Similar presentations:
Anti-tuberculosis immunity. Anti-tuberculosis vaccination and revaccination with BCG. Tuberculin diagnostics. Lecture 2
1.
Anti-tuberculosisimmunity.
Anti-tuberculosis
vaccination and
revaccination with BCG.
Tuberculin diagnostics.
Bashkir State Medical University
Department of Phthisiology
V.E. Izosimova, Associate Professor
2021
1
2.
• We will start at 14:502
3.
Lecture plan1. Anti-tuberculosis immunity and nonspecific
resistance of the organism in tuberculosis.
2. Anti-tuberculosis vaccination and
revaccination with BCG.
3. Tuberculin diagnostics (Mantoux reaction
and Diaskin test).
4. IGRA tests.
3
4.
Let me remind you the following1/3 of the world's
population is infected
(2 billion)
10 million people get
tuberculosis a year
TB is the leading
cause of death in the
world from a bacterial
infectious disease (1,7
million)
4
5.
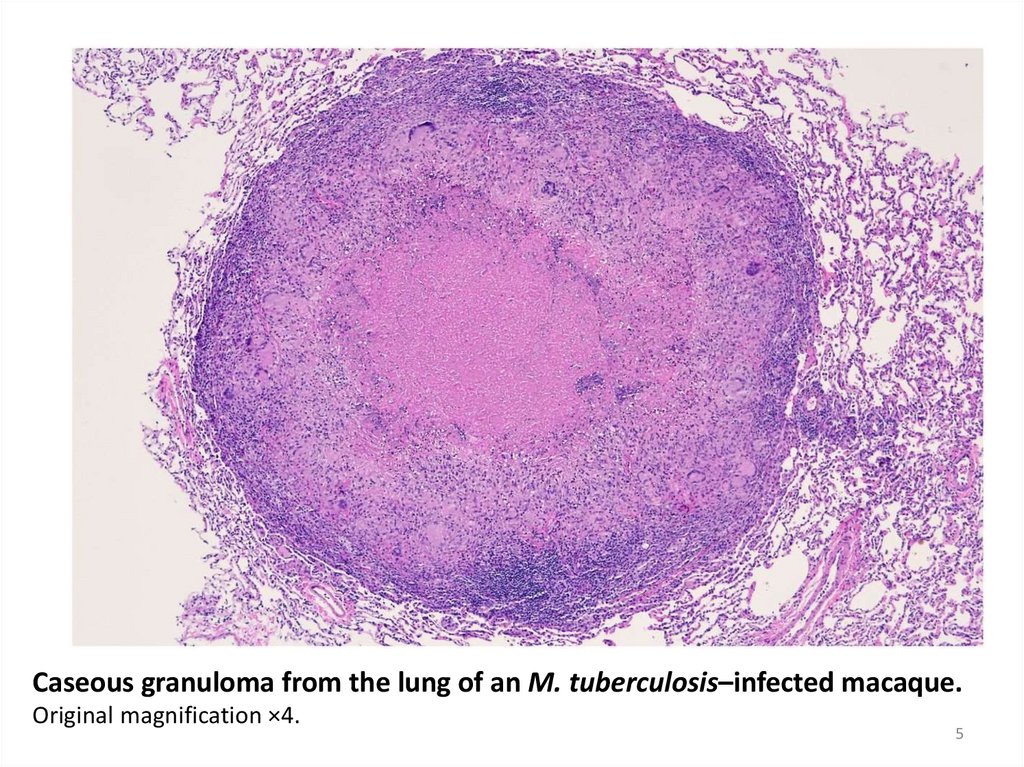
Caseous granuloma from the lung of an M. tuberculosis–infected macaque.Original magnification ×4.
5
6.
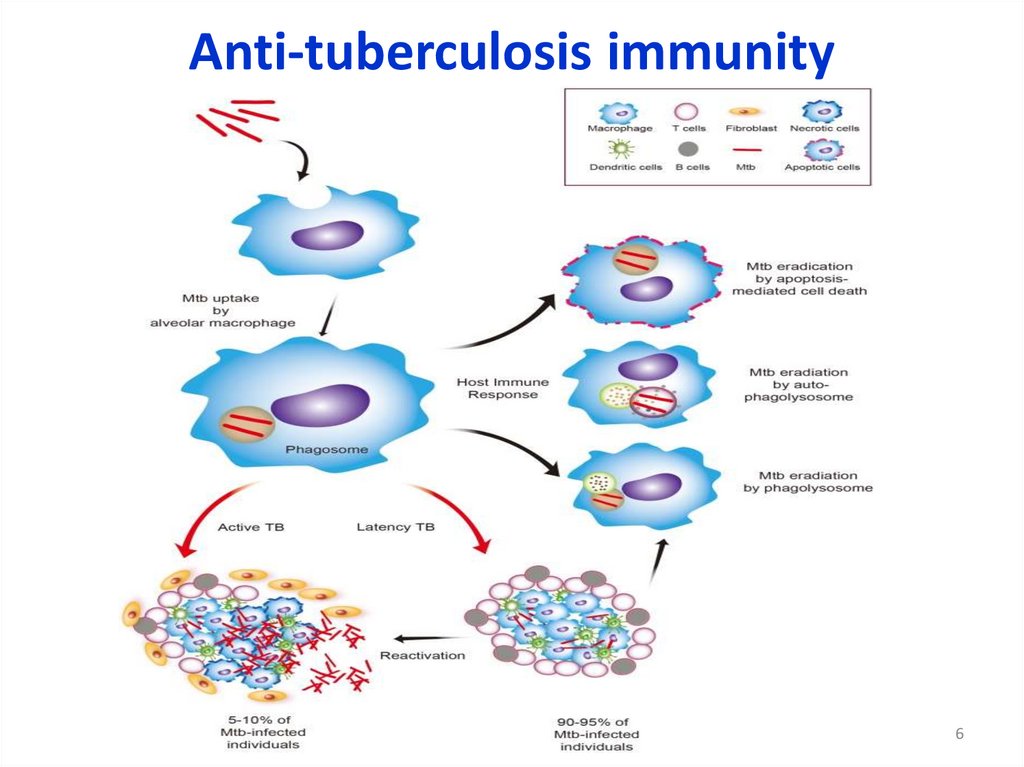
Anti-tuberculosis immunity6
7.
Albert Calmette and Jean-Marie Camille GuérinBCG is a vaccine against tuberculosis, prepared from a strain of
weakened live Mycobacterium bovis, which has practically lost
its virulence for humans, created specially grown in an artificial
environment
7
8.
The BCG vaccineprotects against TB (tuberculosis)
is made from a weakened form of a bacterium
8
9.
The BCG vaccineBCG is a strain of Mycobacterium bovis
This microorganism has been modified in the
vaccine so that it produces immunity against
TB without causing the disease
It is a live vaccine. The bacteria in the vaccine
are still alive but are weakened so that they do
not cause TB disease
9
10.
The BCG vaccineBCG vaccine gives substantial, though not
complete, protection about 70-80%
10
11.
BCG immunization in Russia• BCG (0.05 mg – 0.1 ml)
• BCG-M (0.025 mg – 0.1 ml)
11
12.
BCG immunization is now recommended for:All healthy full-term newborns whatever their weight
and for premature babies who weight is 2 kg and more
Infants (0 to 12 months of age) living in areas where
the annual incidence of TB is significant (greater than
40/100 000)
12
13.
BCG immunization is now recommended for:This indication is valid for newborns of HIV+ mothers, since
it is thought that these babies have a 70% chance not to
be infected with HIV. (90% - mother with ARVD)
In children's clinics - children who have not received
anti-tuberculosis vaccination in the hospital
13
14.
Contraindications to BCG vaccinationAcute diseases (vaccination is postponed until the
end of acute manifestations of the disease and
exacerbation of chronic diseases)
Immunodeficiency states
Malignant neoplasms
14
15.
Contraindications to BCG vaccinationGeneralized BCG - infection
(including lymphadenitis, osteitis of BCG, etiology
identified in other children in the family)
HIV infection in a child
15
16.
Indications for revaccinationhealthy children aged 6-7 years with a
negative reaction for the Mantoux test with 2
TE PPD-L (within 2 years before revaccination,
including the year of revaccination)
16
17.
Vaccination technique17
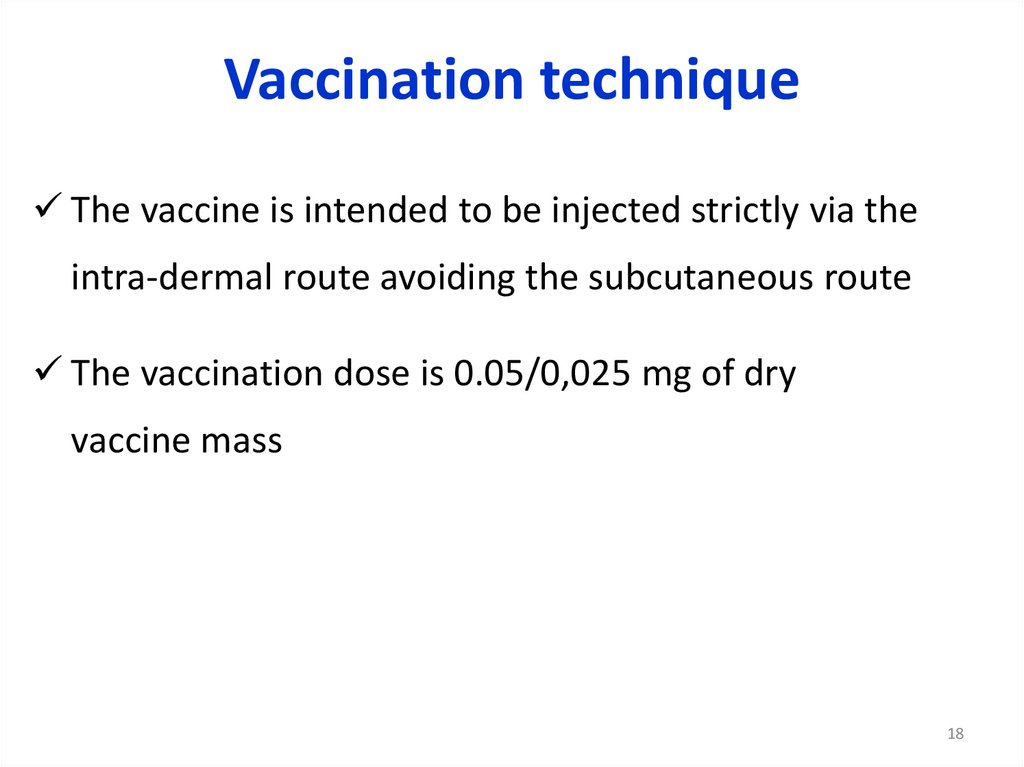
18.
Vaccination techniqueThe vaccine is intended to be injected strictly via the
intra-dermal route avoiding the subcutaneous route
The vaccination dose is 0.05/0,025 mg of dry
vaccine mass
18
19.
Vaccination techniqueThe vaccine should be preferably given with a tuberculin
syringe or 25G/26G sterile needle and syringe
The skin is stretched between thumb and forefinger AT THE
BORDER OF THE UPPER AND THE MIDDLE THIRD of
the outer surface of the LEFT shoulder
Sterile needle (25 G or 26 G) inserted bevel upwards for about
2mm into superficial layers of the dermis (almost parallel with
the surface)
19
20.
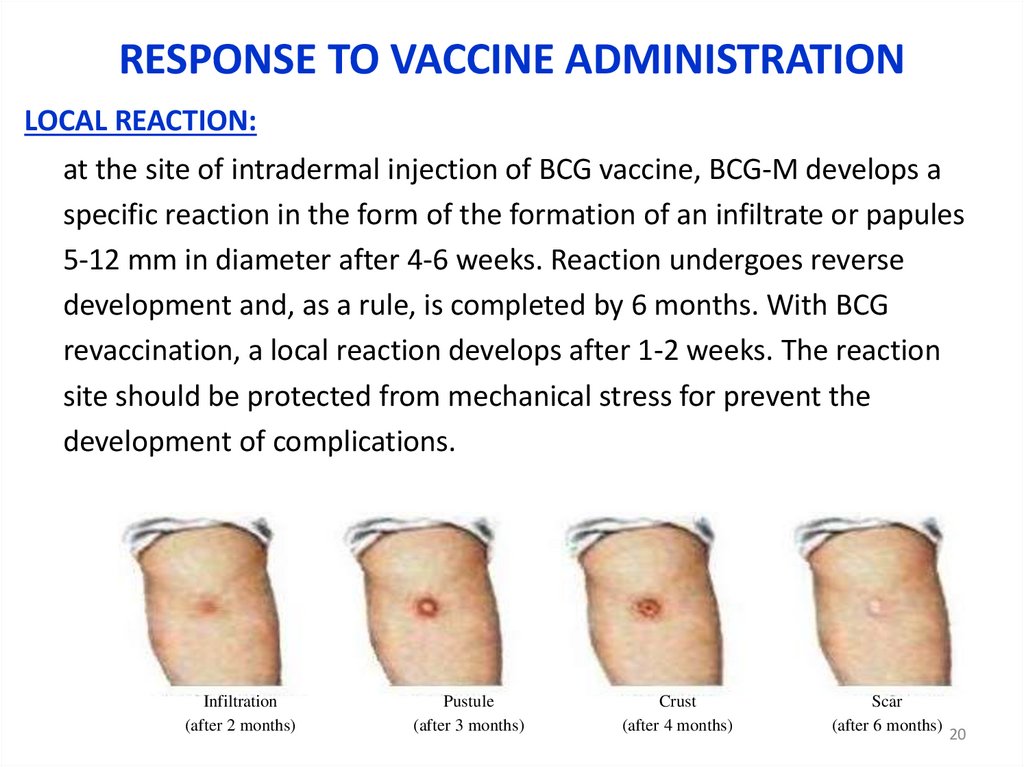
RESPONSE TO VACCINE ADMINISTRATIONLOCAL REACTION:
at the site of intradermal injection of BCG vaccine, BCG-M develops a
specific reaction in the form of the formation of an infiltrate or papules
5-12 mm in diameter after 4-6 weeks. Reaction undergoes reverse
development and, as a rule, is completed by 6 months. With BCG
revaccination, a local reaction develops after 1-2 weeks. The reaction
site should be protected from mechanical stress for prevent the
development of complications.
Infiltration
(after 2 months)
Pustule
(after 3 months)
Crust
(after 4 months)
Scar
(after 6 months)
20
21.
2122.
RESPONSE TO VACCINE ADMINISTRATIONGeneral reaction:
in rare cases, a rise in temperature is possible
body, reaction from peripheral lymph nodes
22
23.
Сomplications of BCG vaccinationCategory 1:
inflammatory lesions developed at the site
administration of the vaccine or in the corresponding
regional lymph nodes - infiltrates, abscesses, fistulas,
ulcers and regional lymphadenitis
Category 2:
inflammatory lesions resulting from hematogenous
spread of bacteria of the vaccine strain outside the
zone vaccine administration - osteitis and soft tissue
isolated abscesses
23
24.
Сomplications of BCG vaccinationCategory 3:
disseminated BCG infection with multiple organ
defeat in congenital immunodeficiency;
Category 4:
post-BCG syndrome - allergic diseases character
that arose after vaccination as a result of specific
sensitization: erythema nodosum, annular
granuloma, rash, keloid, uveitis, etc.
24
25.
Сomplications of BCG vaccinationinflamed skin
subcutaneous
abscess at the BCG
vaccination site
left axillary lymphadenitis
Fistulised suppurative left
axillary lymphadenitis
25
26.
Сomplications of BCG vaccination andtheir management
26
27.
New anti-TB vaccineNew Adjuvant
New way of vaccine
administration
27
28.
Tuberculin testis a diagnostic test to determine specific
sensitization of the body to mycobacterium
tuberculosis
How a specific test is used
in mass examinations population for
tuberculosis (mass tuberculin diagnostics)
for individual examinations (individual
tuberculin diagnostics)
28
29.
Tasks of mass immunodiagnostics(screening for tuberculosis) are
detection of infection with Mycobacterium
tuberculosis
identification of actively metabolizing microbial
population ("Active" tuberculosis infection)
selection of persons for vaccination and
revaccination against tuberculosis
the formation of risk groups for tuberculosis
29
30.
Tasks of individual immunodiagnostics aredifferential diagnosis of post-vaccination immunity
(BCG) and true infection with mycobacterium
tuberculosis
differential diagnosis of tuberculosis and other diseases
determination of the activity of the tuberculous
process
monitoring the effectiveness of treatment of patients
with tuberculosis.
30
31.
Immunodiagnostic tests1. Traditional tuberculin diagnostics –
intradermal test Mantoux with 2 TE PPD
(purified protein derivative)
The drug is a purified liquid tuberculosis allergen
in a standard dilution, the biological activity of
which is measured in tuberculin units (TE).
31
32.
Immunodiagnostic tests2. DIASKINTEST® - is intradermal diagnostic test with
recombinant tuberculosis allergen
DIASKINTEST has been developed in Russia in standard
dilution (CFP10-ESAT6 protein 0.2 μg).
DIASKINTEST®, a complex of recombinant proteins CFP10 and ESAT-6 produced by Echerichia coli BL21
(DE3) / pCFP-ESAT.
32
33.
Immunodiagnostic tests3. Diagnostic tests based on the in vitro release of IFNγ by T-lymphocytes.
QuantiFERON®-TB Gold uses solid phase
immunosorbent assay for measuring antigen-specific
IFN-production by circulating T-cells in whole blood
(under the influence of ESAT-6, CFP-10 and TB7.7
antigens)
T-SPOT.TB, with using the Elispot technique,
determines the number of mononuclear cells of
peripheral blood producing IFN-γ.
33
34.
Test Mantoux. HistoryIn 1890 the Robert Koch discovered
tuberculin
In 1907, the Austrian pediatrician
Clemens Pirke began to use skin
tuberculin vaccination to diagnose
tuberculosis infection. An infected
organism responds to the penetration of
tuberculin into the skin with
characteristic inflammation, while such a
reaction is absent on the skin of a
healthy person
34
35.
Test Mantoux. HistoryIn 1908 Charles Mantoux proposed the use of
tuberculin intradermally for diagnostic purposes.
This method has received general recognition in
medicine as the most accurate
Purified tuberculin PPD (purified protein
derivative) is prepared from a mixture of heatkilled filtrates of human mycobacterium
tuberculosis (M. tuberculosis) and bovine species
(M. bovis). Then the composition is precipitated
with trichloroacetic acid, treated with ethyl
alcohol and ether, and dissolved in a phosphatebuffered solution
35
36.
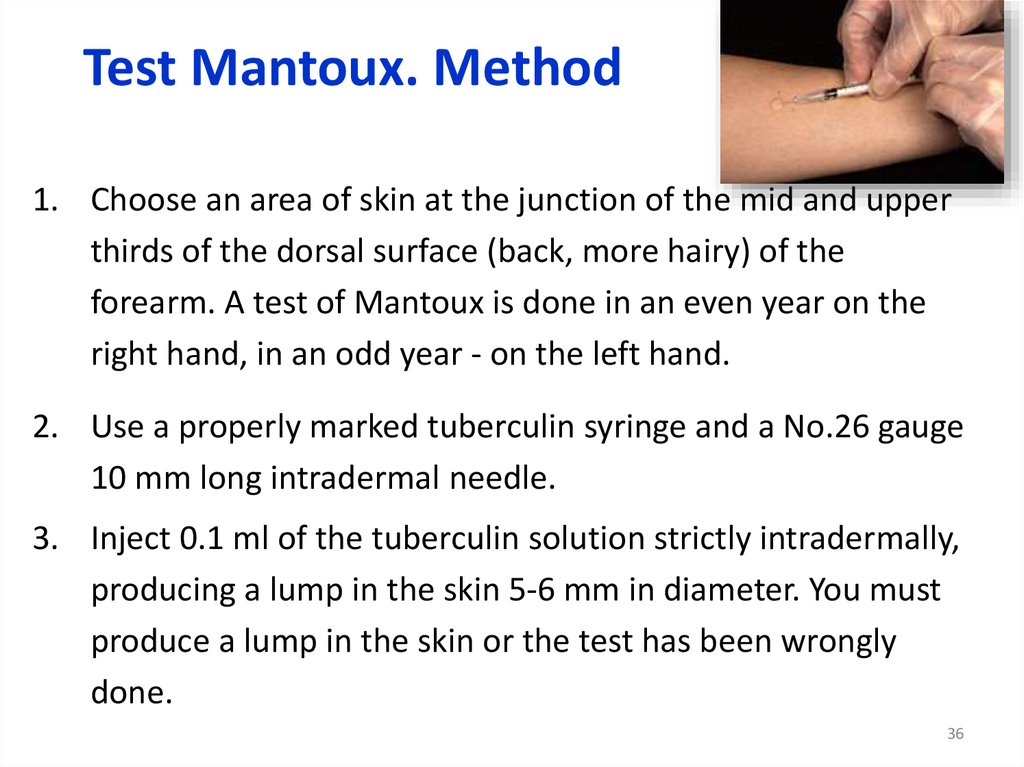
Test Mantoux. Method1. Choose an area of skin at the junction of the mid and upper
thirds of the dorsal surface (back, more hairy) of the
forearm. A test of Mantoux is done in an even year on the
right hand, in an odd year - on the left hand.
2. Use a properly marked tuberculin syringe and a No.26 gauge
10 mm long intradermal needle.
3. Inject 0.1 ml of the tuberculin solution strictly intradermally,
producing a lump in the skin 5-6 mm in diameter. You must
produce a lump in the skin or the test has been wrongly
done.
36
37.
Test Mantoux. Reading the resultRead the test after 48-72 hours. If a
reaction has taken place you will see
an area of erythema (redness) which
may be difficult to see on a dark skin,
and an area of induration (thikening)
of the skin. You can feel the thickening
even when your eyes are closed.
Measure the diameter of induration
across the transverse axis of the arm
Record this diameter carefully, e.g.
‘Mantoux 12 mm’.
37
38.
Test Mantoux. Interpretation• The Mantoux test does not measure immunity to TB but the
degree of hypersensitivity to tuberculin.
• There is no correlation between the size of induration and
likelihood of current active TB disease but the reaction size is
correlated with the future risk of developing TB disease.
• There is no correlation between the size of post-vaccination
Mantoux reactions and protection against TB disease and
routine post-BCG Mantoux testing serves no purpose.
38
39.
Test Mantoux. Interpretation• The results of this test must be interpreted carefully. The
person's medical risk factors determine the size of
induration the result is positive (5 mm, 10 mm, or 15
mm).
• A record should also be made of formation of vesicles,
bullae, lymphangitis, ulceration and necrosis at the test
site. The formation of vesicles, bullae or necrosis at the
test site indicates high degree of tuberculin sensitivity
and thus presence of infection with tubercle bacilli
39
40.
Five mm or more is positive inHIV-positive person
Recent contacts of active tuberculosis cases
Persons with nodular or fibrotic changes on Chest X-ray
consistent with old healed TB
Organ transplant recipients and other immunosuppressed
patients who are on cytotoxic immune-suppressive agents such
as cyclophosphamide or methotrexate.
Patients on long term systemic corticosteroid therapy (> than six
weeks) and those on a dose of prednisone ≥ 15 mg/day or
equivalent.
End stage renal disease
40
41.
Ten mm or more is positive inRecent arrivals (less than five years) from high-prevalence countries
Injectable drug users
Residents and employees of high-risk congregate settings (e.g., prisons,
nursing homes, hospitals, homeless shelters, etc.)
Mycobacteriology lab personnel
Persons with clinical conditions that place them at high risk (e.g.,
diabetes, prolonged corticosteroid therapy, leukemia, end-stage renal
disease, chronic malabsorption syndromes, low body weight, etc.)
Children less than four years of age, or children and adolescents
exposed to adults in high-risk categories
Infants, children, and adolescents exposed to adults in high-risk
categories
41
42.
Fifteen mm or more is positive inPersons with no known risk factors for TB.
Reactions larger than 15 mm are unlikely to be
due to previous BCG vaccination or exposure
to environmental mycobacteria.
42
43.
False-positive resultSome persons may react to the TT even though
they are not infected with M. tuberculosis.
43
44.
False-positive result• Infection with non tuberculous mycobacteria
• Previous BCG vaccination
• Incorrect method
• Incorrect interpretation of reaction
• Incorrect bottle of antigen used
44
45.
False-negative result• Cutaneous anergy (anergy is the inability to react to skin
tests because of a weakened immune system)
• Recent TB infection (within 8-10 weeks of exposure)
• Very old TB infection (many years)
• Very young age (less than six months old)
• Recent live-virus vaccination (e.g., measles and smallpox)
• Overwhelming TB disease
• Some viral illnesses (e.g., measles and chicken pox)
• Incorrect method
• Incorrect interpretation of reaction, insufficient dose and
inadvertent subcutaneous injection.
45
46.
Interpretation in children46
47.
MANTOUX CONVERSIONMantoux conversion is defined as a change
(within a two-year period) of Mantoux
reactivity which meets either of the following
criteria:
• a change from a negative to a positive
reaction
• an increase of ≥ 10 mm.
Conversion has been associated with an annual
incidence of TB disease of 4% in adolescents
or 6% in contacts of smear-positive cases.
47
48.
Test MantouxPost-vaccination and infectious allergy
48
49.
Diaskintest (DST)A skin test using CFP10-ESAT6, produced by
Echerichia coli BL21(DE3)/pCFP-ESAT.
Manufactured by Generium in Russia.
Since 2009, more than 20 million tests have
been performed in Russia, Kazakhstan,
Ukraine, Belarus, Kirgizstan, and
Turkmenistan.
49
50.
Comparison with Test Mantoux50
51.
DiaskintestOnly infectious allergy
51
52.
Interferon-gammarelease assays(IGRA)
Tests on whole blood that can be used to
determine MTB infection.
There are 2 test methods: QuantiFERON-TB
Gold In-Tube; T-SPOT.TB (T-Spot)
52
53.
Interferon-gammarelease assays (IGRA)What is the mechanism of the test?
• The response of the human immune system to
the MTB is determined. White blood cells
produce γ-IFN during mixing with antigens
(substances that can give an immune response)
derived from the MBT in most of patients
infected with MTB.
• Fresh blood samples are shifted with antigens
and controlled reagents.
53
54.
Interferon-gammarelease assays (IGRA)Interpretation of results
• Interpretation of IGRA results is based on the amount of
released γ-IFN or the number of cells that release it. The
results should be reported as standard qualitative (positive,
negative or uncertain) and quantitative interpretation of the
test :
• positive result: TB infection is likely;
• negative result: TB infection is unlikely;
• uncertain result: a certain probability of TB infection;
• cross test result(only T-spot) a certain probability of TB
infection
54
55.
Advantages of IGRA1 visit of a medical institution is
required for a patient to make a test.
Results can be available in 24 hours.
Following tests do not increase the
result. Preliminary vaccination of BCG
does not lead to a false positive result
55
56.
Disadvantages of IGRABlood samples should be processed within 8-30 hours after
taking the material as white blood cells still viable.
Mistakes in taking or transporting blood samples or in
performing and interpreting the analysis may reduce the
effectiveness of the tests.
A small amount of data on use in order to predict the
progression of latent infection to active tuberculosis.
A small amount of test data in: children younger than 5 years;
persons with recent TB contact; immunocompromised patients;
the case of serial testing
56
















































 medicine
medicine