Similar presentations:
Chemical bonding and Molecular Structure
1. 3 – Lecture Chemical bonding and Molecular Structure
Azamat Amzebek2. Topics Covered
Ionic Bonds and CompoundsCovalent Bonds
Lewis Structures
Types of Covalent Bonding
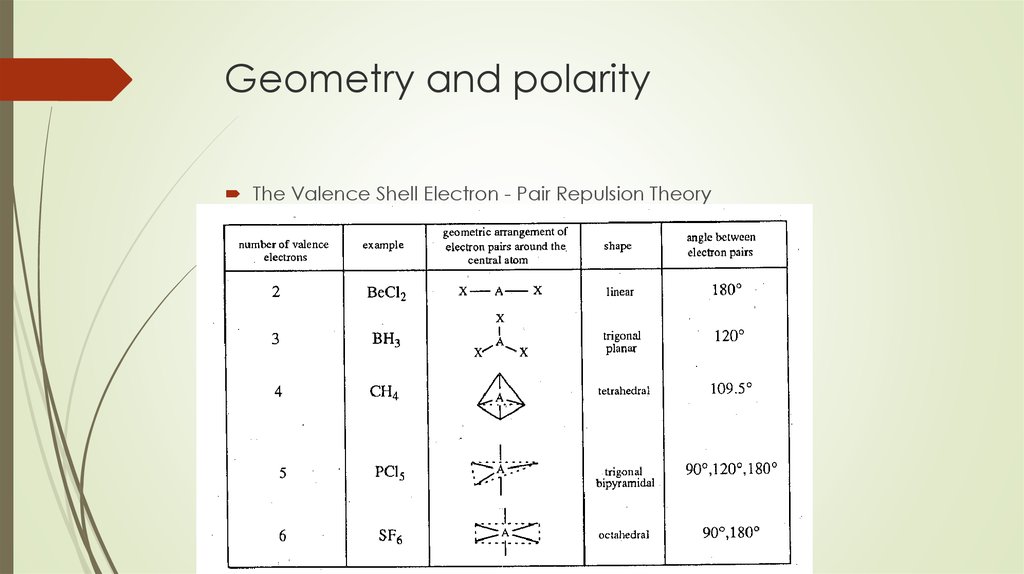
Geometry and Polarity of Covalent Molecules
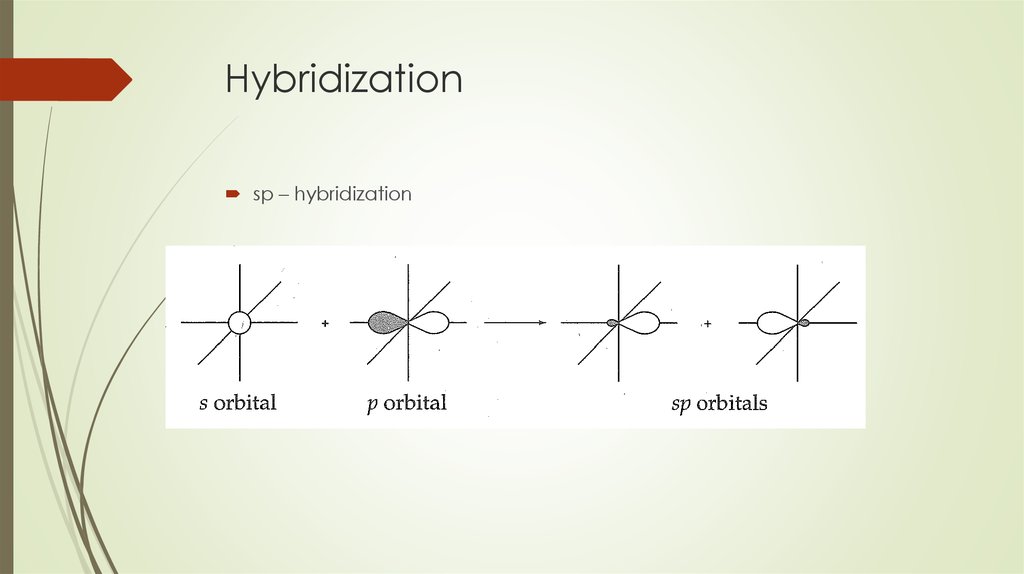
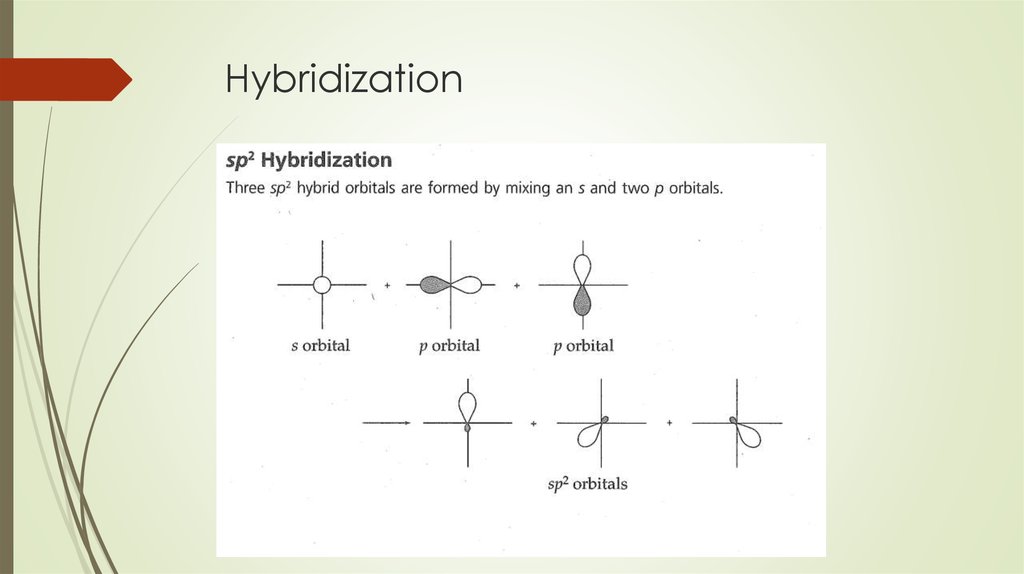
Orbital Hybridization
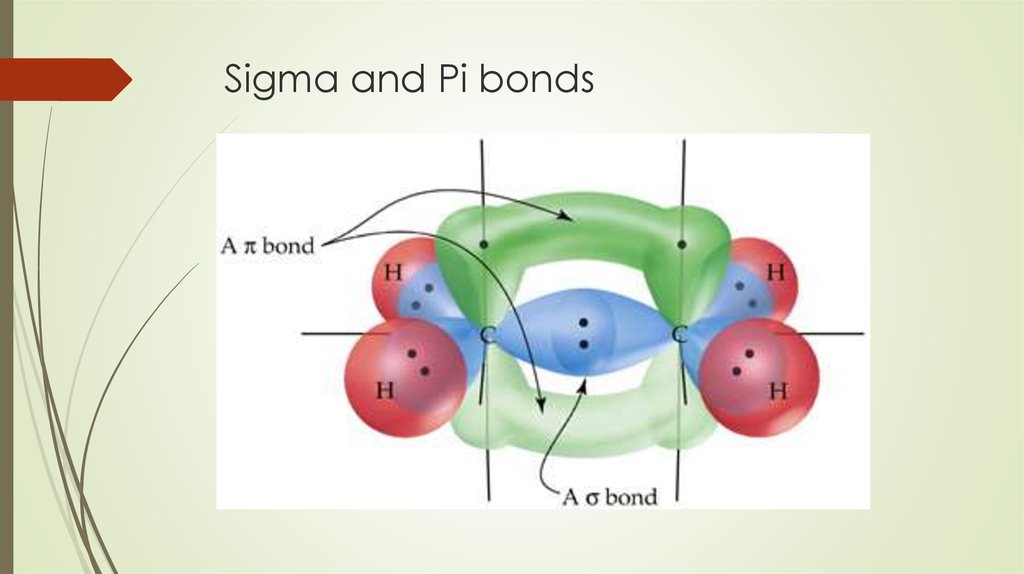
Sigma and Pi Bonds
3. Chemical bond
The atoms in molecules held together by strongattractive forces called chemical bonds
Formed by interaction of valence electrons of
the combining atoms
In the formation chemical bonds atoms bond
according to the octet rule
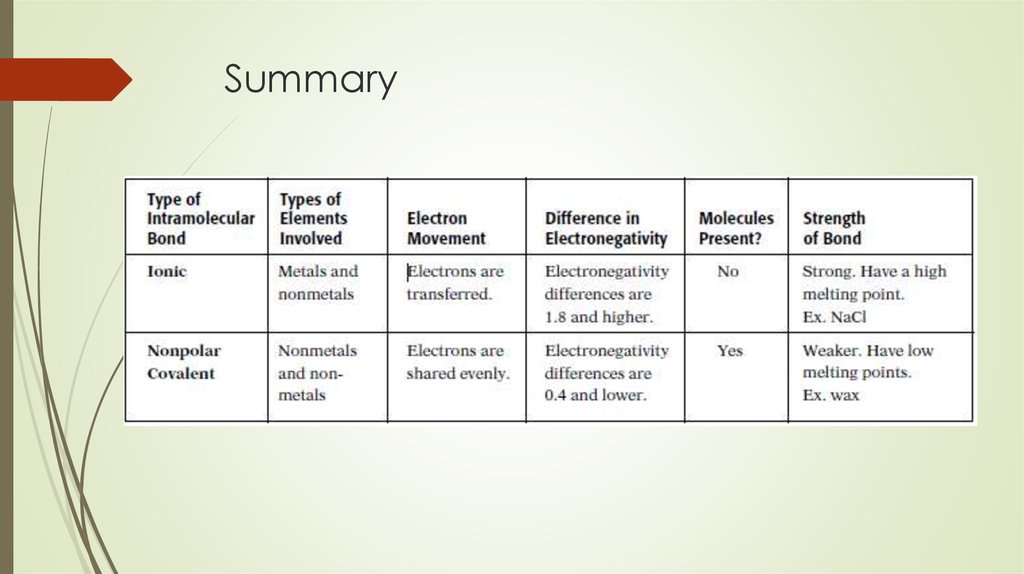
4. Ionic bond
Two atoms with large difference inelectronegativity reactivity, there is complete
electron transfer
High electronegativity == anion
Low electronegativity == cation
5. Ionic bond
These two ions are held together byelectrostatic forces
This force of attraction between the charged
ions is called an ionic bond
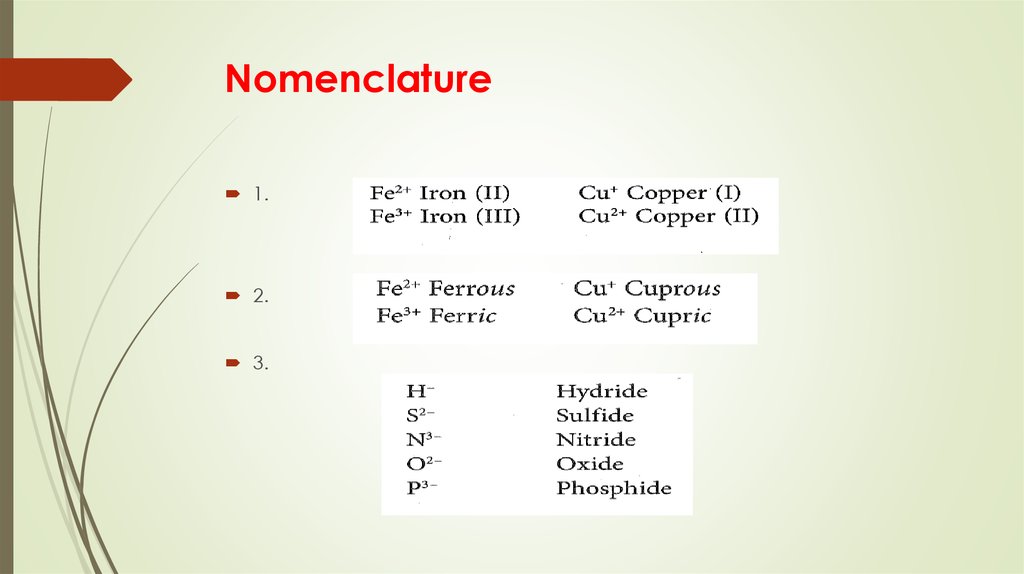
6. Nomenclature
1.2.
3.
7. Nomenclature
4.5.
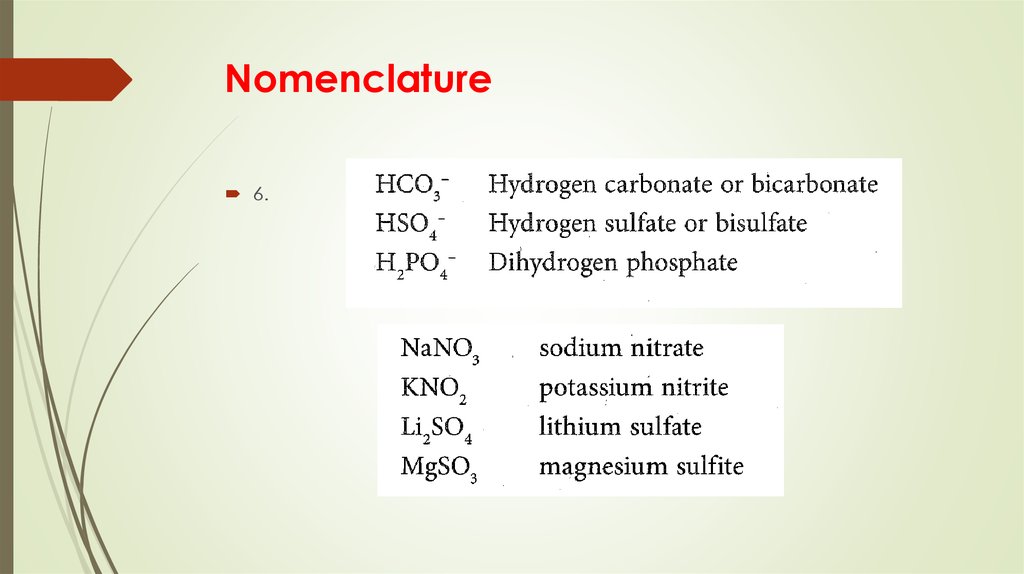
8. Nomenclature
6.9. Ionic bond
High melting and boiling point – strongelectrostatic force
Conduct electricity in liquid and aqueous states
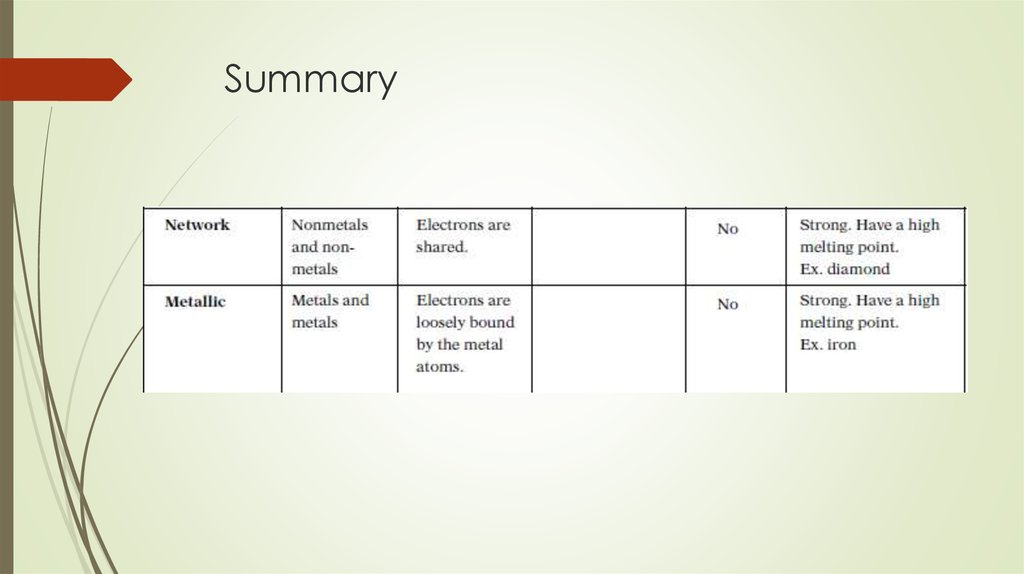
10. Covalent Bonds
Forms when two elements have similarelectronegativities
They achieve a noble gas electron
configuration by sharing electrons in what is
known as a covalent bond
Sometimes need more than one electron to
form octet
11. Covalent bond
Bonds length is the average distance betweenthe two nuclei of the atoms involved
Bond energy is the energy required to separate
two bonded atoms
12. Bonds
Primary bonding – covalent and ionicSecondary – hydrogen and Van der Vaals
13. Lewis Structures
Valence electrons of a covalent bond –bonding electrons
Valence electrons not involved in the covalent
bond – lone electron pairs(nonbonding)
Lewis structure – convenient representation of
bonding and nonbonding electrons in a
molecule
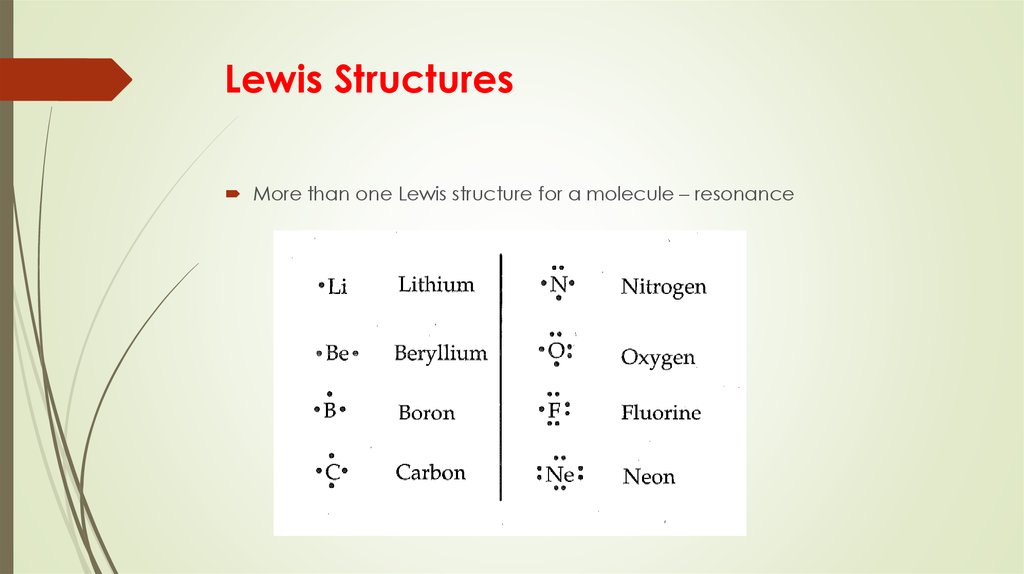
14. Lewis Structures
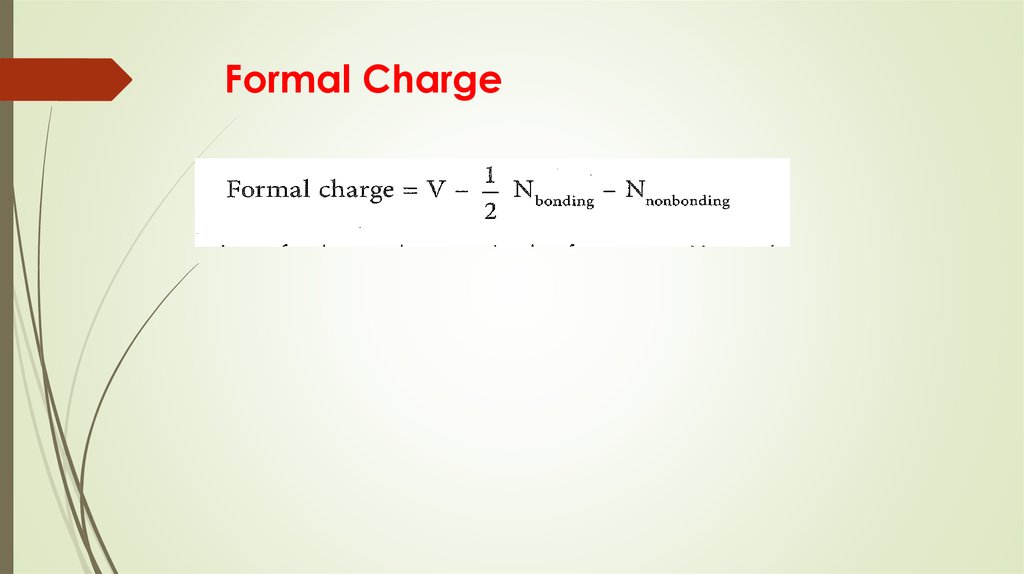
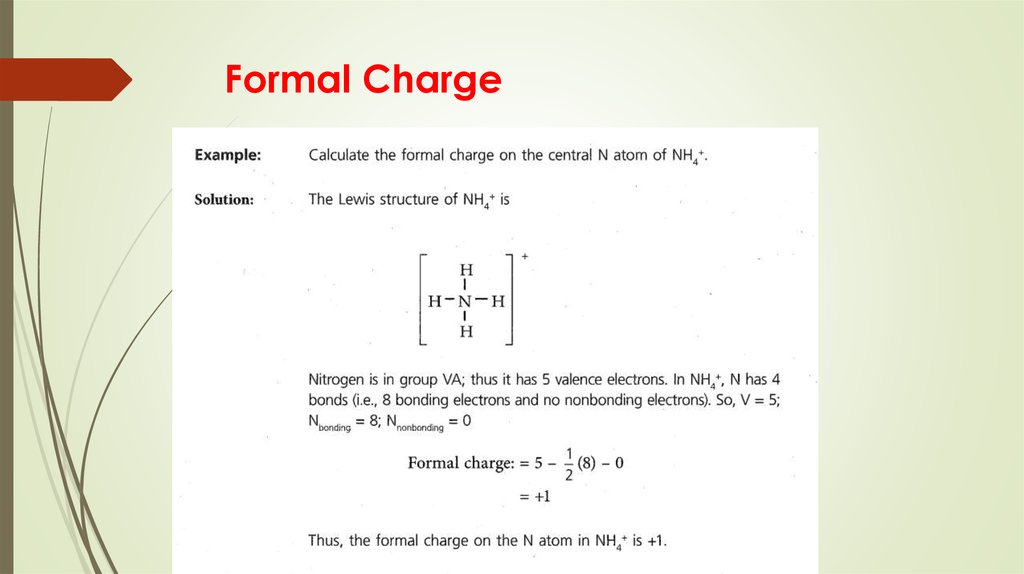
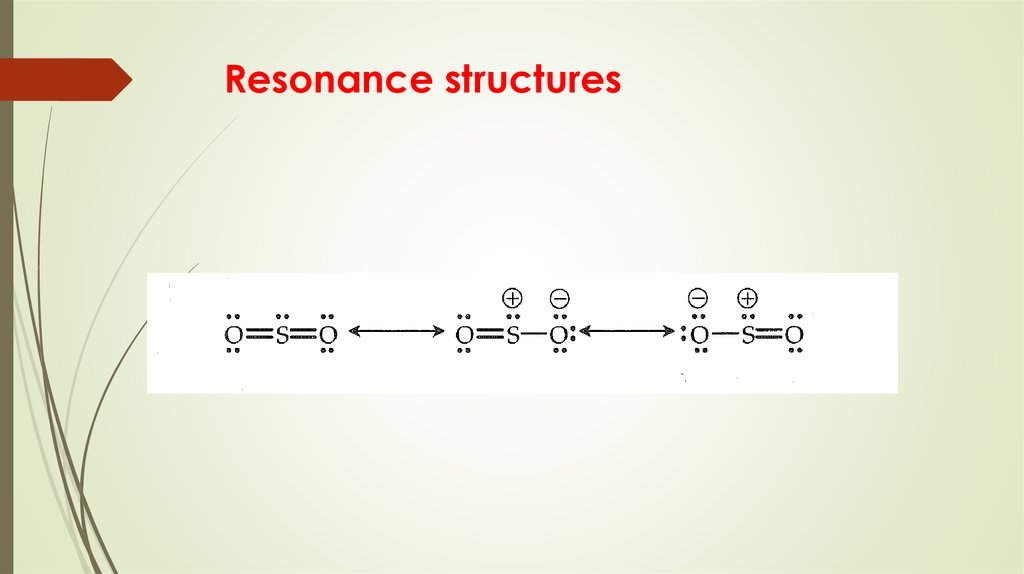
More than one Lewis structure for a molecule – resonance15. Formal Charge
16. Formal Charge
17. Resonance structures
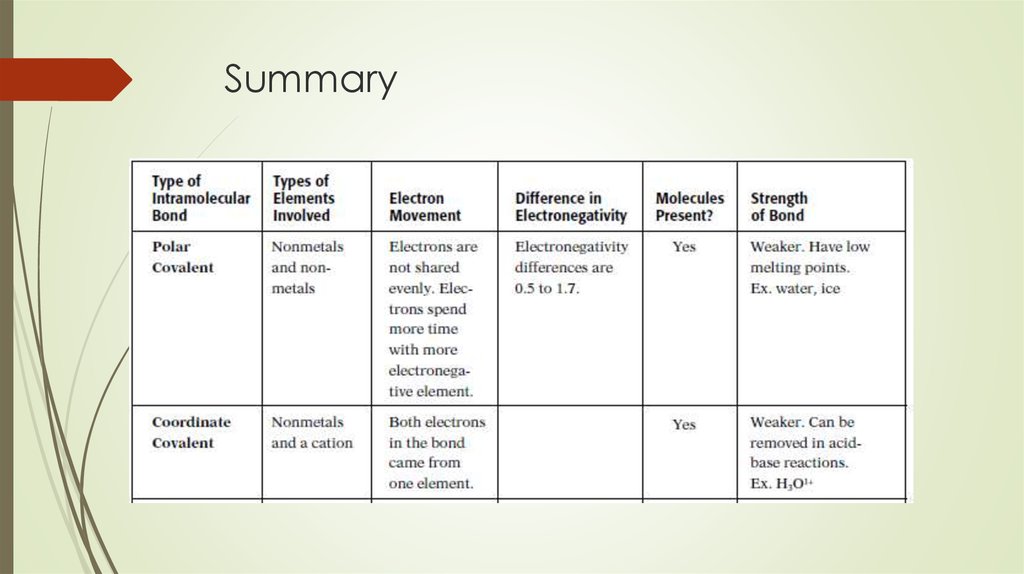
18. Types of covalent bonds
Polar covalent bonds – occurs between atomswith small difference in electronegativity
Nonpolar covalent bond occurs between atoms
that have the same electronegativites
Coordinate Covalent Bond – the shared electron
pair comes from the lone pair of one of the
atoms in the molecules
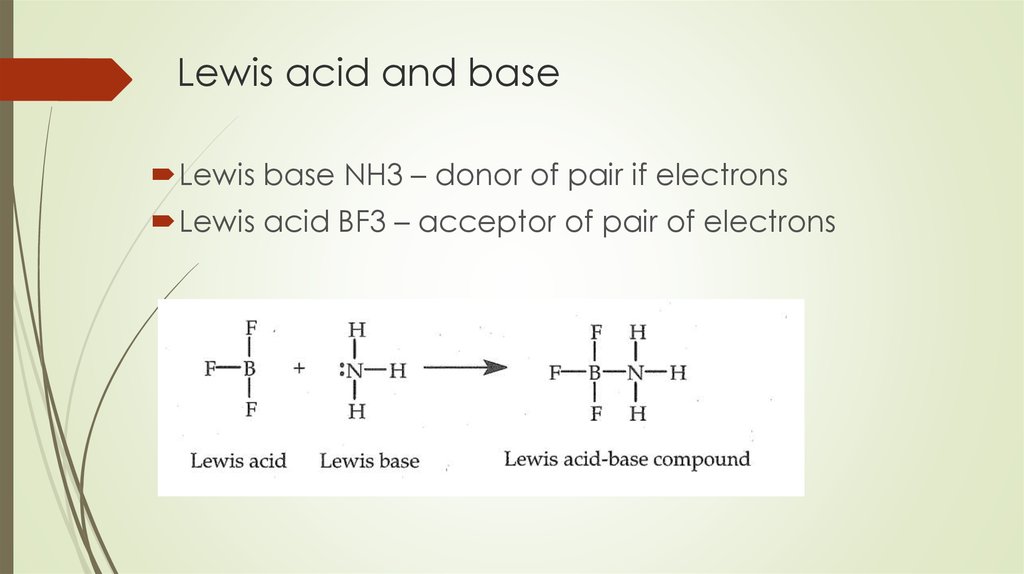
19. Lewis acid and base
Lewis base NH3 – donor of pair if electronsLewis acid BF3 – acceptor of pair of electrons









 chemistry
chemistry








