Similar presentations:
V2 Efferon Group Pitch Deck
1.
Efferon GroupTargeted therapy for inflammatory conditions
Inflammation management across critical care, chronic disease, and longevity
2.
Problem: Inflammation Is a Common Driver of theWorld's Biggest Health Burdens
Inflammation is both
a driver and a hallmark
of many diseases. Gutderived endotoxins in
bloodstream, which
triggers cytokine release
is among the most
common pathways for
inflammation
●Acute Systemic Inflammation
Sepsis, ARDS
●Autoimmune Diseases
Rheumatoid Arthritis, Crohn's Disease,
Psoriasis, Psoriatic arthritis
●Cardiometabolic Diseases
Type 2 Diabetes, NAFLD/NASH
●Neuroinflammation & Age-Related
Diseases Alzheimer’s, Parkinson’s
●Cancer
Hepatocellular Carcinoma, Colorectal
Cancer, Gastric Cancer
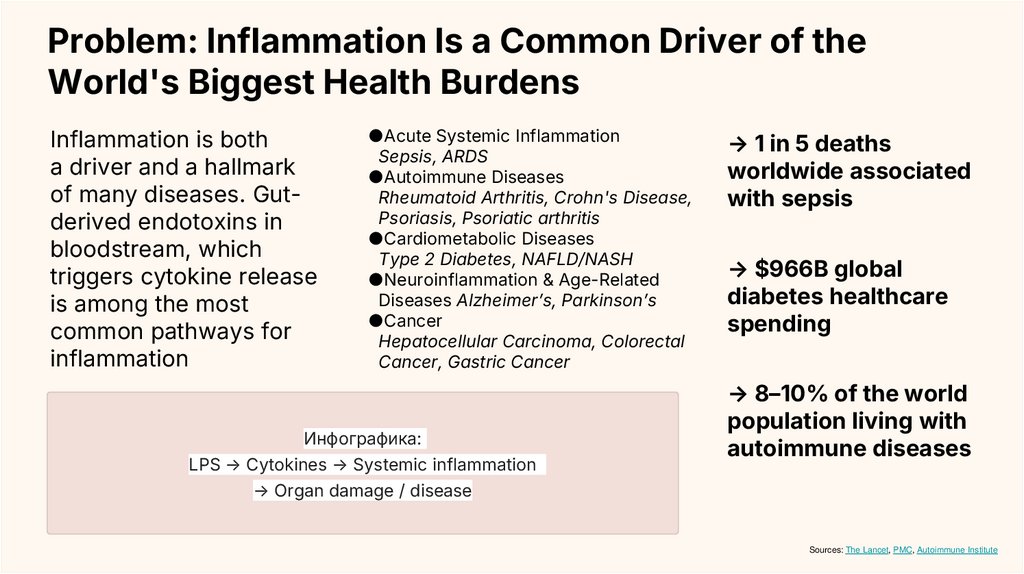
Инфографика:
LPS → Cytokines → Systemic inflammation
→ 1 in 5 deaths
worldwide associated
with sepsis
→ $966B global
diabetes healthcare
spending
→ 8–10% of the world
population living with
autoimmune diseases
→ Organ damage / disease
Sources: The Lancet, PMC, Autoimmune Institute
3.
Solution: A Patented Hemoadsorption Technology forSystemic Inflammation, Already Approved in Europe for
Treatment of Sepsis, Septic Shock, and Cytokine Release
First-in-class, patented
multimodal hemoadsorption therapy
designed to address
systemic inflammation
● Simultaneously removes
endotoxin (LPS) and key proinflammatory cytokines
(e.g., IL-6, TNF-α, IL-1β) to
reduce inflammatory burden
● Well-established, widely used,
safe and tolerable therapy that
integrates into standard
extracorporeal circuits (RRT,
ECMO, CPB)
→ 25,000 patients treated
(including 1000 infants)
→ 3.1x lower mortality in the
ICU
→ 1.2 kg infant (smallest
treated patient)
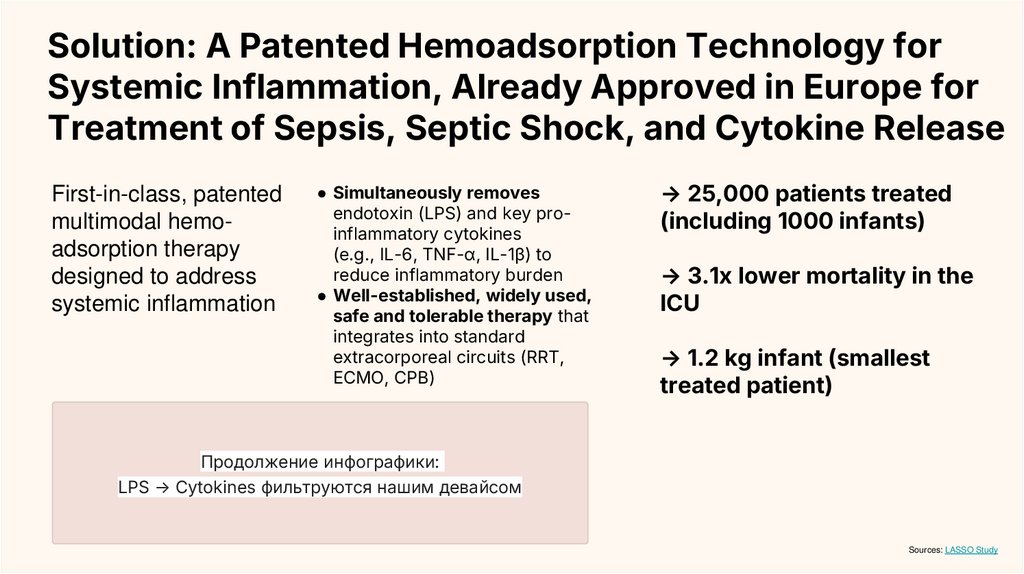
Продолжение инфографики:
LPS → Cytokines фильтруются нашим девайсом
Sources: LASSO Study
4.
Vision: Leading the Future of Inflammation Managementfrom Critical Care to Longevity
Our core hemoadsorption technology enables multiple applications across inflammation-driven
markets—creating flexible partnership and funding options across indications and business models
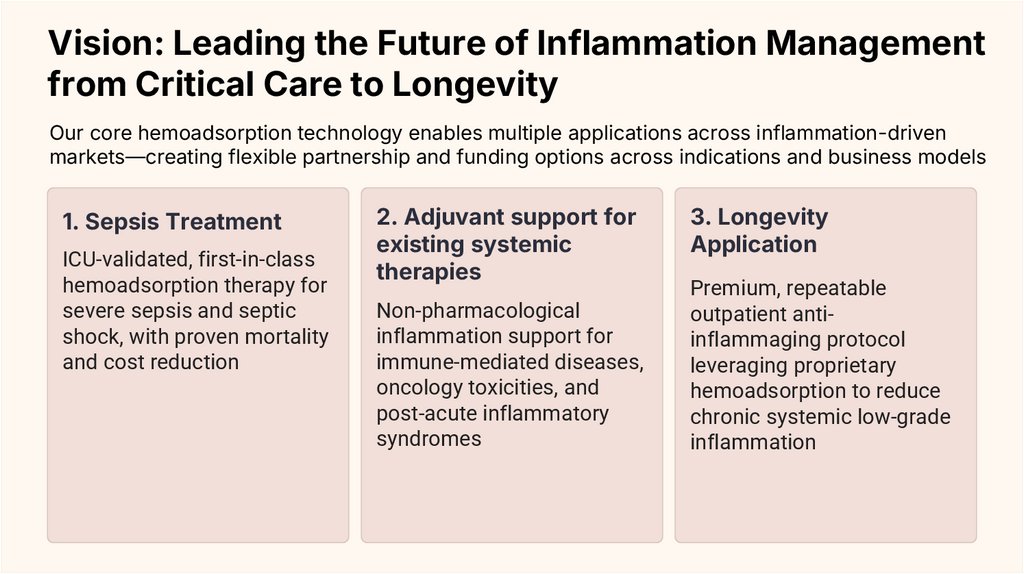
1. Sepsis Treatment
ICU-validated, first-in-class
hemoadsorption therapy for
severe sepsis and septic
shock, with proven mortality
and cost reduction
2. Adjuvant support for
existing systemic
therapies
Non-pharmacological
inflammation support for
immune-mediated diseases,
oncology toxicities, and
post-acute inflammatory
syndromes
3. Longevity
Application
Premium, repeatable
outpatient antiinflammaging protocol
leveraging proprietary
hemoadsorption to reduce
chronic systemic low-grade
inflammation
5.
Sepsis: Massive Unmet Need in a High-Cost, GrowingMarket
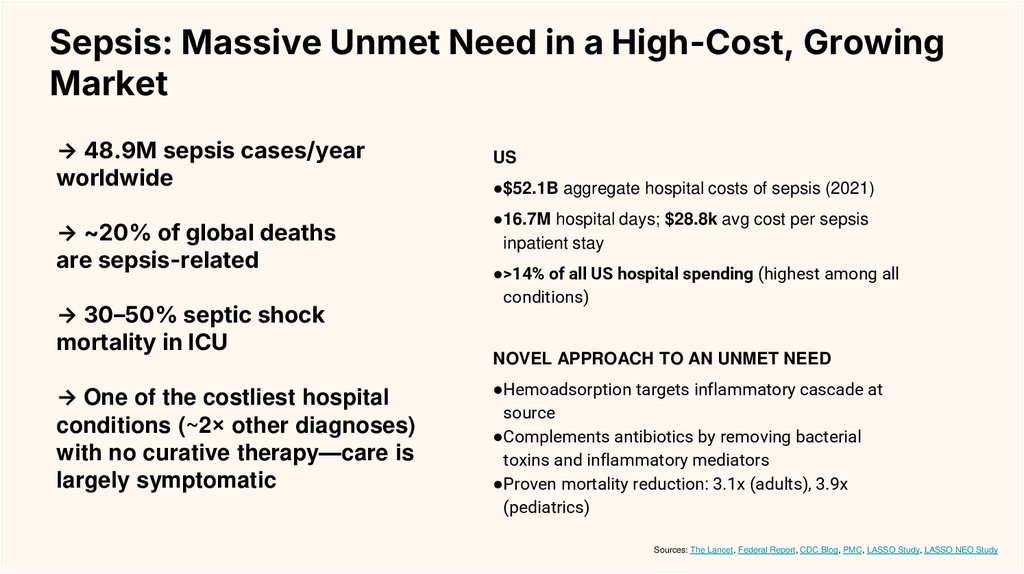
→ 48.9M sepsis cases/year
worldwide
→ ~20% of global deaths
are sepsis-related
→ 30–50% septic shock
mortality in ICU
→ One of the costliest hospital
conditions (~2× other diagnoses)
with no curative therapy—care is
largely symptomatic
US
●$52.1B aggregate hospital costs of sepsis (2021)
●16.7M hospital days; $28.8k avg cost per sepsis
inpatient stay
●>14% of all US hospital spending (highest among all
conditions)
NOVEL APPROACH TO AN UNMET NEED
●Hemoadsorption targets inflammatory cascade at
source
●Complements antibiotics by removing bacterial
toxins and inflammatory mediators
●Proven mortality reduction: 3.1x (adults), 3.9x
(pediatrics)
Sources: The Lancet, Federal Report, CDC Blog, PMC, LASSO Study, LASSO NEO Study
6.
Efferon: ICU-Validated Sepsis Therapy with Real-WorldTraction
●Product Line
- Efferon®LPS (ICU >40 kg, Gram-negative sepsis/septic
shock)
- Efferon® NEO (neonates/peds ≤40 kg, sepsis/septic
shock)
- Efferon®CT (>40 kg, cytokines/myoglobin indications)
●Present in 35+ countries via 29 distributors (EMEA, Asia,
LatAm, Australia)
●Patents across major markets (US, EU, China, Japan,
Korea, India, Israel, Brazil, Canada, Colombia, Mexico, Peru)
●Trademark registered and CE-marked under EU MDR
●Included in “Standard of Therapeutic Apheresis”
(German Society of Nephrology)
●Key partners Hannover Medical School, University
Hospital Essen, AKH Vienna, Brothers of Mercy Linz
●50k units/year today (possible scale up to ~1M units/year)
at our EU MDR–compliant 2,000 m² in-house facility in EU
→ Only therapy with EU MDR
approval specifically for
clinical endpoints of septic
shock
→ Holds ~5–60% market share,
depending on the country
→ 38.7% lowered ICU cost
per patient (€16,656 saved)
→ 3.1× lower early mortality in
adults; 3.9× lower in pediatric
ICU patients
Sources: LASSO Study, LASSO NEO Study
7.
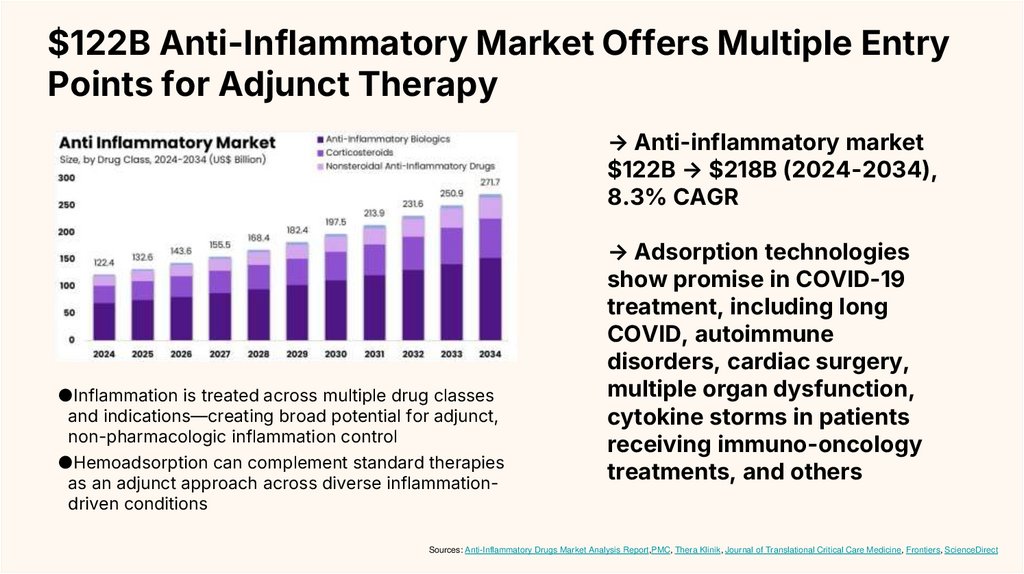
$122B Anti-Inflammatory Market Offers Multiple EntryPoints for Adjunct Therapy
→ Anti-inflammatory market
$122B → $218B (2024-2034),
8.3% CAGR
●Inflammation is treated across multiple drug classes
and indications—creating broad potential for adjunct,
non-pharmacologic inflammation control
●Hemoadsorption can complement standard therapies
as an adjunct approach across diverse inflammationdriven conditions
→ Adsorption technologies
show promise in COVID-19
treatment, including long
COVID, autoimmune
disorders, cardiac surgery,
multiple organ dysfunction,
cytokine storms in patients
receiving immuno-oncology
treatments, and others
Sources: Anti-Inflammatory Drugs Market Analysis Report,PMC, Thera Klinik, Journal of Translational Critical Care Medicine, Frontiers, ScienceDirect
8.
Hemoadsorption as Adjunct Support in InflammationDriven ConditionsImmune-mediated
systemic diseases
Oncology and immunotherapyrelated inflammation
Post-Acute Inflammatory
Syndromes
●Rheumatoid arthritis, SLE,
systemic vasculitis, Crohn’s
disease, Ulcerative colitis,
Psoriasis, Psoriatic arthritis,
Lupus, etc.
●CAR-T and T-cell engager toxicities,
cytokine release syndrome (CRS)
●Post-sepsis syndrome, Long
COVID (PASC), post-infectious
syndromes including posttreatment Lyme disease syndrome
●~1 in 10 people affected by
autoimmune disease
●Immunology therapeutics market
$103B (2024) → $257B (2032)
●Hemoadsorption as adjunct in
selected severe/systemic flares to
lower inflammatory burden
alongside standard therapy
●refractory psoriasis (highmorbidity) — early data suggest
better response + longer remission
on standard treatment
●CAR-T market $5.82B (2025) →
$22.36B (2033)
●Hemoadsorption as an adjunct
therapy to help lower circulating
cytokines in severe or refractory
cytokine-driven toxicity
●May enable safer use of highly
potent T-cell therapies by controlling
cytokine-driven toxicity
●14.1M adults + 2.5M children
survive sepsis annually
●Hemoadsorption as an adjunct
therapy to reduce persistent
systemic inflammatory burden
during recovery and flares
Sources: PMC, Fortune Business Insights, Severe Psoriasis Study, Grand View Research, ScienceDirect, World Sepsis Day
9.
Longevity application: Targeting the Root Cause ofInflamm-aging
OPPORTUNITY
●Chronic disease burden is the main limiter
of healthspan
●Systemic chronic inflammation is a shared
pathway across major age-related diseases
●Age-related increases in gut permeability
let LPS enter the bloodstream, sustaining
cytokine release and chronic low-grade
inflammation
Инфографика:
→ Inflamm-aging is a chronic, lowgrade, progressive inflammation that develops with age and
contributes to age-related diseases
→ Age-related diseases cost $47T
over 15 years in the US alone
→ Longevity & Anti-Aging Market
$268.5B → $455.4B (2024-2030),
~9.2% CAGR
Leaky gut → LPS + Cytokines → Low-grade
inflammation → Age-related diseases
Sources: Business Wire, Market Research Future, Inflamm-aging: An Evolutionary Perspective on Immunosenescence
10.
Multimodal hemoadsorption for repeatable outpatientanti-inflammaging
Safe, repeatable, non-pharmacological
therapy that reduces chronic disease risk,
supports cellular function, improves
energy and resilience, and provides
neuroprotection support
→Safe
ICU-validated safety profile
Potential Therapeutic Effects
→ Multimodal
One session: LPS + key cytokines
●Lowering chronic inflammation
●Reduced oxidative stress
●Improved and better overall well-being
●Better focus and mental clarity
●Better sleep
●Faster recovery after infections, intense
physical effort, or high stress
●Milder seasonal allergies
→ Minimally invasive
Peripheral access, outpatient workflow
→ Repeatable
Designed for life-long use
11.
A three-track plan: scale the core ICU business, explorenon-critical expansion through partnered clinical
programs, and enter premium longevity
1. Sepsis Treatment
Scale manufacturing and expand into
priority global markets
●Automate and expand manufacturing
to increase capacity up to 1M
cartridges per year
●Support commercial expansion into
China and the US with the ICUvalidated sepsis product
●Funding focus: manufacturing
automation, capacity build-up, market
entry execution (China, US)
2. Adjuvant support for
existing systemic
therapies
Definitive proof and expansion into
priority global markets
●Pharma partnerships to run clinical
studies in selected systemic flares
3. Longevity
Application
Build and scale a premium longevity
clinic and franchise model
●Build an anti-inflammaging protocol +
biomarker tracking and analysis AI
software
●Focus on indications where our data
shows the strongest early signals
●Launch a flagship premium clinic in
China (new brand, for HNWI client),
then scale to UAE/Europe/Japan
●Adjunct hemoadsorption to lower
inflammatory burden on top of
standard therapy
●Productize into a franchise model (IT
+ protocols + recurring consumables)
●Funding focus: protocol R&D,
software, flagship clinic launch,
brand/franchise infrastructure
12.
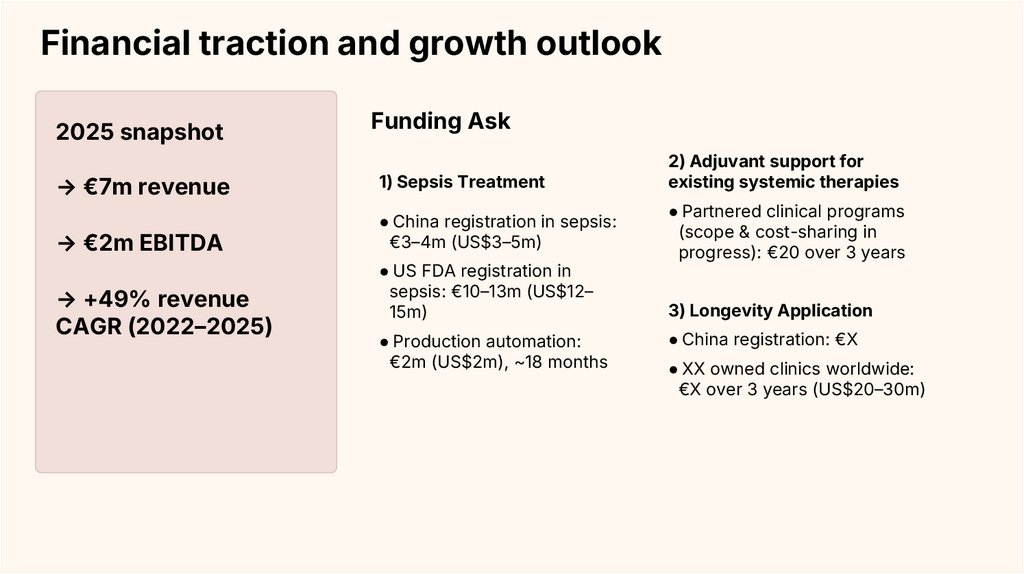
Financial traction and growth outlook2025 snapshot
Funding Ask
→ €7m revenue
1) Sepsis Treatment
→ €2m EBITDA
● China registration in sepsis:
€3–4m (US$3–5m)
→ +49% revenue
CAGR (2022–2025)
● US FDA registration in
sepsis: €10–13m (US$12–
15m)
● Production automation:
€2m (US$2m), ~18 months
2) Adjuvant support for
existing systemic therapies
● Partnered clinical programs
(scope & cost-sharing in
progress): €20 over 3 years
3) Longevity Application
● China registration: €X
● XX owned clinics worldwide:
€X over 3 years (US$20–30m)
13.
An end-to-end leadership mix: science, clinical,commercialization
Dmitriy Romashin
Co-founder, strategy, capital
management, sales & marketing,
global operations
Ivan Bessonov
Co-founder, inventor of the core
technology of proprietary
polymeric adsorbent beads,
device design, clinical research,
production
Maryana Breitman
Chief Strategy & Expansion Officer; a
biotech/pharma strategy leader with
20+ years of experience, including
commercial strategy & innovation at
Pfizer and scientist-entrepreneurship
as co-founder/CEO of MBMR Biolabs
Alexander ShelekhovKravchenko, MD, PhD
Medical Director specializing in
preventive longevity technologies,
leading the launch and scale-up of
advanced longevity clinics
14.
Dmitriy RomashinCEO Efferon Group
dr@efferon.com
+372 5631 0093
15.
Appendix16.
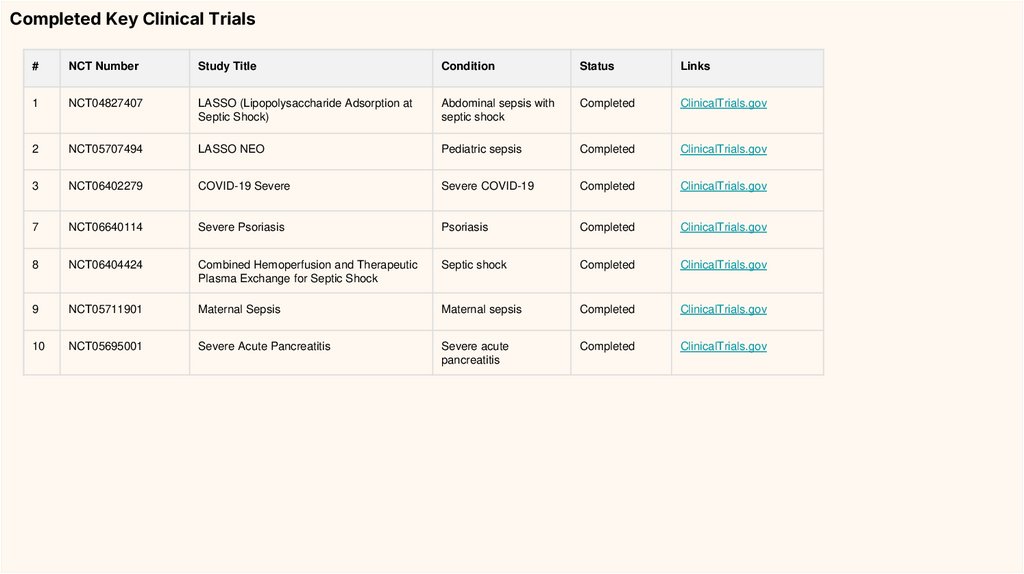
Completed Key Clinical Trials#
NCT Number
Study Title
Condition
Status
Links
1
NCT04827407
LASSO (Lipopolysaccharide Adsorption at
Septic Shock)
Abdominal sepsis with
septic shock
Completed
ClinicalTrials.gov
2
NCT05707494
LASSO NEO
Pediatric sepsis
Completed
ClinicalTrials.gov
3
NCT06402279
COVID-19 Severe
Severe COVID-19
Completed
ClinicalTrials.gov
7
NCT06640114
Severe Psoriasis
Psoriasis
Completed
ClinicalTrials.gov
8
NCT06404424
Combined Hemoperfusion and Therapeutic
Plasma Exchange for Septic Shock
Septic shock
Completed
ClinicalTrials.gov
9
NCT05711901
Maternal Sepsis
Maternal sepsis
Completed
ClinicalTrials.gov
10
NCT05695001
Severe Acute Pancreatitis
Severe acute
pancreatitis
Completed
ClinicalTrials.gov
17.
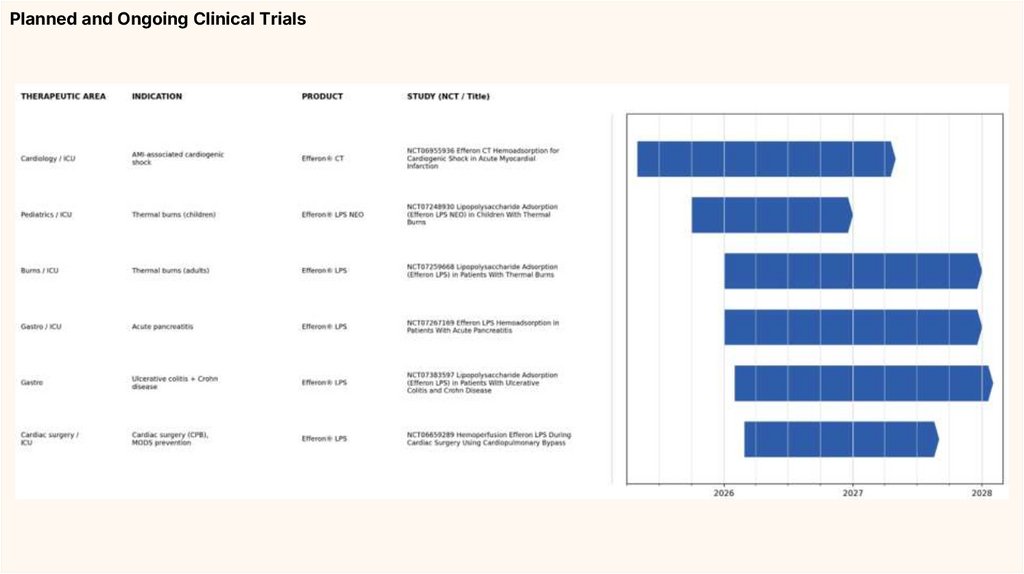
Planned and Ongoing Clinical Trials18.
Publications Listhttps://docs.google.com/spreadshe
ets/d/1gm4Hzlr5QpJHOH7vpVVWp
20QtXF5GJNh/edit?gid=13043215
76#gid=1304321576
19.
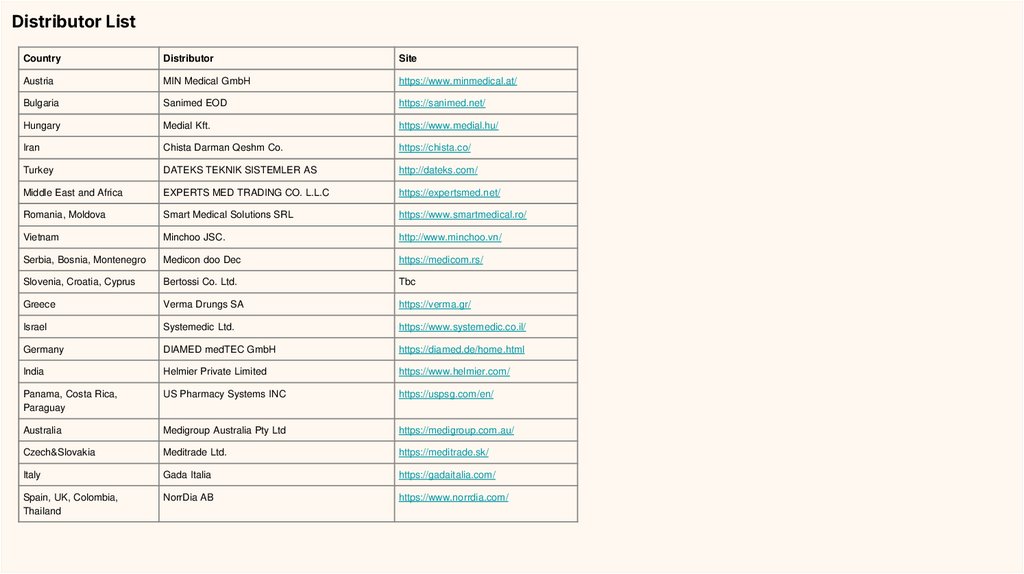
Distributor ListCountry
Distributor
Site
Austria
MIN Medical GmbH
https://www.minmedical.at/
Bulgaria
Sanimed EOD
https://sanimed.net/
Hungary
Medial Kft.
https://www.medial.hu/
Iran
Chista Darman Qeshm Co.
https://chista.co/
Turkey
DATEKS TEKNIK SISTEMLER AS
http://dateks.com/
Middle East and Africa
EXPERTS MED TRADING CO. L.L.C
https://expertsmed.net/
Romania, Moldova
Smart Medical Solutions SRL
https://www.smartmedical.ro/
Vietnam
Minchoo JSC.
http://www.minchoo.vn/
Serbia, Bosnia, Montenegro
Medicon doo Dec
https://medicom.rs/
Slovenia, Croatia, Cyprus
Bertossi Co. Ltd.
Tbc
Greece
Verma Drungs SA
https://verma.gr/
Israel
Systemedic Ltd.
https://www.systemedic.co.il/
Germany
DIAMED medTEC GmbH
https://diamed.de/home.html
India
Helmier Private Limited
https://www.helmier.com/
Panama, Costa Rica,
Paraguay
US Pharmacy Systems INC
https://uspsg.com/en/
Australia
Medigroup Australia Pty Ltd
https://medigroup.com.au/
Czech&Slovakia
Meditrade Ltd.
https://meditrade.sk/
Italy
Gada Italia
https://gadaitalia.com/
Spain, UK, Colombia,
Thailand
NorrDia AB
https://www.norrdia.com/
20.
The LASSO Study: Gold Standard Clinical ValidationMulticenter randomized controlled
trial providing robust evidence for
Efferon's multimodal
hemoadsorption technology
- Rigorous Design Excellence:
Follows international standards for
medical device validation with input
from leading sepsis researchers
(NCT04827407)
- Safety Validation: Comprehensive
safety profile across diverse patient
populations with no device-related
adverse events and excellent
biocompatibility
- Efficacy Demonstration:
Statistically significant improvements
in hemodynamics, organ function,
and inflammatory marker clearance
- Prestigious Publication: Results
published in Shock journal (2023), a
leading peer-reviewed publication in
critical care and sepsis research
Study Design
- Multicenter RCT (NCT04827407), 4
centers in Moscow, March 2021 - May
2022
- 58 patients with abdominal sepsis
and septic shock (Sepsis-3 criteria)
- Randomization 2:1 (38 Efferon LPS
vs 20 control)
- Intervention: 2 hemoperfusion
sessions ≥4h each, 24h apart, within
12h of shock onset
- Primary endpoint: Time to shock
resolution (vasopressor withdrawal)
Key Results
Survival
- 3-day survival: 87% vs 60%
(p=0.012) - significant
- 28-day mortality: 47% vs 55%
(p=0.783) - trend, not significant
Safety
- Zero device-related adverse events
- Circuit thrombosis: <2% (1/74
procedures)
Hemodynamics
- Shock resolution time: 57h vs 101h
control (sHR=2.20, p=0.029) - 44%
faster
- Shock resolved at 72h: 68% vs 45%
- Norepinephrine dose reduction:
0.74→0.16 µg/kg/min at 48h
(p=0.027)
Organ Function
- MV duration: 2.6 days vs 4.8 days
(among survivors)
- Weaning from MV: 57% vs 29%
(sHR=2.5, p=0.037)
- RRT need: decreased from
74%→33% at 72h (p<0.001)
- SOFA score: reduced from 7→3 at
72h (p=0.043 vs control)
21.
Study: Hemoadsorption in Severe PsoriasisStudy Design
- Randomized pilot trial (N=54):
Standard therapy alone vs Standard
therapy + Efferon CT
hemoadsorption in treatmentresistant severe psoriasis patients
(PASI ≥10)
- Hemoadsorption protocol: 190 min
sessions, peripheral access, citrate
anticoagulation.
Key Results
Conclusions
- Hemoadsorption group showed
superior PASI improvement at Day
90 in adjusted analysis
- Identified responder profile (43%
of cohort): age 40-50+,
comorbidities, elevated CRP/IL-6
- Responders achieved -11 PASI
points difference [95%CI 5-17] vs
control
- Safe procedure: 1 adverse event
(device clotting) in 26 patients
- Efferon CT hemoadsorption may
extend remission duration in
treatment-resistant patients
- Predictive biomarkers identified
enable patient selection for future
pivotal trials