Similar presentations:
Alkali metals
1. Slayt 1
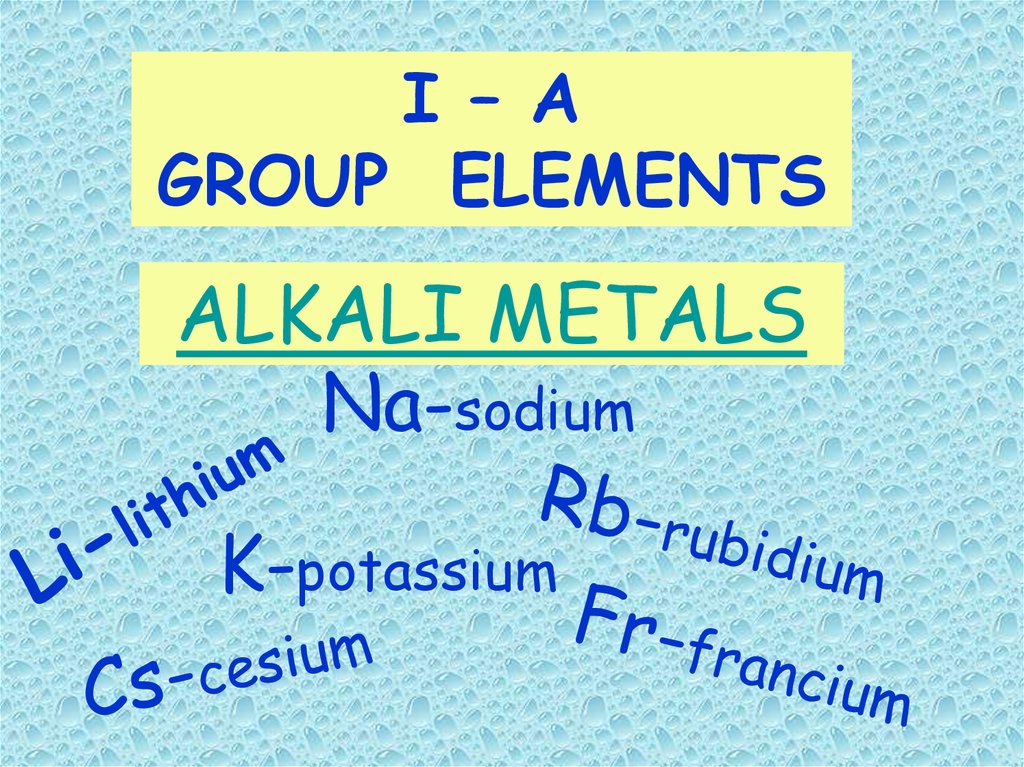
I – AGROUP ELEMENTS
ALKALI METALS
Na-sodium
K-potassium
2. Slayt 2
INTRODUCTIONName of this family comes from the
properties of alkali metals to form
hydroxides with water. Compounds
containing hydroxide ion is basic and
called alkali.
They are very active ,so in
the nature they are not found
in elemental forms. They exist
in various compounds.
3. Slayt 3
GENERAL PROPERTIES OF 1A*By giving their valence electron easily in chemical
reactions, they form +1 charged ions.
* Alkali metals are the elements which have the least
ionization energy and the highest atomic radius, in
each period
* They are a group of most active metals.
* The activity of metals increase from top to bottom
* The element cesium, Cs, is the most active metal
* Francium is a radioactive element
4. Slayt 4
They are solids at roomtemperature.
They
are
soft. They can be cut by a
knife.
Na
5. OCCURRENCE
• Since the alkali metals are the most active metals, theyare not found free in nature, but as compounds.
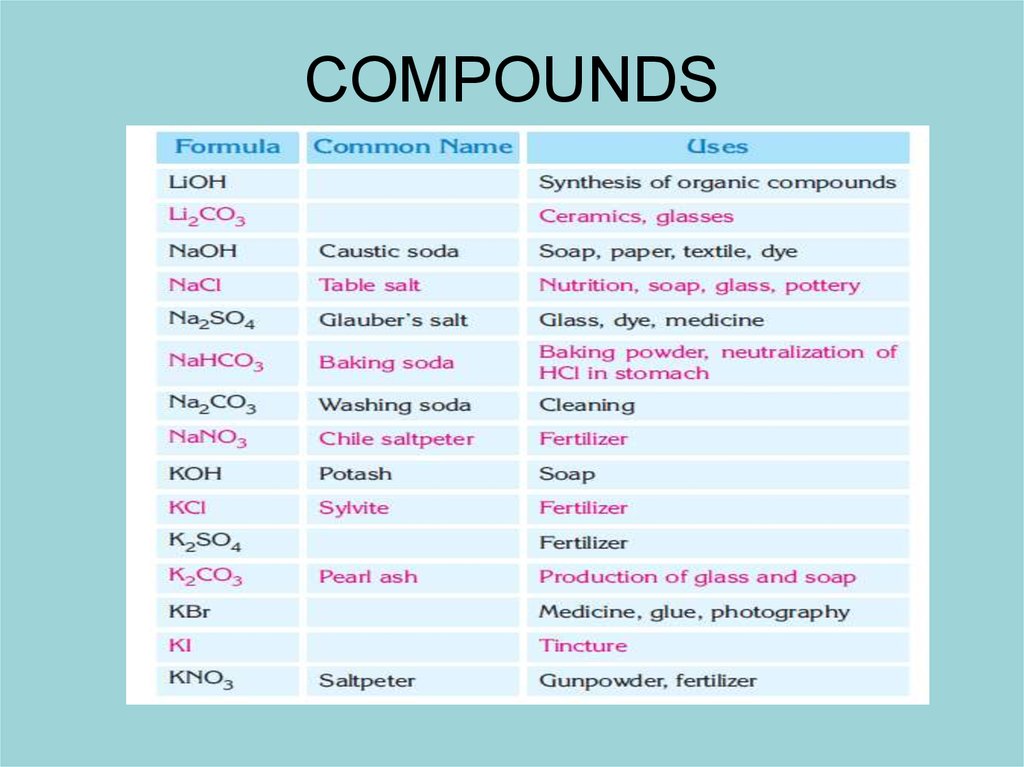
Sodium, Na
• The most important compound of sodium is sodium
chloride, NaCl. The important sodium sources are Chile
saltpeter (NaNO3), washing soda (Na2CO3) and baking
soda (NaHCO3). The most important sodium minerals
are kryolite (Na3AlF6), borax (Na2B4O7.10H2O), sodium
sulfate (Na2SO4) and albite (NaAlSi3O8).
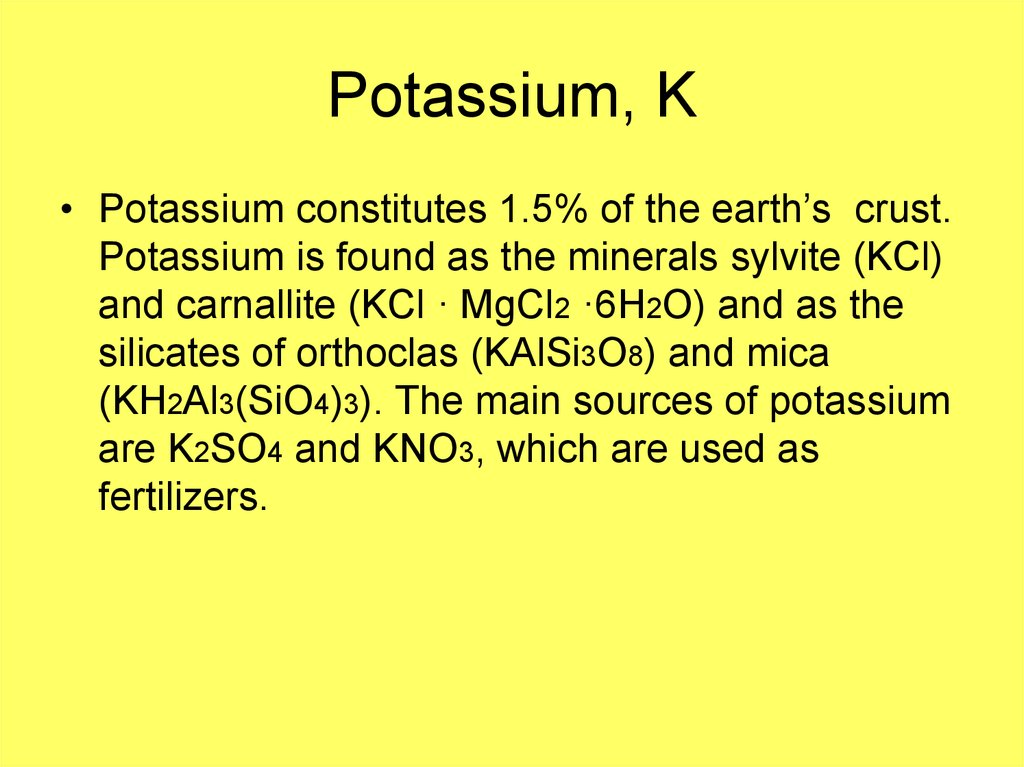
6. Potassium, K
• Potassium constitutes 1.5% of the earth’s crust.Potassium is found as the minerals sylvite (KCl)
and carnallite (KCl · MgCl2 ·6H2O) and as the
silicates of orthoclas (KAlSi3O8) and mica
(KH2Al3(SiO4)3). The main sources of potassium
are K2SO4 and KNO3, which are used as
fertilizers.
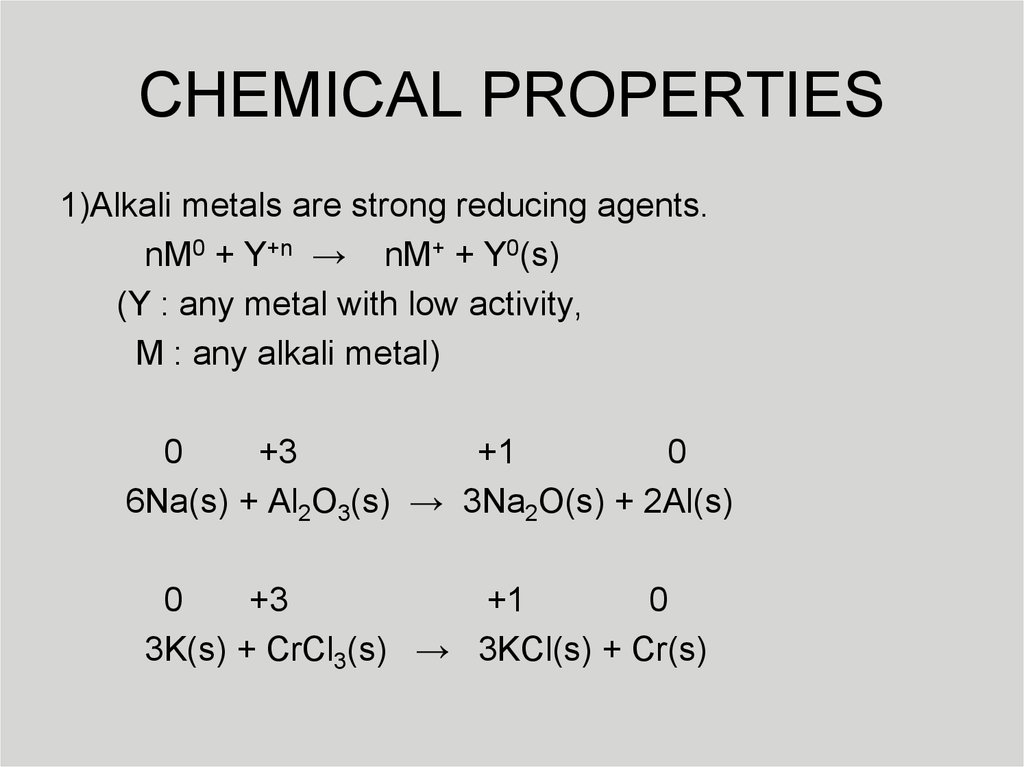
7. CHEMICAL PROPERTIES
1)Alkali metals are strong reducing agents.nM0 + Y+n → nM+ + Y0(s)
(Y : any metal with low activity,
M : any alkali metal)
0
+3
+1
0
6Na(s) + Al2O3(s) → 3Na2O(s) + 2Al(s)
0
+3
+1
0
3K(s) + CrCl3(s) → 3KCl(s) + Cr(s)
8. Slayt 8
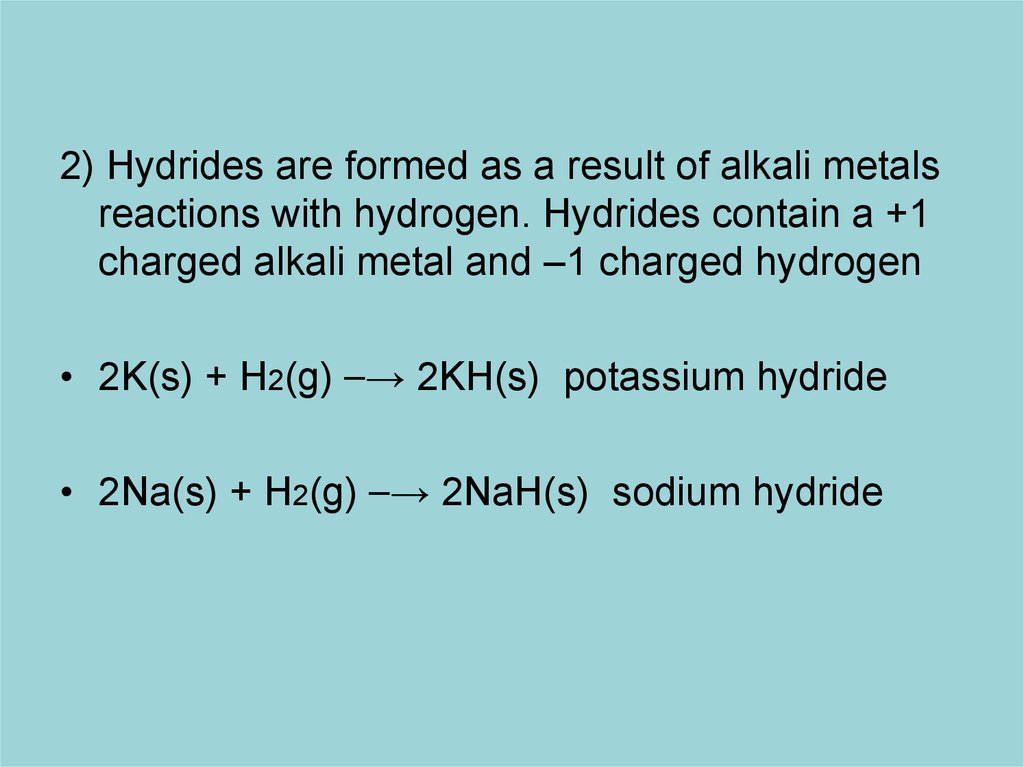
2) Hydrides are formed as a result of alkali metalsreactions with hydrogen. Hydrides contain a +1
charged alkali metal and –1 charged hydrogen
• 2K(s) + H2(g) ⎯→ 2KH(s) potassium hydride
• 2Na(s) + H2(g) ⎯→ 2NaH(s) sodium hydride
9. Slayt 9
3) They react with water violently. As a result ofthis reaction H2 gas and a base solution form.
• 2M(s) + 2H2O(l) → 2MOH(aq) + H2(g) +heat
• 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) + heat
10. Slayt 10
4) They may form oxides, peroxides orsuperoxides by reacting with oxygen in the air.
• As a result of reactions with excess oxygen,
lithium forms oxide, Li2O, sodium forms peroxide
Na2O2 and potassium, rubidium and cesium
form superoxides, such as KO2, RbO2, CsO2.
• 4Li(s) + O2(g) ⎯→ 2Li2O(s)
• 2Na(s) + O2(g) ⎯→ Na2O2(s)
• K(s) + O2(g) ⎯→ KO2(s)
11. Slayt 11
• 5. All of them react with halogens to formalkali halides (salts of alkali metals).
• 2M(s) + X2(g) ⎯→ 2MX(s)
(M : alkali metal, X : halogen,
MX ; alkali metal halides)
• 2Li(s) + F2(g) ⎯→ 2LiF(s)
• 2Na(s) + Cl2(g) ⎯→ 2NaCl(s)
12. Slayt 12
• 6. They do not react with basesM(s) + OH–(aq) ⎯→ No reaction
• 7. When they react with acids, the produce
salts andliberate H2 gas.
• M(s) + HX(aq) ⎯→ MX(aq) + 1/2H2(g)
(HX : Halo acid, MX : salt of an alkali)
• 2K(s) + 2HCl(aq) ⎯→ 2KCl(aq) + H2(g)
• 2Na(s) + 2HBr(aq) ⎯→ 2NaBr(aq) + H2(g)






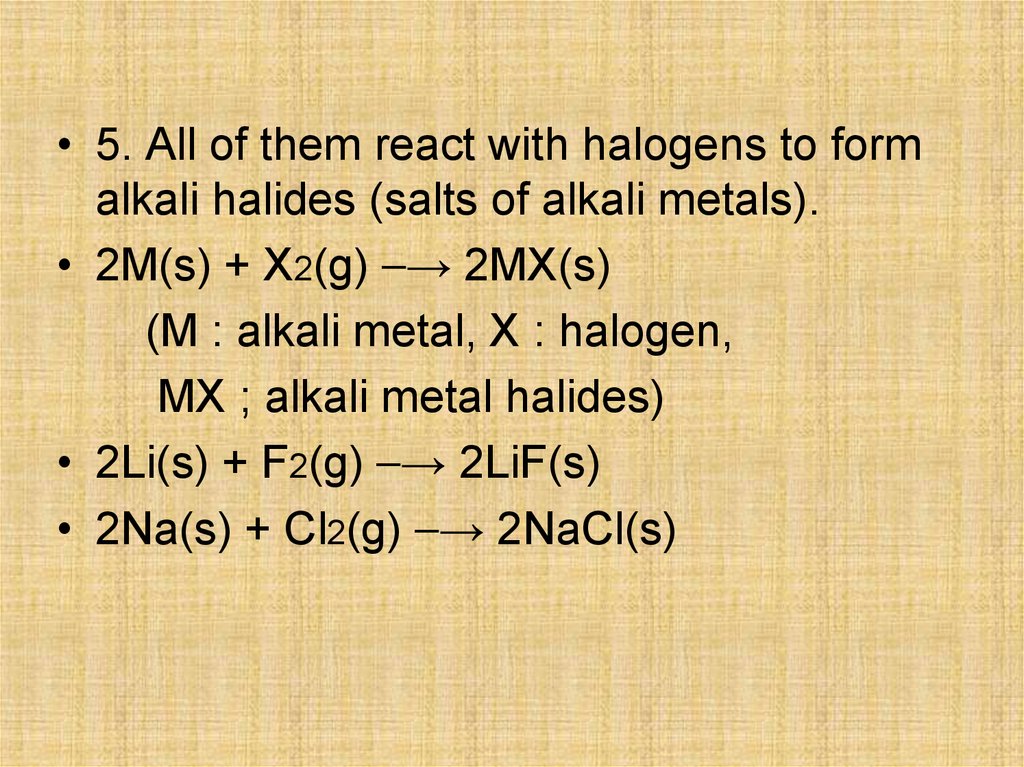

 chemistry
chemistry