Similar presentations:
Acute renal failure (ARF)
1. Acute renal failure (ARF)
2. Definition
DEFINITIONAcute
renal failure
(ARF) is an abrupt
and sudden reduction
in renal function
resulting in the
inability to excrete
metabolic wastes and
maintain proper fluid
& electrolyte balance
3. Definition
DEFINITIONIt
is usually
associated with
oliguria (urine
output <30cc/hr or
<400cc/day), although
urine output may be
normal or increased
BUN & creatinine
values are elevated
4. Functions of the Kidney’s
FUNCTIONS OF THE KIDNEY’SUrine Formation: Formed in the nephrons
through a complex three-step process:
Glomerular filtration (GF)
Tubular reabsorption,
Tubular secretion
Excretion of waste products: eliminates the
body’s metabolic waste products (urea, creatinine,
phosphates, sulfates)
5. Kidney functions
KIDNEY FUNCTIONSControl of water balance: Normal ingestion of
water daily is 1-2L and normally all but 400500mL is excreted in the urine
Osmolality: degree of dilution or concentration of
urine (#particles dissolved/kg urine (glucose & proteins
are osmotically active agents)
Specific Gravity: measurement of the kidney’s ability
to concentrate urine (weight of particles to the weight of
distilled water)
ADH: vasopressin – regulates water excretion and
urine concentration in the tubule by varying the amount
of water reabsorbed.
6. Functions of the Kidney’s
FUNCTIONSOF THE
KIDNEY’S
Regulation of electrolytes: volume of
electrolytes excreted per day is exactly equal to the
volume ingested
Na – allows the kidney to regulate the volume
of body fluids, dependent on aldosterone
(fosters renal reabsorption of Na)
K – kidneys are responsible for excreting more
than 90% of total daily intake
RETENTION OF K IS THE MOST LIFETHREATENING EFFECT OF RENAL
FAILURE
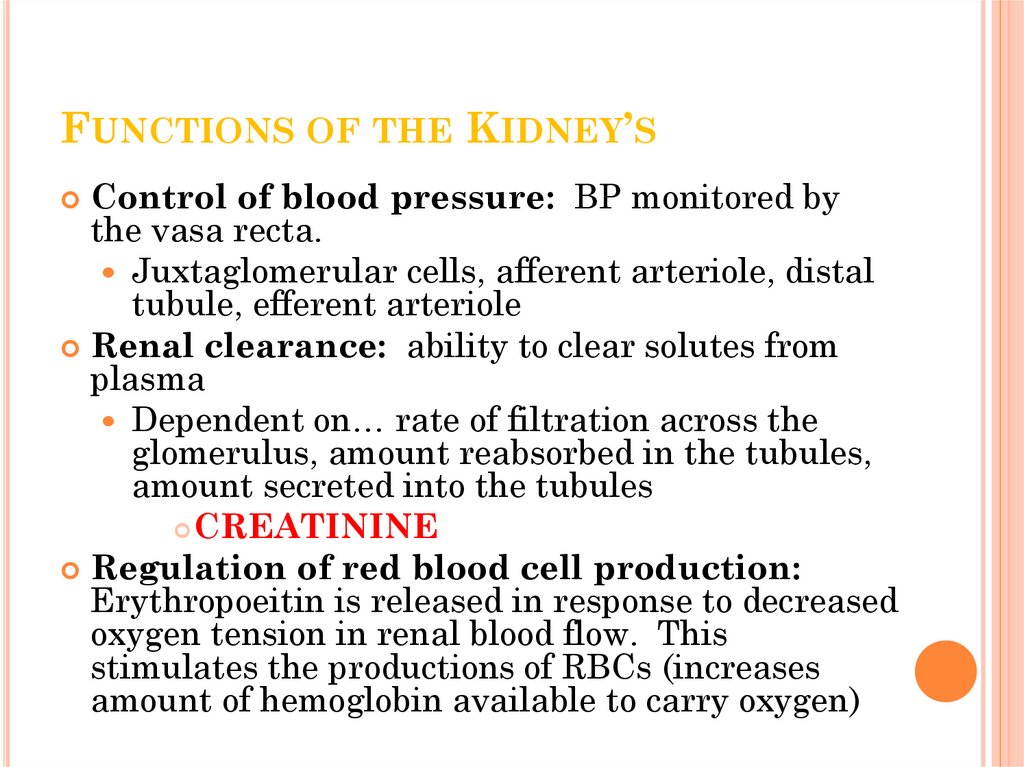
7. Functions of the Kidney’s
FUNCTIONS OF THE KIDNEY’SControl of blood pressure: BP monitored by
the vasa recta.
Juxtaglomerular cells, afferent arteriole, distal
tubule, efferent arteriole
Renal clearance: ability to clear solutes from
plasma
Dependent on… rate of filtration across the
glomerulus, amount reabsorbed in the tubules,
amount secreted into the tubules
CREATININE
Regulation of red blood cell production:
Erythropoeitin is released in response to decreased
oxygen tension in renal blood flow. This
stimulates the productions of RBCs (increases
amount of hemoglobin available to carry oxygen)
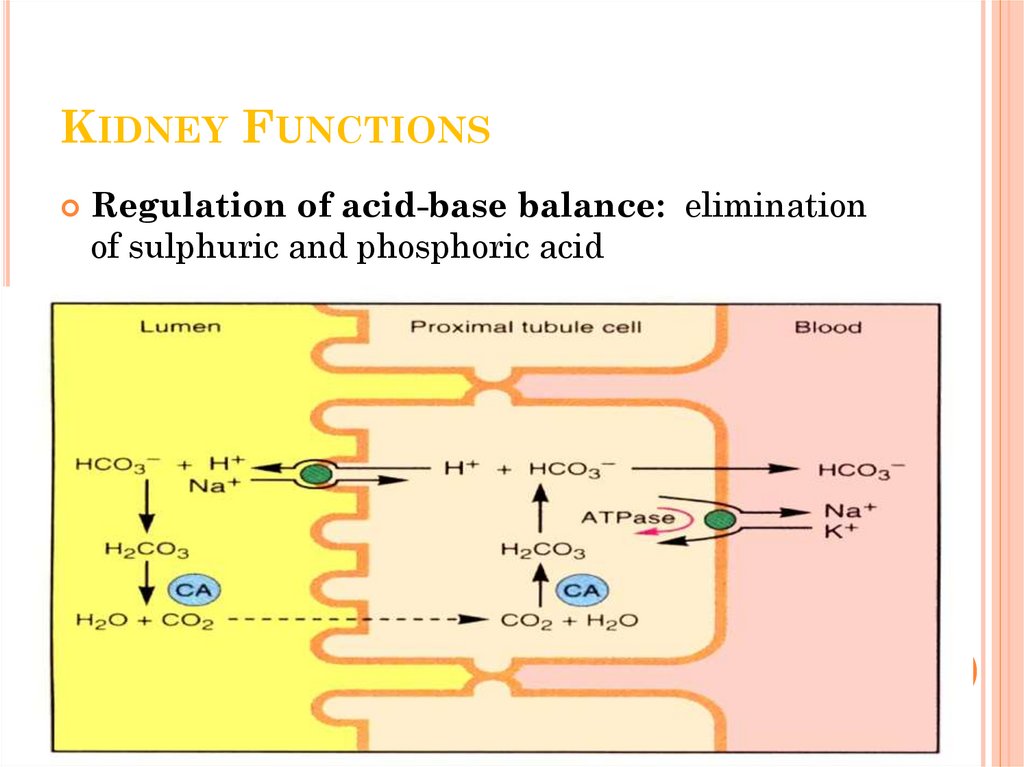
8. Kidney Functions
KIDNEY FUNCTIONSRegulation of acid-base balance: elimination
of sulphuric and phosphoric acid
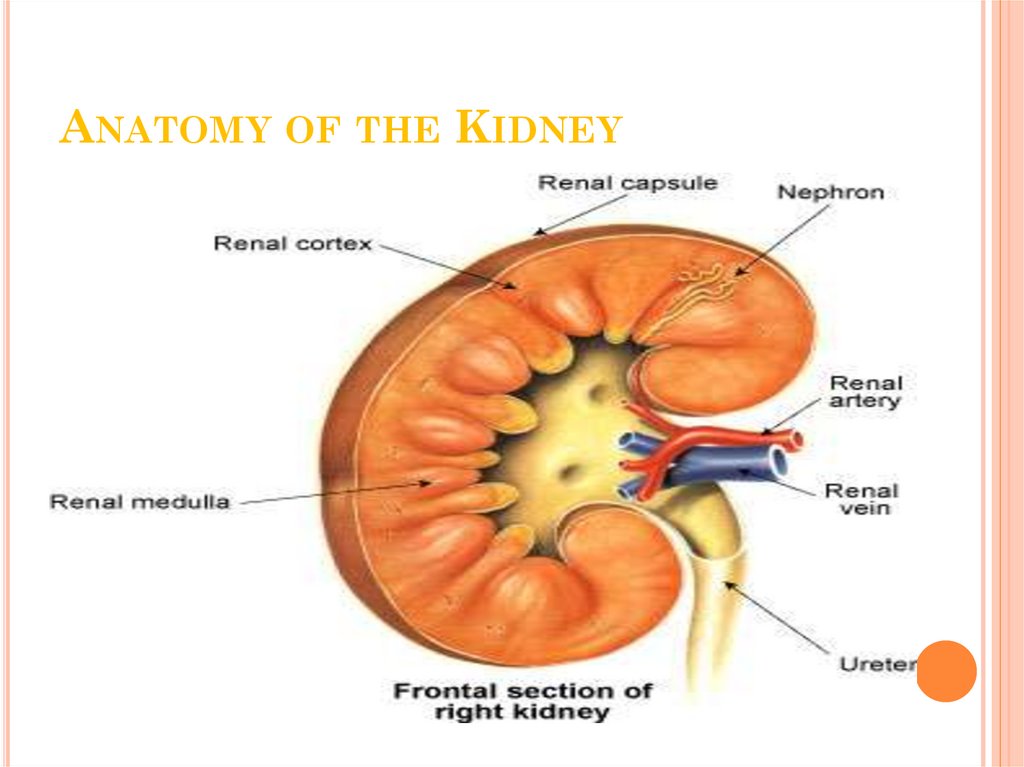
9. Anatomy of the Kidney
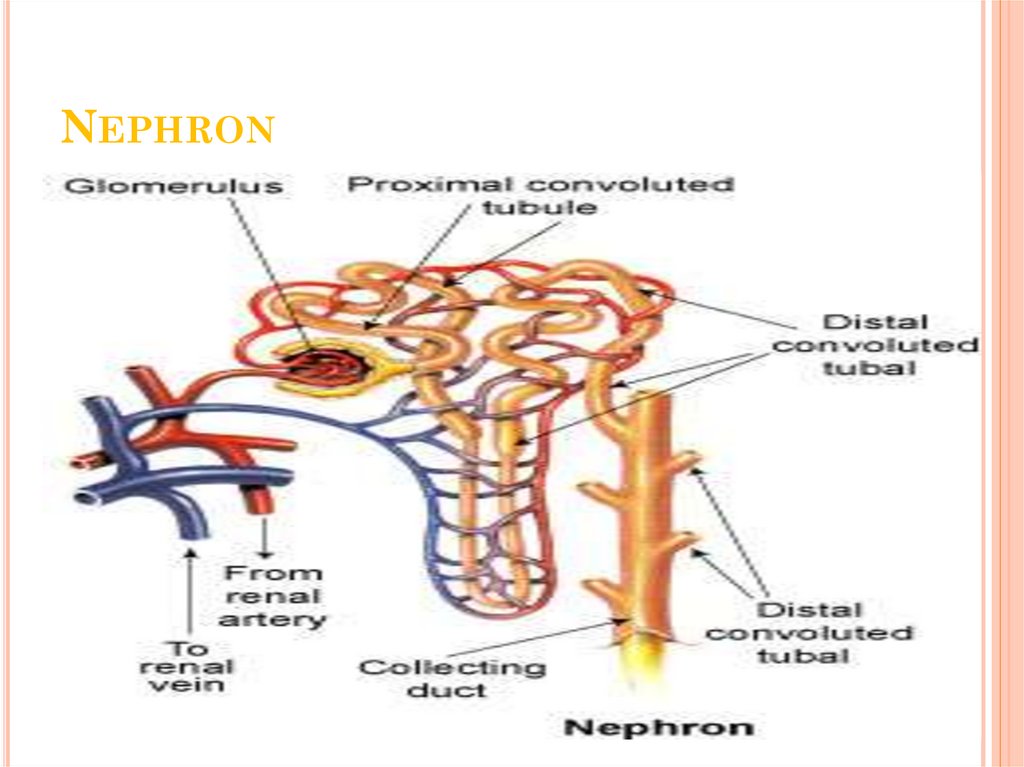
ANATOMY OF THE KIDNEY10. Nephron
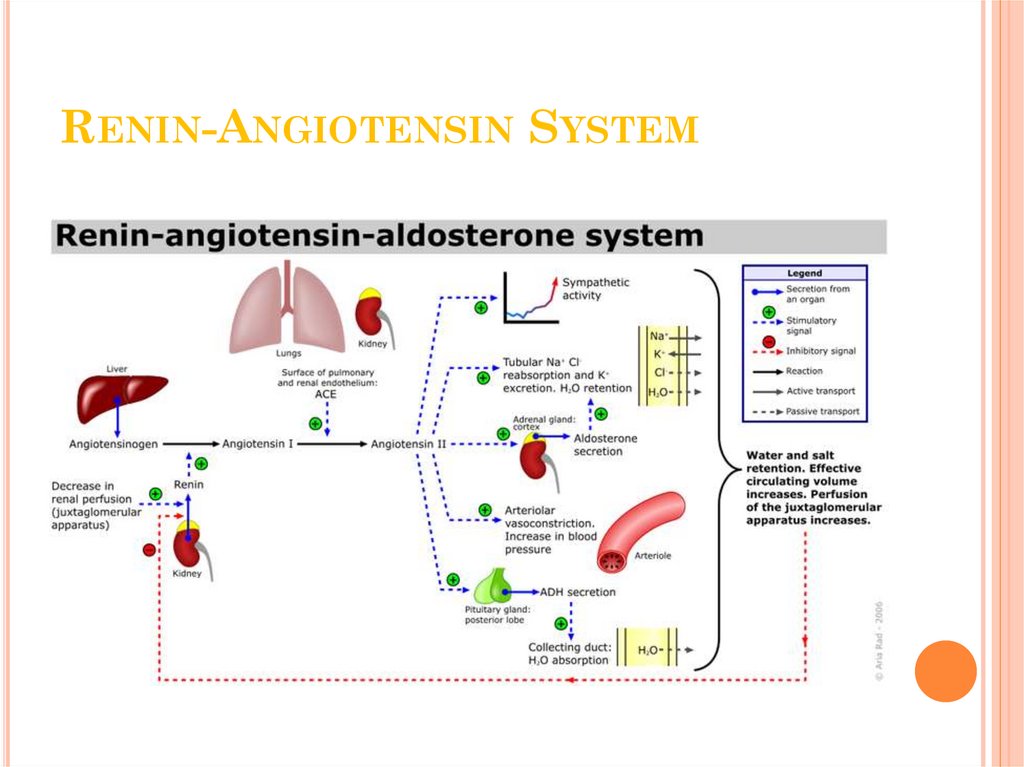
NEPHRON11. Renin-Angiotensin System
RENIN-ANGIOTENSIN SYSTEM12. Pathophysiology
PATHOPHYSIOLOGYGlomerular filtration is caused by
difference between glomerular pressure (70
mm Hg), colloid oncotic pressure (30
mm Hg) and capsular pressure (20 mm Hg).
The effective filtration pressure is
70mmHg -(30mmHg + 20mmHg) = 20mmHg.
Oncotic + capsular pressure must be lower
than glomerular pressure. As a result of the
filtration primary urine is formed.
13. Pathophysiology
PATHOPHYSIOLOGYAs
a result of the filtration primary
urine is formed.
The kidneys produce 180 to 200 l of
filtrate per day.
This fluid is essentially protein-free
and contains mostly crystalloids in
the same concentrations as in the
plasma.
Approximately 99% of the filtrate
must be returned to the vascular
system, while 1% is excreted in the
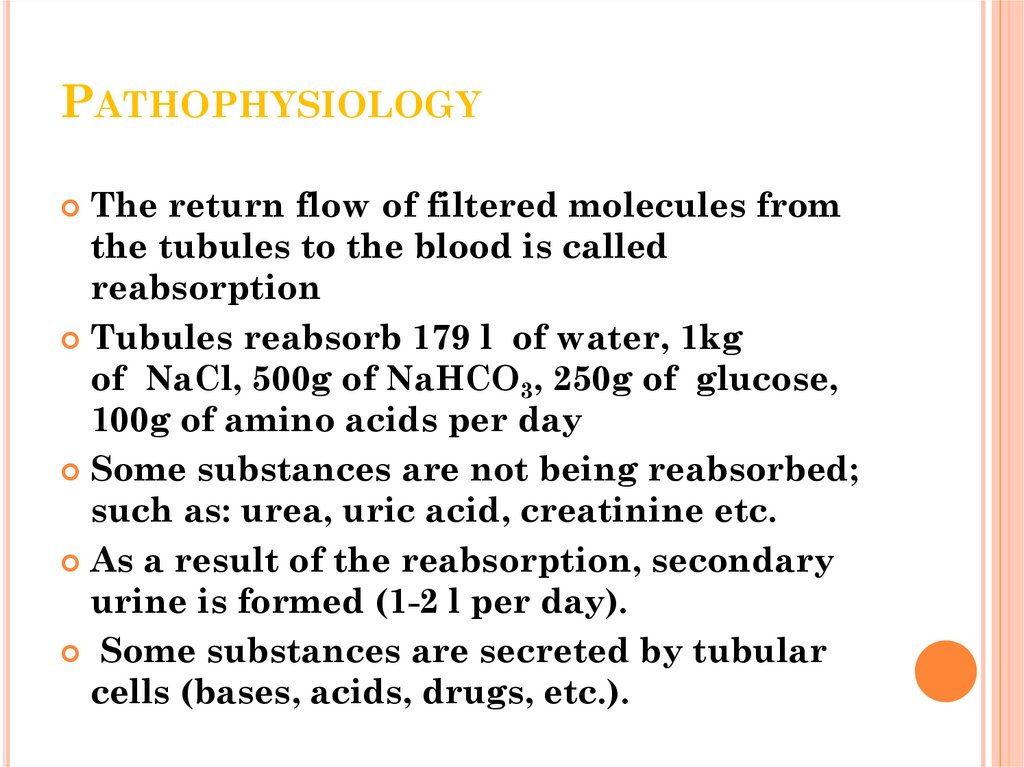
14. Pathophysiology
PATHOPHYSIOLOGYThe return flow of filtered molecules from
the tubules to the blood is called
reabsorption
Tubules reabsorb 179 l of water, 1kg
of NaCl, 500g of NaHCO3, 250g of glucose,
100g of amino acids per day
Some substances are not being reabsorbed;
such as: urea, uric acid, creatinine etc.
As a result of the reabsorption, secondary
urine is formed (1-2 l per day).
Some substances are secreted by tubular
cells (bases, acids, drugs, etc.).
15. Epidemiology of ARF
EPIDEMIOLOGY OF ARFIncidence,
etiology and outcome
varied depending on Population
studied and Definition used
Mostly in-Patient than out –Patient
5-7% of hospital admissions
Mortality varies between 20%-85%
depending on cause
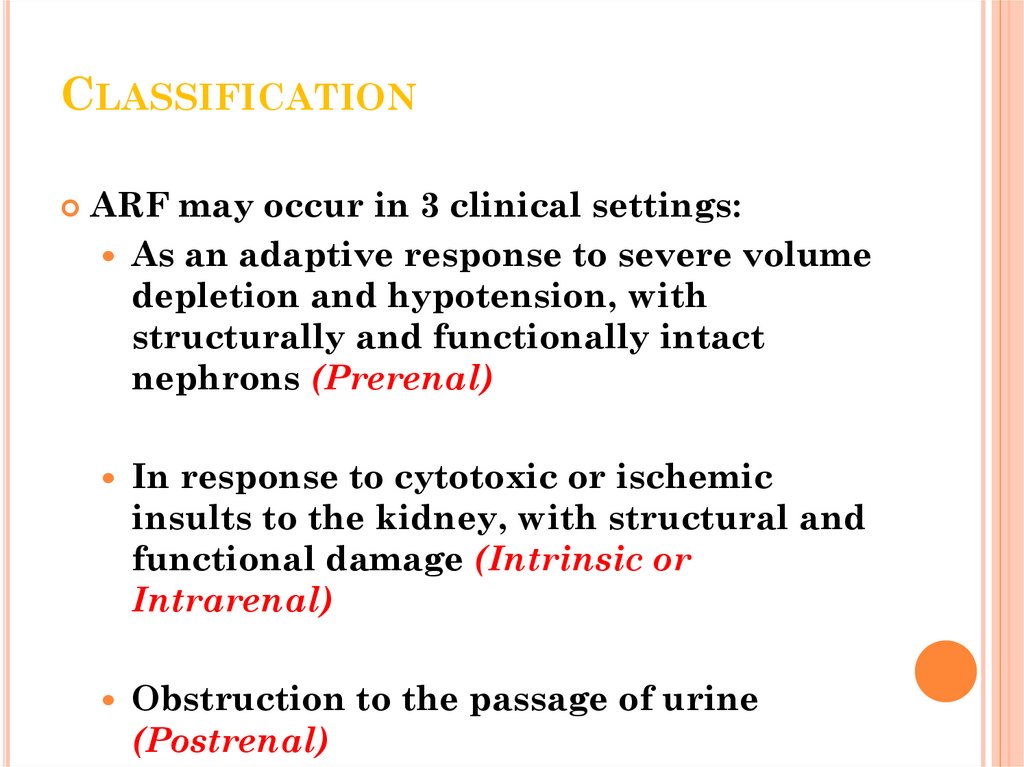
16. Classification
CLASSIFICATIONARF may occur in 3 clinical settings:
As an adaptive response to severe volume
depletion and hypotension, with
structurally and functionally intact
nephrons (Prerenal)
In response to cytotoxic or ischemic
insults to the kidney, with structural and
functional damage (Intrinsic or
Intrarenal)
Obstruction to the passage of urine
(Postrenal)
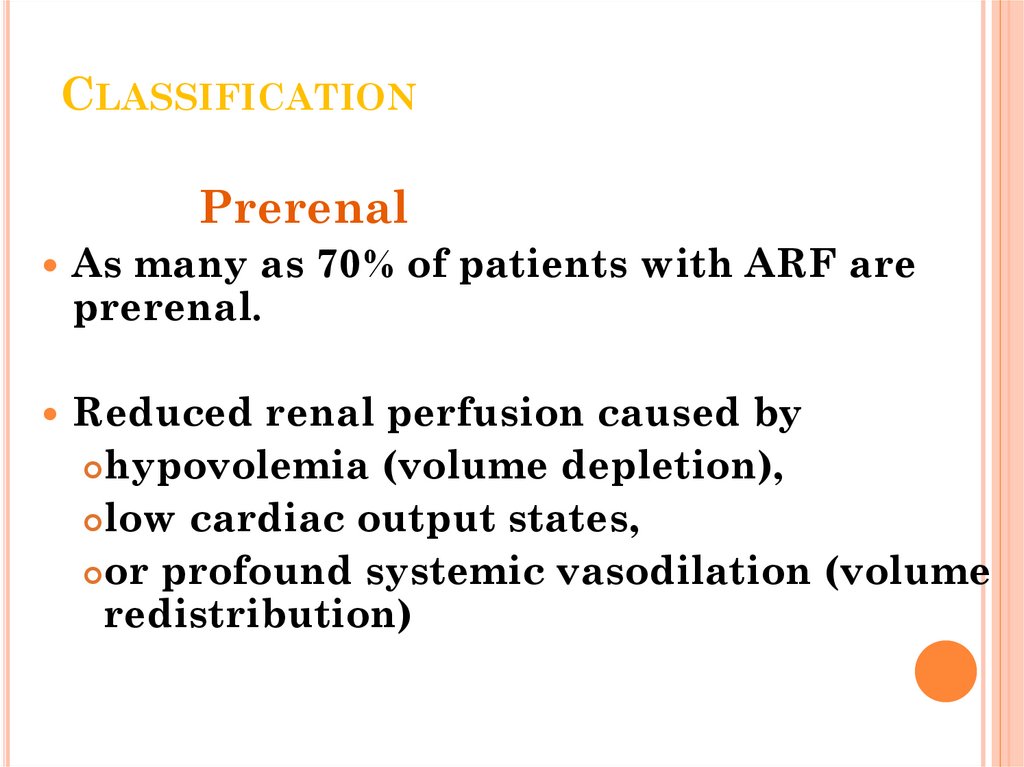
17. Classification
CLASSIFICATIONPrerenal
As many as 70% of patients with ARF are
prerenal.
Reduced renal perfusion caused by
hypovolemia (volume depletion),
low cardiac output states,
or profound systemic vasodilation (volume
redistribution)
18. Classification
CLASSIFICATIONPrerenal
Afferent arteriolar vasodilation and
efferent vasoconstriction of the
glomerular vessels (mediated by dilating
prostaglandins and angiotensin II,
respectively) will initially maintain
glomerular perfusion pressure at a cost
of compromising tubular perfusion.
If renal hypoperfusion persists, acute
tubular necrosis and established renal
failure inevitably develop.
19. Classification
CLASSIFICATIONThe
causes of prerenal ARF include the
following:
volume depletion
gastrointestinal loss,
excessive diuresis,
and salt-wasting nephropathy
volume redistribution (peripheral vasodilation)
peritonitis, burns, pancreatitis,
hypoalbuminemia
reduced cardiac output
pericardial tamponade,
myocardial infarction,
acute/chronic valvular disease,
cardiomyopathies,
20. Classification
CLASSIFICATIONIntrinsic
Intrinsic cases comprise 25% of all acute renal
failure cases.
Most cases (90%) of acute intrinsic renal failure are
acute tubular necrosis caused by renal ischemia
and toxins (including sepsis).
The terminal portion of the proximal tubule and the
ascending limb of the loop of Henle are most at risk
because of their high metabolic activity.
Epithelial casts develop, blocking the tubules and
further impairing function.
Recovery of function is common following acute
tubular necrosis, and is brought about by renal
parenchymal regeneration.
21. Classification
CLASSIFICATIONThe
causes of intrinsic renal failure include:
renal ischemia
renal artery/vein thrombosis
glomerulonephritis
vasculitides
hemolytic uremic syndrome/thrombotic
thrombocytopenic purpura
malignant hypertension
drugs (eg, aminoglycosides, contrast media)
acute tumor lysis syndrome
rhabdomyolysis
allergic interstitial nephritis
acute pyelonephritis.
22. Post-Renal ARF
POST-RENAL ARFObstruction – complete or Partial
Anuria or variable urine output
Recovery depends on duration of obstruction
Conditions Sonogram may not show obstruction,
Retroperitoneal fibrosis
Tumors
Adenopathy
Encasing ureter prevent dilatation
Postrenal causes are typically reversible
23. Phases of Acute Renal Failure
PHASES OF ACUTE RENAL FAILUREClinical progression of reversible RF occurs
in four phases:
Initiation phase
Begins with initial insult and ends
when oliguria develops
Oliguric phase
Accompanied by rise in serum
concentrations of substances usually
excreted by kidneys (urea, creatinine,
ua, organic acids, intracellular cations
[K+ & Mg])
urinary output <400cc/day
May last 1-3 weeks
24. Phases of Acute Renal Failure
PHASES OF ACUTE RENAL FAILUREDiuretic phase
The kidneys begin to recover
Initially produce hypotoniс urine
d/t increase in GFR
Recovery phase
Tubular function restored
Diuresis subsides and kidney
begins to function normally again
25. Diagnosis
DIAGNOSISWhile
a medical history and
physical examination are
important in making a
diagnosis of acute renal
failure, laboratory findings
help to define the
diagnosis.
26. Diagnosis
DIAGNOSISHistory
Observe for disorder that predisposes pt to
ARF
Ask questions about recent illness, infections,
or injuries
Medication history
Urinary patterns
History of GI problems
Psychosocial
Anxious
Family members
27. Clinical Manifestations of ARF
CLINICAL MANIFESTATIONSOF ARF
Cardiovascular
Arrhythmias
BP, N, high or low
Anemia
P, rapid, bounding, or N
Pericardial-type chest pain
Respiratory
Dyspnea
Crackles
Tachypnea
Kussmaul’s respirations
Mental Status
Lethargy
Tremors
Memory loss
Confusion
Musculoskeletal
Muscle spasms
Weakness
28. Clinical Manifestations of ARF
CLINICAL MANIFESTATIONSOF ARF
Genitourinary
Oliguria
Anuria
abN urine colour, clarity, smell
GI
Moist tongue & increased saliva
Dry tongue & mucous membranes
N&V
Integumentary
Moist, warm skin & pitting edema
Decreased skin turgor
bruises
Pallor
Thin, brittle hair & nails
29. Diagnosis
DIAGNOSISOliguria (urine output < 400 mL/day) may or may not
be present. The important laboratory abnormalities
include:
raised urea and creatinine
hyperkalemia
metabolic acidosis.
All of the above problems will be exacerbated if they
are caused or accompanied by the hypercatabolic
state of the systemic inflammatory response syndrome
(SIRS) or sepsis. Urinalysis aids the distinction
between prerenal and intrinsic renal failure.
The urinary bladder must be catheterized and
appropriate imaging (ultrasound, CT) performed to
exclude obstruction of the renal tract.
Autoimmune screens for disorders such as systemic
lupus erythematosus (SLE),Wegener’s granulomatosis,
and Goodpasture syndrome might be indicated,along
30.
The fractional excretion of sodium (FENa+)is considered to be the most reliable
biochemical laboratory discriminator
between prerenal and intrinsicrenal failure,
and is given by:
FENa+ = (UNa/PlNa)/(UCr/PlCr)× 100
where UNa = urea sodium, PlNa = plasma
sodium, UCr = urea creatinine, and PlCr =
plasma creatinine.
The fractional excretion of urea can also be
particularly useful in assessing prerenal
cases.
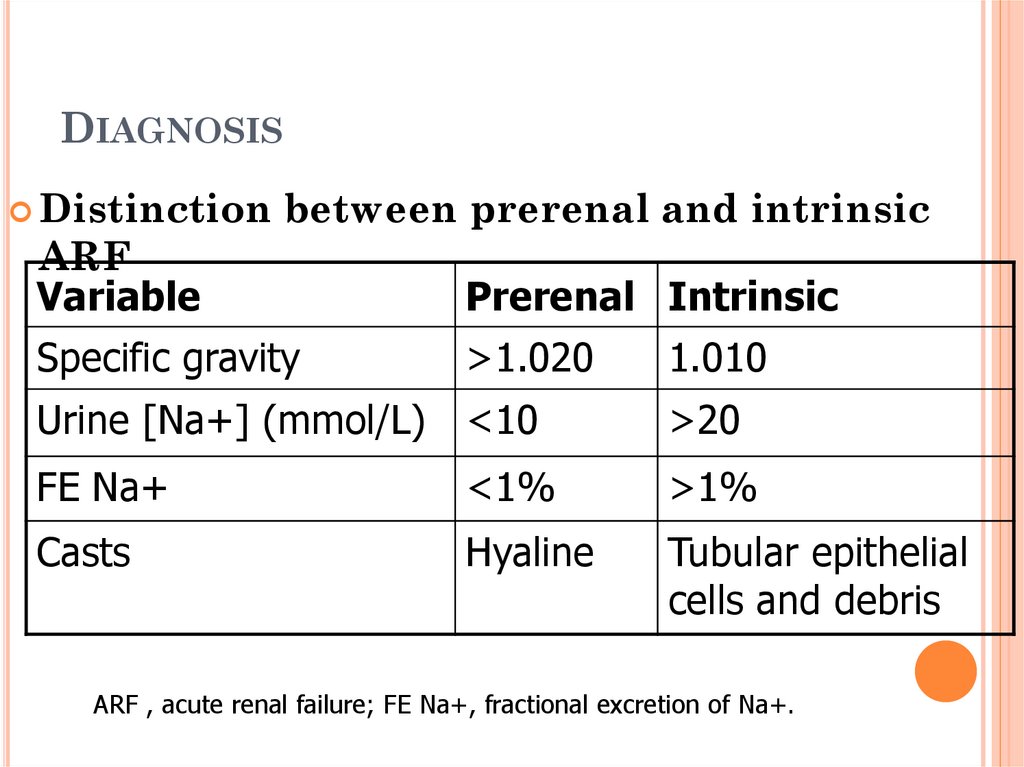
31. Diagnosis
DIAGNOSISDistinction
between prerenal and intrinsic
ARF
Variable
Prerenal Intrinsic
Specific gravity
>1.020
1.010
Urine [Na+] (mmol/L) <10
>20
FE Na+
<1%
>1%
Casts
Hyaline
Tubular epithelial
cells and debris
ARF , acute renal failure; FE Na+, fractional excretion of Na+.
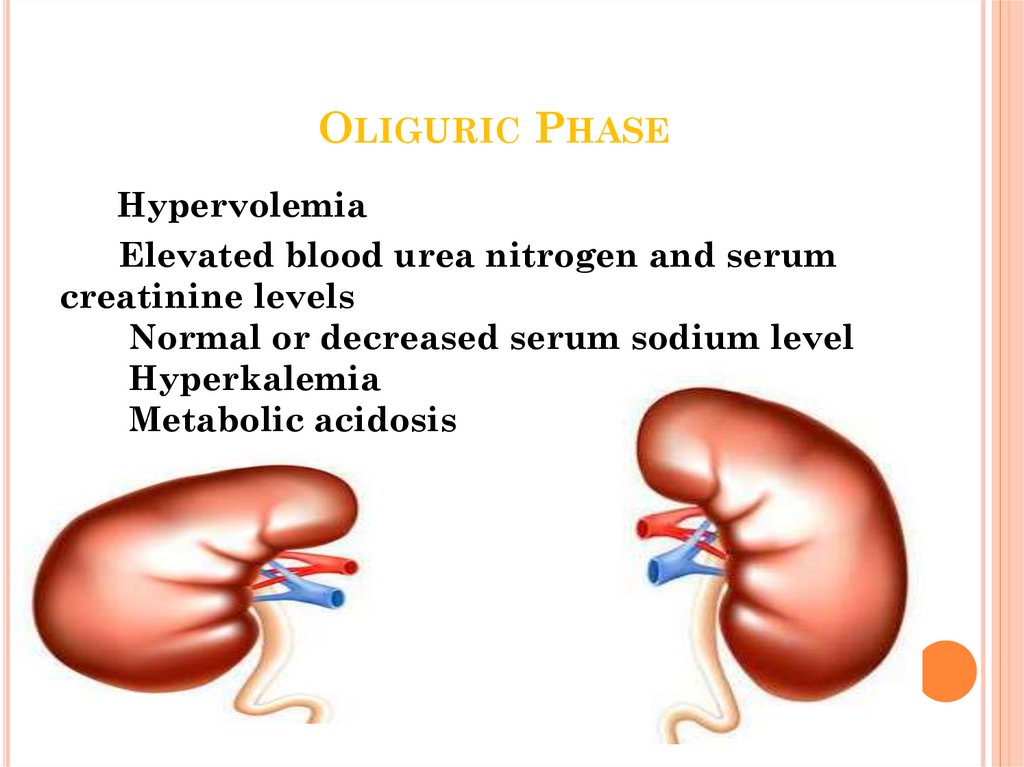
32. Oliguric Phase
OLIGURIC PHASEHypervolemia
Elevated blood urea nitrogen and serum
creatinine levels
Normal or decreased serum sodium level
Hyperkalemia
Metabolic acidosis
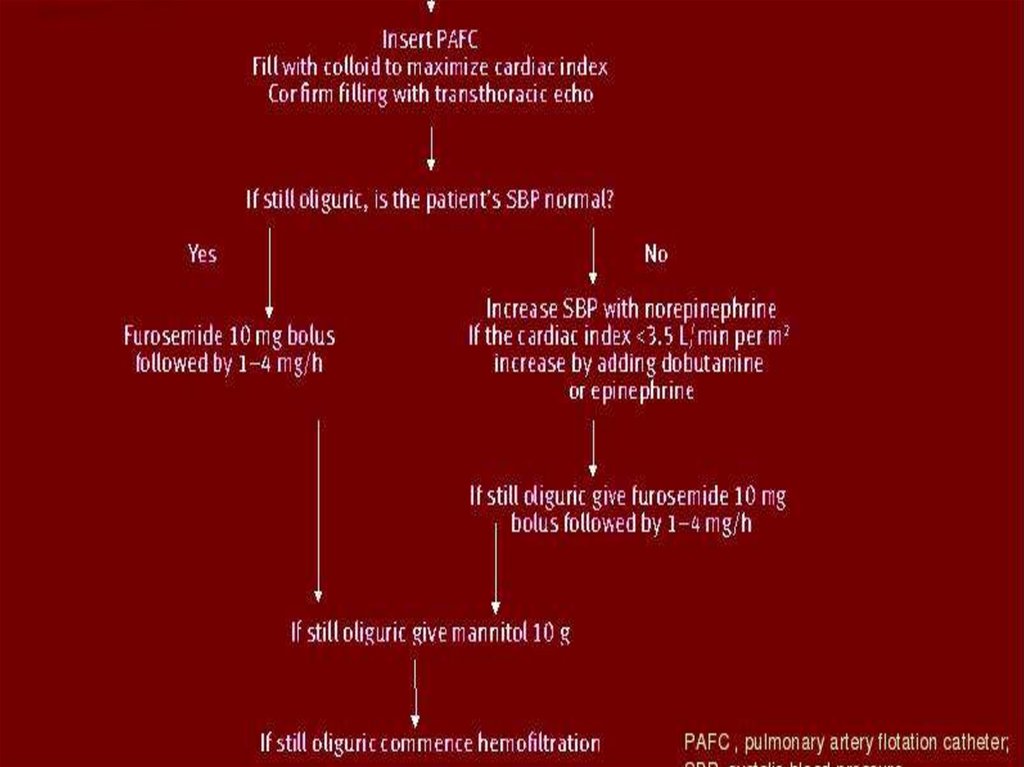
33. Treatment
TREATMENTPrerenal
renal failure
The aim of treatment is to restore renal
perfusion before intrinsic renal failure is
established.
Recent ‘renal rescue’ protocols emphasize
the need for:
invasive monitoring
aggressive fluid resuscitation
restoration of the patient’s systolic blood
pressure to a normal level
avoidance of nephrotoxins
maintenance of adequate oxygenation, with
34.
35. Treatment
TREATMENTIntrinsic
Renal perfusion should be maintained to
eliminate prerenal failure.
Measures should be taken to exclude and treat
obstructive renal failure.
Once intrinsic renal failure is established,
general measures can be adopted and these
are discussed below.
Fluid
renal failure
balance
Restrict fluid intake to 30 mL/h plus losses
(nasogastric, drains, diarrhea, etc) until renal
replacement therapy has been instituted.
36. Treatment
TREATMENTNutritional support
Adequate nutrition is of considerable
importance and should be enteral if at all
possible.
Caloric requirements may be high in
hypercatabolic patients (30–35 kcal/kg
daily).
Protein intake should be restricted to 20–30
g daily
37. Treatment
TREATMENTTreatment of hyperkalemia
Treatment is required if EKG changes are present or
potassium (K+) levels >6.5 mmol/L.
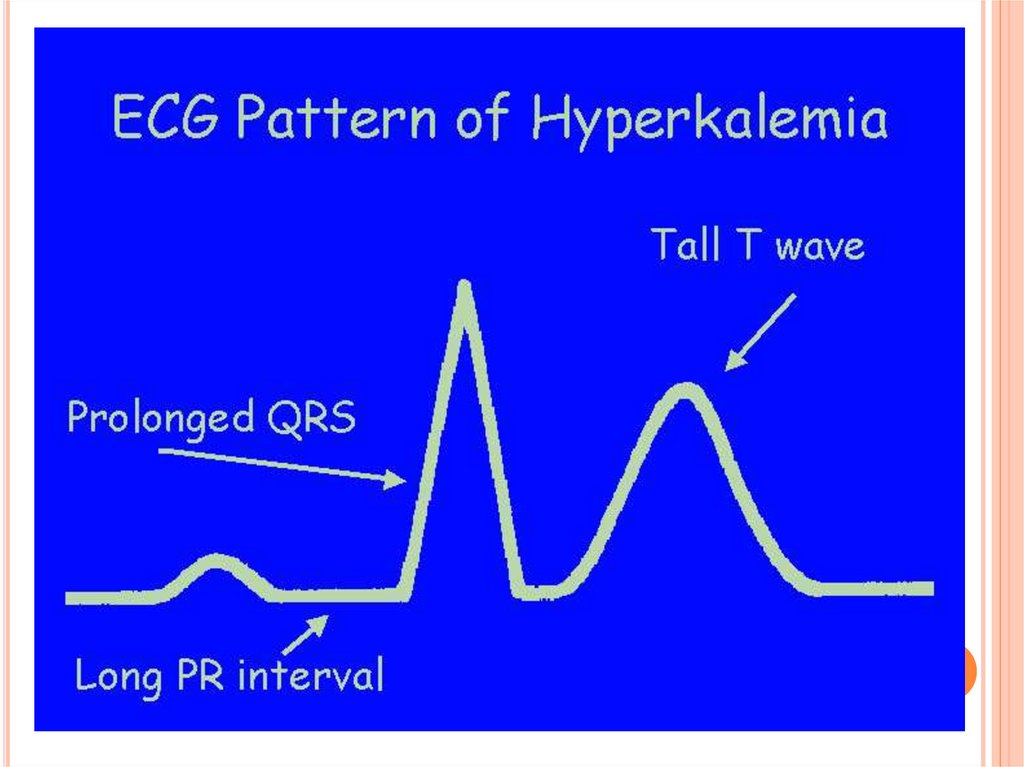
EKG changes signaling hyperkalemia include:
• peaked T waves
• loss of P wave
• broadened QRS complex
• slurring of ST segment into T wave
• sine wave leading to asystole.
38.
39. Treatment
TREATMENTIf
dialysis is not immediately available, the
following measures may be used to
temporarily redistribute K+ from the plasma
or stabilize the myocardium to reduce the
risk of arrhythmias:
• Calcium gluconate (10%) 10 mL i.v. over 5 min (or
20 mL if there is hypocalcemia) will reduce the risk
of arrhythmias.
• Glucose (50%) 50 mL i.v. will stimulate insulin
release, thereby promoting entry of K+ (and
glucose) into cells. If hyperglycemia ensues,
administer 4–12 units of insulin (routine use of
40. Treatment
TREATMENT• If the patient is acidotic, give sodium bicarbonate
(NaHCO3) 50–100 mmol over 1 h but be aware of
the usual risks of bicarbonate administration,
including fluid overload, worsening of
intracellular and cerebrospinal fluid (CSF)
acidosis, acute ionized hypocalcemia, and
increased carbon dioxide production.
• Eliminate any unnecessary K+ administration in
drugs, diet, etc.
41. Treatment
TREATMENTTreatment
of acidosis
Sodium bicarbonate should be used only
when acidosis is severe (pH <7.1), the
patient is symptomatic, or if acidosis is
associated with acute hyperkalemia.
The need for bicarbonate is an indication
for dialysis.
42. Treatment
TREATMENTIdentification and
treatment of sepsis
Commence empiric broadspectrum therapy once
cultures have been taken,
bearing in mind the
potential nephrotoxicity
and reduced elimination of
many antimicrobials.
43. Treatment
TREATMENTCause-specific therapies :
• mannitol/NaHCO3 in acute rhabdomyolysis
• immunosuppression in SLE, Wegener’s
granulomatosis, or Goodpasture syndrome
• plasmapheresis, fresh frozen plasma, and
prostacyclin in the hemolytic uremic
syndrome
44. Hemodialysis
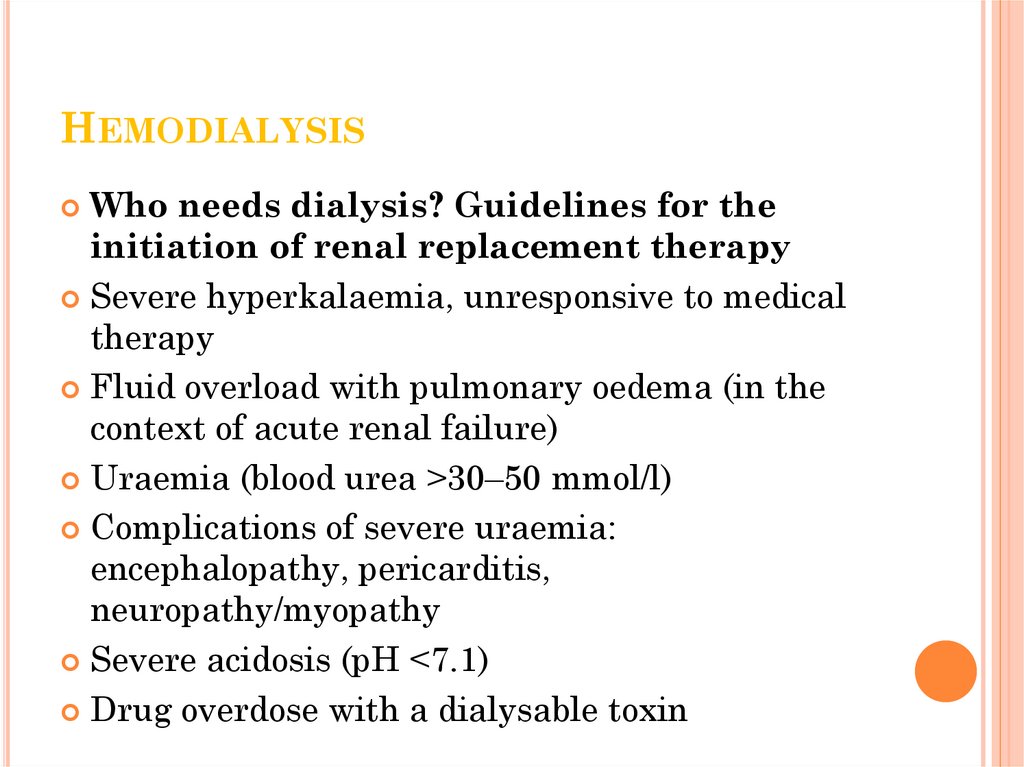
HEMODIALYSISWho needs dialysis? Guidelines for the
initiation of renal replacement therapy
Severe hyperkalaemia, unresponsive to medical
therapy
Fluid overload with pulmonary oedema (in the
context of acute renal failure)
Uraemia (blood urea >30–50 mmol/l)
Complications of severe uraemia:
encephalopathy, pericarditis,
neuropathy/myopathy
Severe acidosis (pH <7.1)
Drug overdose with a dialysable toxin
45. Hemodialysis
HEMODIALYSISDialysis is a type of renal replacement
therapy which is used to provide artificial
replacement for lost kidney function due to
acute or chronic kidney failure
It is a life support treatment, it does not
cure acute or chronic renal failure
May be used for very sick clients who have
suddenly lost kidney function
May be used for stable clients who have
permanently lost kidney function
46. Hemodialysis
HEMODIALYSISHealthy
kidneys remove waste
products (potassium, acid, urea) from
the blood and they also remove
excess fluid in the form of urine
Dialysis has to duplicate both of
these functions
Dialysis – waste removal
Ultrafiltration – fluid removal
47. Principle of Dialysis
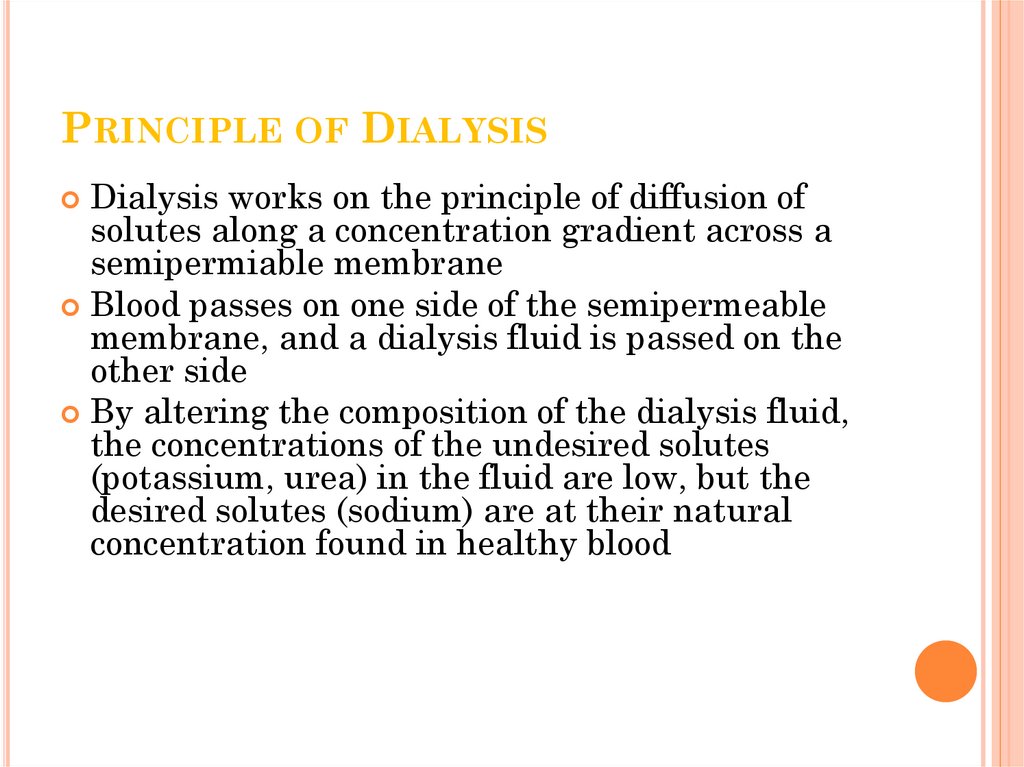
PRINCIPLE OF DIALYSISDialysis works on the principle of diffusion of
solutes along a concentration gradient across a
semipermiable membrane
Blood passes on one side of the semipermeable
membrane, and a dialysis fluid is passed on the
other side
By altering the composition of the dialysis fluid,
the concentrations of the undesired solutes
(potassium, urea) in the fluid are low, but the
desired solutes (sodium) are at their natural
concentration found in healthy blood
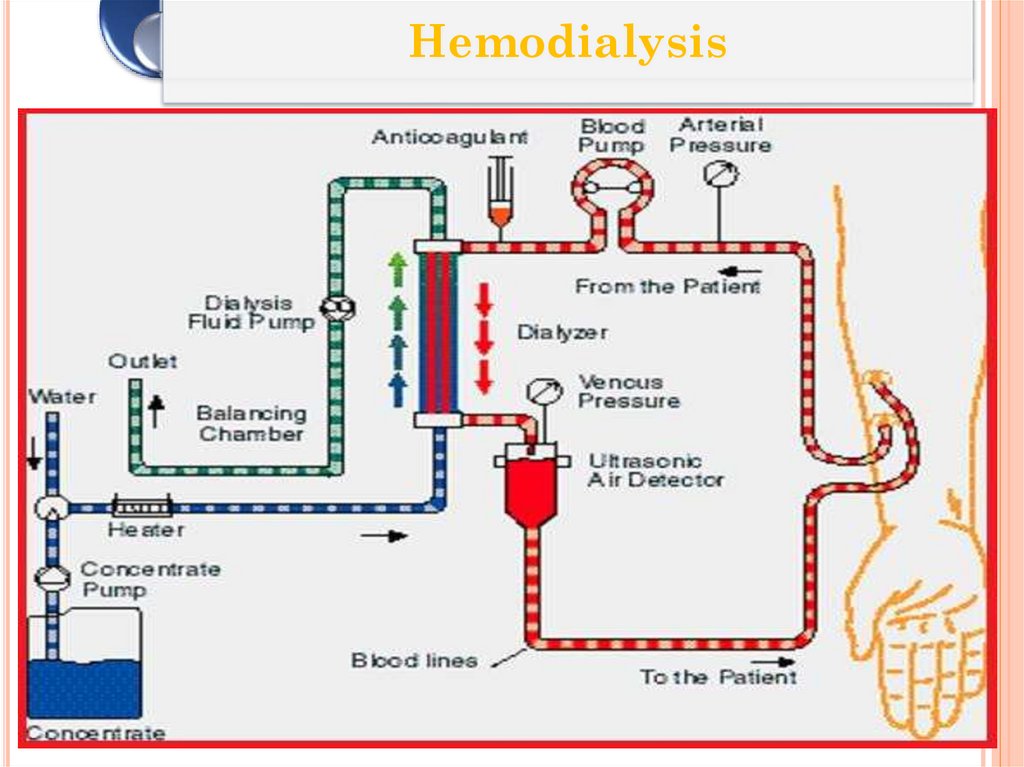
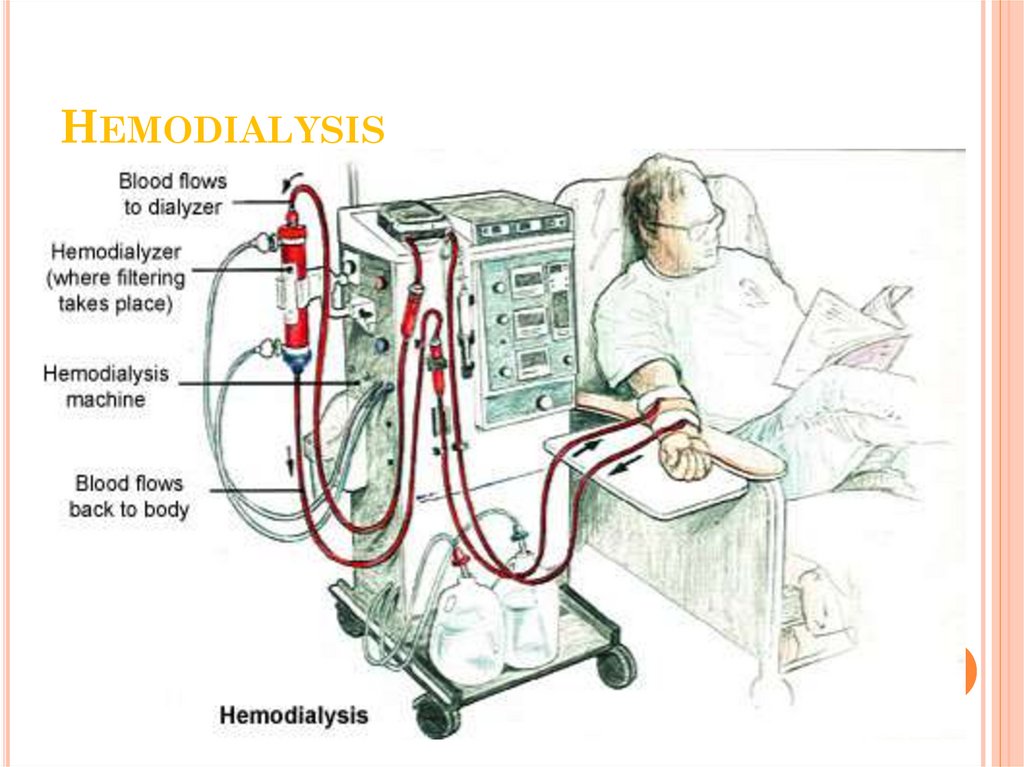
48. Hemodialysis
HEMODIALYSISClient’s blood is passed through a system of
tubing (dialysis circuit) via a machine to a
semipermeable membrane (dialyzer) which has
the dialysis fluid running on the other side
The cleansed blood is then returned via the
circuit back to the body
The dialysis process is very efficient (much
higher than in the natural kidneys), which allows
treatments to take place intermittently (usually 3
times a week), but fairly large volumes of fluid
must be removed in a single treatment which can
be very demanding on a client
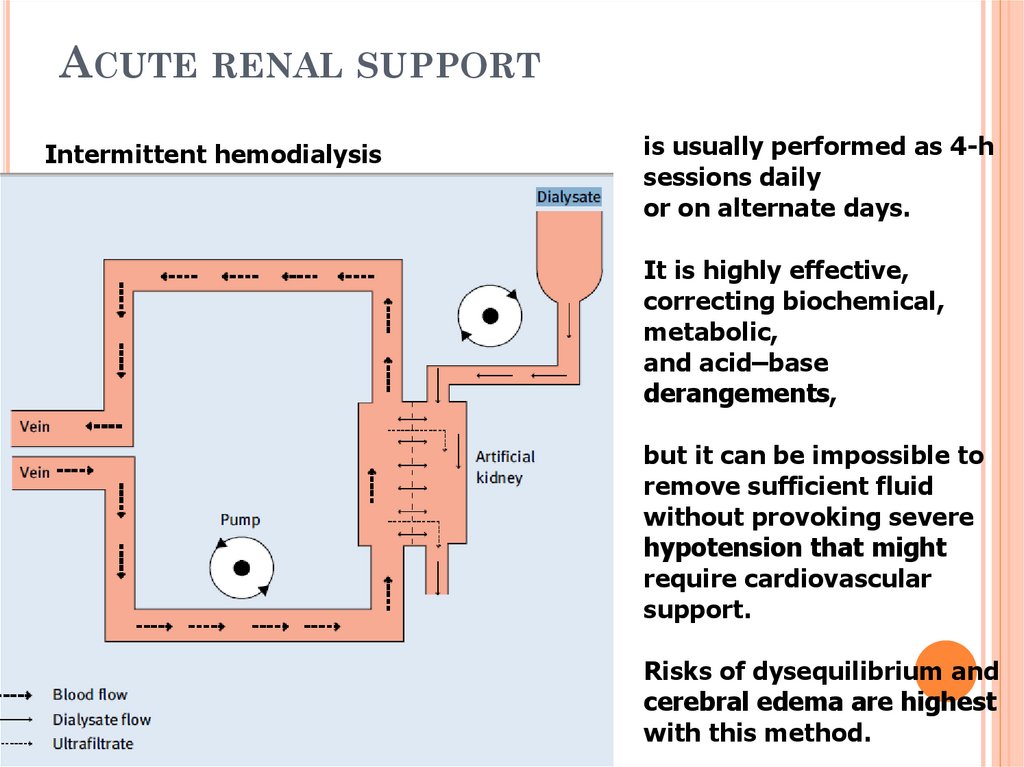
49. Acute renal support
ACUTE RENAL SUPPORTIntermittent hemodialysis
is usually performed as 4-h
sessions daily
or on alternate days.
It is highly effective,
correcting biochemical,
metabolic,
and acid–base
derangements,
but it can be impossible to
remove sufficient fluid
without provoking severe
hypotension that might
require cardiovascular
support.
Risks of dysequilibrium and
cerebral edema are highest
with this method.
50.
Hemodialysis51. Equipment Needed for HD
EQUIPMENT NEEDED FOR HDThe
HD machine performs the
function of pumping the patient's
blood and the dialysate through the
dialyzer.
The newest dialysis machines on the
market are highly computerized and
continuously monitor an array of
safety-critical parameters, including
blood and dialysate flow rates, blood
pressure, heart rate, conductivity,
pH, etc.
If any reading is out of normal range,
an audible alarm will sound to alert
the patient-care technician who is
monitoring the patient.
52. Hemodialysis
HEMODIALYSIS53.
54.
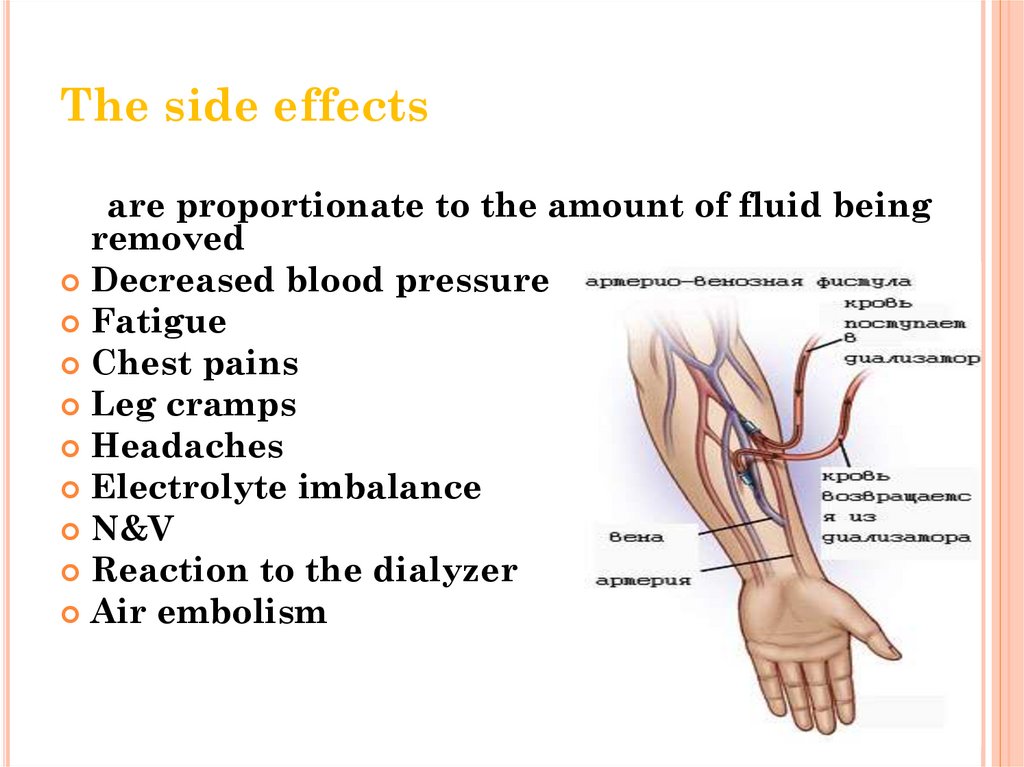
The side effectsare proportionate to the amount of fluid being
removed
Decreased blood pressure
Fatigue
Chest pains
Leg cramps
Headaches
Electrolyte imbalance
N&V
Reaction to the dialyzer
Air embolism
55.
Complications of HDBecause HD requires access to the circulatory system,
clients have a portal of entry for microbes, which could
lead to infection
The risk of infection depends on the type of access used
Bleeding may also occur at the access site
Blood clotting was a serious problem in the past, but
the incidence of this has decreased with the routine
use of anticoagulants (Heparin is the most common)
Anticoagulants also come with their own risk of side effects
and complications
56. Acute renal support
ACUTE RENAL SUPPORTHemodialysis
All variants of hemodialysis share the need
for the following:
• vascular access – this carries the incurrent risks of
complications such as infection or thrombosis
• extracorporeal circuit with artificial kidney –
activation of complement and circulating
neutrophils can lead to cardiorespiratory problems
during dialysis, although this is more of a problem
with cuprophane membranes than with the newer,
more biocompatible membranes (eg, polysulphone
and polyamide)
• anticoagulation – heparin is usually used, although
prostacyclin can be used in the presence of a
coagulopathy.
57. Acute renal support
ACUTE RENAL SUPPORTHemodialysis
Potential problems include the following:
• dysequilibrium syndrome – rapid changes in plasma
osmolality, leading to cerebral edema, and in some
cases intracranial hypertension
• hypovolemia and hypotension
• fluid overload
• hypoxemia – possibly a result of inflammatory
reactions initiated within the pulmonary
microvasculature
• bleeding and vascular access complications.
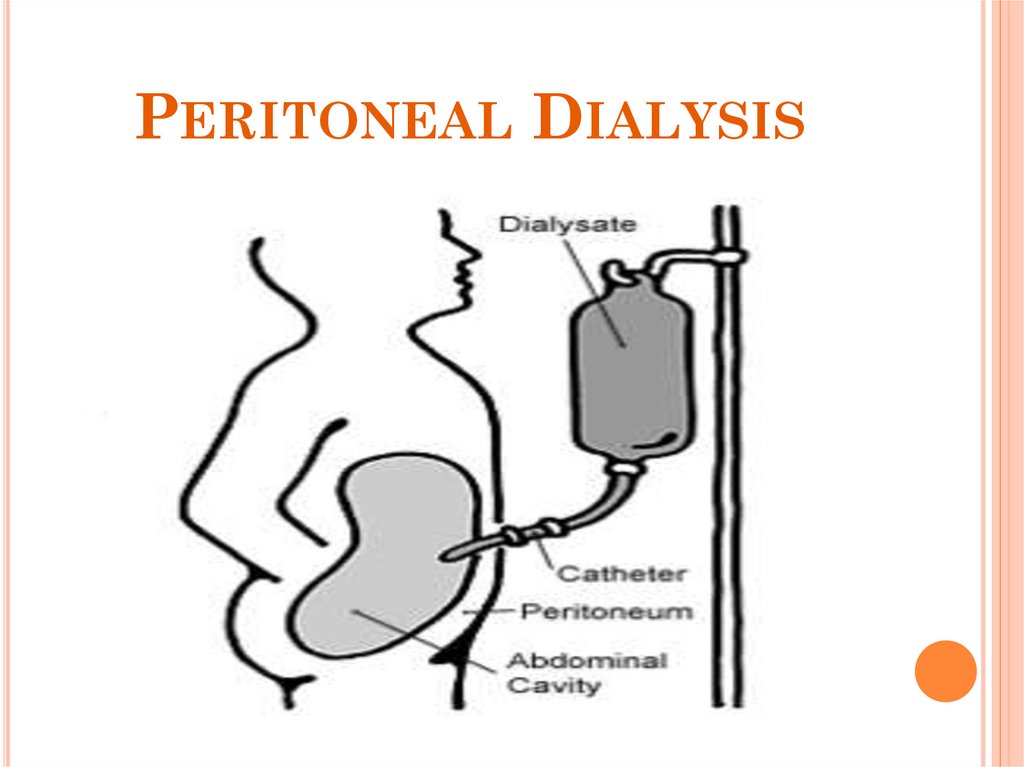
58. Peritoneal Dialysis
PERITONEAL DIALYSIS59. What is Peritoneal Dialysis (PD)?
WHAT IS PERITONEAL DIALYSIS(PD)?
Peritoneal dialysis works by using the
body's peritoneal membrane, which is
inside the abdomen, as a semi-permeable
membrane.
A specially formulated dialysis fluid is
instilled around the membrane, using an
indwelling catheter, then dialysis can
occur, by diffusion
Excess fluid can also be removed by
osmosis, by altering the concentration of
glucose in the fluid.
Dialysis fluid is instilled via a peritoneal
dialysis catheter, which is placed in the
patient's abdomen, running from the
peritoneum out to the surface, near the
navel
60. Advantages of PD
ADVANTAGES OF PDCan be done at home
Relatively easy for the client to learn
Easy to travel with, bags of solution are
easy to take on holiday
Fluid balance is usually easier when the
client is on PD than if the client is on HD
61. Diuretic Phase
DIURETIC PHASEDiuretic phase: The kidneys try to heal and urine
output increases, but tubule scarring and damage
occur.
Gradual decline in blood urea nitrogen and serum
creatinine leveles, but still elevated
Continued low creatinine clearance with improving
glomerular filtration rate
Hypokalemia
Hyponatremia
Hypovolemia
62. Recovery Phase (Convalescent)
RECOVERY PHASE (CONVALESCENT)Tubular edema resolves and renal function
improves.
Increased glomerular filtration rate
Stabilization or continual decline in blood urea
nitrogen and serum creatinine levels toward
normal
Complete recovery (may take 1 to 2 years)
63. Questions?
QUESTIONS?Thank you for listening

























 medicine
medicine