Similar presentations:
Analytical chemistry methods
1. ANALYTICAL CHEMISTRY METHODS
2.
1. Read and translate the international wordsAnalysis, analytical, separation, identification, component,
indication, classical, instrumental, extraction, distillation, apparatus,
absorption, chromatography, electrophoresis, clinical, procedure,
reagent, thermogravimetric, calorimetry, emission, fluorescence,
titration, microscope.
2. Read and translate the verbs
To analyze, to separate, to identify, to determine, to achieve, to
base, to detect, to weight, to add, to measure, to view, to focus on,
to accomplish, to provide, to probe.
3.
3. Match the English word combinations in column A to their Russian equivalents incolumn B
A
1. artificial material
2. precipitation
3. light absorption
4. flame test
5. emission spectrum
6. gravimetric analysis
7. equivalence point
8. electromagnetic radiation
9. mass spectrometry
10.magnetic field
11.electrochemical cell
12.with the unaided eye
13.calorimetry
14.separation process
15.metal ion
16.electroanalytical method
B
a) точка эквивалентности
b) поглощение света
c) электрохимическая ячейка
d) невооруженным глазом
e) магнитное поле
f) искусственный материал
g) ион металла
h) спектр испускания
i) процесс разделения
j) реакция в пламени
k) калориметрия
l) осаждение
m) гравиметрический анализ
n) электромагнитное излучение
o) электроаналитический метод
p) масс-спектрометрия
4.
4. Read and translateAnalytical Chemistry
Analytical chemistry is the study of the separation, identification, and
quantification of the chemical components of natural and artificial
materials. Qualitative analysis gives an indication of the identity of the
chemical species in the sample, while quantitative analysis determines
the amount of one or more of these components. Analytical methods
can be classified as classical and instrumental ones. Classical methods
use separations such as precipitation, extraction, and distillation and
qualitative analysis by color, odor, or melting point. Quantitative
analysis is achieved by measurement of weight or volume. Instrumental
methods use an apparatus to measure physical quantities of the analyte
such as light absorption, fluorescence, or conductivity.
5.
Classical MethodsQualitative analysis determines the presence or absence of a
particular compound, but not its mass or concentration.
Chemical test is designed to prove the existence of a chemical
compound or chemical group with the aid of a specific reagent.
Flame test is a procedure used in chemistry to detect the presence
of certain metal ions, based on each element's characteristic
emission spectrum.
Gravimetric analysis involves determining the amount of material
present by weighing the sample before and/or after some
transformation.
Titration involves the addition of a reactant to a solution being
analyzed until some equivalence point is reached.
6.
Instrumental MethodsSpectroscopy measures the interaction of the molecules with
electromagnetic radiation.
Mass spectrometry measures mass-to-charge ratio of molecules
using electric and magnetic fields.
Electroanalytical methods measure the potential (volts) and/or
current (amps) in an electrochemical cell containing the analyte.
Calorimetry and thermogravimetric analysis measure the
interaction of a material and heat.
Separation processes (chromatography, electrophoresis) are used
to decrease the complexity of material mixtures.
Microscopy is the technical field of using microscopes to view
objects that cannot be seen with the unaided eye.
7.
5. Answer the questions1. What does analytical chemistry study?
2. What are the main classifications of analytical methods?
3. What is the difference between classical and instrumental
methods?
4. What method do you use to determine the presence of certain
materials in a compound?
5. Is microscopy a qualitative or a quantitative method?
6. What does mass spectrometer measure?
7. What properties can we measure using instrumental methods?
8.
6. Read and translateAn optical spectrometer is an instrument used to measure properties
of light over a specific portion of the electromagnetic spectrum,
typically used in spectroscopic analysis to identify materials. The
variable measured is most often the light intensity but could also, for
instance, be the polarization state. The independent variable is usually
the wavelength of the light or a unit directly proportional to the photon
energy, such as wave number or electron volts, which has a reciprocal
relationship to wavelength. A spectrometer is used in spectroscopy for
producing spectral lines and measuring their wavelengths and
intensities. Spectrometer is a term that is applied to instruments that
operate over a very wide range of wavelengths, from gamma rays and
X-rays into the far infrared rays. The majority of spectrophotomers are
used in spectral regions near the visible spectrum.
9.
7. Read and translate the text into the English languageusing the diagrams of atomic force microscope
Атомно-силовой
микроскоп
(АСМ)
–
это
сканирующий
зондовый
микроскоп
высокого
разрешения. Принцип работы АСМ основан на
регистрации
силового
взаимодействия
между
поверхностью исследуемого образца и зондом. В
качестве зонда используется наноразмерное остриё,
располагающееся на конце упругой консоли,
называемой кантилевером. Сила, действующая на зонд
со стороны поверхности, приводит к изгибу консоли.
Появление возвышенностей или впадин под остриём
приводит к изменению силы, действующей на зонд, а,
значит, и изменению величины изгиба кантилевера.
Таким образом, регистрируя величину изгиба, можно
сделать вывод о рельефе поверхности.
10.
8. Find the Russian equivalents for the following phrasesAFM
an instrument of high resolution
operational principle
to be based on
force interaction between
sample surface
a probe
nanosized sharp tip
a deflection of the cantilever
a force acting to the probe
to cause bending of the cantilever
roughness of the surface
to lead to
surface geometry
scanning probe microscope
study sample
9. Find the English equivalents for the following phrases
определенная часть
независимая переменная
интенсивность светового излучения
состояние поляризации
длина волны
прямо пропорционально энергии
волновое число
электрон-вольт
широкий диапазон
рентгеновский
инфракрасный
видимый спектр
прибор
например
11.
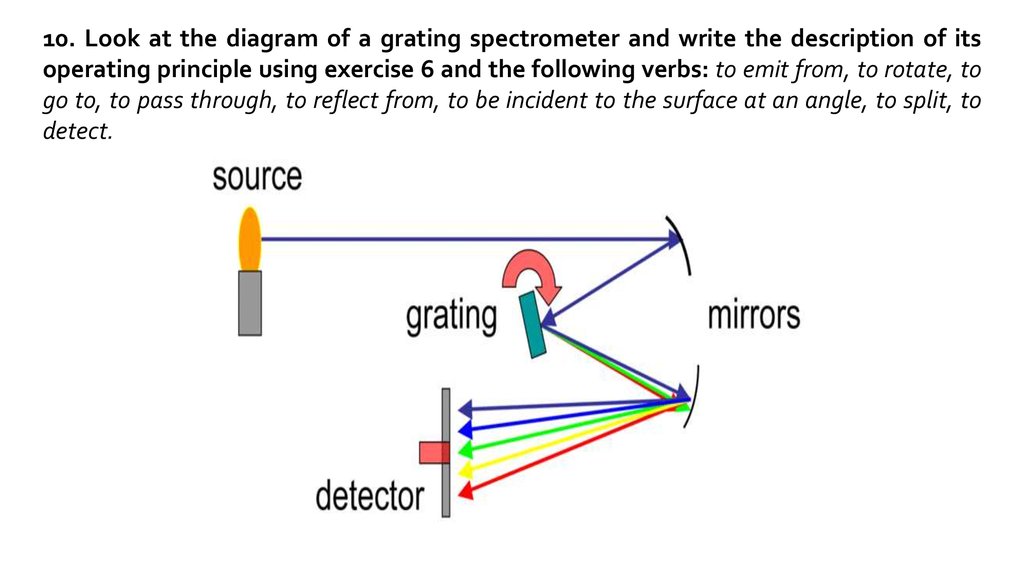
10. Look at the diagram of a grating spectrometer and write the description of itsoperating principle using exercise 6 and the following verbs: to emit from, to rotate, to
go to, to pass through, to reflect from, to be incident to the surface at an angle, to split, to
detect.
12.
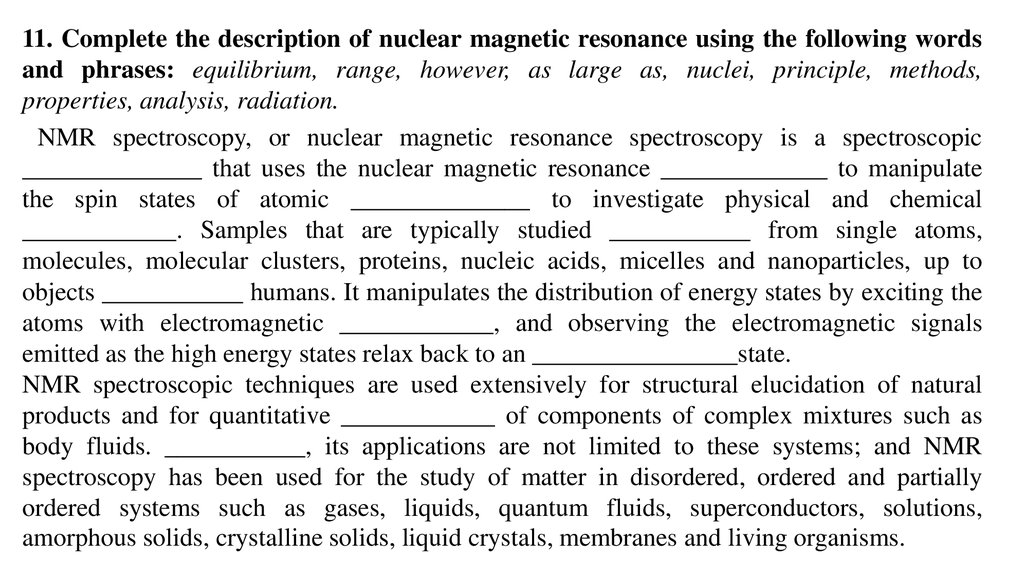
11. Complete the description of nuclear magnetic resonance using the following wordsand phrases: equilibrium, range, however, as large as, nuclei, principle, methods,
properties, analysis, radiation.
NMR spectroscopy, or nuclear magnetic resonance spectroscopy is a spectroscopic
______________ that uses the nuclear magnetic resonance _____________ to manipulate
the spin states of atomic ______________ to investigate physical and chemical
____________. Samples that are typically studied ___________ from single atoms,
molecules, molecular clusters, proteins, nucleic acids, micelles and nanoparticles, up to
objects ___________ humans. It manipulates the distribution of energy states by exciting the
atoms with electromagnetic ____________, and observing the electromagnetic signals
emitted as the high energy states relax back to an ________________state.
NMR spectroscopic techniques are used extensively for structural elucidation of natural
products and for quantitative ____________ of components of complex mixtures such as
body fluids. ___________, its applications are not limited to these systems; and NMR
spectroscopy has been used for the study of matter in disordered, ordered and partially
ordered systems such as gases, liquids, quantum fluids, superconductors, solutions,
amorphous solids, crystalline solids, liquid crystals, membranes and living organisms.
13.
12. Match the English word combinations in column A to their Russian equivalents incolumn B
A
B
1. conductometry
a. анод
2. anode
b. электрическое напряжение
3. electric resistance
с. электролитическая диссоциация
4. weak electrolyte
d. растворенные ионы
5. strong electrolyte
e. раствор
6. electrolytic dissociation
f. кондуктометрия
7. cross-sectional area
g. электросопротивление
8. dissolved ions
h. катод
9. solution
i. площадь поперечного сечения
10. current
j. соль
11. voltage
k. удельная проводимость
12. specific conductivity
l. слабый электролит
13. cathode
m. основание
14. salt
n. электрический ток
15. base
o. сильный электролит
14.
13. Read the text using the terminology from exercise 12 and describe themain principles of conductometry in the English language
Electrolytes are substances that produce free ions when they are placed into
a solvent such as water. Their molecules split up into individual atomic
components, which form ions, in a process called dissociation. Positively
charged ions are cations, and those with a negative charge are anions. Due to
the presence of free ions, electrolyte solutions behave as an electrically
conductive medium.
Common electrolytes consist of salts, acids or bases. Electric properties of
the conductor are described by Ohm's law I = U/R where I corresponds to a
current, U is a voltage and R describes electric resistance. This resistance
depends on the intrinsic properties of a conductor and on its shape as R = ρl/S
where l is a conductor's length and S is a cross-sectional area. Every material is
characterized by a specific resistance, ρ, that is given in units of Ω⋅m (Ω - ohm,
a unit of electric resistance).
15.
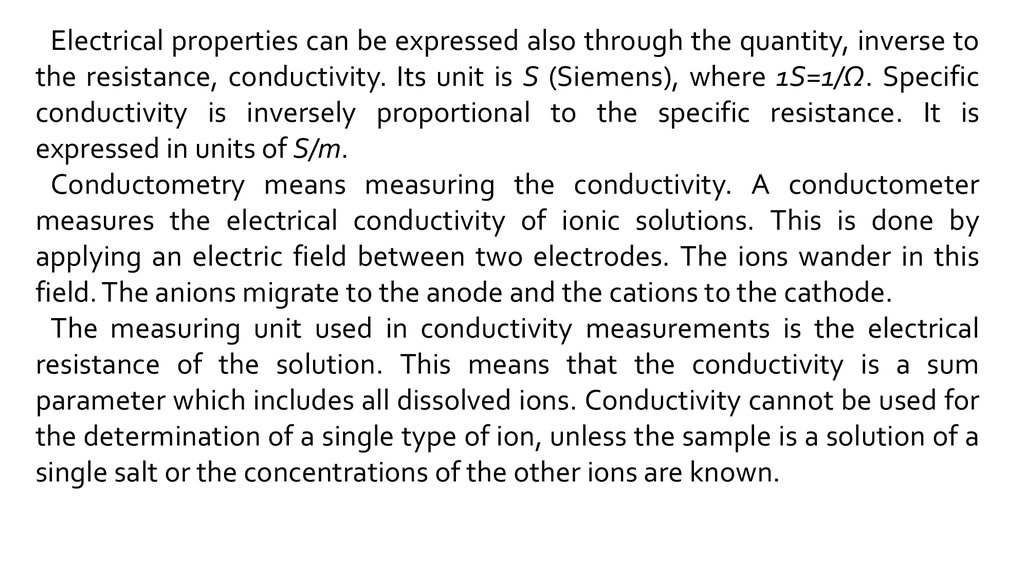
Electrical properties can be expressed also through the quantity, inverse tothe resistance, conductivity. Its unit is S (Siemens), where 1S=1/Ω. Specific
conductivity is inversely proportional to the specific resistance. It is
expressed in units of S/m.
Conductometry means measuring the conductivity. A conductometer
measures the electrical conductivity of ionic solutions. This is done by
applying an electric field between two electrodes. The ions wander in this
field. The anions migrate to the anode and the cations to the cathode.
The measuring unit used in conductivity measurements is the electrical
resistance of the solution. This means that the conductivity is a sum
parameter which includes all dissolved ions. Conductivity cannot be used for
the determination of a single type of ion, unless the sample is a solution of a
single salt or the concentrations of the other ions are known.
16.
17.
1. Briefly describe the experimental process.2.Describe the main parts of the conductometer.
3. What are the modes of the conductometer?
4.Explain what property the engineer measured.
5.What solution did he use?
6.Explain how the engineer analyzed the obtained results.
7. Describe the linear function in the graph.
8.What is the practical application of the presented instrument?
9.Explain what you learnt from the experiment.
18.
15. Translate the following sentences into the English language1. Кондуктометрия – это метод для измерения электропроводности ионных
растворов.
2. Электролит – это вещество, которое проводит электрический ток.
3. Примерами электролитов могут служить водные растворы кислот, солей и
оснований.
4. Удельная электропроводность – это величина обратная удельному
сопротивлению раствора.
5. Электрические свойства проводника описываются законом Ома.
6. Единицей измерения удельной проводимости является См/м.
7. Соль помещают в дистиллированную воду и перемешивают.
8. Для проведения эксперимента используется кондуктометрический датчик.
9. На графике показана линейная зависимость электросопротивления вещества от
времени.
10.Кондуктометрия нашла широкое применение для исследования растворов
твердых и жидких веществ в аналитической химии и на производстве.










 chemistry
chemistry








