟ Ʉɢɩɩ ɚɩɩɚɪɚɬɵɧɚɧ ɫɭɬɟɤ ɠɿɛɟɪɿԙɞɟɪ
ɉɪɨɛɢɪɤɚɥɚɪɞɚ ԕɚɧɞɚɣ ԧɡɝɟɪɿɫɬɟɪ ɛɨɥɚɞɵ" ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ ɬԛɫɫɿɡ ɛɨɥɭɵ
ɠɵɥɞɚɦɞɵԑɵ ɛɿɪɞɟɣ ɛɨɥɚ ɦɚ" ȿɤɿɧɲɿ ɩɪɨɛɢɪɤɚɞɚ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲ
ɪɟɬɿɧɞɟ ɫɭɬɟɤ ɟɤɟɧɿɧ ɚɧɵԕɬɚԙɞɚɪ Ɉɥ ԛɲɿɧ ɛɿɪɿɧɲɿ ɩɪɨɛɢɪɤɚԑɚ
ɦɵɪɵɲɬɵԙ ԛɫɬɿɧɟ KMnO4 ɧɟɦɟɫɟ K2Cr2O7 ԕԝɣɵԙɞɚɪ Ɋɟɚɤɰɢɹ
ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵɩ ɬɨɬɵԑɭ-ɬɨɬɵԕɫɵɡɞɚɧɭ ɪɟɚɤɰɢɹɥɚɪɞɵԙ ɫɯɟɦɚɫɵɧ
ɤԧɪɫɟɬɿԙɞɟɪ
5-ɬԥɠɿɪɢɛɟ Ɉɬɬɟɤɬɿԙ ɚɥɵɧɭ ɠɨɥɞɚɪɵ ɠԥɧɟ ԕɚɫɢɟɬɬɟɪɿ
Ԕԝɪԑɚԕ ɩɪɨɛɢɪɤɚԑɚ ɲɚɦɚɞɚ ɝ KCȱO3 ɫɚɥɵԙɞɚɪ ɲɬɚɬɢɜɤɟ
ɛɟɤɿɬɿԙɞɟɪ ɫɩɢɪɬɨɜɤɚɦɟɧ ԕɵɡɞɵɪɵԙɞɚɪ Ɍԛɡɿɥɝɟɧ ɛɚɥԕɵɦɚɧɵԙ ԛɫɬɿɧɟ
ɤɚɬɚɥɢɡɚɬɨɪ MnO2 ± ԕɨɫɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ȼԧɥɿɧɿɩ ɲɵԕԕɚɧ ɝɚɡɞɵ
ɰɢɥɢɧɞɪɝɟ ɠɢɧɚԙɞɚɪ ɠԥɧɟ ɨɧɵԙ ɠɚɧɵɩ ɬԝɪԑɚɧ ɚԑɚɲ ɠɚԙԕɚɫɵɦɟɧ
ɚɧɵԕɬɚԙɞɚɪ Ԕɚɧɞɚɣ ɝɚɡ ɛԧɥɿɧɟɞɿ" Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
6-ɬԥɠɿɪɢɛɟ Ɉɬɬɟɤɬɿԙ ɚɥɵɧɭ ɠɨɥɞɚɪɵ ɠԥɧɟ ԕɚɫɢɟɬɬɟɪɿ
ɉɪɢɛɨɪɤɚԑɚ ɲɚɦɚɞɚ ɝ KMnO4 ɫɚɥɵԙɞɚɪ ԛɫɬɿɧɟ ɤɚɬɚɥɢɡɚɬɨɪ
(MnO2 ԕɨɫɵԙɞɚɪ ɛԧɥɿɧɝɟɧ ɨɬɬɟɤɬɿ ɰɢɥɢɧɞɪɝɟ ɠɢɧɚԙɞɚɪ ɠɚɧɵɩ ɬԝɪԑɚɧ
ɚԑɚɲ ɠɚԙԕɚɫɵɧ ɫɚɥɵԙɞɚɪ Ɉɫɵ ԕԝɛɵɥɵɫɬɚɪɞɵ ɬԛɫɿɧɞɿɪɿԙɞɟɪ ɠԥɧɟ
ɪɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
7-ɬԥɠɿɪɢɛɟ Ɉɬɬɟɤɬɿԙ ɬɨɬɵԕɬɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɬɟɪɿ
ɚ Ɍɟɦɿɪ ԕɚɫɵԕԕɚ ɤɿɲɤɟɧɬɚɣ ɤԛɤɿɪɬ ɤɪɢɫɬɚɥɵɧ ɠɚɧɞɵɪɵɩ ɨɬɬɟɤ
ɛɚɪ ɵɞɵɫԕɚ ɟɧɝɿɡɿɩ ɤԛɤɿɪɬɬɿԙ ɠɚɧɭɵɧɚ ɚɭɚ ɦɟɧ ɨɬɬɟɤ ԥɫɟɪɿɧ
ɛɚɣԕɚԙɞɚɪ Ʉԛɤɿɪɬ ɠɚɧɵɩ ɛɨɥԑɚɧ ɫɨԙ ɲɚɦɚɥɵ ɦԧɥɲɟɪɞɟ ɫɭ ԕɨɫɵԙɞɚɪ
ɇɟ ɛɚɣԕɚɣɫɵԙɞɚɪ"
ɛ Ɉɫɵ ɬԥɠɿɪɢɛɟɧɿ ԕɚɣɬɚɥɚԙɞɚɪ ɤԛɤɿɪɬɬɿԙ ɨɪɧɵɧɚ ԕɵɡɵɥ
ɮɨɫɮɨɪɞɵ ɩɚɣɞɚɥɚɧɵԙɞɚɪ Ɉɬɬɟɤ ɛɚɪ ɵɞɵɫԕɚ ɠɚɧɵɩ ɬԝɪԑɚɧ ɦɚɝɧɢɣ
ɥɟɧɬɚɫɵɧ ɫɚɥɵԙɞɚɪ Ɇɚɝɧɢɣ ɠɚɧɵɩ ɛɨɥԑɚɧ ɫɨԙ ɫɭ ԕɨɫɵԙɞɚɪ Ʉԛɤɿɪɬ
ɮɨɫɮɨɪ ɠԥɧɟ ɦɚɝɧɢɣ ɨɬɬɟɤɩɟɧ ԕɨɫɵɥɵɩ ԕɚɧɞɚɣ ԕɨɫɵɥɵɫɬɚɪ ɬԛɡɟɞɿ"
Ɉɥɚɪɞɵԙ ɠɚɧԑɚɧ ԕɨɫɵɥɵɫɬɚɪɵ ɫɭɦɟɧ ԥɪɟɤɟɬɬɟɫɤɟɧɞɟ ԕɚɧɞɚɣ ɡɚɬɬɚɪ
ɬԛɡɿɥɟɞɿ" ɍɧɢɜɟɪɫɚɥɞɵ ԕɚԑɚɡɛɟɧ ɨɪɬɚɧɵ ɚɧɵԕɬɚԙɞɚɪ Ɋɟɚɤɰɢɹɥɚɪɞɵԙ
ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
8-ɬԥɠɿɪɢɛɟ ɋɭɬɟɤ ɩɟɪɨɤɫɢɞɿɧɿԙ ɵɞɵɪɚɭɵ
ɉɪɨɛɢɪɤɚԑɚ - ɦɥ -ɬɿɤ ɇ2Ɉ2 ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ ɤɚɬɚɥɢɡɚɬɨɪ
(MnO2 ԕɨɫɵԙɞɚɪ ɋɭɬɟɤ ɩɟɪɨɤɫɢɞɿ ɵɞɵɪɚɣɞɵ ɝɚɡ ɨɬɬɟɤ ɛԧɥɿɧɿɩ
ɲɵԑɚɞɵ Ȼԧɥɿɧɿɩ ɲɵԑɵɩ ɠɚɬԕɚɧ ɨɬɬɟɤɬɿ ɠɚɧɵɩ ɬԝɪԑɚɧ ɚԑɚɲ ɠɚԙԕɚɫɵɦɟɧ
ɬɟɤɫɟɪɿԙɞɟɪ Ʉԧɪɝɟɧɞɟɪɿԙɞɿ ɬԛɫɿɧɞɿɪɿԙɞɟɪ ɠԥɧɟ ɪɟɚɤɰɢɹ ɬɟԙɞɟɭɿɧ
ɠɚɡɵԙɞɚɪ
42
43.
9-ɬԥɠɿɪɢɛɟ ɋɭɬɟɤ ɩɟɪɨɤɫɢɞɿɧɿԙ ɬɨɬɵԕɬɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɿɉɪɨɛɢɪɤɚԑɚ - ɦɥ ԕɨɪԑɚɫɵɧɧɵԙ ɬԝɡ ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ H2S
ԕɵɲԕɵɥɵɧ ԕɨɫɵԙɞɚɪ Ɍԛɡɿɥɝɟɧ ɬԝɧɛɚɧɵ ɮɢɥɶɬɪɞɟɧ ԧɬɤɿɡɿɩ ɫɭɦɟɧ ɠɭɵɩ
ɤɿɲɤɟɧɬɚɣ ɫɚԑɚɬ ɲɵɧɵɧɚ ɚɭɞɚɪɵɩ -ɬɿɤ ɇ2Ɉ2 ԕɨɫɵԙɞɚɪ Ԕɨɪԑɚɫɵɧ
ɫɭɥɶɮɢɞɿ ԕɨɪԑɚɫɵɧ ɫɭɥɶɮɚɬɵɧɚ ɬɨɬɵԑɚɞɵ Ɍԝɧɛɚɧɵԙ ɬԛɫɿ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ"
Ɋɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
ɛ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɦɟɧ ԕɵɲԕɵɥɞɚɧԑɚɧ - ɦɥ -ɬɿɤ ɇ2Ɉ2 ɟɪɿɬɿɧɞɿɫɿɧɟ
Kȱ ɟɪɿɬɿɧɞɿɫɿɧ ԕɨɫɵԙɞɚɪ Ɉɫɵ ɤԧɪɝɟɧɞɟɪɿԙɞɿ ɬԛɫɿɧɞɿɪɿԙɞɟɪ ɠԥɧɟ
ɪɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
10-ɬԥɠɿɪɢɛɟ ɋɭɬɟɤ ɩɟɪɨɤɫɢɞɿɧɿԙ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɿ
ɚ Ʉɚɥɢɣ ɩɟɪɦɚɧɝɚɧɚɬɵɧɵԙ ɟɪɿɬɿɧɞɿɫɿɧɟ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɦɟɧ
ԕɵɲԕɵɥɞɚɧԑɚɧ ɲɚɦɚɞɚ ɦɥ ɇ2Ɉ2 ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ Ɉɫɵɞɚɧ ɧɟ
ɛɚɣԕɚɥɚɞɵ ɫɨɧɵ ɠɚɡɵɩ ɬԛɫɿɧɞɿɪɿԙɞɟɪ
ɛ ɬɚɦɲɵ AgNO3 ɟɪɿɬɿɧɞɿɫɿɧɟ -ɬɿɤ NH4OH ɟɪɿɬɿɧɞɿɫɿɧ ɠԥɧɟ
3%-ɬɿɤ ɇ2Ɉ2 ɟɪɿɬɿɧɞɿɫɿɧ ԕɨɫɵԙɞɚɪ Ɉɫɵɞɚɧ ɧɟ ɛɚɣԕɚɥɚɞɵ"
Ɋɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ 112
Ɍɚԕɵɪɵɛɵ 9ȱȱ ɬɨɩɬɵԙ ɪ-ɷɥɟɦɟɧɬɬɟɪɿ Ƚɚɥɨɝɟɧɞɟɪ ɏɥɨɪ ɛɪɨɦ ɢɨɞ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵԙ ɦɚԕɫɚɬɵ Ƚɚɥɨɝɟɧɞɟɪɞɿԙ ɠԥɧɟ ԕɨɫɵɥɵɫɬɚɪɵɧɵԙ ɚɥɵɧɭ ɠɨɥɞɚɪɵɧ ɠԥɧɟ ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿɧ ɨԕɵɩ ԛɣɪɟɧɭ
Ȼɚԕɵɥɚɭ ɠԝɦɵɫɵɧɵԙ ɫԝɪɚԕɬɚɪɵ ɦɟɧ ɠɚɬɬɵԑɭɥɚɪɵ
1. 9ȱȱ-ɬɨɩɬɵԙ ɪ- ɷɥɟɦɟɧɬɬɟɪɿɧɿԙ ɠɚɥɩɵ ɫɢɩɚɬɬɚɦɚɫɵ
2. Ƚɚɥɨɝɟɧɞɟɪɞɿԙ ɚɬɨɦ ԕԝɪɵɥɵɫɵ ɷɥɟɤɬɪɨɧɞɵԕ ɮɨɪɦɭɥɚɥɚɪɵ Ɍɨɬɵԑɭ
ɞԥɪɟɠɟɥɟɪɿ
3. Ƚɚɥɨɝɟɧɞɟɪɞɿԙ ɬɚɛɢԑɚɬɬɚ ɬɚɪɚɥɭɵ ɚɥɵɧɭ ԥɞɿɫɬɟɪɿ
4. Ƚɚɥɨɝɟɧɞɟɪɞɿԙ ɮɢɡɢɤɚɥɵԕ ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿ
5. Ɇɵɧɚ ԕɚɬɚɪɞɚ ɚ F²Cl²Br²J- ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɬɟɪɿ
ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ" ɛ F2-Cl2-Br2-J2 ɬɨɬɵԕɬɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɿ ԕɚɥɚɣ
ԧɡɝɟɪɟɞɿ" ɨɥ ԧɡɝɟɪɿɫɬɟɪ ɧɟɝɟ ɛɚɣɥɚɧɵɫɬɵ"
6. Ƚɚɥɨɝɟɧɞɟɪɞɿԙ ɨɬɬɟɤɬɿ ԕɨɫɵɥɵɫɬɚɪɵ ɨɤɫɢɞɬɟɪ ɨԑɚɧ ɫԥɣɤɟɫ
ԕɵɲԕɵɥɞɚɪɵ Ԕɵɲԕɵɥɞɚɪɞɵԙ ɚɬɬɚɪɵ ɬԝɡɞɚɪɵ
7. Ɇɵɧɚ ԕɚɬɚɪɞɚ HClOoHClO2oHClO3oHClO4
HBrO oHBrO3
HJOoHJO3oHJO4
Ԕɵɲԕɵɥɞɚɪɞɵԙ ɤԛɲɿ ɛɟɪɿɤɬɟɝɿ ɬɨɬɵԕɬɵɪԑɵɲ-ɬɨɬɵԕɫɵɡɞɚɧɞɚɪԑɵɲ
ԕɚɫɢɟɬɬɟɪɿ ɫɨɥɞɚɧ ɨԙԑɚ ԕɚɪɚɣ ɠɨԑɚɪɵɞɚɧ ɬԧɦɟɧ ԕɚɪɚɣ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ"
43
44.
8. ȿɫɟɩ Ʉɨɧɰɟɧɬɪɚɰɢɹɫɵ ɦɨɥɶ ɥ ɬɟԙ ɯɥɨɪɥɵɥɚɭ ɇɋOɈ ԕɵɲԕɵɥɞɵԙɫɭɬɟɤɬɿɤ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵɧ ɠԥɧɟ ɫɭɬɟɤɬɿɤ ɤԧɪɫɟɬɤɿɲɿɧ ɪɇ
ɟɫɟɩɬɟԙɞɟɪ
9. ȿɫɟɩ Ɍɨɤ ɤԛɲɿ Ⱥ ɛɨɥԑɚɧɞɚ ɛɿɪ ɬԥɭɥɿɤɬɿԙ ɿɲɿɧɞɟ 1D&O ɟɪɿɬɿɧɞɿɫɿ
ɷɥɟɤɬɪɨɥɢɡɞɟɧɝɟɧɞɟ ɤɝ ɯɥɨɪ ɛԧɥɿɧɞɿ ȿɫɟɩɬɟԙɞɟɪ ɚ ɬɨɤ ɛɨɣɵɧɲɚ
ɯɥɨɪɞɵԙ ɲɵԑɵɦɵɧ ɛ ɯɥɨɪɦɟɧ ԕɚɬɚɪ ɛԧɥɿɧɿɩ ɲɵԕԕɚɧ 1D2+ ɩɟɧ ɇ2
ɦɚɫɫɚɥɚɪɵɧ
10.ȿɫɟɩ ɦɥ -ɬɿɤ .ɆQ24 ɟɪɿɬɿɧɞɿɫɿ S ɝ ɫɦ3 ԕɵɲԕɵɥ ɨɪɬɚɞɚ .-
ɟɪɿɬɿɧɞɿɫɿɦɟɧ ɪɟɚɤɰɢɹɥɚɫԕɚɧɞɚ ԕɚɧɲɚ ɝɪɚɦɦ ɢɨɞ ɛԧɥɿɧɟɞɿ"
11.ȿɫɟɩ Ȼɪɨɦ 1D2+ ɟɪɿɬɿɧɞɿɫɿɦɟɧ ԥɪɟɤɟɬɬɟɫɤɟɧɞɟ 1D%U ɠԥɧɟ 1D%U23
ɬԛɡɿɥɟɞɿ ɦ3 14%-ɬɿɤ 1D2CO3 ɟɪɿɬɿɧɞɿɫɿɦɟɧ S ɝ ɫɦ3 ԥɪɟɤɟɬɬɟɫɭ
ԛɲɿɧ ԕɚɧɲɚ ɤɝ ɛɪɨɦ ɠԝɦɫɚɥɚɞɵ ԕɚɧɲɚ ɤԧɥɟɦ ɋɈ2 ɛԧɥɿɧɿɩ ɲɵԑɚɞɵ"
12.ȿɫɟɩ ɦɥ -ɬɿɤ +- S ɝ ɫɦ3 ɟɪɿɬɿɧɞɿɫɿɧ ɬɨɬɵԕɬɵɪɭ ԛɲɿɧ ԕɚɧɲɚ
ɦɥ -ɬɿɤ +-23 S ɝ ɫɦ3 ɠԝɦɫɚɥɚɞɵ" Ԕɚɧɲɚ ɝɪɚɦɦ ɢɨɞ
ɛԧɥɿɧɟɞɿ"
13.Ɍԧɦɟɧɞɟɝɿ ɪɟɚɤɰɢɹɥɚɪɞɵ ɚɹԕɬɚɩ ɤɨɷɮɮɢɰɢɟɧɬɬɟɪɞɿ ԕɨɣɵԙɞɚɪ
ɬɟԙɟɫɬɿɪɿԙɞɟɪ
ɚ Cȱ2 ȱ2+H2Oo
ɛ NaCȱO+MnO2+NaOHo
ɜ KCȱO3+MnO3+KOHo
o
ɝ KCȱO3
ɞ) KNO2 +ȱ .2SO4o
ɟ .ȱ23 .ȱ .2SO4o
ɡ +2O2 .ȱo
ɠ) K2Cr2O7 .ȱ +2SO4o
t
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɬԥɠɿɪɢɛɟɥɟɪ
Ⱥɩɩɚɪɚɬɬɚɪ ɠԥɧɟ ɵɞɵɫɬɚɪ: Ɉɬɬɟɤɩɟɧ ɬɨɥɬɵɪɵɥԑɚɧ ɝɚɡɨɦɟɬɪ
Ʉɢɩɩɚɩɩɚɪɚɬɵ ԕɵɫԕɵɲɵ ɛɚɪ ɲɬɚɬɢɜ ɠɚɧɞɵɪԑɵɲ ɫɵɣɵɦɞɵɥɵԑɵ
100- ɦɥ - ɦɥ ɲɵɧɵ ɰɢɥɢɧɞɪɥɟɪ ɤɪɢɫɬɚɥɥɢɡɚɬɨɪ
ɫɵɣɵɦɞɵɥɵԑɵ - ɦɥ ɫɬɚԕɚɧɞɚɪ ɲɵɧɵ ɩɥɚɫɬɢɧɤɚɥɚɪ ɩɪɨɛɢɪɤɚɥɚɪ
ɫɚɥɵɧԑɚɧ ɲɬɚɬɢɜ
Ɋɟɚɤɬɢɜɬɟɪ ɠԥɧɟ ɦɚɬɟɪɢɚɥɞɚɪ Ɇɵɪɵɲ ɬԛɣɿɪɲɿɤɬɟɪɿ ɚɥɸɦɢɧɢɣ
ɤɟɫɿɧɞɿɥɟɪɿ ɦɵɫ ȱȱ ɨɤɫɢɞɿ &X2 ɫԛɥɝɿ Ʉԛɤɿɪɬ ɦɚɝɧɢɣ ɥɟɧɬɚɫɵ ɚԑɚɲ
ɤԧɦɿɪ ɬԛɣɿɪɿ ԕɵɡɵɥ ɮɨɫɮɨɪ ɬɟɦɿɪ ɫɵɦɵ ɧɚɬɪɢɣ ɩɟɪɨɤɫɢɞɿ ɦɚɪɝɚɧɟɰ
ȱ9 ɨɤɫɢɞɿ ɛɚɪɢɣ ɩɟɪɨɤɫɢɞɿ ɤɚɥɢɣ ɯɥɨɪɚɬɵ ɤɚɥɢɣ ɧɢɬɪɚɬɵ
ɞɢɷɬɢɥɷɮɢɪ ɥɚɤɦɭɫ ԕɚԑɚɡɵ ԕɚɪ ɧɟɦɟɫɟ ɦԝɡ ɫԛɡɝɿ ԕɚԑɚɡɵ ɚԑɚɲ
ɠɚԙԕɚɥɚɪɵ
ȿɪɿɬɿɧɞɿɥɟɪ Ʉԛɤɿɪɬ ԕɵɲԕɵɥɵ +2SO4 ɤԛɣɞɿɪɝɿɲ ɧɚɬɪ 1D2+
ɤɚɥɢɣ ɩɟɪɦɚɧɝɚɧɚɬɵ .0Q24 (0, ɧ ɋɭɬɟɤ ɩɟɪɨɤɫɢɞɿ ɠԥɧɟ
ɤԛɤɿɪɬ ԕɵɲԕɵɥɵ ɧ ɚɦɦɢɚɤ ɧ ɛɚɪɢɣ ɝɢɞɪɨɤɫɢɞɿ ԕɚɧɵԕ ɧɚɬɪɢɣ
ɝɢɞɪɨɤɫɢɞɿ 0, ɧ ɤɚɥɢɣ ɢɨɞɢɞɿ , ɧ ɤɚɥɢɣ ɞɢɯɪɨɦɚɬɵ , ɧ ɤɚɥɢɣ
ɩɟɪɦɚɧɝɚɧɚɬɵ ɤɨɧɰ ɠԥɧɟ ɧ ɦɚɪɝɚɧɟɰ ɫɭɥɶɮɚɬɵ , ɧ ɧɚɬɪɢɣ
44
45.
ɫɭɥɶɮɢɞɿ , ɧ ɤԛɦɿɫ ɧɢɬɪɚɬɵ , ɧ ԕɨɪԑɚɫɵɧ ɧɢɬɪɚɬɵ ɧ ɯɪɨɦȱȱȱ ɧɢɬɪɚɬɵ ɧ ɯɪɨɦ ȱȱȱ ɫɭɥɶɮɚɬɵ ɧ ɤɪɚɯɦɚɥ ɤɥɟɣɫɬɟɪɿ
ɥɚɤɦɭɫ ɮɟɧɨɥɮɬɚɥɟɢɧ
1-ɬԥɠɿɪɢɛɟ Ɍɟɦɿɪ ȱȱ ɢɨɧɵɧɵԙ ɯɥɨɪɦɟɧ ɬɨɬɵԑɭɵ
ɫɬɚɤɚɧԑɚ ɬɟɦɿɪ ȱȱ ɫɭɥɶɮɚɬɵ ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ ɛɿɪɿɧɲɿ
ɫɬɚɤɚɧԑɚ ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭ ɟɤɿɧɲɿɫɿɧɟ-ɯɥɨɪ ɫɭɵɧ ɠԥɧɟ ɟɤɟɭɿɧɟ ɞɟ ɚɦɦɢɚɤ
ɟɪɿɬɿɧɞɿɫɿɧ ԕɨɫɵԙɞɚɪ Ȼɿɪɿɧɲɿ ɫɬɚɤɚɧɞɚ ɬɟɦɿɪ ȱȱ ɟɤɿɧɲɿɫɿɧɞɟ ɬɟɦɿɪ ȱȱȱ
ɝɢɞɪɨɤɫɢɞɬɟɪɿ ɬԛɡɿɥɟɞɿ Ɍԝɧɛɚɧɵԙ ɬԛɫɿɧ ɛɚɣԕɚԙɞɚɪ Ɋɟɚɤɰɢɹ ɬɟԙɞɟɭɥɟɪɿɧ
ɠɚɡɵԙɞɚɪ
2-ɬԥɠɿɪɢɛɟ ɇɟɝɿɡɞɿɤ ɨɪɬɚɞɚ ɯɪɨɦ ȱȱȱ ɢɨɧɵɧɵԙ ɯɥɨɪ ɫɭɵɦɟɧ
ɬɨɬɵԑɭɵ
ɉɪɨɛɢɪɤɚԑɚ - ɬɚɦɲɵ ɯɪɨɦ ȱȱȱ ɫɭɥɶɮɚɬɵ ɟɪɿɬɿɧɞɿɫɿɧ
ɬɚɦɲɵɥɚɬɵɩ ԛɫɬɿɧɟ ɤɚɥɢɣ ɝɢɞɪɨɤɫɢɞɿɧɿԙ ɠԥɧɟ ɯɥɨɪ ɫɭɵɧ ԕԝɣɵԙɞɚɪ
ɉɪɨɛɢɪɤɚɧɵ ԕɵɡɞɵɪɵԙɞɚɪ ɟɪɿɬɿɧɞɿɧɿԙ ɬԛɫɿ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ" Ɍɨɬɵԑɭɬɨɬɵԕɫɵɞɚɧɭ ɪɟɚɤɰɢɹɫɵɧɵԙ ɬɟԙɞɟɭɿɧ ԕԝɪɚɫɬɵɪɵԙɞɚɪ
3-ɬԥɠɿɪɛɢɟ Ƚɚɥɨɝɟɧɞɟɪɞɿԙ ɬɨɬɵԕɬɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɿ
ɩɪɨɛɢɪɤɚԑɚ - ɬɚɦɲɵɞɚɧ ɤԛɤɿɪɬɬɿ ɫɭɬɟɤ ɫɭɵɧ ԕԝɣɵԙɞɚɪ
ɛɿɪɿɧɲɿɫɿɧɟ ± ɯɥɨɪ ɫɭɵɧ ɟɤɿɧɲɿɫɿɧɟ- ɛɪɨɦ ɫɭɵɧ ԛɲɿɧɲɿɫɿɧɟ ± ɢɨɞ ɫɭɵɧ
ɟɪɿɬɿɧɞɿ ɥɚɣɥɚɧԑɚɧɲɚ ԕɨɫɵԙɞɚɪ Ɍɨɬɵԑɭ ±ɬɨɬɵԕɫɵɡɞɚɧɭ ɪɟɚɤɰɢɹɥɚɪɵɧɵԙ
ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
4-ɬԥɠɿɪɢɛɟ Ȼɪɨɦɧɵԙ ɚɥɵɧɭɵ
ɉɪɨɛɢɪɤɚԑɚ 1D%U ɦɟɧ 0Q22 ɤɪɢɫɬɚɥɞɚɪɵɧ ɫɚɥɵԙɞɚɪ ԛɫɬɿɧɟɧ - ɬɚɦɲɵ
ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɧ ԕɨɫɵԙɞɚɪ Ȼԧɥɿɧɝɟɧ ɛɪɨɦɧɵԙ ɛɭɵɧɵԙ
ɬԛɫɿɧ ɛɚɣԕɚԙɞɚɪ Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
5-ɬԥɠɿɪɢɛɟ Ʉɚɥɢɣ ɢɨɞɢɞɿɧɿԙ ɬɨɬɵԑɭɵ
1- ɦɥ .ȱ ɟɪɿɬɿɧɞɿɫɿɧ ɩɪɨɛɢɪɤɚԑɚ ԕԝɣɵɩ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɧ ɠԥɧɟ
3%-ɬɿɤ ɇ2Ɉ2 ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ɋɟɚɤɰɢɹ ɬɟԙɞɟɭɿɧ
ɠɚɡɵɞɚɪ
6-ɬԥɠɿɪɢɛɟ ɂɨɞɬɵԙ ɚɥɵɧɭ ɠɨɥɵ
.ȱ ɤɪɢɫɬɚɥɵɧɚ ɦɚɪɝɚɧɟɰɬɿԙ ȱ9 ɬɨɬɵԑɵɧ ԕɨɫɵɩ ԕɨɫɩɚɧɵ
ɩɪɨɛɢɪɤɚԑɚ ɚɭɞɚɪɵɩ ɫɚɥɵԙɞɚɪ ɛɿɪɧɟɲɟ ɬɚɦɲɵ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ ɤԛɤɿɪɬ
ԕɵɲԕɵɥɵɧ ԕԝɣɵԙɞɚɪ ԕɵɡɞɵɪɵԙɞɚɪ ɂɨɞɬɵԙ ɛԧɥɿɧɝɟɧɿɧ ɛɚɣԕɚԙɞɚɪ
Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
7-ɬԥɠɿɪɢɛɟ ɂɨɞɬɵԙ ԕɚɫɢɟɬɬɟɪɿ
Ԕԝɪԑɚԕ ɩɪɨɛɢɪɤɚԑɚ ɢɨɞɬɵԙ ɤɪɢɫɬɚɥɵɧ ɫɚɥɵԙɞɚɪ ɲɚɦɚɥɵ
ԕɵɡɞɵɪɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" ɂɨɞɬɵԙ ɛɭɥɚɧɭɵɧ ɛɚɣԕɚԙɞɚɪ
8-ɬԥɠɿɪɢɛɟ ɂɨɞɬɵԙ ɫɭɞɚ ɟɪɿɝɿɲɬɿɝɿ
ɉɪɨɛɢɪɤɚԑɚ - ɦɥ ɫɭ ԕԝɣɵԙɞɚɪ ɢɨɞɬɵԙ ɤɪɢɫɬɚɥɞɚɪɵɧ ɫɚɥɵԙɞɚɪ
ɩɪɨɛɢɪɤɚɧɵ ɲɚɣԕɚԙɞɚɪ ȿɪɿɬɿɧɞɿɧɿԙ ɬԛɫɿ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ" ɂɨɞ ɫɭɞɚ ԕɚɥɚɣ
45
46.
ɟɪɢɞɿ" ɋɨɥ ɩɪɨɛɢɪɤɚԑɚ .ȱ ɟɪɿɬɿɧɞɿɫɿɧ ԕɨɫɵԙɞɚɪ ɂɨɞɬɵԙ ɟɪɿɝɿɲɬɿɝɿ .ȱ3ɤɨɦɩɥɟɤɫɬɿ ԕɨɫɵɥɵɫ ɬԛɡɭ ɚɪԕɵɥɵ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ"
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ 113
Ɍɚԕɵɪɵɛɵ 9ȱ ɬɨɩ ɪ-ɷɥɟɦɟɧɬɬɟɪɿ ɏɚɥɶɤɨɝɟɧɞɟɪ
ɤԛɤɿɪɬ ɫɟɥɟɧ ɬɟɥɥɭɪ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɫɚɛɚԕɬɵԙ ɦɚԕɫɚɬɵ Ʉԛɤɿɪɬ ɤԛɤɿɪɬɬɿ ɫɭɬɟɤ ɩɟɧ
ɫɭɥɶɮɢɞɬɟɪɞɿԙ ɚɥɵɧɭ ɠɨɥɞɚɪɵɧ ɠԥɧɟ ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿɧ ɨԕɵɩ
ԛɣɪɟɧɭ Ʉԛɤɿɪɬɬɿԙ ɨɬɬɟɤɬɿ ԕɨɫɵɥɵɫɬɚɪɵɧɵԙ ɚɥɵɧɭ ɠɨɥɞɚɪɵɧ ɠԥɧɟ
ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿɧ ɨԕɵɩ ԛɣɪɟɧɭ.
Ȼɚԕɵɥɚɭ ɠԝɦɵɫɵɧɵԙ ɫԝɪɚԕɬɚɪɵ ɦɟɧ ɠɚɬɬɵԑɭɥɚɪɵ
1. Ʉԛɤɿɪɬ ɬɨɩɲɚɫɵɧɞɚԑɵ ɷɥɟɦɟɧɬɬɟɪɞɿԙ ɠɚɥɩɵ ɫɢɩɚɬɬɚɦɚɫɵ
2. Ʉԛɤɿɪɬ ɫɟɥɟɧ ɬɟɥɥɭɪ ɚɬɨɦɞɚɪɵɧɵԙ ɷɥɟɤɬɪɨɧɞɵԕ ԕԝɪɵɥɵɫɵ
Ԕɚɥɵɩɬɵ ɠԥɧɟ ԕɨɡԑɚɧ ɤԛɣɞɟɝɿ ɬɨɬɵԑɭ ɞԥɪɟɠɟɥɟɪɿ
3. Ʉԛɤɿɪɬɬɿԙ ɬɚɛɢԑɚɬɬɚ ɬɚɪɚɥɭɵ ɚɥɵɧɭ ɠɨɥɞɚɪɵ ԕɨɥɞɚɧɵɥɭɵ S8
ɦɨɥɟɤɭɥɚɫɵɧɵԙ ԕԝɪɵɥɵɫɵ
4. Ʉԛɤɿɪɬɬɿԙ ɫɟɥɟɧɧɿԙ ɬɟɥɥɭɪɞɵԙ ɮɢɡɢɤɚɥɵԕ ɠԥɧɟ ɯɢɦɢɹɥɵԕ
ԕɚɫɢɟɬɬɟɪɿ Ԕɵɲԕɵɥɞɵԕ ɠԥɧɟ ɧɟɝɿɡɞɿɤ ԕɚɫɢɟɬɬɟɪɿ ɦɵɧɚ ԕɚɬɚɪɞɚ SO2SeO2-TeO2 ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ" Ԕɵɲԕɵɥɞɚɪɞɵԙ ɤԛɲɿ ɦɵɧɚ ԕɚɬɚɪɞa:
H2SO3-H2SeO3-H2TeO3 ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ" Ɉɥ ɧɟɝɟ ɛɚɣɥɚɧɵɫɬɵ"
5. Ʉԛɤɿɪɬɬɿ ɫɭɬɟɤ ɚɥɵɧɭ ɠɨɥɞɚɪɵ ɦɨɥɟɤɭɥɚɫɵɧɵԙ ԕԝɪɵɥɵɫɵ ɠԥɧɟ
ɯɢɦɢɹɥɵԕ ɛɚɣɥɚɧɵɫɵ Ȼɟɪɿɤɬɿɝɿ ɠԥɧɟ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɿ
ɦɵɧɚ ԕɚɬɚɪɞɚ H2S-H2Se-H2Te ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ"
6. ɋɭɥɶɮɢɞɬɟɪ ɚɥɵɧɭ ɠɨɥɞɚɪɵ Ԕɵɲԕɵɥɞɚɪɞɚ ɟɪɢɬɿɧ ɠԥɧɟ ɟɪɿɦɟɣɬɿɧ
ɫɭɥɶɮɢɞɬɟɪ ɋɭɥɶɮɢɞɬɟɪɞɿԙ ɝɢɞɪɨɥɢɡɿ Ɇɵɫɚɥ ɤɟɥɬɿɪɿԙɞɟɪ
7. Ɍԧɦɟɧɞɟɝɿ ɪɟɚɤɰɢɹɥɚɪɞɵ ɚɹԕɬɚԙɞɚɪ ɠԥɧɟ ɢɨɧɞɵԕ ɠɚɪɬɵɥɚɣ ɢɨɧɞɵԕ
ɠԥɧɟ ɷɥɟɤɬɪɨɧɞɵԕ-ԥɞɿɫ ɚɪԕɵɥɵ ɬɟԙɟɫɬɿɪɿԙɞɟɪ
ɚ) S+NaOHo
ɛ) S+H2SO4 (k) o
ɜ) H26 &ȱ2+ H2Oo
ɝ) H2S+K2Cr2O7 + H2SO4o
d) H2S+KMnO4+H2SO4o
e) H2S+H2SO4 (k) o
8. ȿɫɟɩ. Ʉԛɤɿɪɬ ԕɵɲԕɵɥɵɦɟɧ ԕɵɲԕɵɥɞɚɧԑɚɧ 0,316 ɝ ɤɚɥɢɣ ɩɟɪɦɚɧɝɚɧɚɬ
ɟɪɿɬɿɧɞɿɫɿɧɟ ɤԛɤɿɪɬɬɿ ɫɭɬɟɤ ɟɪɿɬɿɧɞɿɫɿɧ ԕɨɫԕɚɧ. Ʉԛɤɿɪɬɬɿ ɫɭɬɟɤ ɬɟɦɿɪ
ɫɭɥɶɮɢɞɿɧɟɧ ɠԥɧɟ ԕɵɲԕɵɥɞɚɧ ɬԛɡɿɥɝɟɧ. Ԕɚɧɲɚ ɝɪɚɦɦ ɬɟɦɿɪ ɫɭɥɶɮɢɞɿ
ɠԝɦɫɚɥԑɚɧ?
46
47.
9. Ɍԧɦɟɧɞɟɝɿ ɬԝɡɞɚɪ ɟɪɿɬɿɧɞɿɫɿɧɿԙ: ɚ)Na26 ɛ 1+4)2S ɜ)NaHS ɨɪɬɚɫɵɧɚɧɵԕɬɚԙɞɚɪ.
10.ȿɫɟɩ. 700ɋ 96 ɤɉȺ ԕɵɫɵɦɞɚ, ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɦɟɧ ԕɵɲԕɵɥɞɚɧԑɚɧ,
400ɦɥ 6%-ɬɿɤ KMnO4 ɟɪɿɬɿɧɞɿɫɿɧ (ɪ=1,04 ɝ/ɫɦ3) ɬɨɥɵԕ
ɬɨɬɵԕɫɵɡɞɚɧɞɵɪɭ ԛɲɿɧ, ԕɚɧɲɚ ɤԧɥɟɦ H2S ɠԝɦɫɚɥɚɞɵ?
11.ȿɫɟɩ.
500ɦɥ
K2Cr2O7
ɟɪɿɬɿɧɞɿɫɿɧ
ԕɵɲԕɵɥɞɵԕ
ɨɪɬɚɞɚ
0
ɬɨɬɵԕɫɵɡɞɚɧɞɵɪɭ ԛɲɿɧ, 500 ɦɥ H2S ɠԝɦɫɚɥɞɵ (0 ɋ ɠԥɧɟ 101,3 Ʉɩɚ).
ȿɪɿɬɿɧɞɿɧɿԙ ɧɨɪɦɚɥɶɞɵԕ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵɧ ɟɫɟɩɬɟԙɞɟɪ
12.ȿɫɟɩ ɦɥ -ɬɿɤ ɧɚɬɪɢɣ ɝɢɞɪɨɤɫɢɞɿ ɟɪɿɬɿɧɞɿɫɿɧɟɧ ɥ
ɤԛɤɿɪɬɬɿ ɫɭɬɟɤ ԧɬɤɿɡɿɥɟɞɿ Ԕɚɧɞɚɣ ɬԝɡ ԕɚɧɲɚ ɦԧɥɲɟɪɞɟ ɬԛɡɿɥɝɟɧ"
13.ȿɫɟɩ ɦɵɫ ɯɥɨɪɢɞɿɧɿԙ ɟɪɿɬɿɧɞɿɫɿɧɟɧ ɚɪɬɵԕ ɦԧɥɲɟɪɞɟ H2S
ɟɪɿɬɿɧɞɿɫɿɧ ԧɬɤɿɡɝɟɧɞɟ ɝ ɬԝɧɛɚ ɬԛɡɿɥɞɿ Ɇɵɫ ɯɥɨɪɢɞɿ ɟɪɿɬɿɧɞɿɫɿɧɿԙ
ɩɪɨɰɟɧɬɬɿɤ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵɧ ɚɧɵԕɬɚԙɞɚɪ
14.Ʉԛɤɿɪɬ ȱV ɨɤɫɢɞɿ ɚɥɵɧɭ ɠɨɥɞɚɪɵ ɦɨɥɟɤɭɥɚɫɵɧɵԙ ԕԝɪɵɥɵɫɵ
ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɿ Ʉԛɤɿɪɬɬɿ ԕɵɲԕɵɥ ɚɥɵɧɭɵ ԕԝɪɵɥɵɫɵ ɨɧɵԙ
ɬԝɡɞɚɪɵ ɫɭɥɶɮɢɞɬɟɪɞɿԙ ɬɨɬɵԕɬɵɪԑɵɲɬɵԕ-ɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ
ԕɚɫɢɟɬɬɟɪɿ Ɇɵɫɚɥ ɤɟɥɬɿɪɿԙɞɟɪ
15.Ʉԛɤɿɪɬ ɨɤɫɢɞɿ Vȱ ɚɥɵɧɭ ɠɨɥɞɚɪɵ ɦɨɥɟɤɭɥɚɫɵɧɵԙ ԕԝɪɵɥɵɫɵ
Ʉԛɤɿɪɬ ԕɵɲԕɵɥɵ ɨɧɵԙ ɥɚɛɨɪɚɬɨɪɢɹɞɚ ɠԥɧɟ ԧɧɞɿɪɿɫɬɟ ɚɥɵɧɭ
ɠɨɥɞɚɪɵ Ʉԛɤɿɪɬ ԕɵɲԕɵɥɞɵԙ ɬɨɬɵԕɬɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɿ ɋɭɥɶɮɚɬɬɚɪ
16.Ɍԧɦɟɧɞɟɝɿ ɪɟɚɤɰɢɹɥɚɪɞɵ ɚɹԕɬɚɩ ɢɨɧɞɵԕ-ɷɥɟɤɬɪɨɧɞɵԕ ԥɞɿɫɩɟɧ
ɬɟԙɟɫɬɿɪɿԙɞɟɪ
ɚ) Te + H2SeO4 o
ɛ 1D2SO3+H2O2 o
ɜ H2SO4(k) + Kȱ o
ɝ Te+HCȱO3 + H2O o ɇ2ɌɟɈ6 «
ɞ .0Q24+SO2+H2O o
ɟ .0Q24 + Na2SO3+NaOH o
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɬԥɠɿɪɢɛɟɥɟɪ
Ⱥɩɩɚɪɚɬɬɚɪ ɠԥɧɟ ɵɞɵɫɬɚɪ ɬɟɯɧɢɤɚɥɵԕ ɬɚɪɚɡɵ Ʉɢɩɩ ɚɩɩɚɪɚɬɵ
ԕɵɫԕɵɲɵ ɛɚɪ ɲɬɚɬɢɜ ԕɢɵɧ ɛɚɥԕɢɬɵɧ ɬԛɬɿɤ ɬԛɬɿɝɿ ɛɚɪ ɬɵԑɵɧɞɚɪ ɪɟɡɢɧɚ
ɬԛɬɿɤɬɟɪɿ ɠɚɧɞɵɪԑɵɲ ɮɚɪɮɨɪ ɤɟɥɿɫɿ ɮɚɪɮɨɪ ɬɨɫɬɚԑɚɧɲɚɫɵ ԕɚԕɩɚԕɬɵ
ɮɚɪɮɨɪ ɬɢɝɟɥɿ ɫɵɣɵɦɞɵɥɵԑɵ ɦɥ ɤɨɥɛɚ ɫɵɣɵɦɞɵɥɵԑɵ ɦɥ
ɫɬɚԕɚɧ ɲɵɧɵ ɛɚɧɤɚɫɵ ɤɪɢɫɬɚɥɥɢɡɚɬɨɪ ɜɨɪɨɧɤɚ ɩɪɨɛɢɪɤɚɥɚɪɵ ɛɚɪ
ɲɬɚɬɢɜ ɮɚɪɮɨɪ ԛɲɛԝɪɵɲɵ ɬɢɝɟɥ ԕɵɫԕɵɲɵ ɥɭɩɚ ɚɫɛɟɫ ɬɨɪɵ Ȼɭɧɡɟɧ
ɤɨɥɛɚ ɰɢɥɢɧɞɪɥɟɪ ɬɚɦɲɵɥɚɬԕɵɲ ɜɨɪɨɧɤɚ ɬɟɦɿɪ ԕɚɫɵԕɲɚɫɵ
ɬɟɪɦɨɦɟɬɪ -100ɨɋ
Ɋɟɚɤɬɢɜɬɟɪ ɠԥɧɟ ɦɚɬɟɪɢɚɥɞɚɪ Ʉԛɤɿɪɬ ɬɟɦɿɪ ɦɵɫ ɦɚɝɧɢɣ ɦɵɪɵɲ
ɤԧɦɿɪ ɦɚɝɧɢɣ ɨɤɫɢɞɿ ɮɨɫɮɨɪ 9 ɨɤɫɢɞɿ ɩɢɪɢɬ ɧɚɬɪɢɣ ɫɭɥɶɮɢɬɿ
ɧɚɬɪɢɣ ɯɥɨɪɢɞɿ ɬɟɦɿɪ ȱȱ ɫɭɥɶɮɚɬɵ ɤɚɥɢɣ ɢɨɞɢɞɿ ɦɵɫ
ɫɭɥɶɮɚɬɵ ɤɪɢɫɬɚɥɥɨɝɢɞɪɚɬɵ ɧɚɬɪɢɣ ɫɭɥɶɮɚɬɵ ɧɚɬɪɢɣ ɬɢɨɫɭɥɶɮɚɬɵ
47
48.
ɫɚɯɚɪ ɋ12ɇ22Ɉ11 ɫɩɢɪɬ ԕɚɪ ɧɟɦɟɫɟ ɦԝɡ ɥɚɤɦɭɫ ԕɚԑɚɡɵ ɫԛɡɝɿ ԕɚԑɚɡɵɦɚԕɬɚ ԛɥɤɟɧ ɬɵԑɵɧ
ȿɪɿɬɿɧɞɿɥɟɪ Ɍԝɡ ԕɵɲԕɵɥɵ ɧ ɠԥɧɟ ɫԝɣɵɬ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵ
ɧ ɬɵԑɵɡɞɵԕɬɚɪɵ ɠԥɧɟ ɚɡɨɬ ԕɵɲԕɵɥɵ ɬɵԑɵɡɞɵԑɵ
ɚɦɦɨɧɢɣ ɫɭɥɶɮɢɞɿ ɧ ɄɆɩɈ4 ɧ Ʉ2ɋU2Ɉ7 ɧ ɛɚɪɢɣ ɦɵɪɵɲ
ɚɥɸɦɢɧɢɣ ɦɚɪɝɚɧɟɰ ɤɚɞɦɢɣ ԕɨɪԑɚɫɵɧ ɠԥɧɟ ɦɵɫ ɬԝɡɞɚɪɵ ɮɭɤɫɢɧ
ɥɚɤɦɭɫ
1-ɬԥɠɿɪɢɛɟ Ɇɨɧɨɤɥɢɧɞɿ ɤԛɤɿɪɬɬɿ ɚɥɭ
Ȼɚɪɥɵԕ ɬԥɠɿɪɢɛɟɥɟɪɞɿ ɚɭɚ ɫɨɪԑɵɲ ɲɤɚɮɬɵԙ ɚɫɬɵɧɞɚ ɿɫɬɟԙɞɟɪ
Ɏɚɪɮɨɪ ɬɢɝɟɥɿɧɞɟ ɵɞɵɫɵɧɞɚ ɤԛɤɿɪɬɬɿԙ ԝɧɬɚԑɵɧ ɛɚɥԕɵɬɵԙɞɚɪ ɋɨɞɚɧ
ɤɟɣɿɧ ɨɧɵ ɫɭɵɬɵԙɞɚɪ ɤɪɢɫɬɚɥɞɵ ԕɚɛɵɪɲɚԕɬɚɪɵɧɵԙ ɬԛɡɿɥɭɿɧ ɛɚɣԕɚԙɞɚɪ
Ɍɨɥɵԕ ɫɭɵɦɚԑɚɧ ɫɭɵ ɛɚɪ ɫɬɚɤɚɧԑɚ ԕԝɣɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ"
Ɇɨɧɨɤɥɢɧɞɿ ɤԛɤɿɪɬ ԕɚɧɞɚɣ ɬԛɪɝɟ ɚɭɵɫɚɞɵ"
2-ɬԥɠɿɪɢɛɟ Ʉԛɤɿɪɬɬɿԙ ɦɟɬɚɥɦɟɧ ԥɪɟɤɟɬɬɟɫɭɿ
Ɍɟɯɧɢɤɚɥɵԕ ɬɚɪɚɡɵɞɚ ԝɧɬɚԕ ɬԛɪɿɧɞɟ ɚɥɵɧԑɚɧ ɝ ɤԛɤɿɪɬ ɠԥɧɟ
ɚɥɸɦɢɧɢɣɞɿ ԧɥɲɟԙɞɟɪ ɚɪɚɥɚɫɬɵɪɵԙɞɚɪ ɞɚ ɚɫɛɟɫɬ ɧɟɦɟɫɟ ɦɟɬɚɥɥ
ɩɥɚɫɬɢɧɤɚɫɵɧɚ ɫɚɥɵԙɞɚɪ ԕɵɡɞɵɪԑɚɧ ɲɵɧɵ ɬɚɹԕɩɟɧ ɛɿɪ ɲɟɬɿɧɟɧ
ɚɪɚɥɚɫɬɵɪɵԙɞɚɪ Ȼɚɣԕɚԑɚɧ ԧɡɝɟɪɿɫɬɟɪɞɿ ɬԛɫɿɧɞɿɪɿԙɞɟɪ Ɋɟɚɤɰɢɹɧɵԙ
ɬɟԙɞɟɭɿɧ ɠɡɵԙɞɚɪ
3-ɬԥɠɿɪɢɛɟ Ʉԛɤɿɪɬɬɿԙ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɿ
ɉɪɨɛɢɪɤɚԑɚ - ɦɥ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ ɚɡɨɬ ԕɵɲԕɵɥɵɧ ԕԝɣɵԙɞɚɪ
ɤԛɤɿɪɬ ԕɨɫɵԙɞɚɪ ԕɚɬɬɵ ԕɵɡɞɵɪɵԙɞɚɪ Ɋɟɚɤɰɢɹ ɧԥɬɢɠɟɫɿɧɞɟ ɚɡɨɬ
ԕɵɲԕɵɥɵ ɚɡɨɬ ɨɤɫɢɞɿɧɟ ȱȱ ɬɨɬɵԕɫɵɡɞɚɧɚɞɵ ɤԛɤɿɪɬ ɫɭɥɶɮɚɬ ɢɨɧɵɧɚ
ɞɟɣɿɧ ɬɨɬɵԑɚɞɵ Ɉɧɵ ɚɧɵԕɬɚɭ ԛɲɿɧ %D&ȱ2 ɟɪɿɬɿɧɞɿɫɿɧ ԕɨɫɵԙɞɚɪ
Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
4-ɬԥɠɿɪɢɛɟ Ʉԛɤɿɪɬɬɿ ɫɭɬɟɤ ɚɥɭ ɠɨɥɵ
Ƚɚɡ ԧɬɤɿɡɟɬɿɧ ɬԛɬɿɤɲɟɫɿ ɛɚɪ ɩɪɨɛɢɪɤɚԑɚ ɬɟɦɿɪ ɫɭɥɶɮɢɞɿɧɿԙ 3-4
ɬԛɣɿɪɲɿɝɿɧ ɫɚɥɵԙɞɚɪ - ɦɥ ɫԝɣɵɬɵɥԑɚɧ ɬԝɡ ԕɵɲԕɵɥɵɧɵԙ ɟɪɿɬɿɧɞɿɫɿɧ
ԕԝɣɵԙɞɚɪ ɬɵԑɵɧɦɟɧ ɠɚɛɵԙɞɚɪ Ȼԧɥɿɧɿɩ ɲɵԕԕɚɧ ɝɚɡɞɵ ɠɚɧɞɵɪɵԙɞɚɪ
Ɍɢɝɟɥɶɞɿԙ ԕɚԕɩɚԑɵɧ ɠɚԕɵɧɞɚɬɵɩ ɬԛɡɿɥɝɟɧ ɫɚɪɵ ɬԛɫɬɿ ԕɨɫɵɥɵɫɬɵ
ɛɚɣԕɚԙɞɚɪ Ȼɚɣԕɚԑɚɧ ԕԝɛɵɥɵɫɬɚɪɞɵ ɬԛɫɿɧɞɿɪɿԙɞɟɪ Ɋɟɚɤɰɢɹɥɚɪɞɵԙ
ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
5-ɬԥɠɿɪɢɛɟ Ʉԛɤɿɪɬɬɿ ɫɭɬɟɤ ɟɪɿɬɿɧɞɿɫɿɧɿԙ ԕɵɲԕɵɥɞɵԕ ԕɚɫɢɟɬɿ
ɉɪɨɛɢɪɤɚԑɚ ɤԛɤɿɪɬɬɿ ɫɭɬɟɤ ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ ɨԑɚɧ ɥɚɤɦɭɫ
ԕɨɫɵԙɞɚɪ Ʌɚɤɦɭɫɬɵԙ ɬԛɫɿ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ" Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ
ɠɚɡɵԙɞɚɪ Ʉԛɤɿɪɬɬɿ ɫɭɬɟɤɬɿԙ ɞɢɫɫɨɰɢɚɰɢɹɫɵɧ ɠɚɡɵԙɞɚɪ ɤɟɫɬɟɞɟɧ
ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɭ ɤɨɧɫɬɚɧɬɚɫɵɧɵԙ ɦԥɧɿɧ ɚɧɵԕɬɚԙɞɚɪ +26 ɤԛɲɬɿ ɧɟɦɟɫɟ
ԥɥɫɿɡ ɷɥɟɤɬɪɨɥɢɬɬɟɪɝɟ ɠɚɬɚɬɵɧɵɧ ɛɚɣԕɚԙɞɚɪ
6-ɬԥɠɿɪɢɛɟ ɋɭɥɶɮɢɞɬɟɪɞɿ ɚɥɭ
48
49.
Cu2+, Pb2+, Zn2+, Mn2+ ɬԝɡɞɚɪɞɵԙ ɟɪɿɬɿɧɞɿɫɿ ɛɚɪ ɩɪɨɛɢɪɤɚɥɚɪԑɚɧɚɬɪɢɣ ɫɭɥɶɮɢɞɿɧɿԙ ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ Ɍԛɧɛɚɥɚɪɞɵԙ ɬԛɡɿɥɭɿɧ ɠԥɧɟ
ɨɥɚɪɞɵԙ ɬԛɫɿɧ ɛɚɣԕɚԙɞɚɪ Ɋɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
7-ɬԥɠɿɪɢɛɟ Ʉԛɤɿɪɬ ɨɤɫɢɞɿɧ ȱ9 ɚɥɭ ɠɨɥɵ
ȼɸɪɰ ɤɨɥɛɚɫɵɧɚ ɲɚɦɚɥɵ ɫɭɥɚɧԑɚɧ ɧɚɬɪɢɣ ɫɭɥɶɮɢɬ ɤɪɢɫɬɚɥɵɧ
ɫɚɥɵԙɞɚɪ ɜɨɪɨɧɤɚɞɚɧ ɬɚɦɲɵɥɚɩ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ +2SO4 ԕɨɫɵԙɞɚɪ
Ʉԧɪɝɟɧɞɟɪɿԙɞɿ ɬԛɫɿɧɞɿɪɿԙɞɟɪ ɠԥɧɟ ɠɚɡɵԙɞɚɪ
22-ɫɭɪɟɬ Ʉԛɤɿɪɬ ȱ9 ɨɤɫɢɞɿɧ ɚɥɭ ɫɯɟɦɚɫɵ.
8-ɬԥɠɿɪɢɛɟ Ʉԛɤɿɪɬ ɨɤɫɢɞɿɧɿԙ ȱ9 ɫɭɞɚ ɟɪɭɿ
SO2 ɬɨɥԑɚɧ ɰɢɥɢɧɞɪɞɿ ɫɭɵ ɛɚɪ ɤɪɢɫɬɚɥɢɡɚɬɨɪԑɚ ɚɭɞɚɪɵԙɞɚɪ
ɥɚɤɦɭɫ ԕɨɫɵԙɞɚɪ ɋɭ ɰɢɥɢɧɞɪɝɟ ɤԧɬɟɪɿɥɿɩ ɬԛɫɿɧ ԧɡɝɟɪɬɟɞɿ Ɋɟɚɤɰɢɹɧɵԙ
ɬɟԙɞɟɭɿɧ ɠԥɧɟ ɤԛɤɿɪɬɬɿ ԕɵɲԕɵɥɞɵԙ ɞɢɫɫɨɰɢɚɰɢɹɫɵɧ ɠɚɡɵԙɞɚɪ
9-ɬԥɠɿɪɢɛɟ Ʉԛɤɿɪɬɬɿ ԕɵɲԕɵɥɞɵԙ ɬɨɬɵԑɭ-ɬɨɬɵԕɫɵɡɞɚɧɭ ԕɚɫɢɟɬɿ
ɚ +2SO3 ɧɟɦɟɫɟ ɧɚɬɪɢɣ ɫɭɥɶɮɢɬɿɧɿԙ ɟɪɿɬɿɧɞɿɫɿɧɟ ɤԛɤɿɪɬɬɿ ɫɭɬɟɤ
ɫɭɵɧ ԕɨɫɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ɍɨɬɵԑɭ ± ɬɨɬɵԕɫɵɡɞɚɧɭ ɪɟɚɤɰɢɹɫɵɧɵԙ
ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
ɛ ԛɲ ɩɪɨɛɢɪɤɚ ɚɥɵԙɞɚɪ ɛɿɪɿɧɲɿɫɿɧɟ ɛɪɨɦ ɫɭɵɧ ɟɤɿɧɲɿɫɿɧɟ ±
KMnO4 ɟɪɿɬɿɧɞɿɫɿɧ ԛɲɿɧɲɿɫɿɧɟ ± K2Cr2O7 ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ ɛԥɪɿɧɟ
ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɧ ɨɪɬɚ ԛɲɿɧ ɠԥɧɟ 1D2SO3 ɟɪɿɬɿɧɞɿɫɿɧ ԕɨɫɵԙɞɵɪ ɇɟ
ɛɚɣԕɚɥɚɞɵ" ȿɪɿɬɿɧɞɿɥɟɪɞɿԙ ɬԛɫɿ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ" Ɋɟɚɤɰɢɹɥɚɪɞɵԙ
ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ ɠԥɧɟ ɤɟɫɬɟɞɟɧ 6232- ɢɨɧɵɧɵԙ ɬɨɬɵԑɭɬɨɬɵԕɫɵɡɞɚɧɭ ɩɨɬɟɧɰɢɚɥ ɦԥɧɿɧ ɠɚɡɵԙɞɚɪ
10-ɬԥɠɿɪɢɛɟ Ʉԛɤɿɪɬ ԕɵɲԕɵɥɵɧɵԙ ɬɨɬɵԕɬɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɿ
Ɍԥɠɿɪɢɛɟɥɟɪɞɿ ɚɭɚ ɫɨɪԑɵɲ ɲɤɚɮɬɵԙ ɚɫɬɵɧɞɚ ɿɫɬɟԙɞɟɪ ɚ
ɦɵɪɵɲ ɛ ɦɚɝɧɢɣ ɜ ɦɵɫ ɝ ɚɥɸɦɢɧɢɣɞɿԙ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ ɠԥɧɟ
49
50.
ɫԝɣɵɬɵɥԑɚɧ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɧɚ ԕɚɬɵɧɚɫɵɧ ɛɚɣԕɚԙɞɚɪ Ԕɚɧɞɚɣ ɝɚɡɛԧɥɿɧɟɞɿ" Ԕɚɧɞɚɣ ɚɣɵɪɦɚɲɵɥɵԑɵ ɛɚɣԕɚɥɚɞɵ" Ɇɟɬɚɥɞɚɪɞɵԙ ɤɟɪɧɟɭ
ԕɚɬɚɪɵɧɞɚԑɵ ɨɪɧɵɧ ɛɚɣԕɚԙɞɚɪ Ɋɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ
ɠɚɡɵԙɞɚɪ
11-ɬԥɠɿɪɢɛɟ ɇɚɬɪɢɣ ɬɢɨɫɭɥɶɮɚɬɵɧɵԙ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ
ԕɚɫɢɟɬɿ
ȿɤɿ ɩɪɨɛɢɪɤɚԑɚ ɧɚɬɪɢɣ ɬɢɨɫɭɥɶɮɚɬɵ ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ
ɚ ɛɿɪɿɧɲɿɫɿɧɟ ɫԝɣɵɬɵɥԑɚɧ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɧ ԕɨɫɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ"
Ԕɚɧɞɚɣ ɡɚɬ ɬԝɧɛɚ ɬԛɡɟɞɿ" Ԕɚɧɞɚɣ ɝɚɡ ɛԧɥɿɧɟɞɿ"
ɛ ɟɤɿɧɲɿɫɿɧɟ ± ɯɥɨɪ ɫɭɵɧ ԕɨɫɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ɋɟɚɤɰɢɹɥɚɪɞɵԙ
ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ 114
Ɍɚԕɵɪɵɛɵ 9 ɬɨɩ ɪ-ɷɥɟɦɟɧɬɬɟɪɿ Ⱥɡɨɬ ɮɨɫɮɨɪ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵԙ ɦɚԕɫɚɬɵ Ⱥɡɨɬ ɮɨɫɮɨɪ ɠԥɧɟ ɨɥɚɪɞɵԙ
ԕɨɫɵɥɵɫɬɚɪɵɧɵԙ ɚɥɵɧɭ ɠɨɥɞɚɪɵɧ ɠԥɧɟ ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿɧ ɨԕɵɩ
ԛɣɪɟɧɭ
Ȼɚԕɵɥɚɭ ɫԝɪɚԕɬɚɪɵ ɦɟɧ ɠɚɬɬɵԑɭɥɚɪɵ
1. Ⱥɡɨɬ ɚɬɨɦɵɧɵԙ ɠԥɧɟ ɮɨɫɮɨɪ ɚɬɨɦɵɧɵԙ ɷɥɟɤɬɪɨɧɞɵԕ ԕԝɪɵɥɵɫɵ
ɬɨɬɵԑɭ ɞԥɪɟɠɟɫɿ ɬɚɛɢԑɚɬɬɚ ɤɟɡɞɟɫɭɿ ɚɥɵɧɭ ɠɨɥɞɚɪɵ ԕɨɥɞɚɧɵɥɭɵ
2. Ⱥɡɨɬɬɵԙ ɠԥɧɟ ɮɨɫɮɨɪɞɵԙ ɮɢɡɢɤɚɥɵԕ ɠԥɧɟ ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿ
3. Ⱥɦɦɢɚɤ ɚɥɵɧɭɵ ɦɨɥɟɤɭɥɚɫɵɧɵԙ ԕԝɪɵɥɵɫɵ ɝɢɛɪɢɞɬɟɧɭɿ
ɮɢɡɢɤɚɥɵԕ ɠԥɧɟ ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿ
4. Ɏɨɫɮɨɪɞɵԙ ɨɤɫɢɞɬɟɪɿ ȱȱȱ V ɚɥɵɧɭ ɠɨɥɞɚɪɵ ԕɚɫɢɟɬɬɟɪɿ Ɏɨɫɮɢɧ
ɚɥɵɧɭ ɠɨɥɵ ɨɧɵԙ ԕɚɫɢɟɬɬɟɪɿ
5. Ɏɨɫɮɨɪɞɵԙ ԕɵɲԕɵɥɞɚɪɵ ɨɥɚɪɞɵԙ ɫɬɪɭɤɬɭɪɚɥɵԕ ɮɨɪɦɭɥɚɥɚɪɵ
Ԕɵɲԕɵɥɞɚɪɞɵԙ ɧɟɝɿɡɞɿɝɿ ɦɟɧ ɛɟɪɿɤɬɿɝɿ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ" Ԕɚɧɞɚɣ
ԕɵɲԕɵɥɞɚɪ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬ ɤԧɪɫɟɬɟɞɿ"
6. Ɏɨɫɮɨɪ ɬɵԙɚɣɬԕɵɲɬɚɪɵ ɨɥɚɪɞɵԙ ɚɥɵɧɭ ɠɨɥɞɚɪɵ
7. ȿɫɟɩ ɦɥ - ɬɿɤ NH4OH ɟɪɿɬɿɧɞɿɫɿɧ U ɝ ɫɦ3 ԕɚɣɧɚɬԕɚɧɞɚ
ԕɚɧɲɚ ɤԧɥɟɦ ɚɦɦɢɚɤ ԕ ɠ ɚɥɭԑɚ ɛɨɥɚɞɵ"
8. ȿɫɟɩ ȿɝɟɪ ɞɟ ɨɧɵԙ ɬɢɬɪɿ Ɍ ɬɟԙ ɛɨɥɫɚ -ɬɿɤ U ɝ ɫɦ3)
ɦɥ NH3 ɟɪɿɬɿɧɞɿɫɿɧ ɧɟɣɬɪɚɥɞɚɭ ԛɲɿɧ ԕɚɧɲɚ ɤԧɥɟɦ ɬԝɡ ԕɵɲԕɵɥɵ
ɠԝɦɫɚɥɚɞɵ"
9. ȿɫɟɩ Ɍɢɬɪɿ ɬɟԙ ɛɨɥԑɚɧ ɦɥ ɬԝɡ ԕɵɲԕɵɥɵɧ ɧɟɣɬɪɚɥɞɚɭ ԛɲɿɧ
ɦɥ ɚɦɦɢɚɤ ɟɪɿɬɿɧɞɿɫɿ ԕɚɠɟɬ ɛɨɥɞɵ Ⱥɦɦɢɚɤ ɟɪɿɬɿɧɞɿɫɿɧɿԙ ɬɢɬɪɿɧ
ɠԥɧɟ ɦɨɥɹɪɥɵԕ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵɧ ɟɫɟɩɬɟԙɿɡɞɟɪ
50
51.
10.ȿɫɟɩ ȿɝɟɪ ɝɢɞɪɨɥɢɡɞɟɧ ɬԛɡɿɥɝɟɧ ɡɚɬԕɚ ɬԝɡ ɬԛɡɿɥɭ ԛɲɿɧ ɦɚɫɫɚɥɵԕ ԛɥɟɫɿ14%-ɬɿɤ ɠԥɧɟ ɬɵԑɵɡɞɵԑɵ ɝ ɫɦ3 ɦɥ HCȱ ԕɚɠɟɬ ɛɨɥɫɚ ɦɚɝɧɢɣ
ɧɢɬɪɢɞɿɧɿԙ ԕɚɧɞɚɣ ɦɚɫɫɚɫɵ ɫɭɦɟɧ ɵɞɵɪɚɣɞɵ"
11.Ⱥɡɨɬɬɵԙ ɨɤɫɢɞɬɟɪɿ ȱȱ ȱV, V Ⱥɥɵɧɭɵ ԕԝɪɵɥɵɫɵ ɯɢɦɢɹɥɵԕ
ԕɚɫɢɟɬɬɟɪɿ
12.Ⱥɡɨɬɬɵ ԕɵɲԕɵɥ ɨɧɵԙ ɬԝɡɞɚɪɵ ԕԝɪɵɥɵɫɵ ɚɥɵɧɭ ɠɨɥɞɚɪɵ ɬɨɬɵԑɭɬɨɬɵԕɫɵɡɞɚɧɭ ԕɚɫɢɟɬɬɟɪɿ
13.Ⱥɡɨɬ ԕɵɲԕɵɥɵɧɵԙ ɬԝɡɞɚɪɵ ɚɥɵɧɭɵ ԕɵɡɞɵɪԑɚɧɞɚ ɵɞɵɪɚɭɵ
ԕɨɥɞɚɧɵɥɭɵ
14.ȿɫɟɩ -ɬɿɤ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵ p ɝ ɫɦ3 ɝ NaNO3±ɦɟɧ
ԥɪɟɤɟɬɬɟɫɤɟɧɞɟ ԕɚɧɲɚ ɦɥ ԕɵɲԕɵɥ ɠԝɦɫɚɥɚɞɵ" ȿɝɟɪ ɞɟ ɚɡɨɬ
ԕɵɲɵԕɵɥɵɧɵԙ 4% ɵɞɵɪɚԑɚɧɞɚ, ԕɚɧɲɚ ɝɪɚɦɦ HNO3 ɬԛɡɿɥɟɞɿ?
15.ȿɫɟɩ. Ɇɚɫɫɚɥɵԕ ԛɥɟɫɿ 0,68 (p=1,41 ɝ/ɫɦ3) 500 ɦɥ ɚɡɨɬ ԕɵɲԕɵɥɵɧɚɧ
ԕɚɧɲɚ ɥɢɬɪ 2 ɧɨɪɦɚɥɶɞɵԕ ɟɪɿɬɿɧɞɿ ɞɚɣɵɧɞɚɭԑɚ ɛɨɥɚɞɵ?
16.ȿɫɟɩ. Ɍԧɦɟɧɞɟɝɿ ɛɟɪɿɥɝɟɧ ɡɚɬɬɚɪɞɚɧ: 1) ɮɨɫɮɨɪɞɚɧ, 2) ɤɚɥɶɰɢɣ
ɮɨɫɮɚɬɵɧɚɧ ɮɨɫɮɨɪ ԕɵɲԕɵɥɵɧ ԕɚɥɚɣ ɚɥɭԑɚ ɛɨɥɚɞɵ? ɝ ɇ3ɊɈ4 ɚɥɭ
ԛɲɿɧ ԕɚɧɲɚ ɤɚɥɶɰɢɣ ɮɨɫɮɚɬɵ ɠԝɦɫɚɥɚɞɵ"
17.ȿɫɟɩ Ԕɵɡɵɥ ɮɨɫɮɨɪɞɵԙ ԕԝɪɚɦɵɧɞɚ ɫɭ ɛɨɥɚɞɵ ɝ ɮɨɫɮɨɪɞɵ
ɬɨɬɵԕɬɵɪɭ ԛɲɿɧ ԕɚɧɲɚ ɚɡɨɬ ԕɵɲԕɵɥɵ p ɝ ɫɦ3 ԕɚɠɟɬ ɠԥɧɟ
ԕɚɧɲɚ ɥɢɬɪ ɚɡɨɬ ȱȱ ɨɤɫɢɞɿ ɛԧɥɿɧɿɩ ɲɵԑɚɞɵ"
18.Ɍԧɦɟɧɞɟɝɿ ɪɟɚɤɰɢɹɥɚɪɞɵ ɚɹԕɬɚԙɞɚɪ ɠԥɧɟ ɢɨɧɞɵԕ±ɷɥɟɤɬɪɨɧɞɵԕ ԥɞɿɫɬɿ
ɩɚɣɞɚɥɚɧɵɩ ɤɨɷɮɮɢɰɢɟɧɬɬɟɪɿɧ ԕɨɣɵԙɞɚɪ
ɚ) HN3+KMnO4+H2SO4o N2+ MnSO4 «
ɛ) KNO2+PbO2+H2SO4o
ɜ) N2H4 +J&ȱ2o Hg2&ȱ «
ɝ) N2H4+K2Cr2O7 +&ȱo N2 «
ɞ) H3PO3+AgNO3+H2OoHNO3 «
ɟ) P+HNO3+H2Oo
ɠ) P+Mgo
ɡ) PH3+KMnO4+H2SO4o H3PO4 +«
ɢ) P2H4+KMnO4+H2SO4o
ɤ) Mg3P2 +&ȱo
ɦ) H3PO3 ȱ2+H2Oo
ɡ) Mg3Ɋ2+K2Cr2O7 +&ȱo
ɢ) H3PO2+KMnO4+H2SO4o
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɬԥɠɿɪɢɛɟɥɟɪ
Ⱥɩɩɚɪɚɬɬɚɪ ɠԥɧɟ ɵɞɵɫɬɚɪ: Ԕɵɫԕɵɲɵ ɛɚɪ ɲɬɚɬɢɜ, ɫɵɣɵɦɞɵɥɵԑɵ
50ɦɥ ɛɸɪɟɬɤɚ, ɝɚɡ ԧɬɤɿɡɟɬɿɧ ɬԛɬɿɤɲɟɫɿ ɛɚɪ ɬɵԑɵɧ, ɪɟɡɢɧɚ ɬԛɬɿɤɬɟɪɿ,
ɠɚɧɞɵɪԑɵɲ, Ʉɢɩɩ ɚɩɩɚɪɚɬɵ, ɫɵɣɵɦɞɵɥɵԑɵ 1ɥ ɤɨɥɛɚ, ԕɢɵɧ ɟɪɢɬɿɧ
51
52.
ɬԛɬɿɤ, ɛԝɪɚɧɞɚɥɵ ԕɵɫԕɵɲ, ɮɚɪɮɨɪ ɬɨɫɬɚԑɚɧɲɚ, ɲɵɧɵ ɜɚɧɧɚɫɵ, 20-25ɫɦɲɵɧɵ ɬԛɬɿɤɲɟ, ɰɢɥɢɧɞɪ ɧɟɦɟɫɟ ɩɪɨɛɢɪɤɚɥɚɪ, ɲɵɧɵ ɩɥɚɫɬɢɧɤɚɥɚɪ,
ɲɵɧɵ ɬɚɹԕɲɚɥɚɪ, ɲɩɚɬɟɥɶ.
Ɍɟɯɧɢɤɚɥɵԕ ɬɚɪɚɡɵ, ԕɵɫԕɵɲɵ ɛɚɪ ɲɬɚɬɢɜ, ɠɚɧɞɵɪԑɵɲ, ɯɥɨɪɥɵ
ɤɚɥɶɰɢɣ, ɬԛɬɿɤɲɟ, ɤɨɥɛɚ, ɲɵɧɵ ɜɚɧɧɚɫɵ, ɩɪɨɛɢɪɤɚɥɚɪ, ɟɤɿ ɬԛɬɿɝɿ ɛɚɪ
ɬɵԑɵɧ, ɪɟɡɢɧɚ ɬԛɬɿɤɬɟɪɿ, ɲɵɧɵ ɬԛɬɿɤɲɟ, ɪɟɬɨɪɬɚ, ɚɫɛɟɫɬ ɬɨɪɵ,Ʉɢɩɩ
ɚɩɩɚɪɚɬɵ, ɮɚɪɮɨɪ ɤɟɥɿɫɿ, ɮɚɪɮɨɪ ɬɨɫɬɚԑɚɧɲɚɫɵ, ɜɨɪɨɧɤɚ, ɰɢɥɢɧɞɪɥɟɪ,
ɬɵԑɵɧɵ ɛɚɪ ɩɪɨɛɢɪɤɚ,ɬɢɝɟɥ ԕɵɫԕɵɲɵ, ԕԝɦ ɛɚɧɹɫɵ, ɬɟɦɿɪ ԕɚɫɵԕɲɚ,
ɲɵɧɵ ɩɥɚɫɬɢɧɤɚɫɵ, ɢɿɥɝɟɧ ɬԛɬɿɤɲɟ, ɲɵɧɵ ɬɚɹԕɲɚ, ɩɪɨɛɢɪɤɚɥɚɪɵ ɛɚɪ
ɲɬɚɬɢɜ.
Ɍɟɯɧɢɤɚɥɵԕ ɬɚɪɚɡɵ, ɯɥɨɪ ɚɥɭ ԛɲɿɧ ԕɚɠɟɬ ɩɪɢɛɨɪ, ɪɇ-ɬɵ ɚɧɵԕɬɚɣɬɵɧ
ɩɪɢɛɨɪ, ɫɭ ɛɚɧɹɫɵ, ԕɵɫԕɵɲɵ ɛɚɪ ɲɬɚɬɢɜ, ɠɚɧɞɵɪԑɵɲ, ɮɚɪɮɨɪ ɤɟɥɿɫɿ,
ɮɚɪɮɨɪ ɬɨɫɬɚԑɚɧɲɚɫɵ, ɫɵɣɵɦɞɵɥɵԑɵ 100ɦɥ ɫɬɚԕɚɧ, ɬɢɝɟɥɶ ԕɵɫԕɵɲɵ,
ɜɨɪɨɧɤɚɥɚɪ, ɤɟɫɤɿɲ, ɩɢɧɰɟɬ, ɚɫɛɟɫɬ ɬɨɪɵ, ɲɵɧɵ ɬɚɹԕɲɚ.
Ɋɟɚɤɬɢɜɬɟɪ ɠԥɧɟ ɦɚɬɟɪɢɚɥɞɚɪ: ɬɟɦɿɪ ԝɧɬɚԑɵ, ɦɵɪɵɲ ɬԛɣɿɪɿ, ɦɚɪɝɚɧɟɰ
ȱ9 ɨɤɫɢɞɿ (ɤԛɣɞɿɪɿɥɝɟɧ), ɤɚɥɶɰɢɣ ɝɢɞɪɨɤɫɢɞɿ, ɚɦɦɨɧɢɣ ɧɢɬɪɚɬɵ,
ɚɦɦɨɧɢɣ ɫɭɥɶɮɚɬɵ, ɚɦɦɨɧɢɣ ɯɥɨɪɢɞɿ, ɤɚɥɢɣ ɧɢɬɪɚɬɵ, ɝɢɞɪɚɡɢɧ,
ɝɢɞɪɨɤɫɢɥɚɦɢɧ ɯɥɨɪɢɞɿ, ɚɫɛɟɫɬ ɧɟɦɟɫɟ ɲɵɧɵ ɦɚԕɬɚ, ɥɚɤɦɭɫ ԕɚԑɚɡɵ
(ԕɵɡɵɥ ɧɟ ɤԧɤ ɬԛɫɿ ɫԛɡɝɿ ԕɚԑɚɡɵ ɥɭɱɢɧɚ
Ⱥɥɸɦɢɧɢɣ ɬԛɣɿɪɿ, ɦɵɫ ɠɨԙԕɚɥɚɪɵ ɦɟɧ ɫɵɦɵ, ԕɚɥɚɣɵ ɬԛɣɿɪɿ, ɦɵɪɵɲ
ɬԛɣɿɪɿ, ɬɟɦɿɪ ԝɧɬɚԑɵ, ɤԛɤɿɪɬ, ԕɵɡɵɥ ɮɨɫɮɨɪ, ɦɚɪɝɚɧɟɰ (ȱ9 ɨɤɫɢɞɿ
ɤԛɣɞɿɪɿɥɝɟɧ, ɬɟɦɿɪ ɫɭɥɶɮɢɞɿ, ɤɚɥɢɣ ɧɢɬɪɚɬɵ, ɤɚɥɢɣ ɯɥɨɪɚɬɵ, ɤɚɥɶɰɢɣ
ɯɥɨɪɢɞɿ, ɦɵɫ ɧɢɬɪɚɬɵ,ɧɚɬɪɢɣ ɧɢɬɪɚɬɵ, ɧɚɬɪɢɣ ɯɥɨɪɢɞɿ, ԕɨɪԑɚɫɵɧ
ɧɢɬɪɚɬɵ, ɤԛɦɿɫ ɧɢɬɪɚɬɵ, ɦԝɡ ɧɟɦɟɫɟ ԕɚɪ, ɲɵɧɵ ɦɚԕɬɚ, ɥɚɤɦɭɫ ԕɚԑɚɡɵ,
ɫԛɡɝɿ ԕɚԑɚɡɵ, ɦɚɬɚ ԕɢɵɧɞɵɫɵ.
Ⱥԕ ɠԥɧɟ ԕɵɡɵɥ ɮɨɫɮɨɪ, ɤɚɥɶɰɢɣ ɮɨɫɮɢɞɿ, ɤɚɥɢɣ ɩɟɪɦɚɧɝɚɧɚɬɵ,
ɮɨɫɮɨɪɢɬ, ɧɚɬɪɢɣ ɞɢɝɢɞɪɨɨɪɬɨɮɨɫɮɚɬɵ, ɧɚɬɪɢɣ ɝɢɞɪɨɨɪɬɨɮɨɫɮɚɬɵ,
ɮɨɫɮɨɪ ȱȱȱ ɯɥɨɪɢɞɿ, ɮɨɫɮɨɪ (V) ɯɥɨɪɢɞɿ, ɤԛɤɿɪɬɬɿ ɤԧɦɿɪɬɟɝɿ, ɬɟɦɿɪ
ɩɥɚɫɬɢɧɤɚɫɵ, ԕɚԙɵɥɬɵɪ ɩɥɚɫɬɢɧɤɚɫɵ, ɥɚɤɦɭɫ ԕɚԑɚɡɵ, ɫԛɡɝɿ ԕɚԑɚɡɵ, ɦɚԕɬɚ.
ȿɪɿɬɿɧɞɿɥɟɪ Ʉԛɤɿɪɬ ԕɵɲԕɵɥɵ (ɬɵԑ.1,84 ɠԥɧɟ ɧɚɬɪɢɣ ɝɢɞɪɨɤɫɢɞɿ
(2ɧ ɚɦɦɢɚɤ ɫɭɵ ɠԥɧɟ 2ɧ ɤɚɥɶɰɢɣ ɝɢɞɪɨɤɫɢɞɿ ԕɚɧɵԕ ɚɦɦɨɧɢɣ
ɯɥɨɪɢɞɿ ԕɚɧɵԕԕɚɧ ɠԥɧɟ 2ɧ ɮɟɧɨɥɮɬɚɥɟɢɧ ɩɢɪɨɝɚɥɥɨɥ &6H3(OH)3)
(ɧɟɝɿɡɞɿɤ ɢɨɞ ɫɭɵ
Ⱥɡɨɬ ԕɵɲԕɵɥɵ (ɬɵԑ.1,4, 1:1, ɠԥɧɟ 2ɧ), ɤԛɤɿɪɬ ԕɵɲԕɵɥɵ (ɬɵԑ.1,84 ɠԥɧɟ
2ɧ), ɬԝɡ ԕɵɲԕɵɥɵ (ɬɵԑ.1,19, 1:1, ɠԥɧɟ 2ɧ), ɧɚɬɪɢɣ ɝɢɞɪɨɤɫɢɞɿ(2ɧ),
ɚɦɦɢɚɤ ɫɭɵ (25%), ɛɚɪɢɣ ɯɥɨɪɢɞɿ(1ɧ), ɬɟɦɿɪ (ȱȱ) ɫɭɥɶɮɚɬɵ ԕɚɧɵԕԕɚɧ,
ɤɚɥɢɣ ɢɨɞɢɞɿ (0,1ɧ), ɤɚɥɢɣ ɩɟɪɦɚɧɝɚɧɚɬɵ (0,1ɧ), ɧɚɬɪɢɣ ɧɢɬɪɢɬɿ(1ɧ),
ɥɚɤɦɭɫ, ɤɪɚɯɦɚɥ ɤɥɟɣɫɬɟɪɿ.
Ⱥɡɨɬ ԕɵɲԕɵɥɵ (ɤɨɧɰ,ɬɵԑ.1,412 ɠԥɧɟ 2ɧ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵ ɬԝɡ
ԕɵɲԕɵɥɵ ɤɨɧɰ,ɬɵԑ. 1,19 ɠԥɧɟ 4ɧ ɫɿɪɤɟ ԕɵɲԕɵɥɵ ɧ ɨɪɬɨɮɨɫɮɨɪ
ԕɵɲԕɵɥɵ ɧ ɚɦɦɨɧɢɣ ɦɨɥɢɛɞɚɬɵ ɤɚɥɶɰɢɣ ɯɥɨɪɢɞɿ(2ɧ ɧɚɬɪɢɣ
ɦɟɬɚɮɨɫɮɚɬɵ ɧ ɧɚɬɪɢɣ ɩɢɪɨɮɨɫɮɚɬɵ ɧ ɧɚɬɪɢɣ ɨɪɬɨɮɨɫɮɚɬɵ ɧ),
ɧɚɬɪɢɣ ɞɢɝɢɞɪɨɨɪɬɨɮɨɫɮɚɬɵ ɧ ɧɚɬɪɢɣ ɝɢɞɪɨɨɪɬɨɮɨɫɮɚɬɵ ɧ),
ɧɚɬɪɢɣ ɤɚɪɛɨɧɚɬɵ ɧ ɤԛɦɿɫ ɧɢɬɪɚɬɵ ɧ ɧɚɬɪɢɣ ɚɰɟɬɚɬɵ ɧ ɬɟɦɿɪ
ȱȱȱ ɯɥɨɪɢɞɿ (1ɧ ɚɥɸɦɢɧɢɣ ɫɭɥɶɮɚɬɵ ɧ ɛɟɥɨɤ
52
53.
1-ɬԥɠɿɪɢɛɟ Ⱥɡɨɬɬɵ ɚɥɭ Ⱥɡɨɬɬɵ ɚɥɭ ԛɲɿɧ ɦɵɧɚɧɞɚɣ ɩɪɢɛɨɪ ɠɢɧɚԙɞɚɪ(23-ɫɭɪɟɬ ɩɪɨɛɢɪɤɚԑɚ ɲɚɦɚɞɚ - ɦɥ ɚɦɦɨɧɢɣ ɯɥɨɪɢɞɿ ɦɟɧ ɧɚɬɪɢɣ
ɧɢɬɪɢɬɿɧɿԙ ԕɨɫɩɚɫɵɧ ԕԝɣɵԙɞɚɪ Ԕɨɫɩɚɧɵ ԕɵɡɞɵɪɵԙɞɚɪ Ԕɚɧɞɚɣ ɝɚɡ
ɛԧɥɿɧɟɞɿ"
ɉɪɨɛɢɪɤɚɧɵԙ
ɚɭɡɵɧɚ ɠɚɧɵɩ ɬԝɪԑɚɧ ԝɲԕɵɧɞɵ
ɠɚԕɵɧɞɚɬɵԙɞɚɪ ɇɟ ɛɚɣԕɚɞɵԙɞɚɪ" Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
23-ɫɭɪɟɬ ɋɭɞɚ ɟɪɿɦɟɣɬɿɧ ɝɚɡɞɚɪɞɵ ɚɥɭԑɚ ɚɪɧɚɥԑɚɧ ɩɪɢɛɨɪ
1-ɉɪɨɛɢɪɤɚ ԕɨɫɩɚɦɟɧ, 2- Ƚɚɡ ɠɢɧɚɥɚɬɵɧ ɬɪɭɛɤɚ -ɋɭɦɟɧ ɩɪɨɛɢɪɤɚ,
4-Ʉɪɢɫɬɚɥɢɡɚɬɨɪ ɫɭɦɟɧ, 5- ɋɩɢɪɬɨɜɤɚ, 6-ɒɬɚɬɢɜ
2-ɬԥɠɿɪɢɛɟ Ⱥɦɦɢɚɤɬɵ ɚɥɭ Ⱥɭɚ ɫɨɪԑɵɲ ɲɤɚɮɬɚ ɠԝɦɵɫ ɿɫɬɟԙɿɡɞɟɪ
Ɍɢɝɟɥɶɝɟ ɝ ɲɚɦɚɞɚ NH4Cȱ ɠԥɧɟ ɝ ɋɚ Ɉɇ 2 ԕɨɫɩɚ ɞɚɣɵɧɞɚԙɞɚɪ
Ԕɨɫɩɚɧɵ ɠɚԕɫɵ ɚɪɚɥɚɫɬɵɪɵԙɞɚɪ ɚɦɦɢɚɤɬɵԙ ɬԛɡɿɥɝɟɧɿɧ ɢɿɫɿɧɟɧ
ɛɚɣԕɚԙɞɚɪ Ԕɨɫɩɚɧɵ ɩɪɨɛɢɪɤɚԑɚ ɚɭɵɫɬɵɪɵɩ ɨɧɵԙ ɚɭɵɡɵɧ ɢɦɟɤ
ɬԛɬɿɤɲɟɫɿ ɛɚɪ ɬɵԑɵɧɦɟɧ ɠɚɭɵɩ ɲɬɚɬɢɜɤɟ ɨɪɧɚɬɵԙɞɚɪ 24-ɫɭɪɟɬ.
1. ɉɪɨɛɢɪɤɚ ԕɨɫɩɚɦɟɧ
2. Ⱥɦɦɢɚɤ ɛԧɥɿɧɝɟɧ
3. ɒɬɚɬɢɜ
4. ɋɩɢɪɬɨɜɤɚ
-
24-ɫɭɪɟɬ. Ⱥɦɦɢɚɤɬɵ ɚɥɭԑɚ ɚɪɧɚɥԑɚɧ ɩɪɢɛɨɪ
53
54.
Ԕɨɫɩɚɫɵ ɛɚɪ ɩɪɨɛɢɪɤɚɧɵ ԕɵɡɞɵɪɵԙɞɚɪ Ɍԛɬɿɤɲɟɧɿԙ ɚɭɡɵɧɚ ɫɭԑɚɛɚɬɵɪɵɥԑɚɧ ɥɚɤɦɭɫ ԕɚԑɚɡɵɧ ɠɚԕɵɧɞɚɬɵԙɞɚɪ ɧɟ ɛɚɣԕɚɥɚɞɵ" Ɍԛɬɿɤɲɟɧɿԙ
ԝɲɵɧɚ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ ɬԝɡ ԕɵɲԕɵɥɵɧɚ ɛɚɬɵɪɵɥԑɚɧ ɲɵɧɵ ɬɚɹԕɲɚɧɵ
ɠɚԕɵɧɞɚɬɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ɋɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
4-ɬԥɠɿɪɢɛɟ Ⱥɦɦɢɚɤɬɵԙ ɬɨɬɵԕɫɵɡɞɚɧɞɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɿ
ɩɪɨɛɢɪɤɚԑɚ ɛԧɥɟɤ-ɛԧɥɟɤ - ɬɚɦɲɵɞɚɧ ɛɪɨɦ ɫɭɵ ɠԥɧɟ .0Q24
ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ ɟɤɟɭɿɧɟ ɞɟ - ɬɚɦɲɵ -ɬɿɤ ɚɦɦɢɚɤ ɟɪɿɬɿɧɞɿɫɿɧ
ԕɨɫɵԙɞɚɪ ɚɪɚɥɚɫɬɵɪɵԙɞɚɪ ɲɚɦɚɥɵ ԕɵɡɞɵɪɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ"
Ɋɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ ɟɝɟɪ ɞɟ ɚɦɦɢɚɤ ɚɡɨɬԕɚ ɞɟɣɿɧ ɚɥ
KMnO4 ɦɚɪɝɚɧɟɰɬɿԙ ɨɤɫɢɞɿɧɟ ȱ9 ɞɟɣɿɧ ɬɨɬɵԕɫɵɡɞɚɧɚɬɵɧ ɛɨɥɫɚ
5-ɬԥɠɿɪɢɛɟ Ⱥɦɦɨɧɢɣ ɬԝɡɞɚɪɵɧɵԙ ɝɢɞɪɨɥɢɡɿ
ɩɪɨɛɢɪɤɚԑɚ - ɬɚɦɲɵ ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭ ɠԥɧɟ - ɬɚɦɲɵ ɥɚɤɦɭɫ
ԕԝɣɵԙɞɚɪ Ȼɿɪ ɩɪɨɛɢɪɤɚԑɚ 1+4&ȱ ɤɪɢɫɬɚɥɵɧ ɟɤɿɧɲɿɫɿɧɟ ± NH4NO3
ԕɨɫɵԙɞɚɪ Ⱥɪɚɥɚɫɬɵɪɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ʌɚɤɦɭɫ ɬԛɫɿɧ ԧɡɝɟɪɬɟɦɟ"
Ɍԛɡɞɚɪɞɵԙ ɝɢɞɪɨɥɢɡɿɧɿԙ ɪɟɚɤɰɢɹɥɚɪɵɧɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
6-ɬԥɠɿɪɢɛɟ Ⱥɦɦɨɧɢɣ ɬԝɡɵɧɵԙ ɵɞɵɪɚɭɵ Ⱥɭɚ ɫɨɪԑɵɲ ɲɤɚɮɬɚ
ɠԝɦɵɫ ɿɫɬɟԙɞɟɪ
ԕԝɪԑɚԕ ɩɪɨɛɢɪɤɚԑɚ ɚɡɞɚԑɚɧ ɦԧɥɲɟɪɞɟ ԝɧɬɚԕɬɚɥԑɚɧ ԕɚɬɬɵ 1+4&ȱ,
(NH4)2SO4 ɠԥɧɟ 1+4)2Cr2O7 ɬԝɡɞɚɪɵɧ ɫɚɥɵԙɞɚɪ ɉɪɨɛɢɪɤɚɥɚɪɞɵ
ɲɬɚɬɢɜɤɟ ɜɟɪɬɢɤɚɥɶ ɤԛɣɿɧɞɟ ɛɟɤɿɬɿԙɞɟɪ ɠԥɧɟ ԕɵɡɞɵɪɵԙɞɚɪ
ɉɪɨɛɢɪɤɚɧɵԙ ɚɭɡɵɧɚ ɫɭԑɚ ɛɚɬɵɪɵɥԑɚɧ ɥɚɤɦɭɫ ԕɚԑɚɡɵɧ ɠɚԕɵɧɞɚɬɵԙɞɚɪ
ɇɟ ɛɚɣԕɚɥɚɞɵ" Ɉɫɵ ɪɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
7-ɬԥɠɿɪɢɛɟ Ƚɢɞɪɚɡɢɧɧɿԙ ԕɚɫɢɟɬɿ
ȿɤɿ ɩɪɨɛɢɪɤɚԑɚ ɦɥ ɫɭ ԕԝɣɵԙɞɚɪ ɠԥɧɟ - ɤɪɢɫɬɚɥɞɵ ɝɢɞɪɚɡɢɧ
ɫɭɥɶɮɚɬɵɧ ԕɨɫɵԙɞɚɪ Ȼɿɪ ɩɪɨɛɢɪɤɚԑɚ ɢɨɞ ɫɭɵɧ ɟɤɿɧɲɿɫɿɧɟ ɦɵɫ
ɯɥɨɪɢɞɿɧɿԙ ɟɪɿɬɿɧɞɿɫɿɧ ԕɨɫɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ⱥɡɨɬɬɵԙ ɬɨɬɵԑɭ
ɞԥɪɟɠɟɫɿ ɝɢɞɪɚɡɢɧɞɟ ԕɚɧɲɚԑɚ ɬɟԙ ɛɨɥɚɞɵ"
8-ɬԥɠɿɪɢɛɟ Ƚɢɞɪɨɤɫɢɥɚɦɢɧɧɿԙ ԕɚɫɢɟɬɿ
4- ɦɥ ɫɭɞɚ ɝɢɞɪɨɤɫɢɥɚɦɢɧ ɯɥɨɪɢɞɿɧɿԙ ɛɿɪɧɟɲɟ ɤɪɢɫɬɚɥɵɧ
ɟɪɿɬɿԙɞɟɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ɉɫɵ ɪɟɚɤɰɢɹ ɝɢɞɪɨɤɫɢɥɚɦɢɧɧɿԙ ԕɚɧɞɚɣ
ԕɚɫɢɟɬɿɧ ɤԧɪɫɟɬɟɞɿ" Ⱥɡɨɬɬɵԙ ɬɨɬɵԑɭ ɞԥɪɟɠɟɫɿ ԕɚɧɲɚԑɚ ɬɟԙ ɛɨɥɚɞɵ"
Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
9-ɬԥɠɿɪɢɛɟ Ⱥɡɨɬ ȱȱ ɨɤɫɢɞɿɧ ɚɥɭ ɠɨɥɵ Ⱥɭɚ ɫɨɪԑɵɲ ɲɤɚɮɬɚ ɠԝɦɵɫ
ɿɫɬɟԙɞɟɪ
Ɇɵɧɚ 25-ɫɭɪɟɬɬɟ ɤԧɪɫɟɬɿɥɝɟɧ ɩɪɢɛɨɪɞɵ ɠɢɧɚԙɞɚɪ ɉɪɨɛɢɪɤɚԑɚ ɦɵɫ
ɧɟɦɟɫɟ ɥɚɬɭɧɧɵԙ ԕɚɛɵɪɲɚԕɬɚɪɵɧ ɫɚɥɵɩ ɨɧɵԙ ԛɫɬɿɧɟ ɫԝɣɵɬɵɥԑɚɧ
ɚɡɨɬ ԕɵɲԕɵɥɵɧ ԕԝɣɵԙɞɚɪ Ȼԧɥɿɧɿɩ ɲɵԑɵɩ ɠɚɬԕɚɧ ɚɡɨɬ ȱȱ ɨɤɫɢɞɿɧ
ɝɚɡɞɵԙ ɚɭɡɵ ɲɵɧɵ ɩɥɚɫɬɢɧɤɚɦɟɧ ɠɚɛɵɥɚɬɵɧ ɟɤɿ ɰɢɥɢɧɞɪɝɟ ɠɢɧɚԙɞɚɪ
54
55.
Ȼɿɪɿɧɲɿ ɰɢɥɢɧɞɪɞɿԙ ԛɫɬɿɧ ɠɚɭɵɩ ɬԝɪԑɚɧ ɲɵɧɵɧɵ ɚɥɵɩ ɬɚɫɬɚɩ ɚԕ ԕɚԑɚɡɠɚɛɵԙɞɚɪ ɷɤɪɚɧ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ƚɚɡɞɵԙ ɬԛɫɿ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ" ȿɤɿɧɲɿ
ɰɢɥɢɧɞɪɝɟ ɠɚɧɵɩ ɬԝɪԑɚɧ ɚԑɚɲ ɠɚԙԕɚɫɵɧ ɠɚԕɵɧɞɚɬɵԙɞɚɪ ɇɟ
ɛɚɣԕɚɥɚɞɵ" Ȼɚɣԕɚԑɚɧ ԕԝɛɵɥɵɫɬɚɪɞɵ ɬԛɫɿɧɞɿɪɿԙɞɟɪ ɠԥɧɟ ɪɟɚɤɰɢɹɥɚɪɞɵԙ
ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
25-cɭɪɟɬ Ⱥɡɨɬ ɨɤɫɢɞɿɧ ɚɥɭ ɩɪɢɛɨɪɵ
10-ɬԥɠɿɪɢɛɟ Ⱥɡɨɬɬɵԙ ȱ9 ɨɤɫɢɞɿɧɿԙ ɚɥɵɧɭɵ
Ⱥɭɚ ɫɨɪԑɵɲ ɲɤɚɮɬɵԙ ɚɫɬɵɧɞɚ ɠԝɦɵɫ ɿɫɬɟԙɞɟɪ ɉɪɨɛɢɪɤɚԑɚ ɦɵɫ
ԕɚɛɵɪɲɚԕɬɚɪɵɧ ɫɚɥɵɩ ԛɫɬɿɧɟ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ - ɦɥ ɚɡɨɬ ԕɵɲԕɵɥɵɧ
ԕԝɣɵԙɞɚɪ Ԕɨԙɵɪ ɬԛɫɬɿ ɝɚɡ ɛԧɥɿɧɟɞɿ ɨɧɵ ɰɢɥɢɧɞɪɝɟ ɠɢɧɚɩ ɚɥɵԙɞɚɪ
ɨɞɚɧ ɤɟɣɿɧ ɛɿɪɧɟɲɟ ɦɥ ɫɭ ԕɨɫɵԙɞɚɪ ԕɚɬɬɵ ɚɪɚɥɚɫɬɵɪɵԙɞɚɪ ɇɟ
ɛɚɣԕɚɥɚɞɵ" Ⱥɥɵɧԑɚɧ ɟɪɿɬɿɧɞɿɝɟ ɛɿɪ-ɟɤɿ ɬɚɦɲɵ ɥɚɤɦɭɫ ԕɨɫɵԙɞɚɪ
Ɋɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
11-ɬԥɠɿɪɢɛɟ Ⱥɡɨɬɬɵ ԕɵɲԕɵɥɞɵԙ ɬԛɡɿɥɭɿ ɦɟɧ ɵɞɵɪɚɭɵ
ɉɪɨɛɢɪɤɚɞɚԑɵ - ɦɥ ɧɚɬɪɢɣ ɧɢɬɪɢɬɿɧɿԙ ɟɪɿɬɿɧɞɿɫɿɧɟ
ɫԝɣɵɬɵɥԑɚɧ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɧ ԕɨɫɵԙɞɚɪ ȿɪɿɬɿɧɞɿɧɿԙ ɬԛɫɿɧ ɠԥɧɟ
ɛԧɥɿɧɿɩ ɲɵԕԕɚɧ ɝɚɡɞɵԙ ɢɿɫɿɧ ɛɚɣԕɵɥɚԙɞɚɪ Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ
ɠɚɡɵԙɞɚɪ
12-ɬԥɠɿɪɢɛɟ Ⱥɡɨɬɬɵ ԕɵɲԕɵɥ ɬԝɡɞɚɪɵɧɵԙ ɬɨɬɵԑɭ-ɬɨɬɵԕɫɵɡɞɚɧɭ
ԕɚɫɢɟɬɬɟɪɿ
ɚ ɩɪɨɛɢɪɤɚԑɚ .ȱ ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵɩ ɨɧɵԙ ԛɫɬɿɧɟ 1D1Ɉ2 ɠԥɧɟ
ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɧ ԕɨɫɵԙɞɚɪ Ԕɚɧɞɚɣ ԧɡɝɟɪɿɫɬɟɪ ɛɚɣԕɚɥɚɞɵ"
ɛ ɉɪɨɛɢɪɤɚԑɚ .0Q24 ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɦɟɧ
ԕɵɲԕɵɥɞɚԙɞɚɪ ɠԥɧɟ ɚɡɞɚԑɚɧ ɦԧɥɲɟɪɞɟ 1D122 ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɵɪ ɇɟ
ɛɚɣԕɚɥɚɞɵ" Ȼɚɪɥɵԕ ɠԛɪɝɟɧ ɪɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
13-ɬԥɠɿɪɢɛɟ Ⱥɡɨɬ ԕɵɲԕɵɥɵɧɵԙ ɬɨɬɵԕɬɵɪԑɵɲɬɵԕ ԕɚɫɢɟɬɿ
Ⱥɭɚ ɫɨɪԑɵɲ ɲɤɚɮɬɚ ɠԝɦɵɫ ɿɫɬɟԙɞɟɪ
ɚ ɩɪɨɛɢɪɤɚԑɚ - ɬɚɦɲɵ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ ɚɡɨɬ ԕɵɲԕɵɥɵɧ
ԕԝɣɵԙɞɚɪ ɲɬɚɬɢɜɤɟ ɛɟɤɿɬɿԙɞɟɪ ԕɵɡɞɵɪɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ȼԧɥɿɧɿɩ
ɲɵԕԕɚɧ ɝɚɡɞɵ ɠɚɧɵɩ ɬԝɪԑɚɧ ɚԑɚ ɠɚԙԕɚɫɵɦɟɧ ɬɟɤɫɟɪɿԙɞɟɪ Ԕɚɧɞɚɣ
55
56.
ԧɡɝɟɪɿɫɬɟɪ ɛɚɣԕɚɥɚɞɵ" Ⱥɡɨɬ ԕɵɲԕɵɥɵɧɵԙ ɵɞɵɪɚɭ ɪɟɚɤɰɢɹɫɵɧɵԙɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
ɛ ɛɿɪɿɧɲɿ ɩɪɨɛɢɪɤɚԑɚ ɦɵɪɵɲ ɦɟɬɚɥɞɵ ɟɤɿɧɲɿɫɿɧɟ ԕɚɥɚɣɵ
ɦɟɬɚɥɵɧ ɫɚɥɵԙɞɚɪ ɟɤɿ ɩɪɨɛɢɪɤɚԑɚ ɞɚ - ɬɚɦɲɵ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ ɚɡɨɬ
ԕɵɲԕɵɥɵɧ ԕԝɣɵԙɞɚɪ Ԕɚɧɞɚɣ ɝɚɡ ɛԧɥɿɧɿɩ ɲɵԑɚɞɵ" Ɋɟɚɤɰɢɹɥɚɪɞɵԙ
ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
ɜ ɩɪɨɛɢɪɤɚԑɚ ɧ - ɦɥ ɬԝɡ ԕɵɲԕɵɥɵɧ ԕԝɣɵԙɞɚɪ ɨԑɚɧ ɚɥɸɦɢɧɢɣ
ɦɟɬɚɥɥ ɫɚɥɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ⱥɥɸɦɢɧɢɣ ɦɟɬɚɥɞɵ ԕɵɲԕɵɥɞɚɧ ɚɥɵɩ
ɫɭɦɟɧ ɠɭɵɩ ɮɢɥɶɬɪ ԕɚԑɚɡɵɦɟɧ ԕԝɪԑɚɬɵɩ ɚɡɨɬ ԕɵɲԕɵɥɵ ɟɪɿɬɿɧɞɿɫɿɧɟ
ɫɚɥɵԙɞɚɪ - ɦɢɧɭɬ ԧɬɤɟɧ ɫɨԙ ɚɥɸɦɢɧɢɣ ɦɟɬɚɥɞɵ ԕɵɲԕɵɥɞɚɧ ɚɥɵɩ
ɫɭɦɟɧ ɠɭɵɩ ԕɚɣɬɚɞɚɧ ɬԝɡ ԕɵɲԕɵɥɵ ɟɪɿɬɿɧɞɿɫɿɧɟ ɫɚɥɵԙɞɚɪ Ƚɚɡ ɫɭɬɟɤ
ɛԧɥɿɧɟɞɿ ɦɟ" Ȼɚɪɥɵԕ ɩɪɨɰɟɫɬɟɪɞɿ ɬԛɫɿɧɞɿɪɿԙɞɟɪ
ɝ ɉɪɨɛɢɪɤɚԑɚ ɬɟɦɿɪɞɿԙ ԕɚɛɵɪɲɚԕɬɚɪɵɧ ɫɚɥɵԙɞɚɪ ԛɫɬɿɧɟ
ɫԝɣɵɬɵɥԑɚɧ ɚɡɨɬ ԕɵɲԕɵɥɵɧ ԕԝɣɵԙɞɚɪ Ԕɚɧɞɚɣ ɝɚɡ ɛԧɥɿɧɟɞɿ"
Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
ɞ ɩɪɨɛɢɪɤɚԑɚ ɫԝɣɵɬɵɥԑɚɧ ɚɡɨɬ ԕɵɲԕɵɥɵɧ ԕԝɣɵԙɞɚɪ ɛɿɪ
ɩɪɨɛɢɪɤɚԑɚ ɦɵɪɵɲ ± ɦɟɬɚɥɥ ɟɤɿɧɲɿɫɿɧɟ-ԕɚɥɚɣɵ ɦɟɬɚɥɥ ɫɚɥɵԙɞɚɪ
ɛɿɪɧɟɲɟ ɦɢɧɭɬ ԕɚɬɬɵ ɚɪɚɥɚɫɬɵɪɵԙɞɚɪ ȿɪɿɬɿɧɞɿɧɿ ɦɟɬɚɥɞɚɪɞɚɧ ɛԧɥɿɩ
ɚɥɵԙɞɚɪ ԕԝɪɚɦɵɧɞɚ 1+4+ ɢɨɧɵ ɛɚɪ ɟɤɟɧɿɧ ɞԥɥɟɥɞɟԙɞɟɪ Ɋɟɚɤɰɢɹɥɚɪɞɵԙ
ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
ɟ ɉɪɨɛɢɪɤɚԑɚ ɦɵɫ ɫɭɥɶɮɢɞɿɧ ɫɚɥɵԙɞɚɪ ԛɫɬɿɧɟ - ɦɥ
ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ +123 ԕԝɣɵԙɞɚɪ Ɋɟɚɤɰɢɹ ɧԥɬɢɠɟɫɿɧɞɟ ɦɵɫ ɫɭɥɶɮɢɞɿ
ɟɪɿɞɿ Ɉɫɵɧɵ ɬԛɫɿɧɞɿɪɿԙɞɟɪ ɠԥɧɟ ɪɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
14-ɬԥɠɿɪɢɛɟ Ɏɨɫɮɨɪ ɝɢɞɪɢɞɿ ɠԥɧɟ ɨɧɵԙ ԕɚɫɢɟɬɬɟɪɿ
Ɏɨɫɮɨɪ ɬɢɝɟɥɶɝɟ ɤɚɥɶɰɢɣ ɮɨɫɮɨɪɢɞɿɧɿԙ ɋɚ3Ɋ2 ɤɿɲɤɟɧɬɚɣ ɛɿɪ
ɛԧɥɿɝɿɧ ɫɚɥɵԙɞɚɪ ɧ ɬԝɡ ԕɵɲԕɵɥɵɧ ԕԝɣɵԙɞɚɪ Ԕɚɧɞɚɣ ɝɚɡ ɛԧɥɿɧɟɞɿ"
Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
15-ɬԥɠɿɪɢɛɟ Ɏɨɫɮɨɪ 9 ɨɤɫɢɞɿɧ ɚɥɭ ɠɨɥɵ
Ɏɚɪɮɨɪ ɵɞɵɫɬɵ ɚɫɛɟɫɬ ɫɟɬɤɚԑɚ ԕɨɣɵɩ - ɝ ԕɵɡɵɥ ɮɨɫɮɨɪ
ɿɲɿɧɟ ɫɚɥɵԙɞɚɪ ɕɞɵɫɬɵԙ ԛɫɬɿɧɟɧ ɫɦ ɠɨԑɚɪɵ ԕԝɪԑɚԕ ɜɨɪɨɧɤɚ
ɛɟɤɿɬɿԙɞɟɪ Ɏɨɫɮɨɪɞɵ ԕɵɡɞɵɪԑɚɧ ɲɵɧɵ ɬɚɹԕɩɟɧ ɠɚɧɞɵɪɵԙɞɚɪ ɇɟ
ɛɚɣԕɚɥɚɞɵ" ȼɨɪɨɧɤɚɧɵԙ ɛɟɬɿɧɞɟ ԕɚɧɞɚɣ ԕɨɫɵɥɵɫ ɬԛɡɿɥɟɞɿ" Ɋɟɚɤɰɢɹɧɵԙ
ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
16-ɬԥɠɿɪɢɛɟ Ɏɨɫɮɨɪ ԕɵɲԕɵɥɞɚɪɵɧɵԙ ɚɥɵɧɭ ɠɨɥɞɚɪɵ
Ⱥɭɚ ɫɨɪԑɵɲ ɲɤɚɮɬɵԙ ɚɫɬɵɧɞɚ ɠԝɦɵɫ ɿɫɬɟԙɞɟɪ
ɚ ɋɚԑɚɬ ɱɚɫɨɜɨɟ ɲɵɧɵɫɵɧɚ ɮɨɫɮɨɪ ɚɧɝɢɞɪɢɞɿɧ Ɋ2Ɉ5 ɫɚɥɵԙɞɚɪ
Ⱥɭɚɧɵԙ ԥɫɟɪɿɧ ɛɚɣԕɚԙɞɚɪ Ԕɚɧɞɚɣ ԧɡɝɟɪɿɫ ɛɨɥɚɞɵ" Ɋɟɚɤɰɢɹ ɬɟԙɞɟɭɿɧ
ɠɚɡɵԙɞɚɪ
ɛ ɩɪɨɛɢɪɤɚԑɚ ԝɧɬɚԕɬɚɥԑɚɧ ɤɚɥɶɰɢɣ ɮɨɫɮɨɪɢɬɿɧ ɫɚɥɵԙɞɚɪ ԛɫɬɿɧɟ
ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɧ ԕԝɣɵԙɞɚɪ Ԕɨɫɩɚɧɵ ԕɚɣɧɚɬɵԙɞɚɪ ɬԝɧɛɚɧɵ
ɮɢɥɶɬɪɚɰɢɹ ɚɪԕɵɥɵ ɛԧɥɿԙɞɟɪ ɚɦɦɨɧɢɣ ɦɨɥɢɛɞɚɬɵ ɟɪɿɬɿɧɞɿɫɿɧɞɟ ɮɨɫɮɨɪ
56
57.
ԕɵɲԕɵɥɵ ɛɚɪ ɟɤɟɧɿɧ ɚɧɵԕɬɚԙɞɚɪ ɦɨɥɢɛɞɟɧ ɚɦɦɨɧɢɣ ɟɪɿɬɿɧɞɿɫɿɧɩɚɣɞɚɥɚɧɵɩ Ɋɚɟɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
17-ɬԥɠɿɪɢɛɟ Ɏɨɫɮɨɪ ԕɵɲԕɵɥɵɧɵԙ ɬԝɡɞɚɪɵ
ɚ ɩɪɨɛɢɪɤɚԑɚ ɮɨɫɮɨɪ ԕɵɲԕɵɥɵɧɵԙ ɬԝɡɞɚɪɵɧ ɚɥɵԙɞɚɪ ɛɿɪɿɧɲɿ
ɩɪɨɛɢɪɤɚԑɚ ± ɧɚɬɪɢɣ ɮɨɫɮɚɬɵɧ ɟɤɿɧɲɿɫɿɧɟ-ɧɚɬɪɢɣ ɝɢɞɪɨɮɨɫɮɚɬɵɧ
ԛɲɿɧɲɿɫɿɧɟ ɧɚɬɪɢɣ ɞɢɝɢɞɪɨɮɨɫɮɚɬɵɧ Ȼɚɪɥɵԕ ɩɪɨɛɢɪɤɚԑɚ ɥɚɤɦɭɫ
ԕԝɣɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" ɍɧɢɜɟɪɫɚɥ ɢɧɞɢɤɚɬɨɪ ɚɪԕɵɥɵ ɪɇ ɚɧɵԕɬɚԙɞɚɪ
Ƚɢɞɪɨɥɢɡ ɪɟɚɤɰɢɹɥɚɪɵɧɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
ɛ Ʉɚɥɶɰɢɣ ɮɨɫɮɚɬɵɧ ɚɥɭ ɠɨɥɵ Ʌɚɛɨɪɚɬɨɪɢɹɞɚԑɵ ɪɟɚɤɬɢɜɬɟɪɞɿ
ɩɚɣɞɚɥɚɧɵɩ ɤɚɥɶɰɢɣɞɿԙ ɮɨɫɮɚɬɵɧ ɝɢɞɪɨ ɠԥɧɟ ɞɢɝɢɞɪɨɮɨɫɮɚɬɬɚɪɵɧ
ɚɥɵԙɞɚɪ Ɋɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ Ɍԛɡɿɥɝɟɧ ɬԝɡɞɚɪɞɵԙ
ɫɭɞɚ ɟɪɭɿɧ ɛɚɣԕɚԙɞɚɪ CaHPO4 ɬԝɧɛɚԑɚ ɫɿɪɤɟ ԕɵɲԕɵɥɵɧ ԕɨɫɵԙɞɚɪ ɇɟ
ɛɚɣԕɚɥɚɞɵ" Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫ 115
Ɍɚԕɵɪɵɛɵ ȱ9 ɬɨɩ ɪ-ɷɥɟɦɟɧɬɬɟɪɿ Ʉԧɦɿɪɬɟɤ ɤɪɟɦɧɢɣ
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɠԝɦɵɫɬɵԙ ɦɚԕɫɚɬɵ Ʉԧɦɿɪɬɟɤ ɤɪɟɦɧɢɣ ɠԥɧɟ
ɨɥɚɪɞɵԙ ԕɨɫɵɥɵɫɬɚɪɵɧɵԙ ɚɥɵɧɭ ɠɨɥɞɚɪɵɧ ɠԥɧɟ ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿɧ
ɨԕɵɩ ԛɣɪɟɧɭ
Ȼɚԕɵɥɚɭ ɠԝɦɵɫɵɧɵԙ ɫԝɪɚԕɬɚɪɵ ɦɟɧ ɠɚɬɬɵԑɭɥɚɪɵ
1. Ʉԧɦɿɪɬɟɤ ɬɨɩɲɚɫɵɧɵԙ ɷɥɟɦɟɧɬɬɟɪɿɧɿԙ ɠɚɥɩɵ ɫɢɩɚɬɬɚɦɚɫɵ
2. Ʉԧɦɿɪɬɟɤ ɠԥɧɟ ɤɪɟɦɧɢɣ ɚɬɨɦɵɧɵԙ ɷɥɟɤɬɪɨɧɞɵԕ ԕԝɪɵɥɵɫɵ ɬɨɬɵԑɭ
ɞԥɪɟɠɟɫɿ ɝɢɛɪɢɞɬɟɧɭ ɬԛɪɥɟɪɿ Ʉԧɦɿɪɬɟɤ ԕɨɫɵɥɵɫɬɚɪɵɧɵԙ ԥɪ ɬԛɪɥɟɪɿ
Ɉɧɵԙ ɫɟɛɟɩɬɟɪɿ
3. Ʉԧɦɿɪɬɟɤ ɠԥɧɟ ɤɪɟɦɧɢɣ Ⱥɥɥɨɬɪɨɩɢɹɥɵԕ ɬԛɪ ԧɡɝɟɪɿɫɬɟɪɿ ɮɢɡɢɤɚɥɵԕ
ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿ ԕɨɥɞɚɧɵɥɭɵ
4. Ʉԧɦɿɪɬɟɤɬɿԙ ɠԥɧɟ ɤɪɟɦɧɢɣɞɿԙ ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿ Ⱥɦɦɨɧɢɣ
ɤɚɪɛɢɞɿ Ⱥɥɵɧɭ ɠɨɥɞɚɪɵ ԕɨɥɞɚɧɵɥɭɵ ɫɭɦɟɧ ԥɪɟɤɟɬɬɟɫɭɿ
5. Ʉԧɦɿɪɬɟɤɬɿԙ ɨɤɫɢɞɬɟɪɿ ȱȱ ȱ9 ɨɥɚɪɞɵԙ ԕԝɪɵɥɵɫɵ ɚɥɭ ɠɨɥɞɚɪɵ
ɮɢɡɢɤɚɥɵԕ ɠԥɧɟ ɯɢɦɢɹɥɵԕ ԕɚɫɢɟɬɬɟɪɿ
6. ɒɵɧɵ ɰɟɦɟɧɬ ɫɢɬɚɥɞɚɪ ɨɥɚɪɞɵԙ ԕɨɥɞɚɧɵɥɭɵ
7. ȿɫɟɩ ɝ ɫɭɞɚ ɝ ɤɪɢɫɬɚɥɞɵԕ ɫɨɞɚ ɟɪɿɝɟɧ ȿɝɟɪ ɞɟ U ɝ ɫɦ3
ɬɟԙ ɛɨɥɫɚ ɫɭɫɵɡ ɬԝɡɞɵԙ ɟɪɿɬɿɧɞɿɫɿɧɿԙ ɬɢɬɪɿɧ ɦɚɫɫɚɥɵԕ ԛɥɟɫɿɧ
ɦɨɥɹɪɥɵԕ ɠԥɧɟ ɷɤɜɢɜɚɥɟɧɬɬɿɤ ɤɨɧɰɟɧɬɪɚɰɢɹɫɵɧ ɟɫɟɩɬɟԙɞɟɪ"
8. ȿɫɟɩ ȿɝɟɪ ɞɟ ɤԧɦɿɪ ԕɵɲԕɵɥɵ ɬɟɤ ԕɚɧɚ ɛɿɪɿɧɲɿ ɫɚɬɵɦɟɧ
ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɫɚ ɦɨɥɹɪɥɵԕ ɤԧɦɿɪ ԕɵɲԕɵɥɵ ɟɪɿɬɿɧɞɿɫɿɧɿԙ ɪɇ
ɠԥɧɟ D ɟɫɟɩɬɟԙɞɟɪ"
9. ȿɫɟɩ ȿɝɟɪ ɞɟ ɚ ɵɞɵɪɚɬԕɚɧɞɚ ɛ ԕɵɲԕɵɥ ԕɨɫԕɚɧɞɚ ɝ NaHCO3
ԕ ɠ ԕɚɧɲɚ ɤԧɥɟɦ ɋɈ2 ɚɥɭԑɚ ɛɨɥɚɞɵ"
10.ȿɫɟɩ Ʉɚɥɶɰɢɣ ɤɚɪɛɢɞɿ ɦɵɧɚ ɪɟɚɤɰɢɹ ɚɪԕɵɥɵ ɬԛɡɿɥɟɞɿ
57
58.
ɋɚɈ ɋoɋɚɋ2 ɋɈɝ ɋɚɋ2 ɚɥɭ ԛɲɿɧ ԕɚɧɲɚ ɋɚɈ ɠԝɦɫɚɥɚɞɵ" Ԕɚɧɞɚɣ ɤԧɥɟɦ ԕ ɠ
ɋɈ ɛԧɥɿɧɟɞɿ"
11.ȿɫɟɩ 0ɋ ԕɵɫɵɦɵ Ɋ ɦɦ ɫɵɧ ɛɚԑ ɦ3ɫɭɬɟɤ ɚɥɭ ԛɲɿɧ ԕɚɧɲɚ
ɤԧɥɟɦ ɦɚɫɫɚɥɵԕ ԛɥɟɫɿ ±ɬɿɤ ɪ ɝ ɫɦ3/ NaOɇ ɟɪɿɬɿɧɞɿɫɿ ɠԥɧɟ
ԕɚɧɲɚ ɤɝ ɤɪɟɦɧɢɣ ɠԝɦɫɚɥɚɞɵ" 6ɿ 1D2+ +2+Na26ɿ23
12.ȿɫɟɩ -ɬɿɤ ɝ ɧɚɬɪɢɣ ɫɢɥɢɤɚɬɵɧ ɞɚɣɵɧɞɚɭ ԛɲɿɧ ԕɚɧɲɚ
Na2SɿO3 ɇ2Ɉ ԕɚɠɟɬ"
13.Ɍԧɦɟɧɞɟɝɿ ɪɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ԕԝɪɚɫɬɵɪɵԙɞɚɪ
6ɿo6ɿ+4o6ɿ22 oNa26ɿ23oH26ɿ23oNa26ɿ2O5
Ʌɚɛɨɪɚɬɨɪɢɹɥɵԕ ɬԥɠɿɪɢɛɟɥɟɪ
Ⱥɩɩɚɪɚɬɬɚɪ ɠԥɧɟ ɵɞɵɫɬɚɪ Ɍɟɯɧɢɤɚɥɵԕ ɬɚɪɚɡɵ Ʉɢɩɩ ɚɩɩɚɪɚɬɵ
ɫɵɣɵɦɞɵɥɵԑɵ ɦɥ ԧɥɲɟɭɿɲ ɰɢɥɢɧɞɪ ɠɚɧɞɵɪԑɵɲ ɮɚɪɮɨɪ ɤɟɥɿ
ɰɢɥɢɧɞɪ ɩɪɨɛɢɪɤɚɫɵ ɛɚɪ ɲɬɚɬɢɜ ɬɢɝɟɥɶ ԕɵɫԕɵɲɬɚɪɵ ɤɟɫɤɿɲ ɬɟɦɿɪ
ԕɚɫɵԕɲɚ ɲɵɧɵ ɬɚɹԕɲɚ ɲɵɧɵ ɩɥɚɫɬɢɧɤɚɥɚɪ ɤɪɢɫɬɚɥɥɢɡɚɬɨɪ ԕɵɫԕɵɲɵ
ɛɚɪ ɬɟɦɿɪ ɲɬɚɬɢɜ ɜɨɪɨɧɤɚ ɬԛɬɿɤɲɟ
Ɍɟɯɧɢɤɚɥɵԕ ɬɚɪɚɡɵ Ʉɢɩɩ ɚɩɩɚɪɚɬɵ ԕɵɫԕɵɲɵ ɛɚɪ ɲɬɚɬɢɜ
ɠɚɧɞɵɪԑɵɲ ɮɚɪɮɨɪ ɤɟɥɿɫɿ ɮɚɪɮɨɪ ԝɲɛԝɪɵɲɵ ɫɵɣɵɦɞɵɥɵԑɵ ɦɥ
ɫɬɚԕɚɧɞɚɪ ɜɨɪɨɧɤɚ ɬɟɦɿɪ ɬɢɝɟɥɿ ɩɪɨɛɢɪɤɚɥɚɪɵ ɛɚɪ ɲɬɚɬɢɜ ɲɵɧɵ
ɬɚɹԕɲɚ
Ɋɟɚɤɬɢɜɬɟɪ ɠԥɧɟ ɦɚɬɟɪɢɚɥɞɚɪ ɚԑɚɲ ɠɚԙԕɚɥɚɪɵ ɚɤɬɢɜɬɟɥɿɧɝɟɧ ɚԑɚɲ
ɤԧɦɿɪ ɦɚɝɧɢɣ ɥɟɧɬɚɫɵ ԕɵɡɵɥ ɮɨɫɮɨɪ ɦɵɫ ȱȱ ɨɤɫɢɞɿ ɧɚɬɪɨɧ ԥɝɿ ɬɟɦɿɪ
ȱȱ ɫɭɥɶɮɢɞɿ ɤɚɥɶɰɢɣ ɤɚɪɛɢɞɿ ɤɚɥɶɰɢɣ ɤɚɪɛɨɧɚɬɵ ɦɪɚɦɨɪ ɧɟɦɟɫɟ ɛɨɪ
ɦɚɝɧɢɣ ɤɚɪɛɨɧɚɬɵ ɧɚɬɪɢɣ ɝɢɞɪɨɤɚɪɛɨɧɚɬɵ ɧɚɬɪɢɣ ɤɚɪɛɨɧɚɬɵ ɧɚɬɪɢɣ
ɚɰɟɬɚɬɵ ɷɬɢɥ ɫɩɢɪɬɿ ԕԝɦɵɪɫԕɚ ԕɵɲԕɵɥɵ ԕɵɦɵɡɞɵԕ ԕɵɲԕɵɥɵ ɥɚɤɦɭɫ
ԕɚԑɚɡɵ ɦɚԕɬɚ ɫԛɡɝɿ ԕɚԑɚɡɵ ɫɵɣɵɦɞɵɥɵԑɵ ɦɥ ɫɬɚԕɚɧɞɚɪ
Ɇɚɝɧɢɣ ԝɧɬɚԑɵ ɤɜɚɪɰ ԝɧɬɚԑɵ ɧɚɬɪɢɣ ɝɢɞɪɨɤɫɢɞɿ ɤɚɥɢɣ ɤɚɪɛɨɧɚɬɵ
ɤɚɥɶɰɢɣ ɮɬɨɪɢɞɿ ɦɪɚɦɨɪ ɧɟɦɟɫɟ ɛɨɪ ɧɚɬɪɢɣ ɤɚɪɛɨɧɚɬɵ ɬɟɦɿɪ
ԕɚԙɵɥɬɵɪɵ ɥɚɤɦɭɫ ԕɚԑɚɡɵ ɫԛɡɝɿ ԕɚԑɚɡɵ ɲɵɧɵ ɬɚɹԕɲɚ ɫɚԕɬɚɧɞɵɪԑɵɲ
ɤԧɡɿɥɞɿɪɿɤ
ȿɪɿɬɿɧɞɿɥɟɪ Ʉԛɤɿɪɬ ԕɵɲԕɵɥɵ ɤɨɧɰ ɫԝɣɵɬɵɥԑɚɧ ɬԝɡ ԕɵɲԕɵɥɵ
ɧ ɧɚɬɪɢɣ ɝɢɞɪɨɤɫɢɞɿ ɧ ɚɦɦɢɚɤ ɧ ɤɚɥɢɣ ɤɚɪɛɨɧɚɬɵ ɧ
ɧɚɬɪɢɣ ɝɢɞɪɨɤɚɪɛɨɧɚɬɵ ɧ ɧɚɬɪɢɣ ɤɚɪɛɨɧɚɬɵ ɧ ԕɨɪԑɚɫɵɧ ɧɢɬɪɚɬɵ
ɧ ɧ ɤɚɥɢɣ ɢɨɞɢɞɿ ɧ ɤԛɦɿɫ ɧɢɬɪɚɬɵ ɧ ɤɚɥɶɰɢɣ
ɝɢɞɪɨɤɫɢɞɿ ԕɚɧɵԕ ɥɚɤɦɭɫɬɵԙ ɛɟɣɬɚɪɚɩ ɟɪɿɬɿɧɞɿɫɿ ɮɭɤɫɢɧ ɟɪɿɬɿɧɞɿɫɿ
ԕɨɪԑɚɫɵɧ ɚɰɟɬɚɬɵ ɧ ɧɚɬɪɢɣ ɫɭɥɶɮɢɞɿ ɧ
Ʉԛɤɿɪɬ ԕɵɲԕɵɥɵ ɤɨɧɰ ɬɵԑ ɬԝɡ ԕɵɲԕɵɥɵ ɤɨɧɰ ɬɵԑ ɠԥɧɟ
ɫԝɣɵɬ ɧɚɬɪɢɣ ɝɢɞɪɨɤɫɢɞɿ ɚɦɦɨɧɢɣ ɯɥɨɪɢɞɿ ԕɚɧɵԕԕɚɧ ɧɚɬɪɢɣ
ɫɢɥɢɤɚɬɵ ɤɨɧɰ ɮɟɧɨɥɮɬɚɥɟɢɧ ɟɪɿɬɿɧɞɿɫɿ
58
59.
1-ɬԥɠɿɪɢɛɟ Ʉԧɦɿɪɬɟɤ ɨɤɫɢɞɿɧ ȱȱ ɚɥɭ ɠɨɥɵɚ Ʉɢɩɩ ɚɩɩɚɪɚɬɵɧɚ ɢɡɜɟɫɬɶ ɚԕ ɬɚɫɬɵԙ ɛԧɥɲɟɤɬɟɪɿɧ ɫɚɥɵԙɞɚɪ
ɬԝɡ ԕɵɲԕɵɥɵɧ ԕɨɫɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ɍԝɡ ԕɵɲԕɵɥɵɧɵԙ ɨɪɧɵɧɚ
ɤԛɤɿɪɬ ԕɵɲԕɵɥɵɧ ɩɚɣɞɚɥɚɧɭԑɚ ɛɨɥɚ ɦɚ"
ɛ Ʉɢɩɩ ɚɩɩɚɪɚɬɵɧɞɚԑɵ ɛԧɥɿɧɿɩ ɲɵԕԕɚɧ ɝɚɡɞɵ ɫɭɵ ɛɚɪ ɩɪɨɛɢɪɤɚԑɚ
ɠɿɛɟɪɿԙɞɟɪ ɉɚɣɞɚ ɛɨɥԑɚɧ ɟɪɿɬɿɧɞɿɧɿ ɢɧɞɢɤɚɬɨɪɦɟɧ ɬɟɤɫɟɪɿԙɞɟɪ Ʉɢɩɩ
ɚɩɩɚɪɚɬɵɧɞɚ ɠԛɪɟɬɿɧ ɠԥɧɟ ɋɈ2 ɫɭɞɚ ɟɪɭ ɪɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ
ɠɚɡɵԙɞɚɪ
ɜ ɋɭɞɚ ɟɪɿɝɟɧ ɋɈ2 ɟɪɿɬɿɧɞɿɝɟ ɥɚɤɦɭɫ ԕԝɣɵԙɞɚɪ ԕɚɣɧɚɬɵԙɞɚɪ ɇɟ
ɛɚɣԕɚɥɚɞɵ" ȿɪɿɬɿɧɞɿɧɿԙ ɬԛɫɿ ԕɚɥɚɣ ԧɡɝɟɪɟɞɿ" Ɍɟɩɟ-ɬɟԙɞɿɤɬɿԙ ɬɟԙɞɟɭɿɧ
ɠɚɡɵԙɞɚɪ
ɝ Ȼɿɪ ɫɬɚɤɚɧɞɵ ɋɈ2- ɦɟɧ ɬɨɥɬɵɪɵԙɞɚɪ ɬɟɦɿɪ ԕɚɫɵԕɬɚ ԕɵɡɵɥ
ɮɨɫɮɨɪɞɵ ɠɚɧɞɵɪɵɩ ɫɬɚɤɚɧԑɚ ɫɚɥɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ɍԛɡɿɥɝɟɧ ɡɚɬɬɵ
ɫɭɞɚ ɟɪɿɬɿԙɞɟɪ ɥɚɤɦɭɫ ɚɪԕɵɥɵ ɬɟɤɫɟɪɿԙɞɟɪ Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ
ɠɚɡɵԙɞɚɪ
2-ɬԥɠɿɪɢɛɟ Ʉԧɦɿɪ ԕɵɲԕɵɥɵɧɵԙ ɬԝɡɞɚɪɵɧɵԙ ɬԛɡɿɥɭɿ
ɚ ɉɪɨɛɢɪɤɚԑɚ ɚԕ ԥɤɬɿԙ ɫɭɵɧ ԕԝɣɵԙɞɚɪ - ɦɢɧ Ʉɢɩɩ
ɚɩɩɚɪɚɬɵɧɚɧ ɋɈ2 ɝɚɡ ɠɿɛɟɪɿԙɞɟɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ
ɠɚɡɵԙɞɚɪ Ɍԛɡɿɥɝɟɧ ɬԝɡɞɚɪɞɵԙ ɫɬɪɭɤɬɭɪɚɥɵԕ ɮɨɪɦɭɥɚɫɵɧ ɠɚɡɵԙɞɚɪ
ɠԥɧɟ ɫɭɞɚ ɟɪɿɝɿɲɬɿɝɿɧ ɛɚɣԕɚԙɞɚɪ Ɍԛɡɿɥɝɟɧ ɟɪɿɬɿɧɞɿɧɿ ɤɟɥɟɫɿ ɬԥɠɿɪɢɛɟɝɟ
ɫɚԕɬɚԙɞɚɪ
ɛ - ɬԥɠɿɪɢɛɟɞɟɝɿ ɟɪɿɬɿɧɞɿɧɿ ɩɪɨɛɢɪɤɚԑɚ ɛԧɥɿԙɞɟɪ ɛɿɪ
ɩɪɨɛɢɪɤɚɧɵ ԕɵɡɞɵɪɵԙɞɚɪ ɟɤɿɧɲɿɝɟ ԥɤ ɫɭɵɧ ԕԝɣɵԙɞɚɪ Ԕɚɧɞɚɣ
ԧɡɝɟɪɿɫɬɟɪ ɛɚɣԕɚɥɚɞɵ" Ɋɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
ɜ ɉɪɨɛɢɪɤɚɧɵ ɋɈ2- ɦɟɧ ɬɨɥɬɵɪɵɩ ɬɵԑɵɧɦɟɧ ɠɚɭɵɩ 1D2+
ɟɪɿɬɿɧɞɿɫɿ ɛɚɪ ɤɪɢɫɬɚɥɢɡɚɬɨɪԑɚ ɚɭɞɚɪɵԙɞɚɪ ɬɵԑɵɧɞɵ ɚɲɵԙɞɚɪ
Ԧɡɝɟɪɿɫɬɟɪɞɿ ɬԛɫɿɧɞɿɪɿԙɞɟɪ Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
3-ɬԥɠɿɪɢɛɟ Ʉԧɦɿɪ ԕɵɲԕɵɥɵ ɬԝɡɞɚɪɵɧɵԙ ԕɚɫɢɟɬɬɟɪɿ
ɚ Ɍԧɦɟɧɞɟɝɿ ɬԝɡɞɚɪɞɵ 1D2SO4, MgCO3, CaCO3 ɫɭԑɚ +&ȱ ɠԥɧɟ
CH3&22+ ԕɚɬɵɧɚɫɵɧ ɛɚɣԕɚԙɞɚɪ Ɋɟɚɤɰɢɹɥɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ
ɦɨɥɟɤɭɥɚɥɵԕ ɠԥɧɟ ɢɨɧɞɵԕ ɬԛɪɿɧɞɟ ɠɚɡɵԙɞɚɪ
ɛ Ɇɵɧɚ ɬԝɡɞɚɪɞɵԙ NaHCO3, Na2CO3, CaCO3 ɚɭɡɵ ɢɦɟɤ ɬԛɬɿɤɲɟɫɿ
ɛɚɪ ԥɪ ԕɚɣɫɵɫɵɧ ɩɪɨɛɢɪɤɚԑɚ ɫɚɥɵԙɞɚɪ ԕɵɡɞɵɪɵԙɞɚɪ Ɍɪɭɛɤɚɧɵԙ ԝɲɵɧ
ԥɤ ɫɭɵ ɛɚɪ ɩɪɨɛɢɪɤɚԑɚ ɛɚɬɵɪɵԙɞɚɪ Ԕɚɧɞɚɣ ԧɡɝɟɪɿɫɬɟɪ ɠԛɪɟɞɿ"
Ɋɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ
4-ɬԥɠɿɪɢɛɟ Ʉɚɪɛɨɧɚɬ ɬԝɡɞɚɪɵɧɵԙ ɝɢɞɪɨɥɢɡɿ
Ʌɚɤɦɭɫɬɵԙ ɤԧɦɟɝɿɦɟɧ ɟɪɿɬɿɧɞɿɧɿԙ ɪɟɚɤɰɢɹɫɵɧ ɬɟɤɫɟɪɿԙɞɟɪ
ɚ ɇɚɬɪɢɣ ɤɚɪɛɨɧɚɬɵɧɵԙ ɛ ɧɚɬɪɢɣ ɝɢɞɪɨɤɚɪɛɨɧɚɬɵɧɵԙ ɜ ɤɚɥɢɣ
ɤɚɪɛɨɧɚɬɵɧɵԙ.
Ƚɢɞɪɨɥɢɡ ɪɟɚɤɰɢɹɥɚɪɵɧɵԙ ɦɨɥɟɤɭɥɚɥɵԕ ɠԥɧɟ ɢɨɧɞɵԕ-ɦɨɥɟɤɭɥɚɥɵԕ
ɬԛɪɞɟ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ ɭɧɢɜɟɪɫɚɥɞɵ ɢɧɞɢɤɚɬɨɪɞɵԙ ɠԥɧɟ ɪɇ±
59
60.
ɦɟɬɪɞɿԙ ɤԧɦɟɝɿɦɟɧ ɟɪɿɬɿɧɞɿɧɿԙ ɪɇ-ɵɧ ɚɧɵԕɬɚԙɞɚɪ Ɍԝɡɞɵԙ ԕɚɣɫɵɫɵɚɪɬɵԕ ɝɢɞɪɨɥɢɡɞɟɧɟɞɿ"
5-ɬԥɠɿɪɢɛɟ Ʉɪɟɦɧɢɣ ɦɟɧ ɫɢɥɚɧɞɚɪɞɵԙ ɚɥɵɧɭ ɠɨɥɞɚɪɵ
ɉɪɨɛɢɪɤɚԑɚ ԝɧɬɚԕɬɚɥԑɚɧ ɦɚɝɧɢɣ ɦɟɧ ɬɚɡɚ ԕԝɪԑɚԕ ɦɚɣɞɚ ԕԝɦ
ԕɨɫɩɚɫɵɧ ɫɚɥɵԙɞɚɪ ɉɪɨɛɢɪɤɚɧɵ ɲɬɚɬɢɜɤɟ ɛɟɤɿɬɿԙɞɟɪ Ʉԧɡɞɿ
ɫɚԕɬɚɣɬɵɧ ɤԧɡɿɥɞɿɪɿɤ ɩɚɣɞɚɥɚɧɵԙɞɚɪ ɉɪɨɛɢɪɤɚɧɵ ԕɨɫɩɚɦɟɧ ɚɥɞɵɦɟɧ
ɲɚɦɚɥɵ ɤɟɣɿɧ ԕɚɬɬɵ ԕɵɡɞɵɪɵԙɞɚɪ Ԕɨɫɩɚ ԕɚɬɬɵ ԕɵɡԑɚɧ ɫɨԙ
ɫɩɢɪɬɨɜɤɚɧɵ ɚɥɵɩ ԕɨɣɵԙɞɚɪ ɞɟɦɟɤ ɠɵɥɭ ɛԧɥɿɧɟɞɿ ɇɟ ɛɚɣԕɚɥɚɞɵ"
Ȼɚɣԕɚԑɚɧ ԕԝɛɵɥɵɫɬɚɪɞɵ ɬԛɫɿɧɞɿɪɿԙɞɟɪ ɪɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
ɹԑɧɢ ɪɟɚɤɰɢɹ ɧԥɬɢɠɟɫɿɧɞɟ ɤɪɟɦɧɢɣ ɨɧɵԙ ɨɤɫɢɞɿ ɠԥɧɟ ɦɚɝɧɢɣ ɫɢɥɢɰɢɞɿ
ɬԛɡɿɥɟɞɿ ɉɪɨɛɢɪɤɚɧɵ ɫɭɵɬɵɩ ɬԛɡɿɥɝɟɧ ɡɚɬɬɚɪɞɵ ɮɚɪɮɨɪ ɬɢɝɟɥɶɞɟ
ԝɧɬɚԕɬɚɩ ɤɿɲɤɟɧɬɚɣ ɛԧɥɲɟɤɬɟɪɿɧ ɬԝɡ ԕɵɲԕɵɥɵ ɛɚɪ ɫɬɚɤɚɧԑɚ ɫɚɥɵԙɞɚɪ
Ʉɪɟɦɧɢɣ ɨɤɫɢɞɿ ȱV ɦɚɝɧɢɣ ɫɢɥɢɰɢɞɿ ɬԝɡ ԕɵɲԕɵɥɵɦɟɧ ԥɪɟɤɟɬɬɟɫɟɞɿ
Ɋɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ ɠɚɡɵԙɞɚɪ Ɍԛɡɿɥɝɟɧ ɫɢɥɚɧ ɠɚɧԑɚɧɞɚ SɿO2 ɚԕ
ɬԛɬɿɧ ɪɟɬɿɧɞɟ ɛԧɥɿɧɟɞɿ Ɋɟɚɤɰɢɹɧɵ ɚɹԕɬɚԑɚɧ ɫɨԙ ɬԝɧɛɚɧɵ ԝɧɬɚԕɬɚɥԑɚɧ
Sɿ ɮɢɥɶɬɪ ɚɪԕɵɥɵ ɛԧɥɿԙɞɟɪ ɞɢɫɬɢɥɞɟɧɝɟɧ ɫɭɦɟɧ ɠɭɵԙɞɚɪ
ԕԝɪԑɚɬɵԙɞɚɪ Ɍԛɫɿɧ ɛɚɣԕɚԙɞɚɪ
6-ɬԥɠɿɪɢɛɟ ɒɚɦɚɞɚ -ɲɿ ɬԥɠɿɪɢɛɟɞɟ ɬԛɡɿɥɝɟɧ ԝɧɬɚԕ ɤɪɟɦɧɢɣɞɿ
ɩɪɨɛɢɪɤɚԑɚ ɫɚɥɵԙɞɚɪ - ɦɥ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ NaOH ԕԝɣɵԙɞɚɪ ɇɟ
ɛɚɣԕɚɥɚɞɵ" Ȼԧɥɿɧɿɩ ɲɵԕԕɚɧ ɝɚɡɞɵ ɠɚɧɞɵɪɵԙɞɚɪ Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ
ɠɚɡɵԙɞɚɪ
7-ɬԥɠɿɪɢɛɟ Ʉɪɟɦɧɢɣ ԕɵɲԕɵɥɵɧɵԙ ɚɥɵɧɭ ɠɨɥɵ
ɚ ɦɥ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ ɧɚɬɪɢɣ ɫɢɥɢɤɚɬ ɟɪɿɬɿɧɞɿɫɿɧɟ ɬɟɡ ɚɪɚɞɚ
2- ɦɥ ɫԝɣɵɬɵɥԑɚɧ ɬԝɡ ԕɵɲԕɵɥɵɧ ԕԝɣɵԙɞɚɪ ɲɵɧɵ ɬɚɹԕɩɟɧ
ɠɚԕɫɵ ɚɪɚɥɚɫɬɵɪɵԙɞɚɪ Ƚɟɥɶɞɿԙ ɬԛɡɿɥɝɟɧɿɧ ԕɚɥɚɣ ɬԛɫɿɧɞɿɪɭɝɟ ɛɨɥɚɞɵ"
Ɋɟɚɤɰɢɹɧɵԙ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
ɛ - ɦɥ ɧɚɬɪɢɣ ɫɢɥɢɤɚɬ ɟɪɿɬɿɧɞɿɫɿɧɟ ɲɚɦɚɞɚ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ ɬԝɡ
ԕɵɲԕɵɥɵɧ ԕԝɣɵԙɞɚɪ Ɍԛɡɿɥɝɟɧ ɤɪɟɦɧɢɣ ԕɵɲԕɵɥɵ ɤɨɥɥɨɢɞ ɬԛɪɿɧɞɟ
ɛɨɥɚɞɵ ɨɫɵ ɟɪɿɬɿɧɞɿɧɿ ԕɚɣɧɚԑɚɧɲɚ ԕɚɬɬɵ ԕɵɡɞɵɪɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ"
ɜ Na2SɿO3 ɟɪɿɬɿɧɞɿɫɿɧɟ Ʉɢɩɩ ɚɩɩɚɪɚɬɵɧɚɧ ɋɈ2 ɝɚɡ ɠɿɛɟɪɿԙɞɟɪ
Ʉɪɟɦɧɢɣ ԕɵɲԕɵɥɵ ɬԛɡɿɥɟɞɿ Ɋɟɚɤɰɢɹ ɬɟԙɞɟɭɿɧ ɠɚɡɵԙɞɚɪ
Ԕɵɲԕɵɥɞɚɪɞɵԙ
ɞɢɫɫɨɰɢɚɰɢɹɥɚɧɭ
ɤɨɧɫɬɚɧɬɚɫɵɧɵԙ
ɦԥɧɞɟɪɿɧ
ɫɚɥɵɫɬɵɪɵԙɞɚɪ Ԕɚɣɫɵɫɵ ԥɥɫɿɡ ɷɥɟɤɬɪɨɥɢɬɤɟ ɠɚɬɚɞɵ"
8-ɬԥɠɿɪɢɛɟ ɚ Ʌɚɤɦɭɫɬɵԙ ɤԧɦɟɝɿɦɟɧ ɧɚɬɪɢɣ ɫɢɥɢɤɚɬɵ ɟɪɿɬɿɧɞɿɫɿɧɿԙ
ɪɟɚɤɰɢɹ ɨɪɬɚɫɵɧ ɬɟɤɫɟɪɿԙɞɟɪ ɪɟɚɤɰɢɹ ɬɟԙɞɟɭɿɧ ɦɨɥɟɤɭɥɚɥɵԕ ɠԥɧɟ
ɢɨɧɞɵԕ ɬԛɪɿɧɞɟ ɠɚɡɵԙɞɚɪ
ɛ - ɦɥ ɤɨɧɰɟɧɬɪɥɟɧɝɟɧ Na2SɿO3 ɟɪɿɬɿɧɞɿɫɿɧɟ ԕɚɧɵԕԕɚɧ - ɦɥ NH4Cȱ
ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ Ƚɚɡ ɛԧɥɿɧɟɞɿ ɬԝɧɛɚ ɬԛɡɿɥɟɞɿ Ɋɟɚɤɰɢɹɥɚɪɞɵԙ
ɬɟԙɞɟɭɥɟɪɿɧ ɢɨɧɞɵԕ ɬԛɪɿɧɞɟ ɠɚɡɵԙɞɚɪ
60
61.
9-ɬԥɠɿɪɢɛɟ ɀɟɤɟ ɩɪɨɛɢɪɤɚԑɚ ɤɚɥɶɰɢɣ ɬɟɦɿɪ ɤɨɛɚɥɶɬ ɧɢɤɟɥɶɬԝɡɞɚɪɵɧɵԙ ɟɪɿɬɿɧɞɿɫɿɧɟ ɚɡɞɚԑɚɧ ɦԧɥɲɟɪɞɟ ɧɚɬɪɢɣ ɫɢɥɢɤɚɬɵɧɵԙ
ɟɪɿɬɿɧɞɿɫɿɧ ԕԝɣɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ" Ɋɟɚɤɰɢɹɥɚɪɞɵԙ ɬɟԙɞɟɭɥɟɪɿɧ
ɠɚɡɵԙɞɚɪ Ⱥɥɵɧԑɚɧ ɡɚɬɬɚɪɞɵԙ ɟɪɿɝɿɲɬɿɝɿɧ ɬɟɤɫɟɪɿԙɞɟɪ
10-ɬԥɠɿɪɢɛɟ ɇɚɬɪɢɣ ɫɢɥɢɤɚɬɵ ɟɪɿɬɿɧɞɿɫɿɧɟ ɦɟɬɚɧɧɵԙ ɛԧɥɲɟɝɿɧ
ɛɚɬɵɪɵԙɞɚɪ ԕԝɪԑɚɬɵԙɞɚɪ ɨɬԕɚ ɠɚɧɞɵɪɵԙɞɚɪ ɇɟ ɛɚɣԕɚɥɚɞɵ"
ɋɚɥɵɫɬɵɪɭ ԛɲɿɧ ɧɚɬɪɢɣ ɫɢɥɢɤɚɬ ɟɪɿɬɿɧɞɿɫɿɧɟ ɛɚɬɵɪɵɥԑɚɧ ɦɚɬɚɧɵ
ɠɚɧɞɵɪɵԙɞɚɪ
Ԥɞɟɛɢɟɬɬɟɪ ɬɿɡɿɦɿ
ȻLɪLɦɠɚɧɨɜ Ȼ Ⱥ ɇԝɪɚɯɦɟɬɨɜ ɇ ɇ ɀɚɥɩɵ ɯɢɦɢɹ Ⱥɥɦɚɬɵ ɠ
Ⱥɯɚɧɛɚɟɜ Ʉ ɏɢɦɢɹ ɧɟɝLɡɞɟɪL Ⱥɥɦɚɬɵ
Ԧɬɟɥɛɚɟɜ Ȼ Ɍ ɏɢɦɢɹ ɒɵɦɤɟɧɬ
ɒɨԕɵɛɚɟɜ ɀ Ȼɟɣɨɪɝɚɧɢɤɚɥɵԕ ɠԥɧɟ ɚɧɚɥɢɬɢɤɚɥɵԕ ɯɢɦɢɹ Ⱥɥɦɚɬɵ
Ȼɿɥɿɦ ɠ
5. Ⱥɯɦɟɬɨɜ ɇ ɋ Ɉɛɳɚɹ ɢ ɧɟɨɪɝɚɧɢɱɟɫɤɚɹ ɯɢɦɢɹ Ɇ ȼɵɫɲɚɹ ɲɤɨɥɚ,
2001
6. Ʉɚɪɚɩɟɬɶɹɧɰ Ɇ ɏ Ⱦɪɚɤɢɧ ɋ ɂ Ɉɛɳɚɹ ɢ ɧɟɨɪɝɚɧɢɱɟɫɤɚɹ ɯɢɦɢɹ Ɇ
ɏɢɦɢɹ 2003
7. ɋɬɟɩɢɧ Ȼ Ⱦ ɐɜɟɬɤɨɜ Ⱥ Ⱥ ɇɟɨɪɝɚɧɢɱɟɫɤɚɹ ɯɢɦɢɹ Ɇ ȼɵɫɲɚɹ
ɲɤɨɥɚ 2000
8. Ⱥɯɚɧɛɚɟɜ Ʉ ɏɢɦɢɹ, Ⱥɥɦɚɬɵ Ɇɟɤɬɟɩ 2007
9. ɇɟɤɪɚɫɨɜ Ȼ ȼ Ɉɫɧɨɜɵ ɨɛɳɟɣ ɯɢɦɢɢ Ɍ - Ɇ ɏɢɦɢɹ 2001 ɝ
ɋɬɚɦԕԝɥɨɜ Ԧ ɋ Ɍԝɪɬɚɛɚɟɜ ɋ Ʉ ԤɲLɪɨɜ Ԥ Ɇ ɀɚɥɩɵ ɠԥɧɟ ɛɟɣɨɪɝɚɧɢɤɚɥɵԕ ɯɢɦɢɹ ɠɚɬɬɵԑɭɥɚɪ ɦɟɧ ɟɫɟɩɬɟɪ ɠɢɧɚԑɵ ɒɵɦɤɟɧɬ
ɄLɬɚɩ 1997
11. ɋɬɚɦԕԝɥ Ԧ ɋ ɉɪɚɥɢɟɜ ɋ ɀ Ɍԝɪɬɚɛɚɟɜ ɋ Ԕ ɀɚɥɩɵ ɠԥɧɟ ɛɟɣɨɪɝɚɧɢɤɚɥɵԕ ɯɢɦɢɹ ɩɪɚɤɬɢɤɭɦɵ ɒɵɦɤɟɧɬ ɠ
12. ɒɨԕɵɛɚɟɜ ɀ Ԥ Ԕɚɪɚɠɚɧɨɜɚ Ⱦ Ԥ ɏɢɦɢɹ ɟɫɟɩɬɟɪɿ ɦɟɧ
ɠɚɬɬɵԑɭɥɚɪɵ Ⱥɥɦɚɬɵ
61
62.
Ʉɟɪɿɦɛɚɟɜɚ Ʉԛɥԥɲ Ɂԥɭɿɪɛɟɤԕɵɡɵɋɬɚɦԕԝɥ Ԧɬɟɦɛɟɤ ɋɚԑɵɪԝɥɵ
ɀɚɥɩɵ ɯɢɦɢɹ
Ɉԕɭ-ԥɞɿɫɬɟɦɟɥɿɤ ɧԝɫԕɚɭ
Ɋɟɞɚɤɬɨɪɵ ± Ʉɟɪɿɦɛɚɟɜɚ Ʉ Ɂ
Ɍɟɯɧɢɤɚɥɵԕ ɪɟɞɚɤɬɨɪ ± Ɍɭɥɟɝɟɧɨɜ Ԑɚɛɢɬ
Ɏɨɪɦɚɬ ɯ Ƚɚɪɧɢɬɭɪɚ 7LPHV 1HZ 5RPDQ
Ȼɚɫɩɚ ɬɚɛɚԑɵ Ɍɢɪɚɠɵ ɞɚɧɚ ʋ ɡɚɤɚɡ
ɒɵɦɤɟɧɬ ԕɚɥɚɫɵ Ȼɚɣɬԝɪɫɵɧɨɜ ɤԧɲɟɫɿ ԛɣ
Ȼɚɫ ԑɢɦɚɪɚɬɵ
62







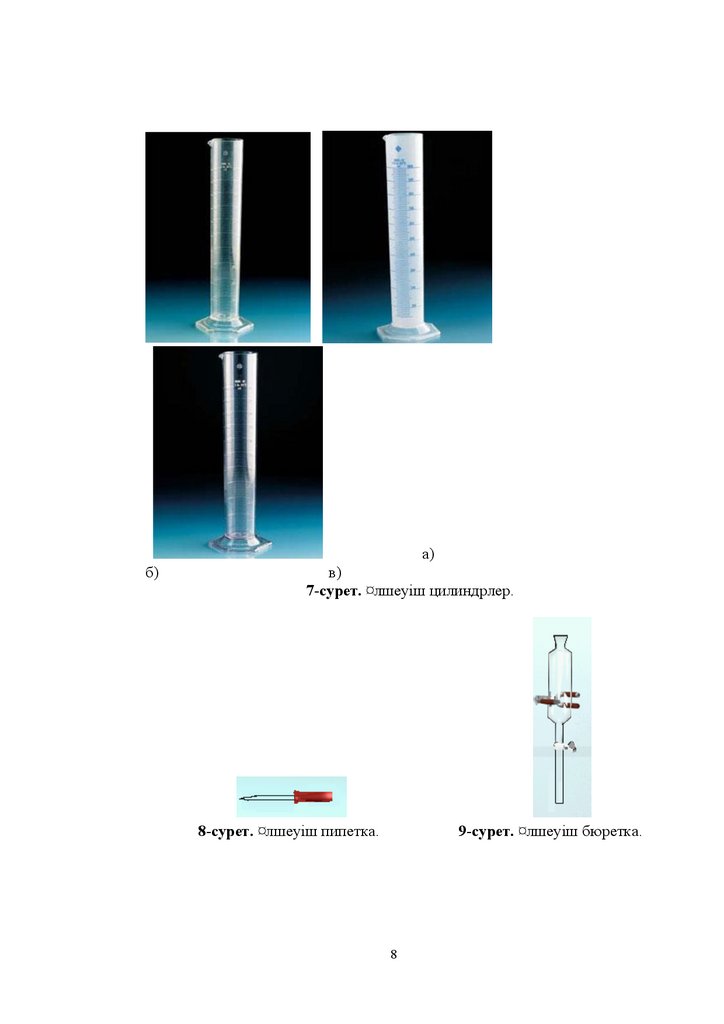








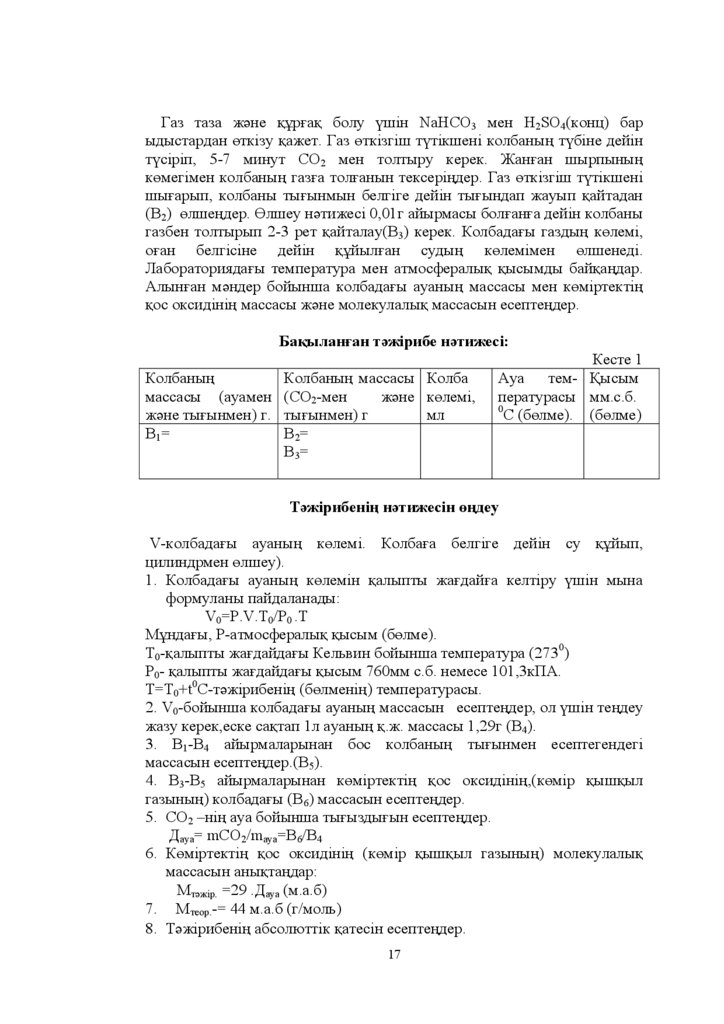


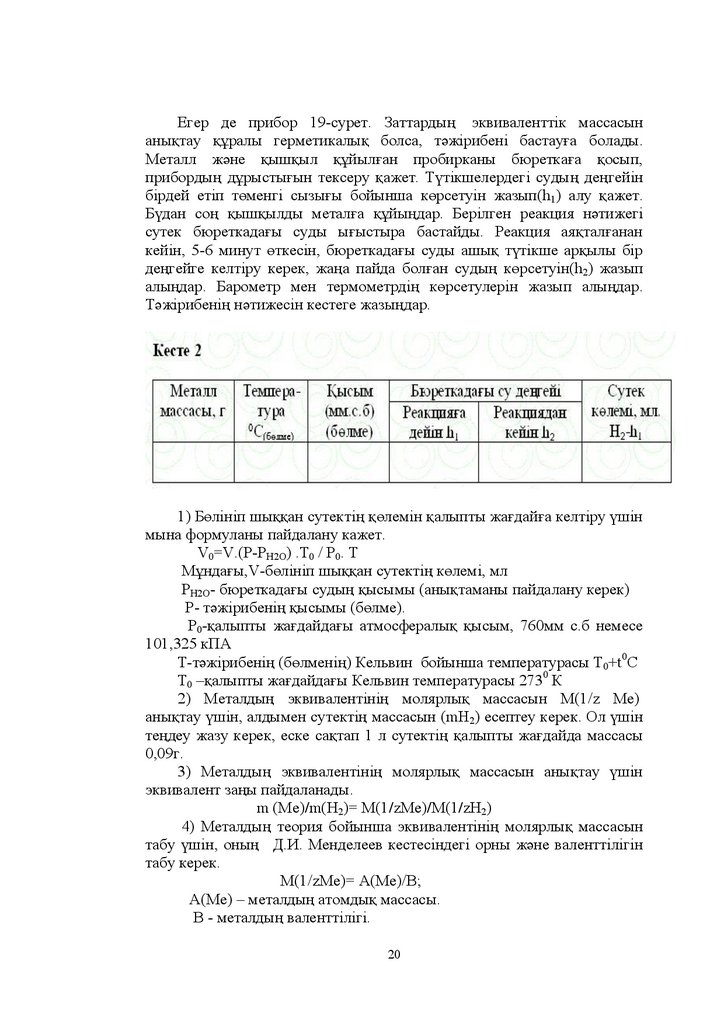





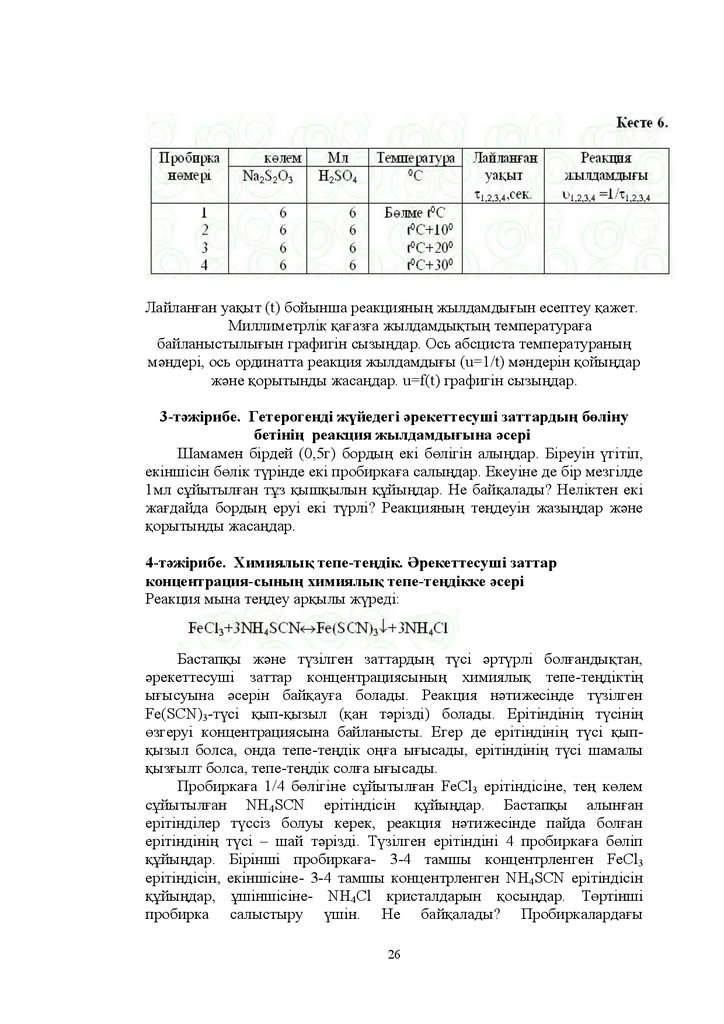





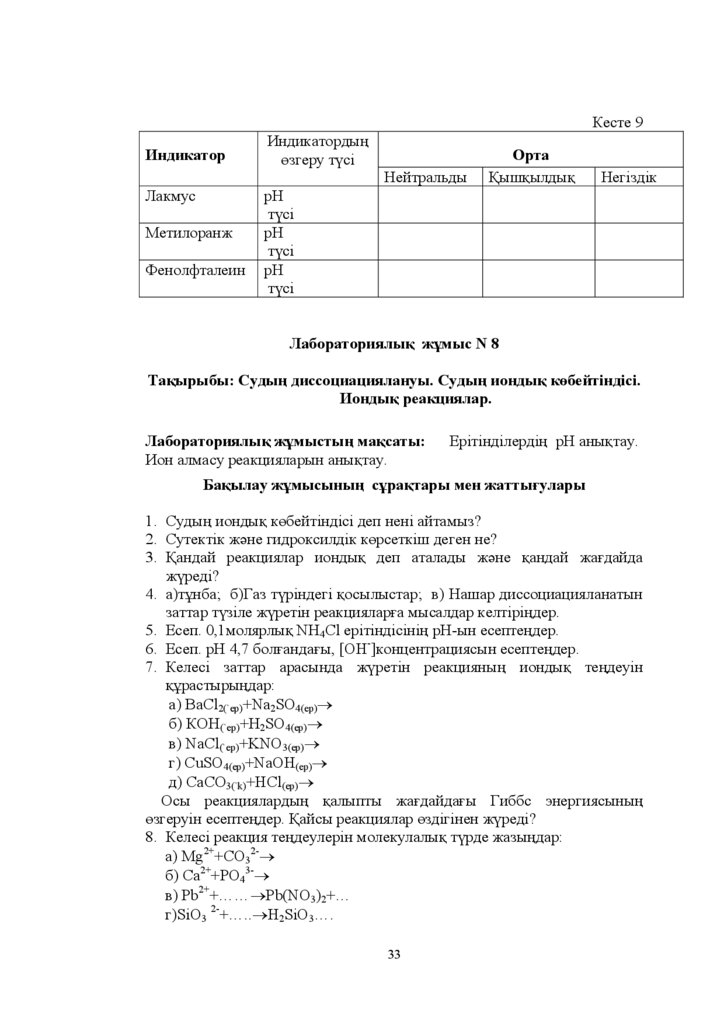



















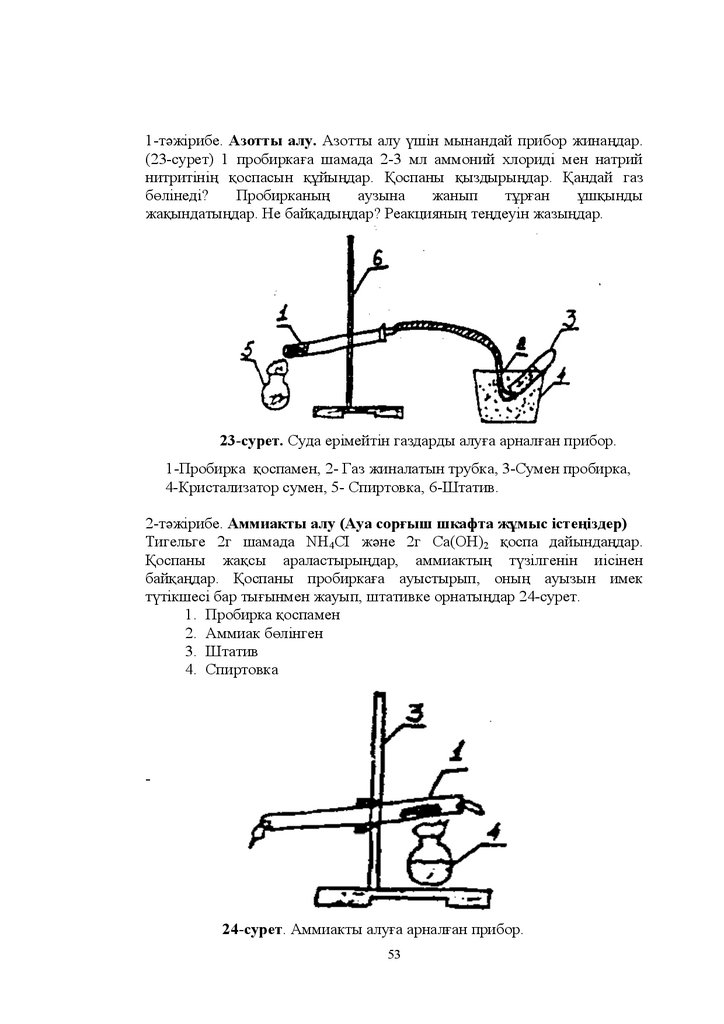











 chemistry
chemistry








