Similar presentations:
Obtaining oxygen
1. obtaining oxygen
2. Obtaining oxygen in industry
Liquid air distillationair
air
The principle of working of the
membrane cartridge
Liquid air
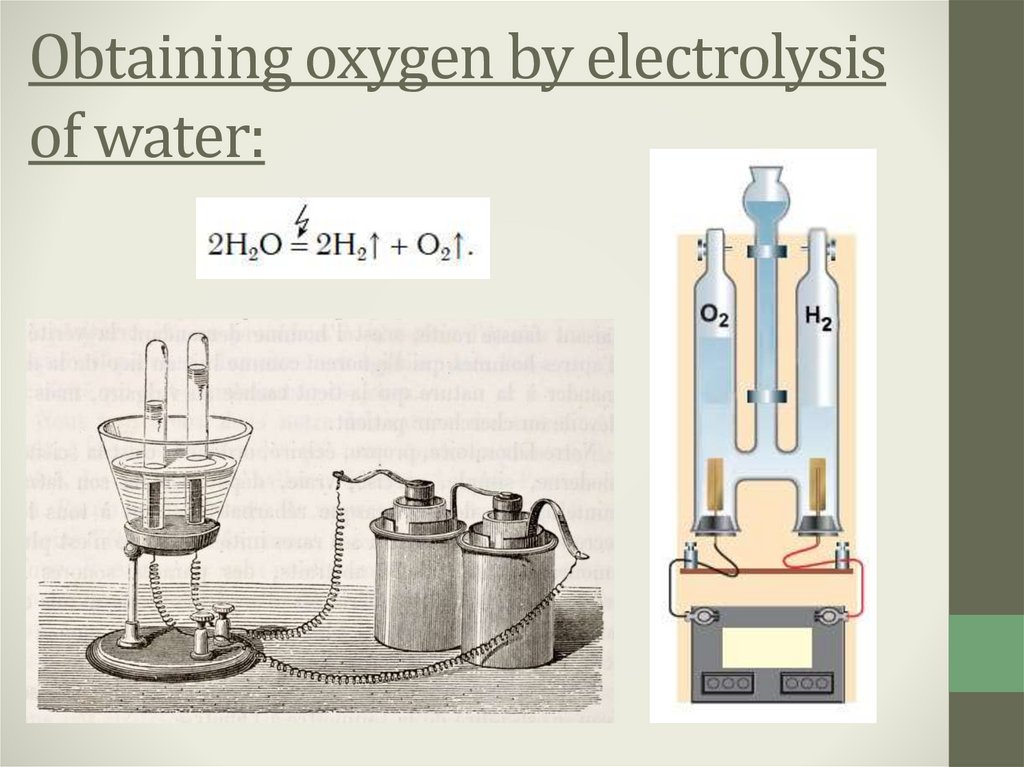
3. Laboratory oxygen obtaining
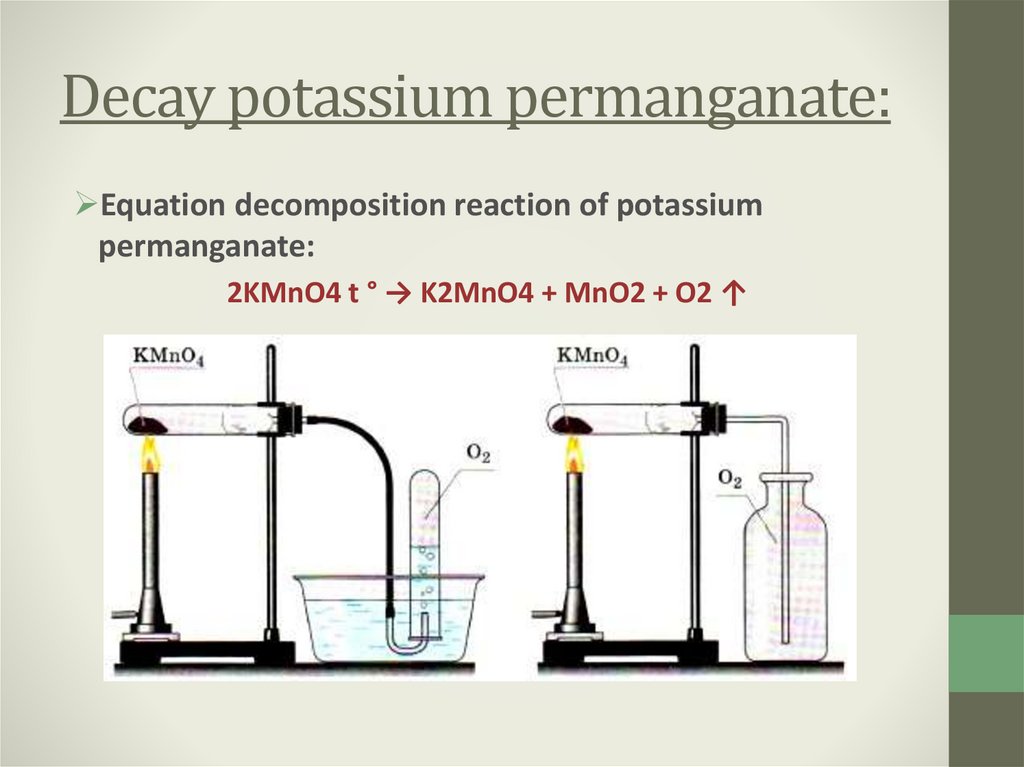
4. Decay potassium permanganate:
Equation decomposition reaction of potassiumpermanganate:
2KMnO4 t ° → K2MnO4 + MnO2 + O2 ↑
5.
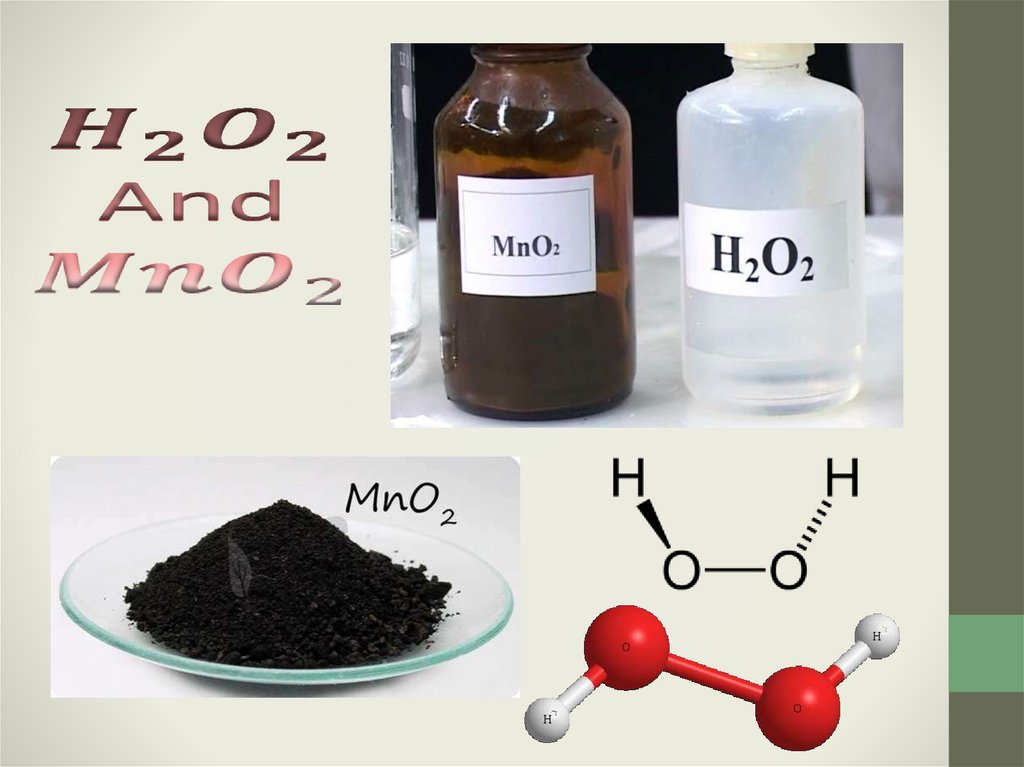
6. Decay Hydrogen Peroxide:
Equation decompositionreaction of hydrogen peroxide:
7.
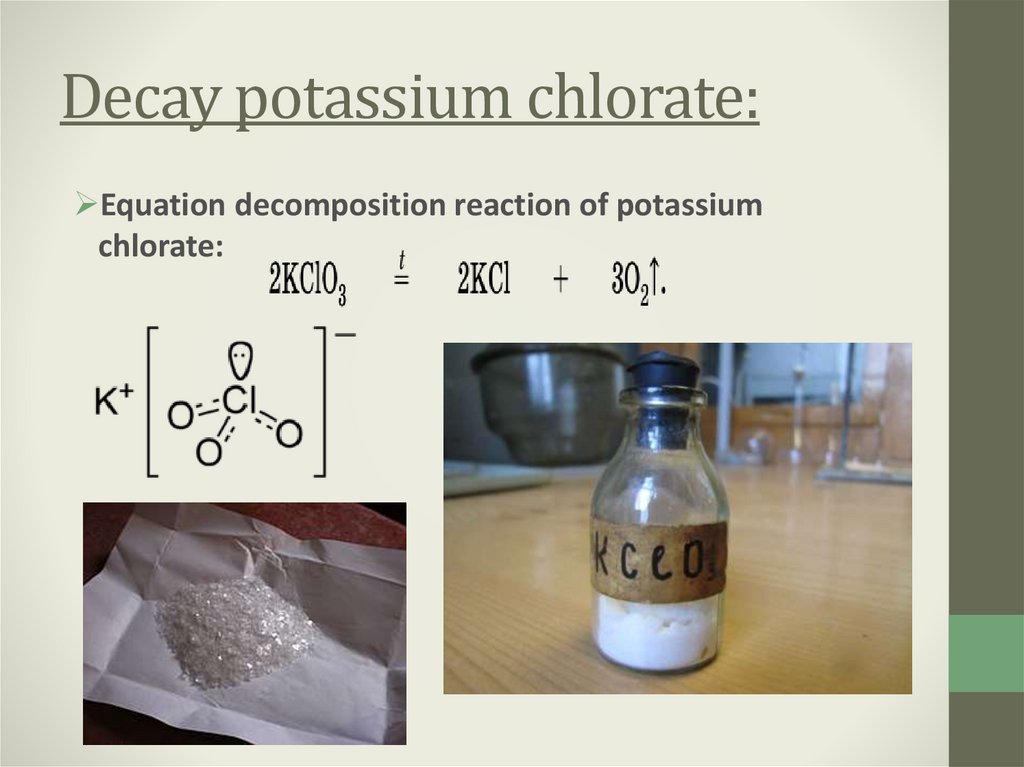
8. Decay potassium chlorate:
Equation decomposition reaction of potassiumchlorate:
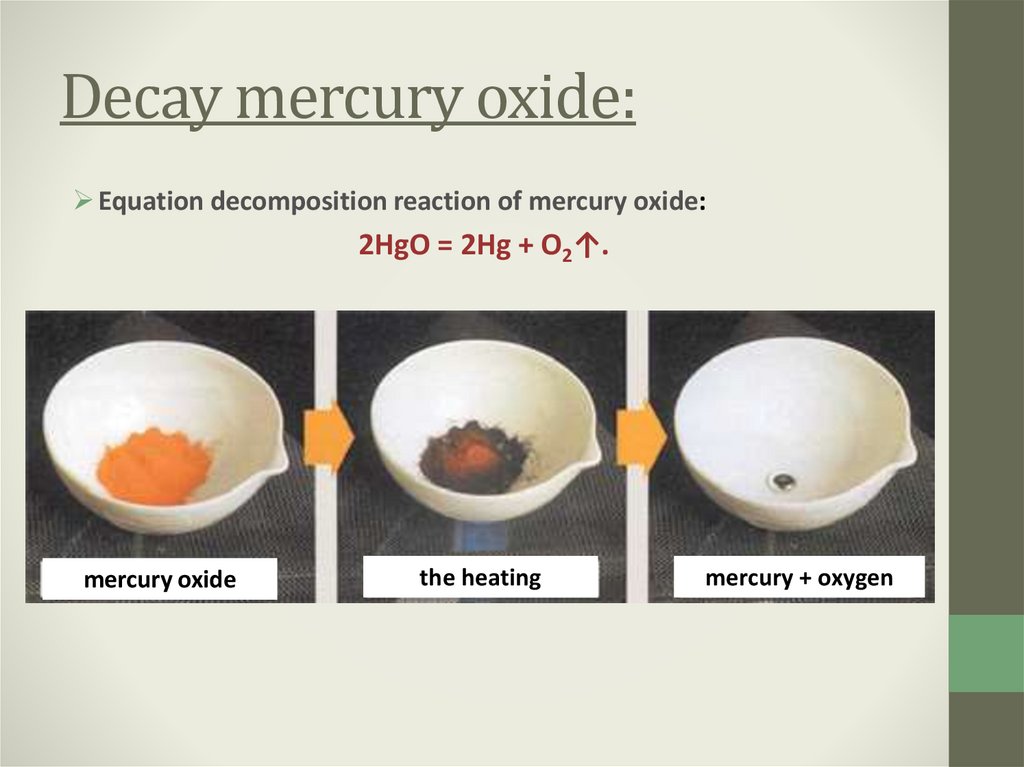
9. Decay mercury oxide:
Equation decomposition reaction of mercury oxide:2HgO = 2Hg + О2↑.
mercury oxide
the heating
mercury + oxygen
10.
Priestley's experience with the decomposition ofmercury oxide: a - using a lens; b - when heated in vitro.
b)







 chemistry
chemistry