Similar presentations:
Theoretical principles of bioenergetics
1. Zaporizhzhya State Medical University Analytical Chemistry Department THEORETICAL PRINCIPLES OF BIOENERGETICS Lecturer: Monaykina Yulia Vitalievna 2016
2.
Chemical thermodynamics studies theconditions of chemical systems stability and
the laws under which they change to achieve
maximum stability – the state of chemical
equilibrium.
It allows foreseeing a definite chemical
reaction proceeding in a given chemical
system, as well as the direction of equilibrium
shift in the system in accordance with the
change of temperature, pressure and agents
concentration.
3.
Bioenergetics is a field ofthermodynamics that deals with
biosystems.
The energy of reactions is studied by the
branch of thermodynamics called
thermochemistry or chemical
thermodynamics.
4.
Thermodynamic system – is a body or agroup of bodies, which interact whith
each other and are separated from the
environment by a real or imaginary
surface.
5.
Isolated system is a system which exchangesneither mass nor energy with environment.
Closed system is a system which exchanges
energy but doesn’t exchange mass with
environment.
Open system is a system which exchanges both
mass and energy with environment.
All biological systems are open systems
6.
Homogeneous system is a system, whichconsists of only one phase.
Heterogeneous system consists of several
phases.
Phase is a part of a system, which is
separated by the interfacial area while its
crossing the properties change stepwise.
7.
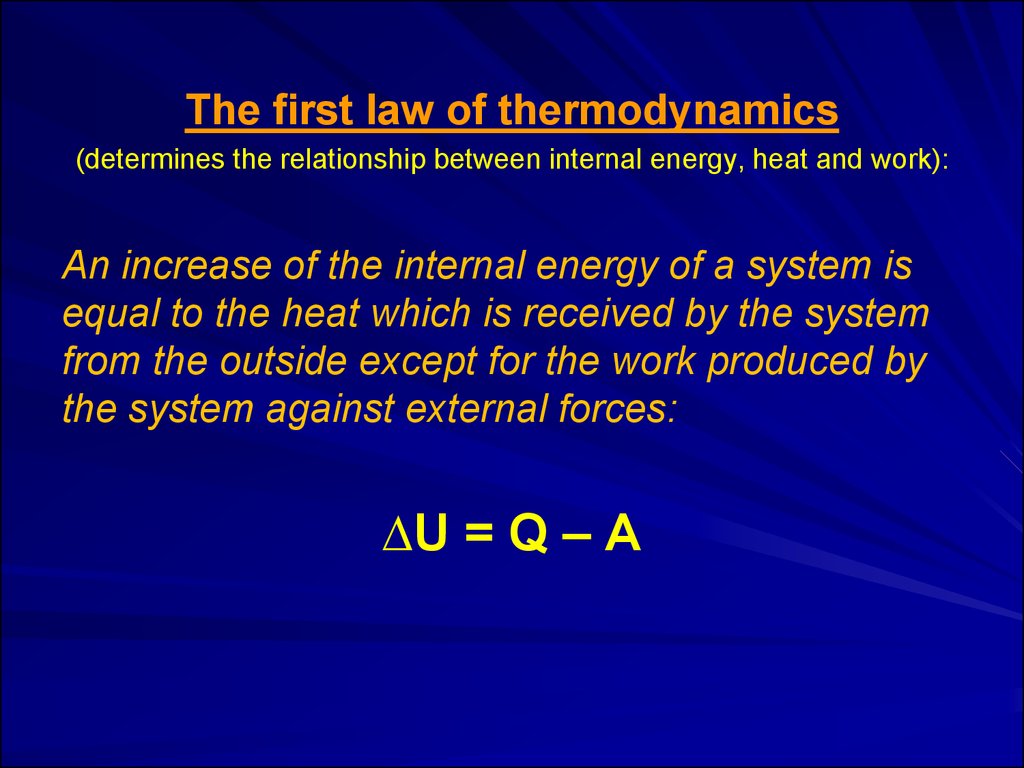
The first law of thermodynamics(determines the relationship between internal energy, heat and work):
An increase of the internal energy of a system is
equal to the heat which is received by the system
from the outside except for the work produced by
the system against external forces:
∆U = Q – A
8.
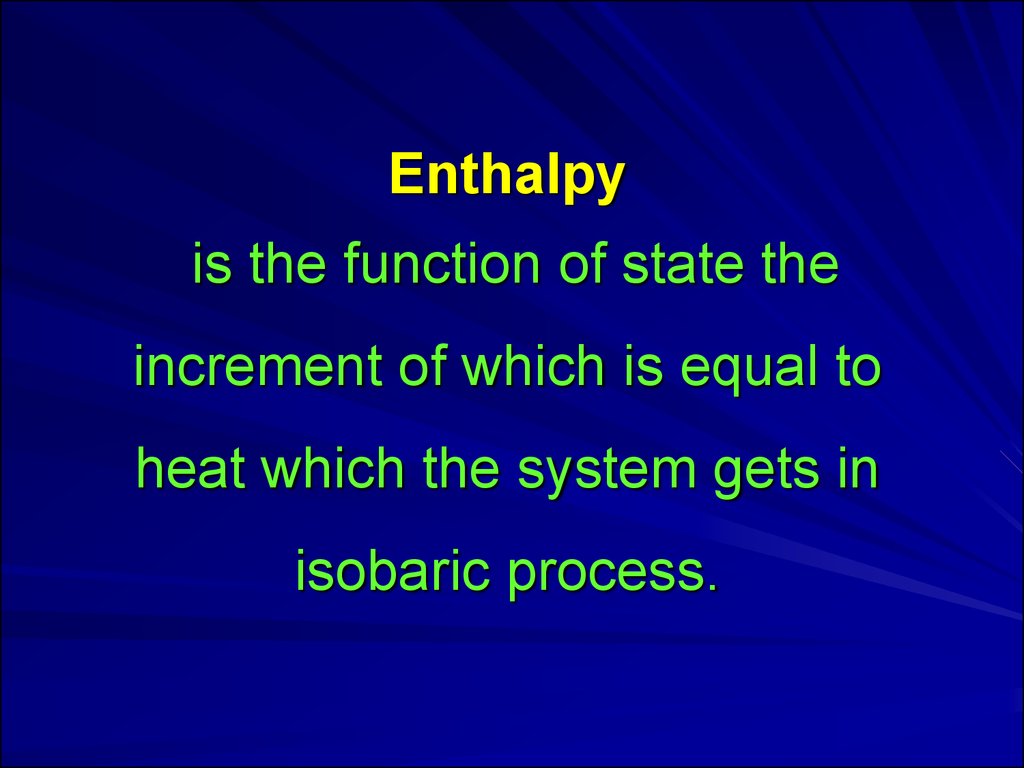
Enthalpyis the function of state the
increment of which is equal to
heat which the system gets in
isobaric process.
9.
Hess’s lawThe heat of a reaction is independent of the
way, in which this reaction occurs, and only
depends upon the initial and final states of a
system.
10.
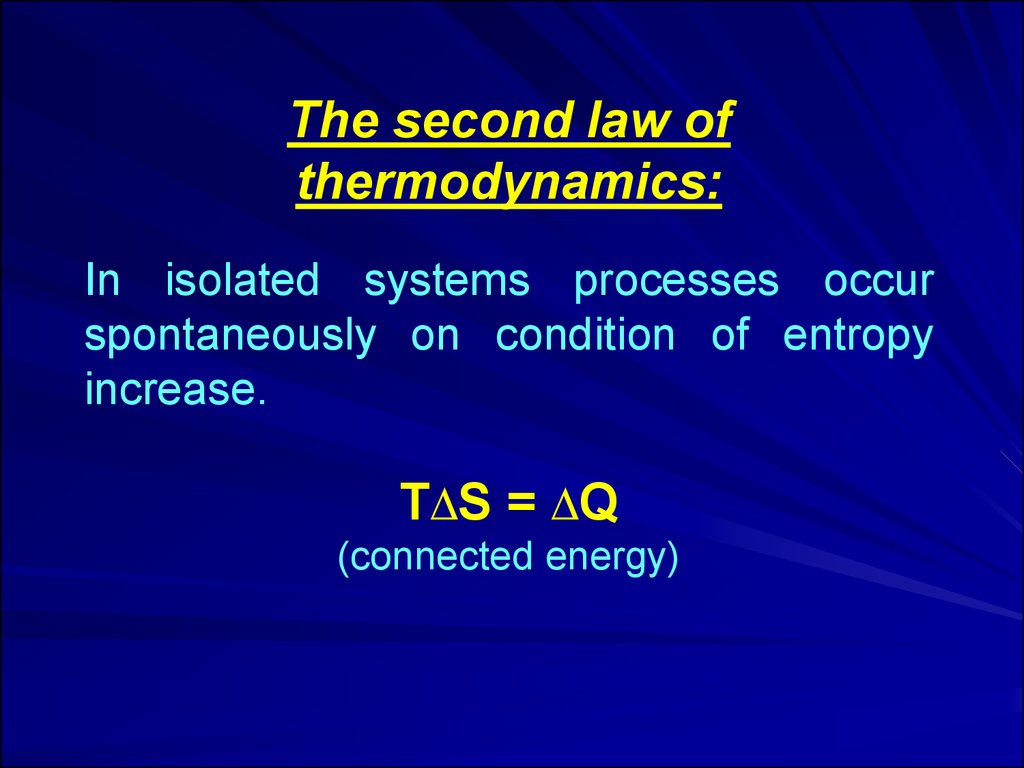
The second law ofthermodynamics:
In isolated systems processes occur
spontaneously on condition of entropy
increase.
Т∆S = ∆Q
(connected energy)
11.
Living organismsare open systems that exchange their
matter and energy with the
environment.








 physics
physics








