Similar presentations:
Unipharma - professional pharmaceutical wholesale company
1. COMPANY INTRODUCTION for Evalar 08th October 2019
UNIPHARMA Introduction - 10th May 20182.
Company overviewSTRATEGY
UNIPHARMA - Professional pharmaceutical wholesale company which is driven by its
customer = pharmacist Is to keep the No.1 position in every well-run pharmacy
that means No.1 among the Slovak wholesale pharmaceutical distributors.
By continual achievement of following goals :
- having the best sortiment, service, seriousness, serveability, stability
-
keeping acceptable profitability for it´s shareholders
continuously building a company of more generations of and for pharmaceutists
UNIPHARMA Introduction - 20th February 2019
2
3.
Company overview – Company historyUNIPHARMA (UP) was established 11.11. 1992 as a pharmaceutical
company with main focus on the wholesale distribution of
pharmaceutical goods in Slovakia market
1992 – 1995 Unipharma Ltd.
Since 1995 is Unipharma joint stock company
CEO / Chairman of the board of directors RNDr. Tomislav Jurik, CSc.
Unipharma basic philosophy is to supports its clients – public
pharmaceutists, of whom many are at the same time shareholders (806)
of the company.
The founding
Unipharma
of Unipharma ltd.
is transformed to
Business started
a joint-stock
at very small building company, moving
in Kos rented by the
to bigger rented
(bunder - pharmacist
building
Mr. Tomislav Jurik
in Prievidza
(actual Chairman
of the board & CEO)
Unipharma
accomplished
building of its
very first own
Distribution Center
in Bojnice
Unipharma
accomplished
building of
an affiliate
Distribution Center
in Bratislava
Unipharma
bought the Hospital
Kosice-Saca as.
1st private
Unipharma
accomplished
building
of an affiliate
Distribution
Center
in Prešov
Unipharma
Unipharma bought
bought the
the Hospital
Hospital
Bánovce
Handlová - 2nd 3rd private hospital,
private hospital, ltd
ltd.
UNIPHARMA Introduction - 20th February 2019
Unipharma build
its
own Medical Diagnostic Centre
- Uniklinika of
Cardinal
Korec in Prievidza
YEARS
3
4.
Company overview – annual salesAnnual sales was 485,5 mil. EUR (2018)
UNIPHARMA Introduction - 20th February 2019
4
5.
Company overview – Company values = 5xSservice
sortiment
stability
seriousness
reliability
UNIPHARMA Introduction - 20th February 2019
5
6.
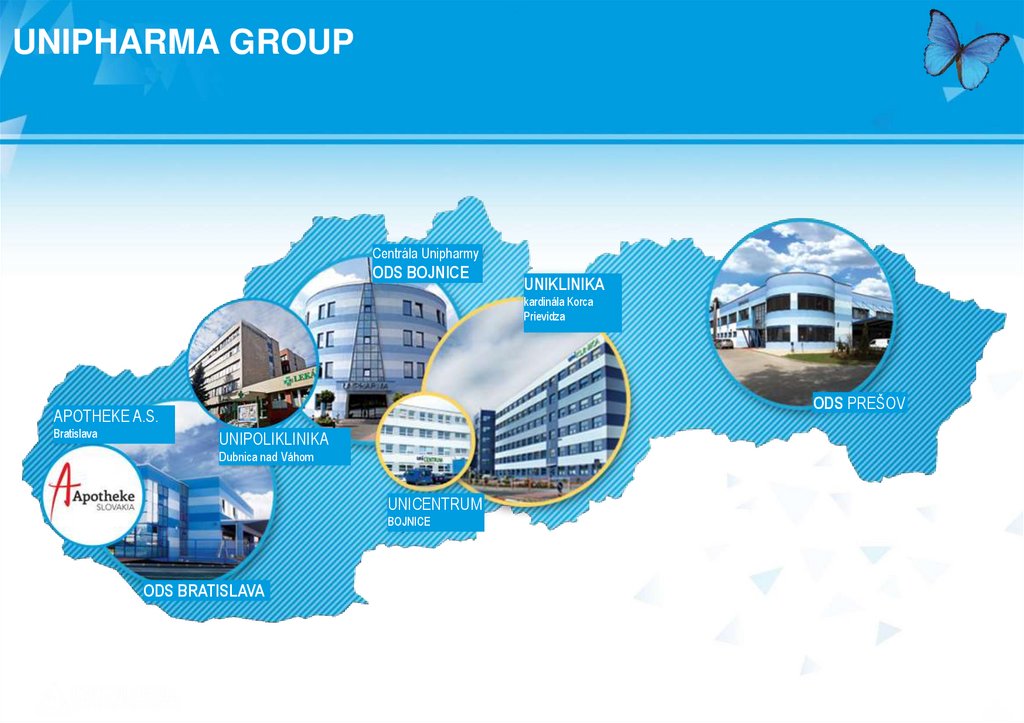
UNIPHARMA GROUPCentrála Unipharmy
ODS BOJNICE
UNIKLINIKA
kardinála Korca
Prievidza
ODS PREŠOV
APOTHEKE A.S.
Bratislava
UNIPOLIKLINIKA
Dubnica nad Váhom
UNICENTRUM
BOJNICE
ODS BRATISLAVA
UNIPHARMA Introduction - 20th February 2019
6
7.
UNIPHARMA IntroductionNational information center of
Slovak republic issued the
Certificate
„Reliable partner”
in public procurement
(2014 & 2016 & 2017)
UNIPHARMA Introduction - 20th February 2019
7
8.
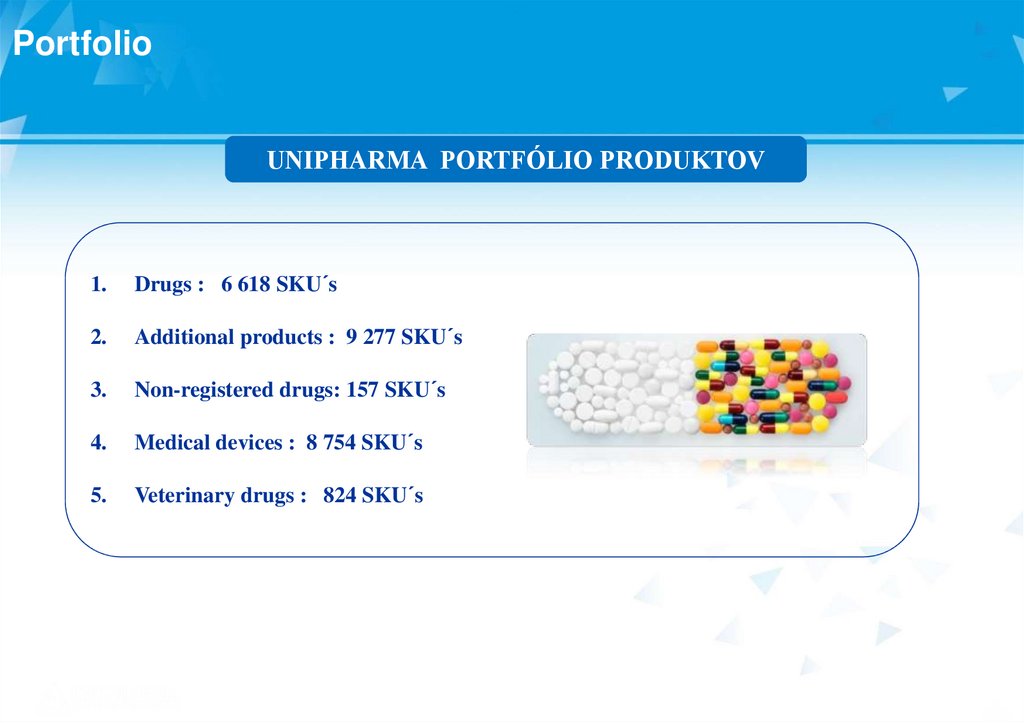
PortfolioUNIPHARMA PORTFÓLIO PRODUKTOV
1.
Drugs : 6 618 SKU´s
2.
Additional products : 9 277 SKU´s
3.
Non-registered drugs: 157 SKU´s
4.
Medical devices : 8 754 SKU´s
5.
Veterinary drugs : 824 SKU´s
UNIPHARMA Proposal and Quotation details
8
9.
Marketing activities – „Lekárnik“Scientific magazine for pharmasists and UNIPHARMA clients
UNIPHARMA Introduction - 20th February 2019
9
10.
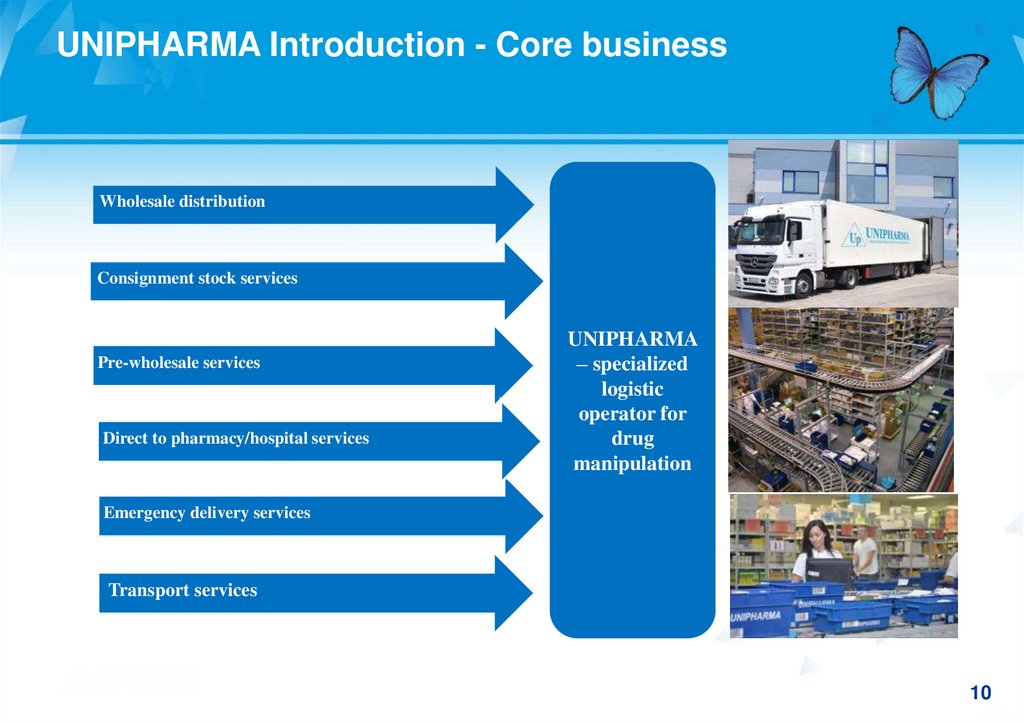
UNIPHARMA Introduction - Core businessWholesale distribution
Consignment stock services
Pre-wholesale services
Direct to pharmacy/hospital services
UNIPHARMA
– specialized
logistic
operator for
drug
manipulation
Emergency delivery services
Transport services
10
UNIPHARMA Introduction - 20th February 2019
10
11.
„Plus lekáreň“ associationNumber of partner pharmacies:
508
UNIPHARMA Introduction - 20th February 2019
11
12.
„Plus lekáreň“ associationUNIPHARMA Introduction - 20th February 2019
12
13.
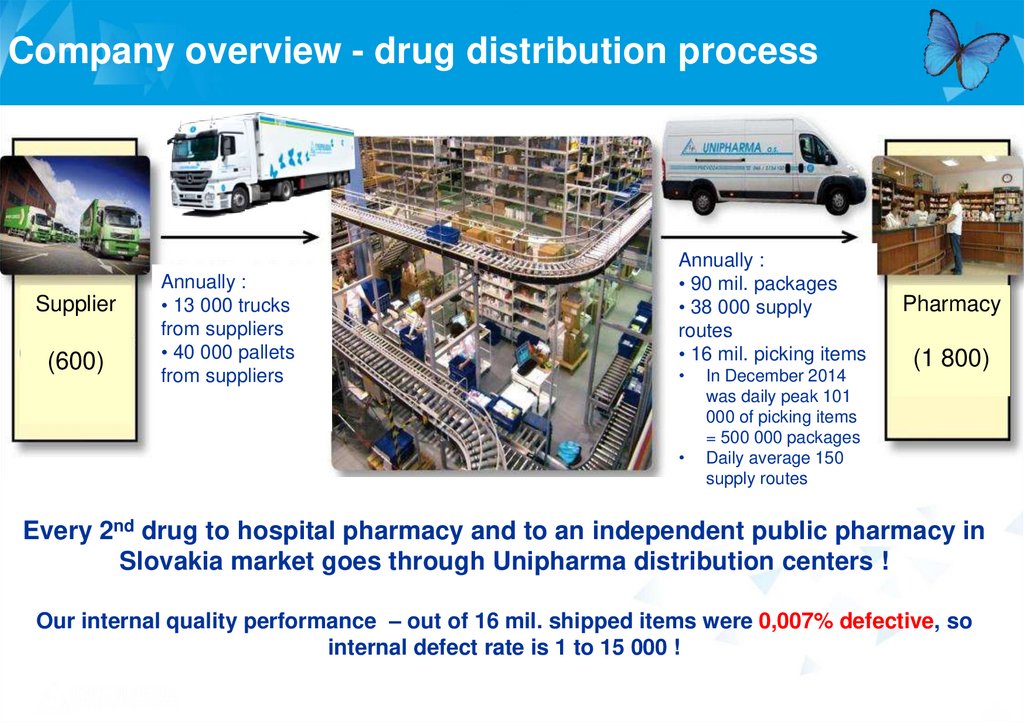
Company overview - drug distribution processSupplier
(600)
Annually :
• 13 000 trucks
from suppliers
• 40 000 pallets
from suppliers
Annually :
• 90 mil. packages
• 38 000 supply
routes
• 16 mil. picking items
In December 2014
was daily peak 101
000 of picking items
= 500 000 packages
Daily average 150
supply routes
Pharmacy
(1 800)
Every 2nd drug to hospital pharmacy and to an independent public pharmacy in
Slovakia market goes through Unipharma distribution centers !
Our internal quality performance – out of 16 mil. shipped items were 0,007% defective, so
internal defect rate is 1 to 15 000 !
UNIPHARMA Introduction - 20th February 2019
13
14.
Warehousing – Distribution centersRNDr. Igor Šuňal, PhD.
Distribution division director &
ODS Bojnice
Headquarters UNIPHARMA ODS Bojnice (1999)
PharmDr. Ondrej
Takáč
ODS Prešov Director
Bc. Marcel Svrček
ODS Bratislava Director
Affiliate Bratislava (2002)
Affiliate Prešov (2004)
Total warehouses capacity : 13 000 pallets positions and 20 000 square metres.
UNIPHARMA Introduction - 20th February 2019
14
15.
NEW WAREHOUSE IN BOJNICEUNIPHARMA Introduction - 20th February 2019
15
15
16.
Warehousing – Smoooth process flowAVERAGE LEAD-TIME FROM RECEIVING CUSTOMER ORDER UNTIL SHIPMENT IS 40 MINUTES !
UNIPHARMA Introduction - 20th February 2019
16
17.
Warehousing – receiving inspection processGOODS-IN DOCK
TEMPERATURE
INSPECTION
VISUAL INSPECTION
Receiving inspection
• Warehouse operator performs receiving inspection and
together with driver of supplier check following :
• Basic data: supplier, type, quantity
• Temperature inspection and visual inspection (no
damages etc.)
• Release of received goods is performed by QP
UNIPHARMA Introduction - 20th February 2019
17
18.
Warehousing – GOODS RECEIPTION• UNIPHARMA has batches
tracking tool assured by Oracle
eBS connected on-line to reader
devices
• Serialization
• ORACLE IS enables 100% on-line traceability from
• Actually our information
receiving inspection until shipment and delivery of each
system Oracle fully support
product
unique serial number scanning
using GS1 2D Data Matrix.
UNIPHARMA Introduction - 20th February 2019
18
19.
Warehousing – MODERN DISTRIBUTION CENTERSUNIPHARMA Introduction - 20th February 2019
19
20.
Warehousing – Employees periodical GDP testing & traningWarehouse operators and drivers are periodically trained, educated and tested
according to GDP + SOP´s requirements.
UNIPHARMA Introduction - 20th February 2019
20
21.
Warehousing – COLD-CHAIN PRODUCTS FLOW• All chilled products are considered as highest priority and are
immediately stored in chilling boxes at reception and as well as at
expedition gates .
Cold chain products are stored in
chilling box (from +2 °C up to +
8°C)
• QP is responsible to download and
evaluate data-loggers data during chilled
products receiving inspection process.
• In case that temperature was found out-of
tolerance during receiving inspection, all
products shall be stored immediately at
Quarantine area located inside chilling box.
UNIPHARMA Introduction - 20th February 2019
21
22.
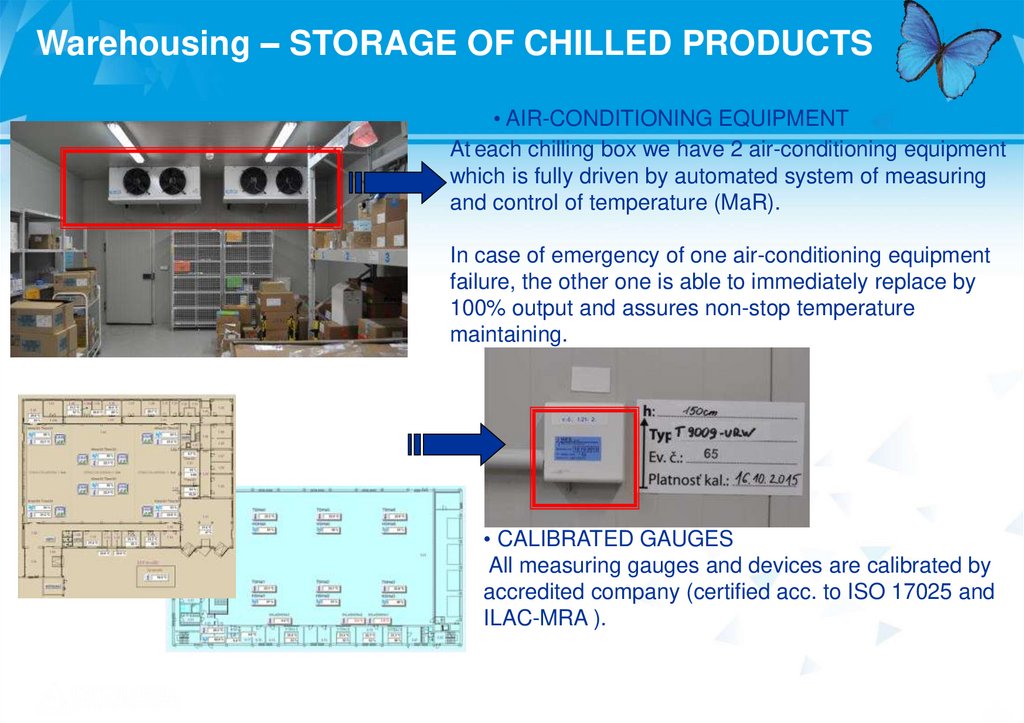
Warehousing – STORAGE OF CHILLED PRODUCTS• AIR-CONDITIONING EQUIPMENT
At each chilling box we have 2 air-conditioning equipment
which is fully driven by automated system of measuring
and control of temperature (MaR).
In case of emergency of one air-conditioning equipment
failure, the other one is able to immediately replace by
100% output and assures non-stop temperature
maintaining.
• CALIBRATED GAUGES
All measuring gauges and devices are calibrated by
accredited company (certified acc. to ISO 17025 and
ILAC-MRA ).
UNIPHARMA Introduction - 20th February 2019
22
23.
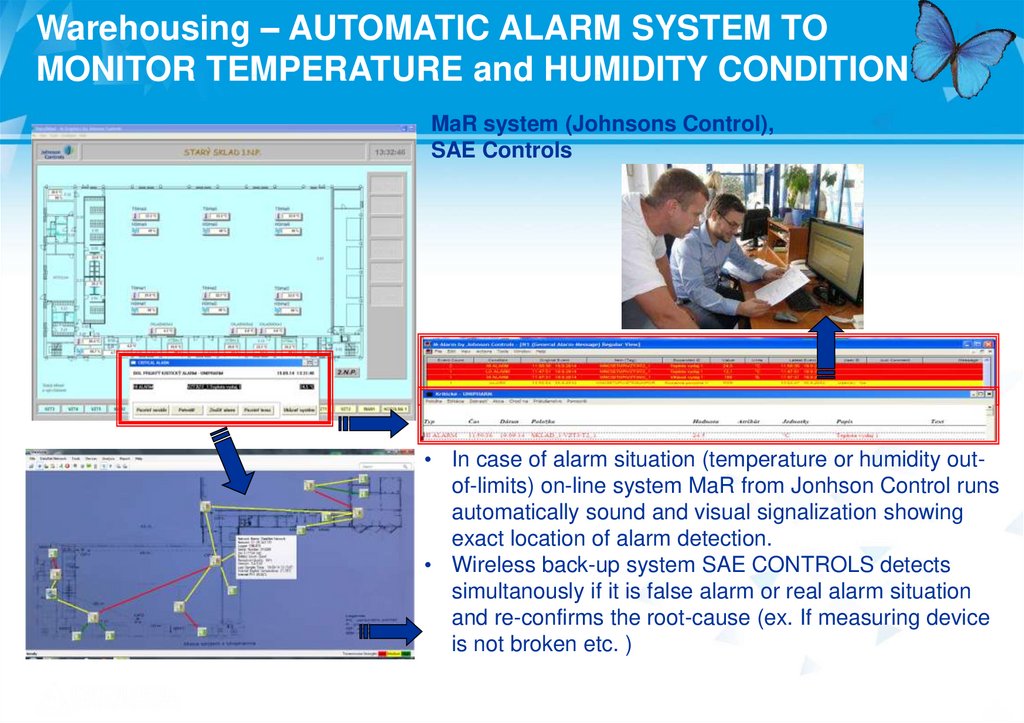
Warehousing – AUTOMATIC ALARM SYSTEM TOMONITOR TEMPERATURE and HUMIDITY CONDITION
MaR system (Johnsons Control),
SAE Controls
• In case of alarm situation (temperature or humidity outof-limits) on-line system MaR from Jonhson Control runs
automatically sound and visual signalization showing
exact location of alarm detection.
• Wireless back-up system SAE CONTROLS detects
simultanously if it is false alarm or real alarm situation
and re-confirms the root-cause (ex. If measuring device
is not broken etc. )
UNIPHARMA Introduction - 20th February 2019
23
24.
Warehousing – QUARANTINE MANAGEMENT• Nonconforming (returned, refused, products for
disposal) or recalled products are stored in locked
Quarantine areas. QP together with Purchasing and
Warehouse leaders are promptly solving each
complaint.
• Nonconforming products are blocked in IS Oracle as
„NON-SEALABLE“ on special location in Quarantine.
UNIPHARMA Introduction - 20th February 2019
24
25.
Warehousing – CLEANING / SANITATIONQualified Unipharma personnel performs sanitation of all warehouse
premises and returnable packages for pharmacies.
UNIPHARMA Introduction - 20th February 2019
25
26.
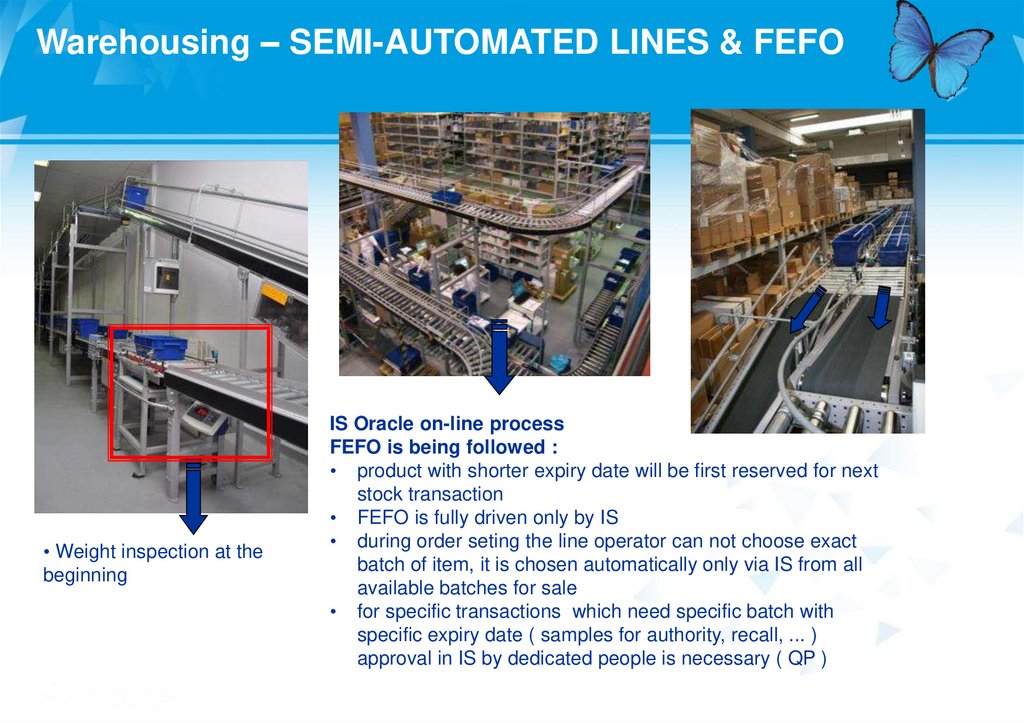
Warehousing – SEMI-AUTOMATED LINES & FEFO• Weight inspection at the
beginning
IS Oracle on-line process
FEFO is being followed :
• product with shorter expiry date will be first reserved for next
stock transaction
• FEFO is fully driven only by IS
• during order seting the line operator can not choose exact
batch of item, it is chosen automatically only via IS from all
available batches for sale
• for specific transactions which need specific batch with
specific expiry date ( samples for authority, recall, ... )
approval in IS by dedicated people is necessary ( QP )
UNIPHARMA Introduction - 20th February 2019
26
27.
Warehousing – SEMI-AUTOMATED LINE PROCESSEach product has dedicated
specific location
UNIPHARMA Introduction - 20th February 2019
27
28.
Warehousing – SEMI-AUTOMATED LINE PROCESSBar-code-reader
automatically
detects failures
for ex. wrong
location of picked
product of wrong
product type
UNIPHARMA Introduction - 20th February 2019
28
29.
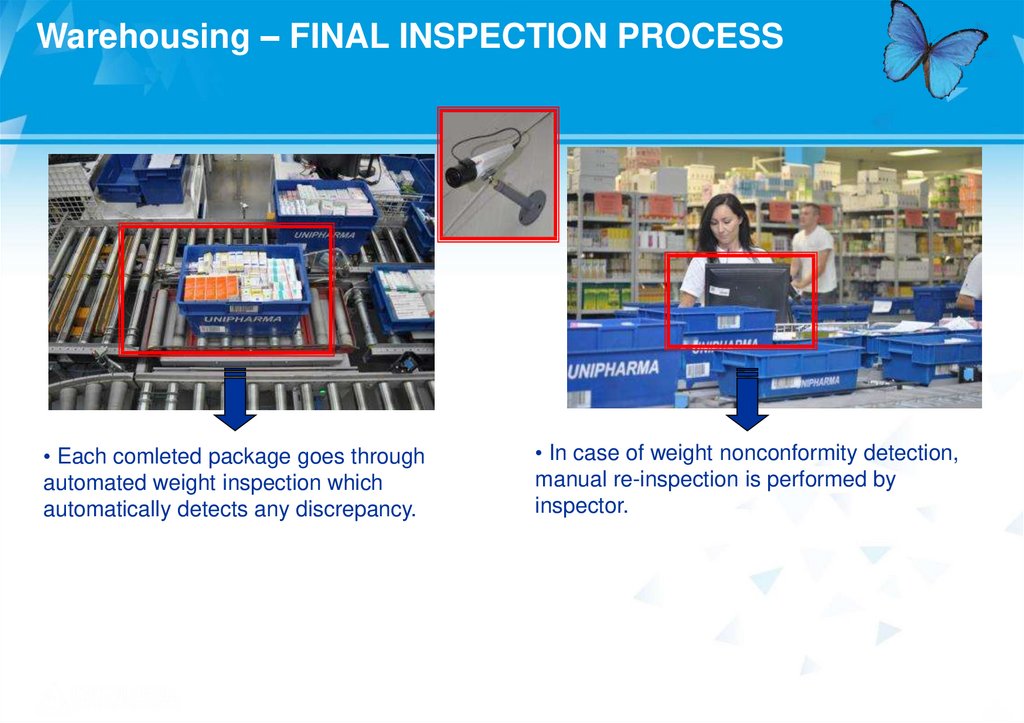
Warehousing – FINAL INSPECTION PROCESS• Each comleted package goes through
automated weight inspection which
automatically detects any discrepancy.
• In case of weight nonconformity detection,
manual re-inspection is performed by
inspector.
UNIPHARMA Introduction - 20th February 2019
29
30.
Warehousing – FINAL INSPECTION PROCESSFINAL INSPECTION
At all warehouse are
located VIDEO
CAMERAs to have
30 days traceability
of all processes in
case complaints.
• Quality performance: out of 16 mil. delivered packaged just 0,007% defect rate,
so defectivity is 1 to 15 000 items !
UNIPHARMA Introduction - 20th February 2019
30
31.
EXPEDITION (PICK & PACK)Released and packed packages are moved to expedition gate
UNIPHARMA Introduction - 20th February 2019
31
32.
Warehousing– description of Warehouse Management System
Dedicated location at expedition
goods-out gate
Shipment loading
• Drivers also use bar-code-reader to automatically
check completion of each delivery
• In case of missing boxes or incorrect supply route, IS
will automatically stop the transaction and shows
„ALERT“ message through bar-code-reader, so driver
can not continue transaction as long as mistake is not
being corrected
UNIPHARMA Introduction - 20th February 2019
32
32
33.
Warehousing– description of Warehouse Management System
Delivery to customers
Automated bar-code reader device
connected to IS Oracle assures mistakeproof system during loading process. In
ca of missing package, delivery route can
not be closed.
TERMOBOX with calibrated
gauge connected to GPS online software for safe
transportation of cold chain
products
UNIPHARMA Introduction - 20th February 2019
33
33
34.
Warehousing– description of Warehouse Management System
Delivery to customers
• All UNIPHARMA vehicles are equipped by automatic locking system
which assures locking of the vehicle during delivery to each pharmacy
UNIPHARMA Introduction - 20th February 2019
34
34
35.
Warehousing– description of Warehouse Management System
Delivery to customers
• All UNIPHARMA vehicles are equipped by isothermic device which
assures heating and cooling of vehicle to achieve required temperature + they
are equipped by GPS on-line system to have non-stop monitoring of
temperature and on-time delivery performance
• Our vehicles are validated and has valid ATP certificate.
UNIPHARMA Introduction - 20th February 2019
35
35
36.
Warehousing– description of Warehouse Management System
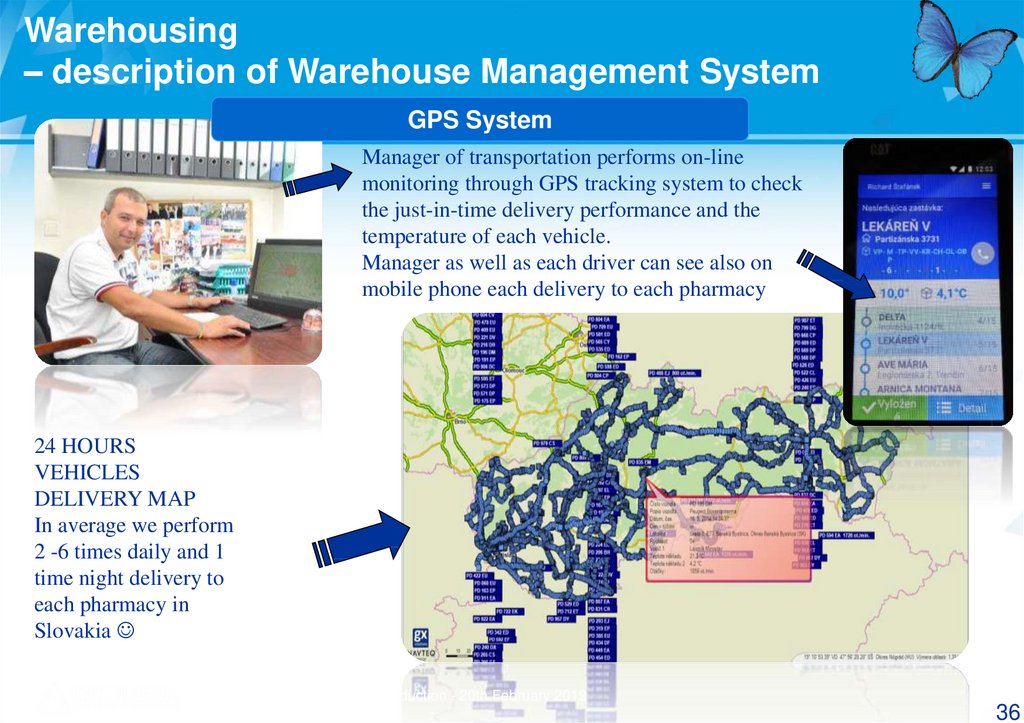
GPS System
Manager of transportation performs on-line
monitoring through GPS tracking system to check
the just-in-time delivery performance and the
temperature of each vehicle.
Manager as well as each driver can see also on
mobile phone each delivery to each pharmacy
24 HOURS
VEHICLES
DELIVERY MAP
In average we perform
2 -6 times daily and 1
time night delivery to
each pharmacy in
Slovakia
UNIPHARMA Introduction - 20th February 2019
36
36




























 marketing
marketing medicine
medicine








