Similar presentations:
IPV - The renewal of a classical tool for Polio eradication (Moscow Chumakov Institute Dec 2014)
1. IPV: the renewal of a classical tool for polio eradication
Emmanuel Vidor, MDMedical Affairs
Sanofi Pasteur, Lyon, France
1
2. Poliomyelitis global epidemiological situation (Year To Date Oct. 2014)
23. Poliomyelitis global epidemiological situation (as of Nov. 2014)
• WPV– Type 2 circulation is eradicated since 1999
• Direct effect of the good performance of tOPV against PV2
– Type 3: no AFP cases since Nov. 2012 (Nigeria): Eradicated?
– Type 1: areas of transmission are diminishing
• High reduction of the genetic diversity of circulating viruses
• Strong reduction in the number of cases
• VDPV
– No more active cVDPV type 1 and 3 outbreaks
– Two active cVDPV type 2 outbreaks
• Nigeria (since 2005): declining slowly (the last AFP onset on Aug. 2014)
• Pakistan (since 2012): seems to progress (the last AFP onset on June 2014)
• In 2014, the total number of AFP cases due to WPV1 (more than
247) will be lower than VAPP cases (several hundreds) and
cVDPV cases (more than 39)
http://www.polioeradication.org/Dataandmonitoring/Poliothisweek.aspx
3
4. Some key characteristics of OPV are challenging the eradication of the circulation of polioviruses and control of paralytic poliomyelitis
• The by-dose (and cumulated) and by-serotype SC and gutimmunity installment in vaccinees is inconsistent and low in
many settings and therefore vaccine effectiveness is sub-optimal
despite vaccination programs relying on > 10 consecutive OPV
administrations up to 5 years of age
– Host-related: concomitant viral, bacterial or parasitic enteric coinfections; The gut microbiome; Environmental Enteropathy syndrome;
Post-birth short-lived inhibitory factors in some ethnicities / settings
– Vaccine-related: intra-vaccine viral interference (type 2 the best replicant
+++)
– Prevalence of maternally-transmitted Abs
• The use of mOPV1, mOPV3 and bOPV1&3 do improve the
immune responses against polioviruses types 1 and 3, and
therefore do improve their effectiveness
4
5. Vaccine-Derived Polioviruses (VDPV): An inevitable consequence of a sub-optimal use of OPV
VDPV: presents more than 1% genomic divergence from the parental
Sabin poliovirus in their VP1 sequence (0.6% for type 2)
– Accumulation of mutations (1% nucleotide change / yr) or recombination
with other enteroviruses of the replicating and/or inter-human circulating
Sabin virus within the vaccinee’s guts or within vaccinee’s contact’s guts
– When detected, prove that such lineage might circulate since at least 1 yr
– Reverted to neurovirulence (or paralyticogenicity) and transmissibility
cVDPV is a VDPV which finds adequate conditions for a sustained
transmission in a human community and responsible for at least >2
AFP cases
–
–
–
–
–
Low vaccination coverage
Poor sanitation conditions
High population density
Lack of competition from WPV circulation
Tropical conditions
5
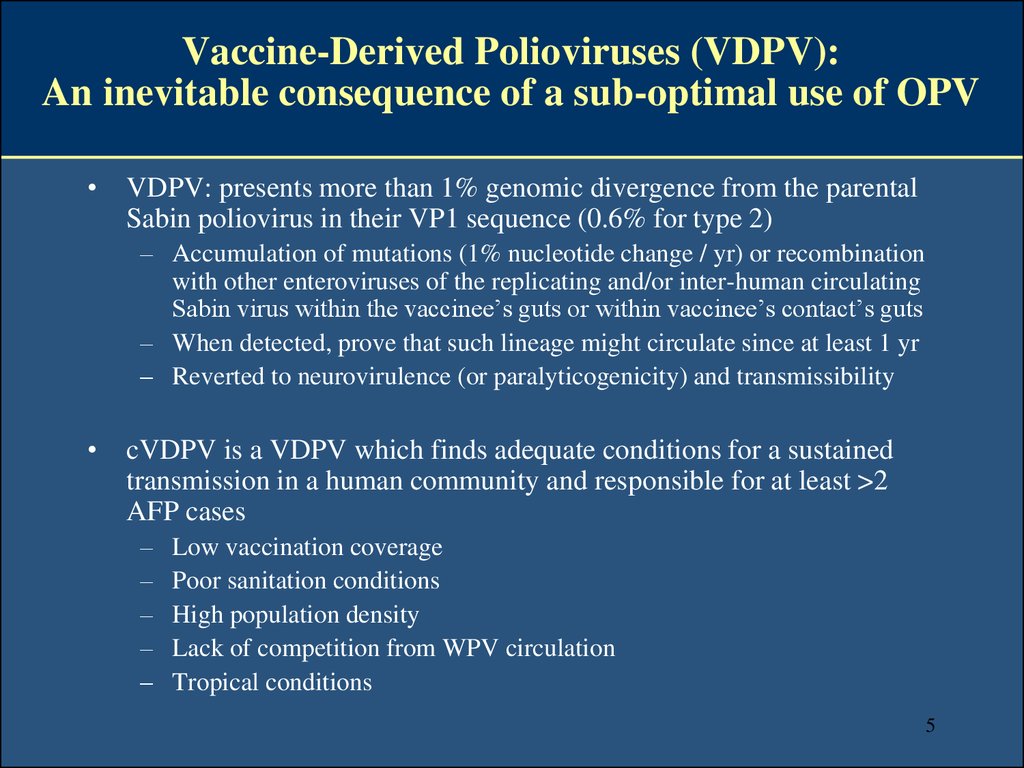
6. Since 2000, 23 cVDPV outbreaks (14 type 2; 6 type 1; 3 type 3) have been responsible for ~ 700 AFP cases - 2 outbreaks still active in 2014 -
Since 2000, 23 cVDPV outbreaks (14 type 2; 6 type 1;3 type 3) have been responsible for ~ 700 AFP cases
- 2 outbreaks still active in 2014 AFGHANISTAN
2010-13
cVDPV2
CHAD
2010
2012-13
cVDPV2
DOR / HAITI
2000-01
cVDPV1
PAKISTAN
2013-14
cVDPV2
INDIA
2009-10
cVDPV2
CHINA
2004
cVDPV1
ETHIOPIA
2008-09
cVDPV2
ETHIOPIA
2009-10
cVDPV3
MYANMAR
2006-07
cVDPV1
CAMBODIA
2005-06
cVDPV3
NIGER
2006
2009-11
2013
cVDPV2
PHILIPPINES
2001
cVDPV1
YEMEN
2011
cVDPV2
SOMALIA
2008-13
cVDPV2
NIGERIA
2005-14
cVDPV2
YEMEN
2012
cVDPV3
KENYA
2012
cVDPV2
CAMEROON
2013
cVDPV2
DR CONGO
2008-12
cVDPV2
MOZAMBIQUE
2011
cVDPV1
MADAGASCAR
2005
cVDPV2
INDONESIA
2005
cVDPV1
MADAGASCAR
2001-02
cVDPV2
http://www.polioeradication.org/Dataandmonitoring/Poliothisweek.aspx
6
7. VAPP: a rare but serious and inevitable Adverse Event associated with OPV
AFP with neurologic deficit 60 days after onset caused by a vaccine-related virus
Diffusion from the gut of a mutant virus having re-acquired the neurovirulence
phenotype & clinically indistinguishable from AFP induced by WPVs
May affect both OPV vaccinees & contacts of recent OPV recipients
– Most of the time occur in primo-infected
Risk factors
– First-dose recipients, as progressive immunity decreases the risk (except birth)
– Contact to OPV recipients
– Level of exposure to WPVs
Protective factors: Maternally transmitted Abs and birth OPV vaccination
Type 3 (50%) > Type 2 (40%) > Type 1 (10%)
Incidence
– One case (in recipients and contacts of recipients) per 2,9 - 3,3 Mdose distributed
– 2 to 4 cases per million birth cohorts
– One case per 1,4 M 1st-time recipients (US) or 2,8 M 1st-time recipients (India)
Leads to 250 to 500 of VAPP cases per year on a global basis (estimation)
7
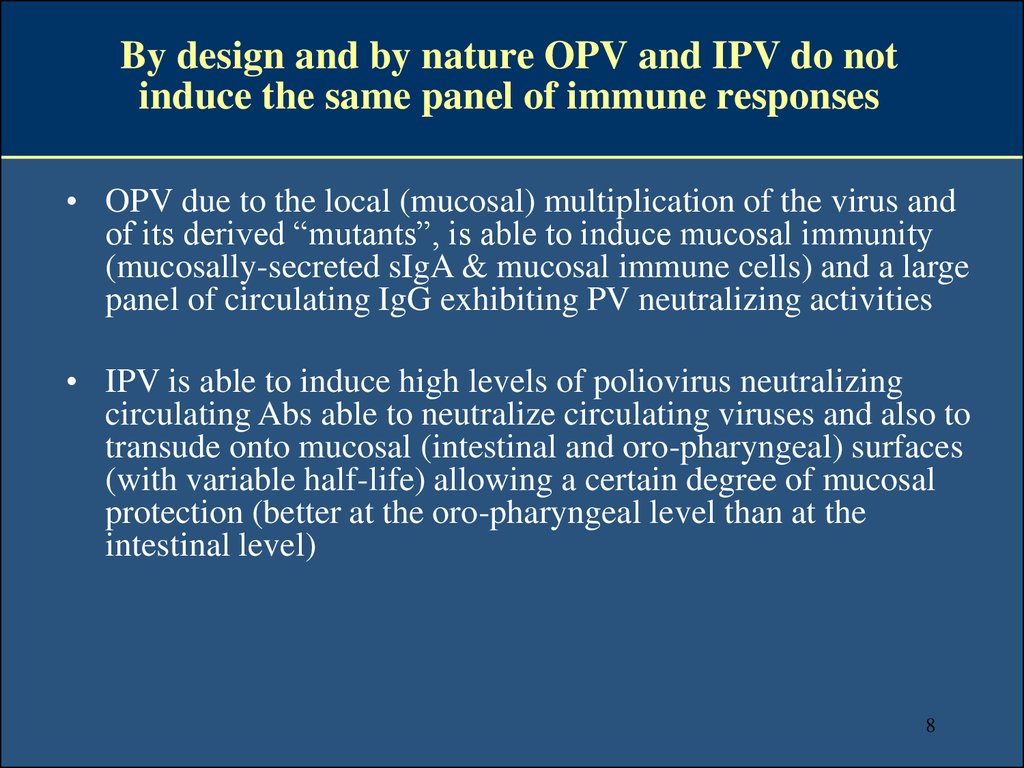
8. By design and by nature OPV and IPV do not induce the same panel of immune responses
• OPV due to the local (mucosal) multiplication of the virus andof its derived “mutants”, is able to induce mucosal immunity
(mucosally-secreted sIgA & mucosal immune cells) and a large
panel of circulating IgG exhibiting PV neutralizing activities
• IPV is able to induce high levels of poliovirus neutralizing
circulating Abs able to neutralize circulating viruses and also to
transude onto mucosal (intestinal and oro-pharyngeal) surfaces
(with variable half-life) allowing a certain degree of mucosal
protection (better at the oro-pharyngeal level than at the
intestinal level)
8
9. Consequently herd protection effect conferred by OPV or IPV are not of the same magnitude
• OPV- and IPV-vaccinees cannot resist to a poliovirusinfection but OPV-vaccinees will be infected for much
shorter durations than IPV-vaccinees
• The epidemiological consequences of this vary
according to the ecological conditions prevailing in the
affected communities
• In many situations, an IPV-based program do have no effect
on preventing the installation of a silent sustained PV
transmission: Israel, Q2-3 2013
– Whereas in other situations, it does: France 2000-2014: no
isolation of WPV in environmental samples, regular
isolations of imported Sabin-PV and no AFP cases and no
evidence of spread within communities
Duintjer-Tebbens RJ & al. Risk Analysis 2014; 33: 544-605
Shulman LM & al. Euro Surveill. 2014; 19: pii=20709
Antona D & al. Eur J Microbiol Infect Dis 2007; 26: 403-412and Antona D & al. BEH 2010; 48: 489-493
9
10. SAGE recommendations: at least 1 dose of IPV in routine schedule at least end of 2015
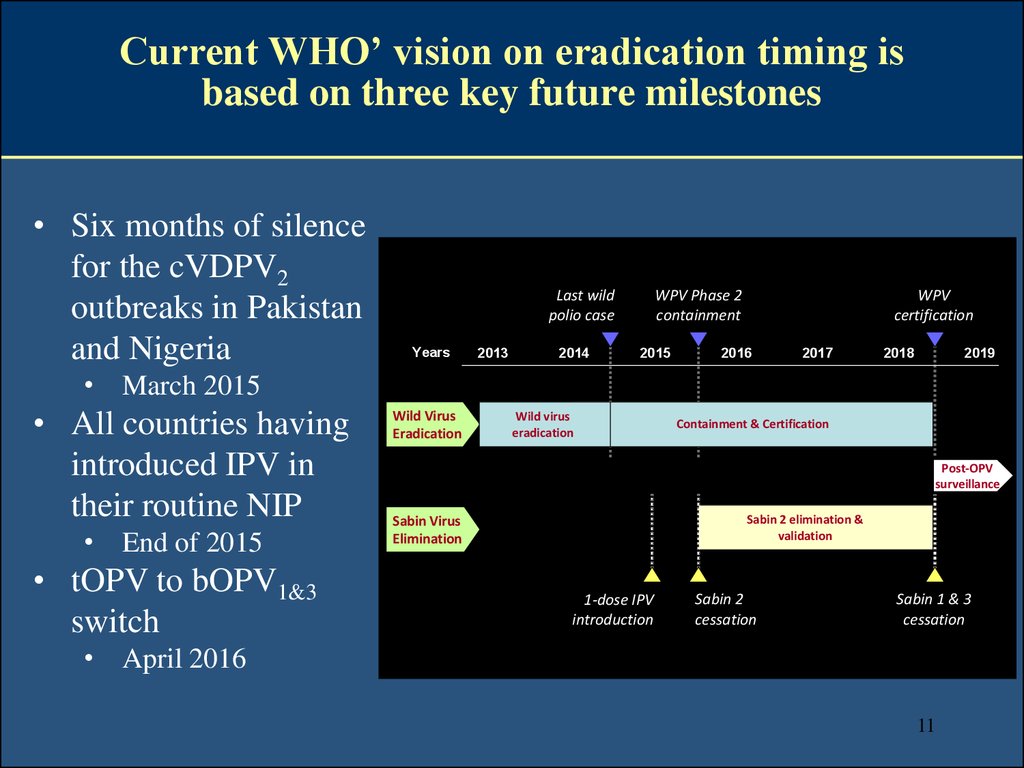
1011. Current WHO’ vision on eradication timing is based on three key future milestones
• Six months of silencefor the cVDPV2
outbreaks in Pakistan
and Nigeria
Last wild
polio case
Years
2013
2014
WPV Phase 2
containment
2015
WPV
certification
2016
2017
2018
2019
• March 2015
• All countries having
introduced IPV in
their routine NIP
• End of 2015
• tOPV to bOPV1&3
switch
Wild Virus
Eradication
Wild virus
eradication
Containment & Certification
Post-OPV
surveillance
Sabin 2 elimination &
validation
Sabin Virus
Elimination
1-dose IPV
introduction
Sabin 2
cessation
Sabin 1 & 3
cessation
• April 2016
11
12.
Following the pioneering work done at RIVM,Ch.Mérieux at the Mérieux Institute drove the large-scale
industrialization of the modern IPV (eIPV)
In the early 1970s
1. Safety of OPV in question
2. Efficacy in routine immunization with
OPV SC sub-optimal in the tropics
3. Very limited IPV industrial capacity
The challenges
1. To really scale up the industrial IPV
production
2. To improve and standardize potency (in
vitro antigenicity assay)
3. To demonstrate immunogenicity and
efficacy both in developed and
developing countries
4. To license products as broadly as
possible
“The father founders”
Mérieux Foundation, Veyrier-du-Lac, 1978
Charles Mérieux
Hans Cohen
Jonas Salk
and the RIVM scientist A. van Wezel
12
13. The Target Product Profile of modern IPV: Sufficiently immunogenic when given 2 times with a long interval (6 month) in developing countries
Several dose-response (using 2- or 4-fold increase incremental antigen
contents) clinical trials conducted in 1977-80 with IPV antigens
combined or not with DTwP vaccine
Ags and vaccines prepared by RIVM (PMKC) and Mérieux Institute
(PMKC from wild monkeys, then tertiary MKC from captive-bred
monkeys and then Vero)
13
14. The efficacy of modern IPVs against AFP due to WPV has been demonstrated several times through field studies
Melnick calculated an efficacy of 96% through two polio seasons in
Houston
In Senegal, two doses of a DTwP-IPV combination vaccine were
given in the Kolda area, which subsequently suffered an outbreak of
WPV1
–
In the North Arcot region of India, J. John compared OPV in one
district with IPV vaccination in two other districts
–
–
A case-control analysis revealed an efficacy for one dose of 36% (95% CI: 0% to 67%)
and for two doses of 89% (95% CI: 62% to 97%)
Vaccination coverage with three doses rose to 85% to 90% in the OPV districts and 75%
to 80% in the IPV districts
Case-control analysis revealed an efficacy of 92% for IPV and 66% for OPV
During the introduction of IPV into Canada, efficacy of the vaccine
was calculated at more than 90%
Melnick JL & al. JAMA 1961; 175: 1159-1162; Stoeckel P & al. Rev Infect Dis 1984; 6(S2): S463-S466;
CDC. MMWR 1988; 37: 257-259; John T. 1992. World Conference on Poliomyelitis and Measles, New
Delhi. Varughese PV & al. Can J Public Health 1989; 80: 363-368
14
15. While it is true that IPV-containing products do induce immunological priming after 1st dose, public health impact not yet known
• One single IM IPV dose in naive 4-month old infants seroconverts32%-63% (all types)
• 97%-98% of infants who didn't seroconvert after single IPV dose
evidenced immunological priming (against all types)
100%
80%
Not immune
SC post 2nd dose
Primed
SC post 1st dose
60%
40%
20%
0%
ID
Resik S & al. NEJM 2013
IM
P1
ID
IM
P2
ID
IM
P3
15
16. IPV-containing products do induce seroconversion and seroprotective levels in almost all subjects after two doses
Vidor E & al. PIDJ 1997; 16: 312-32216
17. Two doses of IPV during the 1st year of life reduce prevalence and duration (intensity?) of PV intestinal excretion (all types) following tOPV challenge compared to 1st time tOPV-recipients
Viral shedding of any type after different schedules of vaccination with oral poliovirus vaccine(OPV) and inactivated poliovirus vaccine (IPV).
1st time tOPV-recipients
Laassri M et al. J Infect Dis. 2005;192:2092-2098
•76% of 2-dose IPVprimed excreted PV
vs 92% of 1st time
tOPV recipents at 1
week
•37% of 2-dose IPVprimed excreted PV
vs 81% of 1st time
tOPV recipents at 1
week
© 2005 by the Infectious Diseases Society of America
Rennels & al. PIDJ 2000; 19: 417-23; Laassri M & al. JID 2005; 92: 2092-8; Laassri M & al. 2006; 193: 1344-9
17
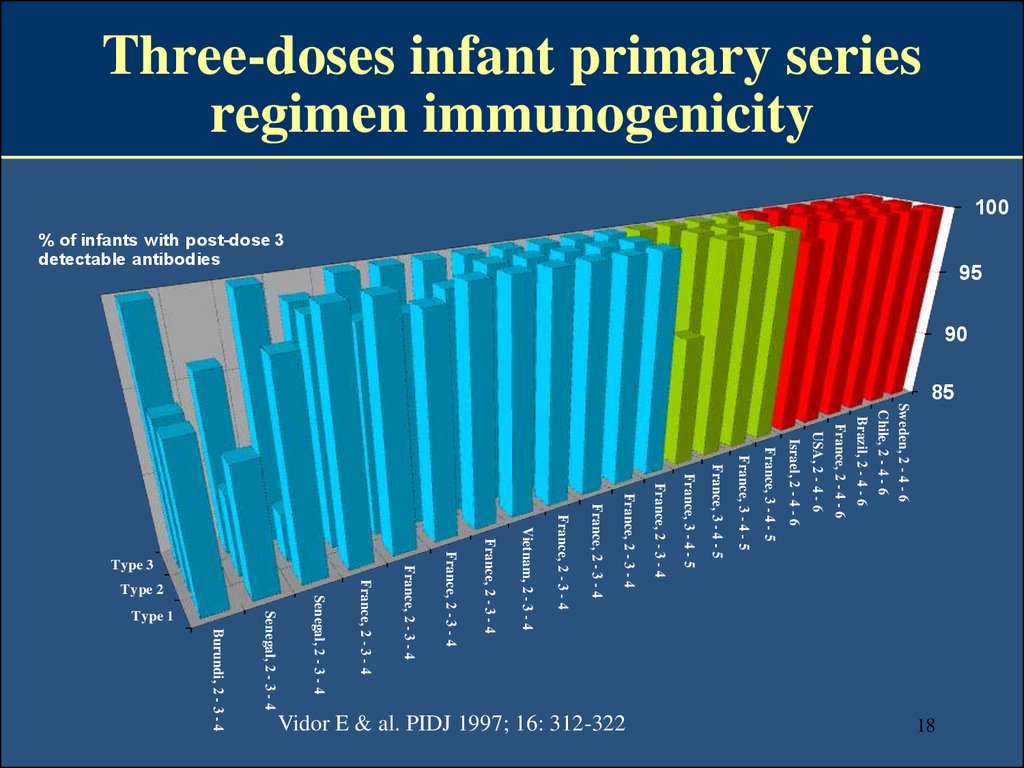
18. Three-doses infant primary series regimen immunogenicity
10085
Sweden, 2 - 4 - 6
Chile, 2 - 4 - 6
Brazil, 2 - 4 - 6
France, 2 - 4 - 6
USA, 2 - 4 - 6
Israel, 2 - 4 - 6
France, 3 - 4 - 5
France, 3 - 4 - 5
France, 3 - 4 - 5
France, 3 - 4 - 5
18
Burundi, 2 - 3 - 4
Vidor E & al. PIDJ 1997; 16: 312-322
France, 2 - 3 - 4
France, 2 - 3 - 4
France, 2 - 3 - 4
France, 2 - 3 - 4
Vietnam, 2 - 3 - 4
France, 2 - 3 - 4
France, 2 - 3 - 4
Type 3
France, 2 - 3 - 4
France, 2 - 3 - 4
Type 1
Senegal, 2 - 3 - 4
Senegal, 2 - 3 - 4
Type 2
95
% of infants with post-dose 3
detectable antibodies
90
19. Multiple factors drive the immunogenicity of IPV-containing vaccines when used for infant primary immunization
Multiple factors drive the immunogenicity of IPVcontaining vaccines when used for infant primaryimmunization
+
• Number of primary series injections (3 > 2 > 1)
• Age at first injection (older [3 months or later] > younger [6
weeks of age] > birth)
• Interval between doses (more than 2 months > 2 mo > 1 mo)
• Ecological context (presence of passively transmitted Abs from
mother)
• Presence of an aluminium salt adjuvant in the vaccine
formulation (IPV-containing adjuvanted multivalent
combination products > standalone un-adsorbed IPV)
-
19
-
20. Different IPV-followed-by-OPV sequential regimen have been documented
13 trials with several IPV-containing vaccines in 7 countries since 1986
– USA (5), China (3), UK, Brazil, Mexico, Taiwan & Guatemala
1 or 2 IPV followed by 1 or 2 OPV administered as « 2+1 », « 3+1 » or
« 3+0 » regimen
No OPV given at birth in all cases
Several types of study design
– Non-randomized descriptive-only licensing or launch studies or RCTs
between sequential schedules and IPV-only and / or OPV-only schedules
– Some trials have investigated prevalence, intensity, duration and genetics of
PV intestinal excretion after OPV vaccination / challenge
Several types of IPV-containing vaccines
– IPV stand-alone (Vero-IPV or MRC5-IPV)
– wP-based combinations
– aP-based combinations
20
21. IPV-followed-by-OPV regimen is the most relevant from a Public Health perspective in still-at-risk communities
Address most (if not all) VAPP cases
Combine the full benefit of both vaccines in terms of breath of
individual responses (mucosal and humoral)
The incorporation of at least one dose of IPV at the start of the
immunization schedule increase post-primary series Ab levels
compared to OPV-only schedules
I-I-O primary series during the first year of life schedule is the best
performer in terms of absolute post-primary series antibody levels
(versus OPV-only)
≥ 2 doses of IPV during the first year of life seems able to reduce
prevalence and duration (intensity?) of PV intestinal excretion (all
types) following PV infection compared to 1st time tOPV-recipients
– Much stronger effect on oro-pharyngeal excretion
The use of OPV in populations around the "uncovered" will allow their
"indirect vaccination" if environmental / ecological conditions are
adequate
21
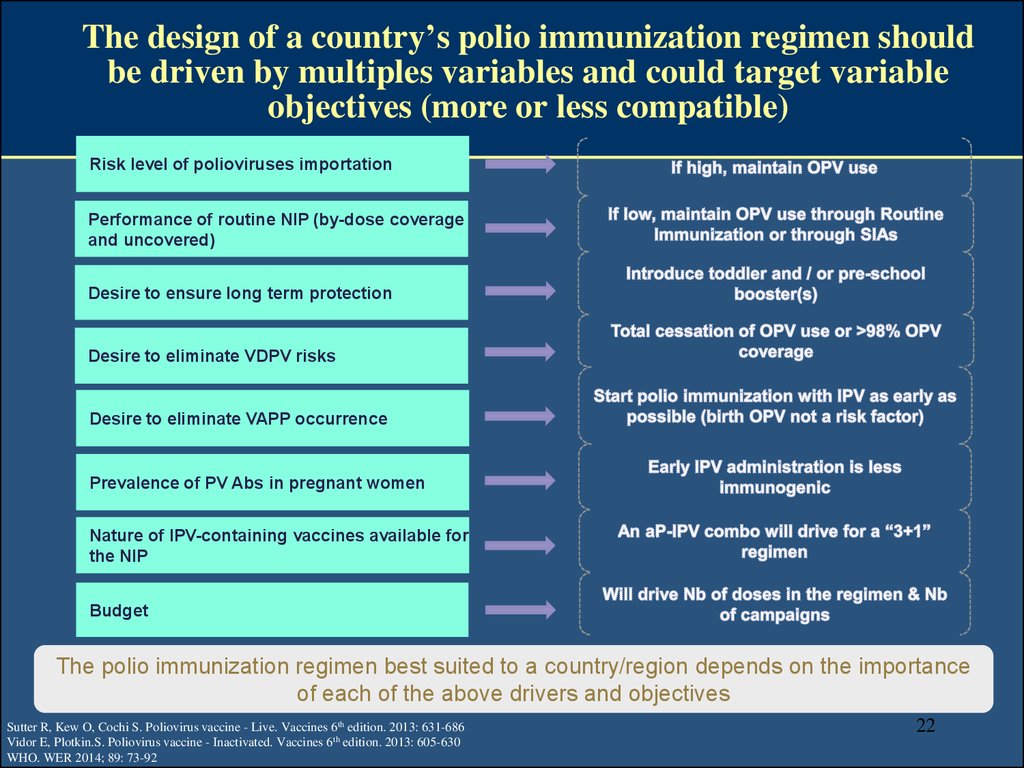
22. The design of a country’s polio immunization regimen should be driven by multiples variables and could target variable objectives (more or less compatible)
Risk level of polioviruses importationPerformance of routine NIP (by-dose coverage
and uncovered)
Desire to ensure long term protection
Desire to eliminate VDPV risks
Desire to eliminate VAPP occurrence
Prevalence of PV Abs in pregnant women
Nature of IPV-containing vaccines available for
the NIP
Budget
The polio immunization regimen best suited to a country/region depends on the importance
of each of the above drivers and objectives
Sutter R, Kew O, Cochi S. Poliovirus vaccine - Live. Vaccines 6th edition. 2013: 631-686
Vidor E, Plotkin.S. Poliovirus vaccine - Inactivated. Vaccines 6th edition. 2013: 605-630
WHO. WER 2014; 89: 73-92
22
23. As of Nov. 2014 more than 70 countries have introduced IPV in their routine publicly funded infant National Immunization Programs (and much more countries in their private markets)
IPV-only(46 countries)
- IPV-only supplemented with OPV SIAs
- IPV-followed-by-OPV Sequential
- Mixed / Combined IPV / OPV
Sutter R & al. Poliovirus vaccine-Live. Plotkin SA, Orenstein WA, Offit P (eds), 6th edition, in Vaccines. W.B. Saunders
Company, Philadelphia, PA, 2012
Vidor E & al. Poliovirus vaccine-Inactivated. Plotkin SA, Orenstein WA, Offit P (eds), 6th edition, in Vaccines. W.B.
Saunders Company, Philadelphia, PA, 2012
(24 countries)
23
24. From all the experience and data accumulated through clinical trials, introduction programs and routine use of IPV-containing vaccines over 35 years, it is Sanofi Pasteur’s conviction that …
• A “2-dose IPV IM-dosing regimen” should be the backbone ofall polio immunization regimen
– All IPV dosing(s) done on the top of this backbone IPV regimen will
improve duration of immunity and maximize mucosal protection
• Every opportunity of catching 0 to 2 years old infants living in
LICs and LMICs should be used to administer as early as
possible this backbone regimen whatever the number and type of
OPV administered before, during or after these IPV dosing
opportunities
• All OPV dosing(s) done on the top of this backbone IPV regimen
will re-enforce the quality of the intestinal immunity at the
individual level and at the community level (if ecological
conditions permit) and therefore will decrease the chain of
poliovirus transmission
24
25. Conclusions
• OPV limitations drive the need for evolution• A large and diverse experience has been accumulated with
IPV-containing vaccines
• A 2-dose IPV IM-dosing regimen should be the backbone of
all polio immunization regimens
• Multiples drivers govern the choice of the polio
immunization regimen to be used in each situation
• The most medically- and epidemiologically-sounded regimen
in polio-afflicted communities is the IPV-followed-by-OPV
sequential schedule made of I – I – O – O given as a “3+1”
• Many countries have already adopted IPV with different
approaches in terms of immunization regimen each one
adapted to specific country situation and objectives
25


















 medicine
medicine