Similar presentations:
enthalpy and enthalpy changes
1. Energetics
2. What is Energetics?
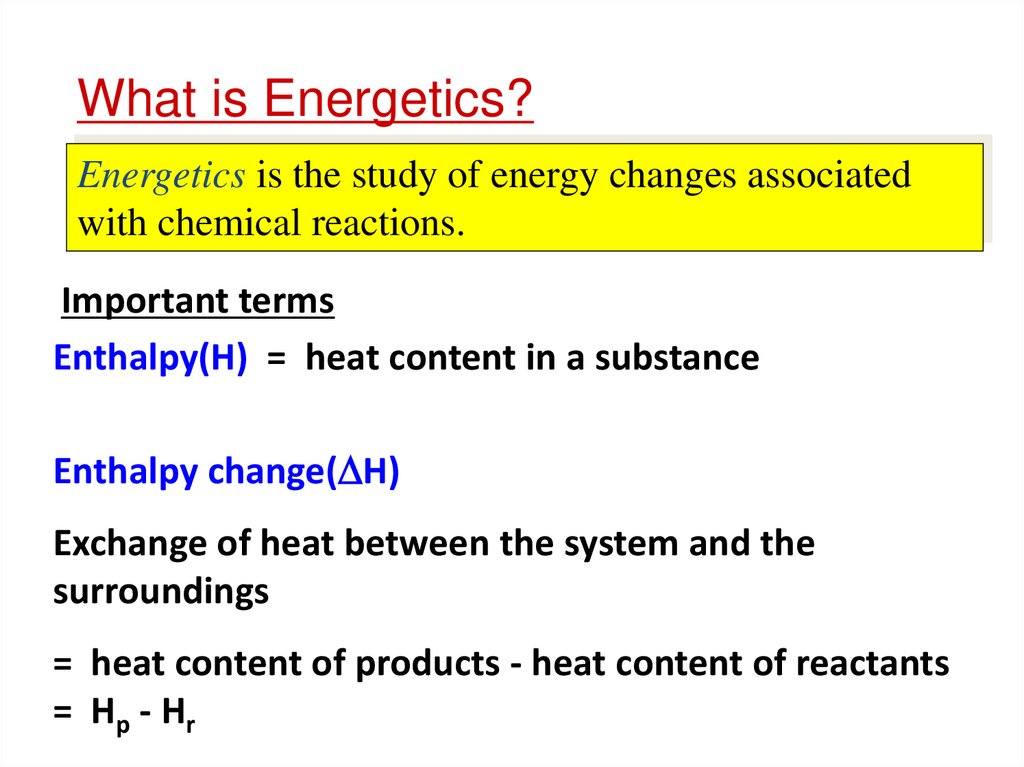
Energetics is the study of energy changes associatedwith chemical reactions.
Important terms
Enthalpy(H) = heat content in a substance
Enthalpy change( H)
Exchange of heat between the system and the
surroundings
= heat content of products - heat content of reactants
= Hp - Hr
3. Recall endothermic and exothermic reactions
What do you understand by:• Endothermic
• Exothermic
• https://www.youtube.com/watch?v=
BTDRtSGNMtM
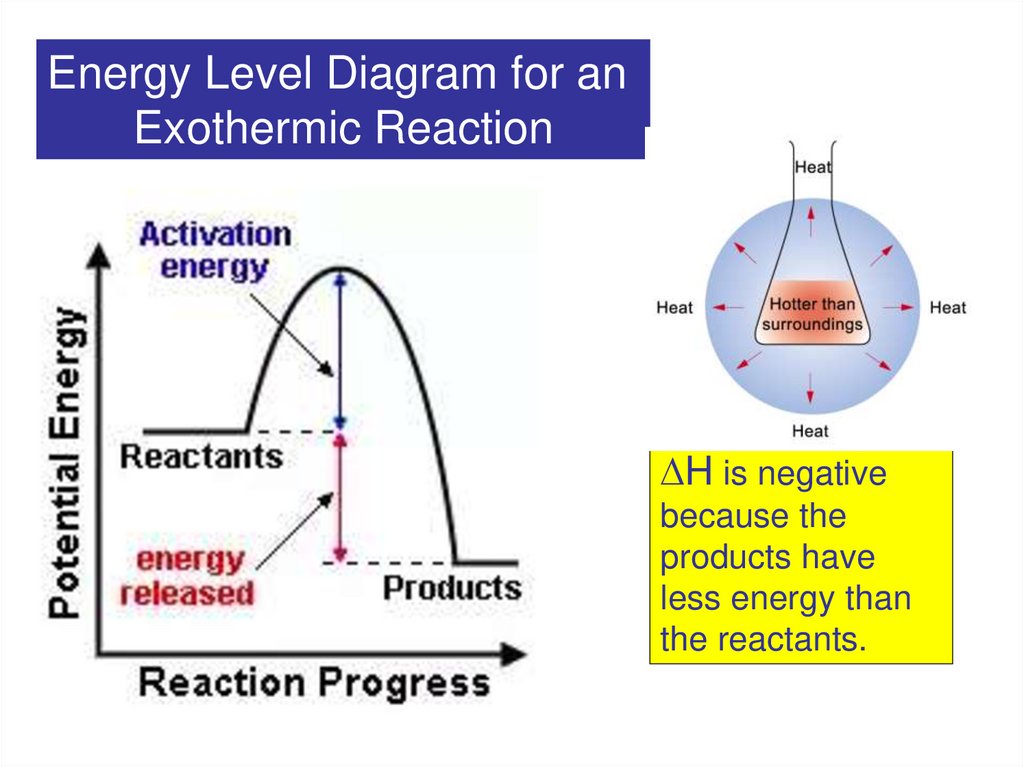
4.
Energy Level Diagram for anExothermic
Exothermic
Reaction
Reaction
2.
H is negative
because the
products have
less energy than
the reactants.
5. Exothermic reactions
• A change that gives out heat energy.• There is increase in temperature
• Potential energy (enthalpy) is converted into
heat energy.
• The products are more stable than the
reactants
• The enthalpy change is negative
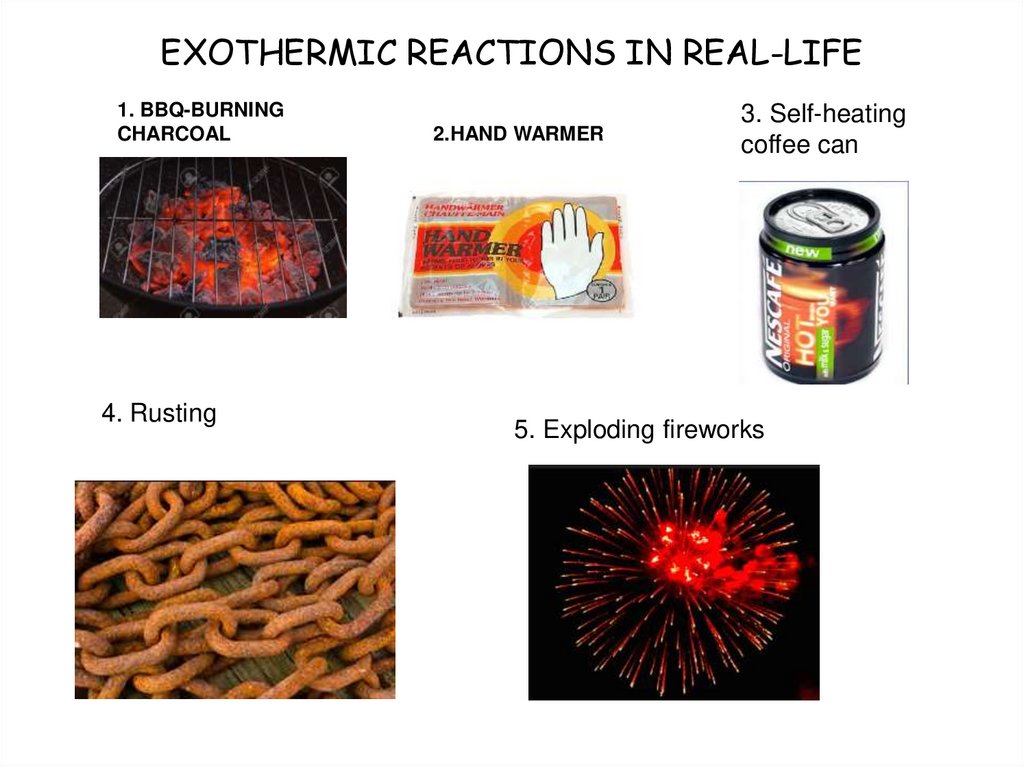
6. EXOTHERMIC REACTIONS IN REAL-LIFE
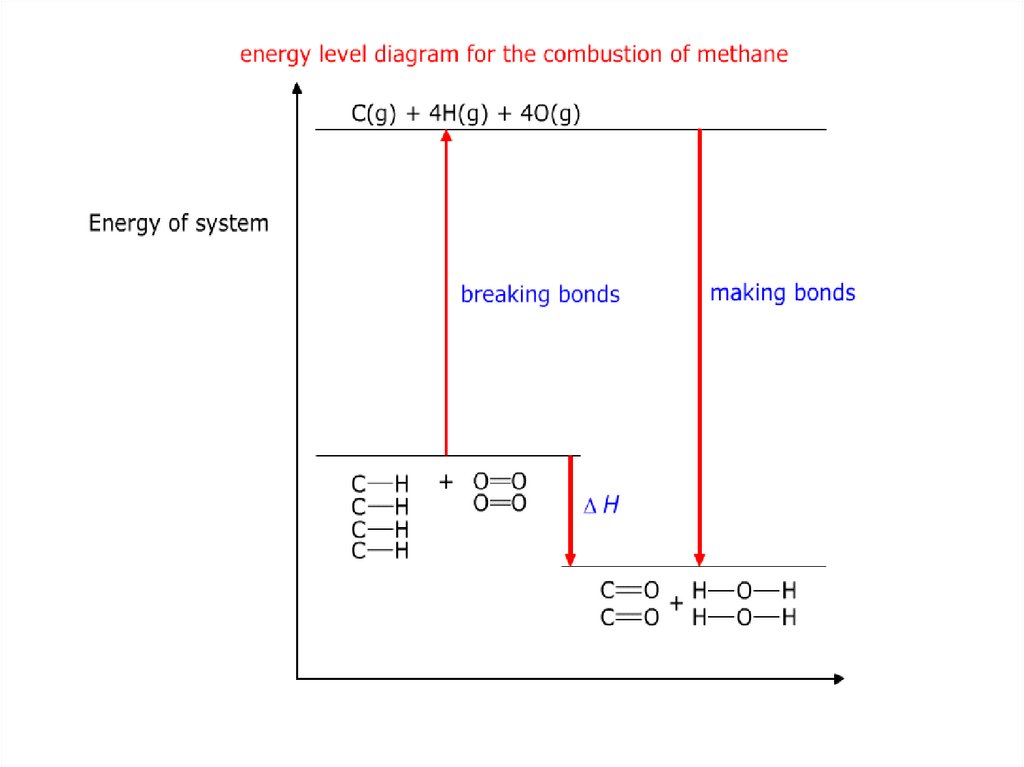
1. BBQ-BURNINGCHARCOAL
4. Rusting
2.HAND WARMER
3. Self-heating
coffee can
5. Exploding fireworks
7. Endothermic reactions
• A change that takes in heat energy from thesurrounding.
• There is decrease in temperature
• Heat energy (enthalpy) is converted into
potential energy.
• The reactants are more stable than the
products
• The enthalpy change is positive
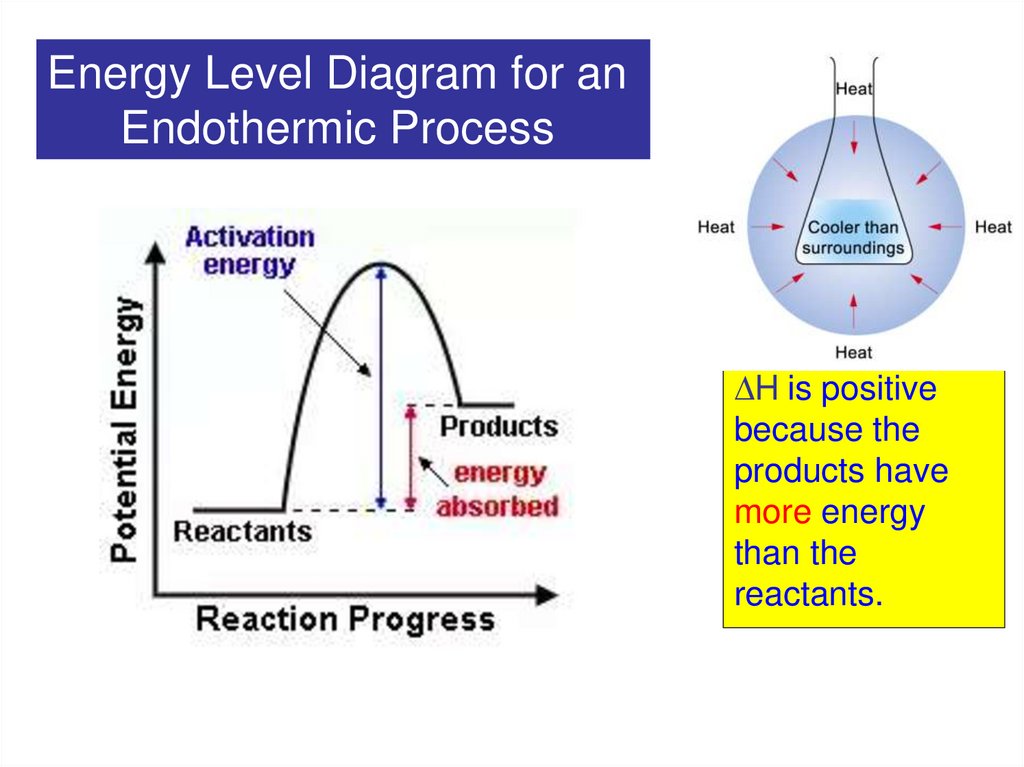
8.
Energy Level Diagram for anEndothermic Process
H is positive
because the
products have
more energy
than the
reactants.
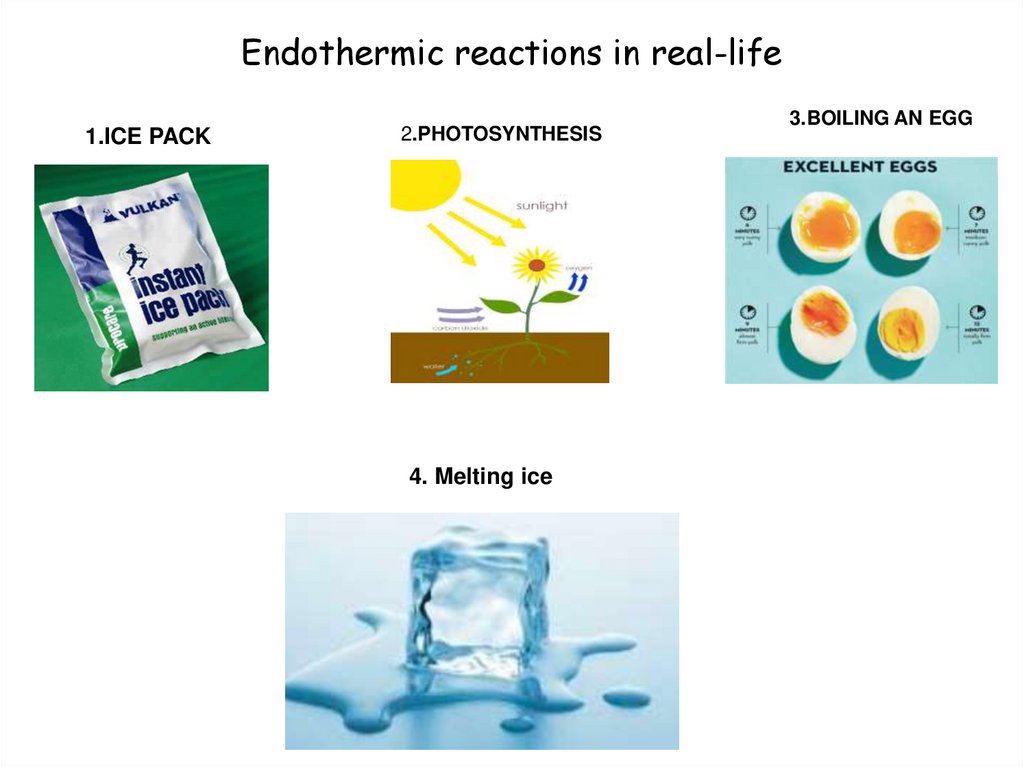
9. Endothermic reactions in real-life
1.ICE PACK2.PHOTOSYNTHESIS
4. Melting ice
3.BOILING AN EGG
10.
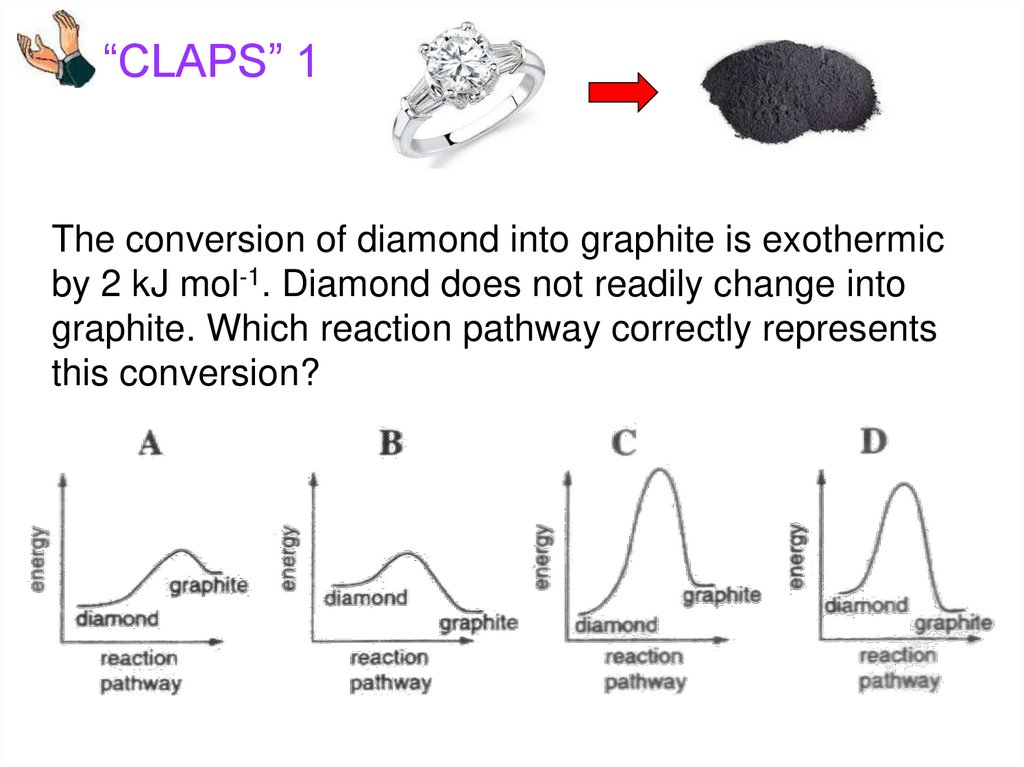
“CLAPS” 1The conversion of diamond into graphite is exothermic
by 2 kJ mol-1. Diamond does not readily change into
graphite. Which reaction pathway correctly represents
this conversion?
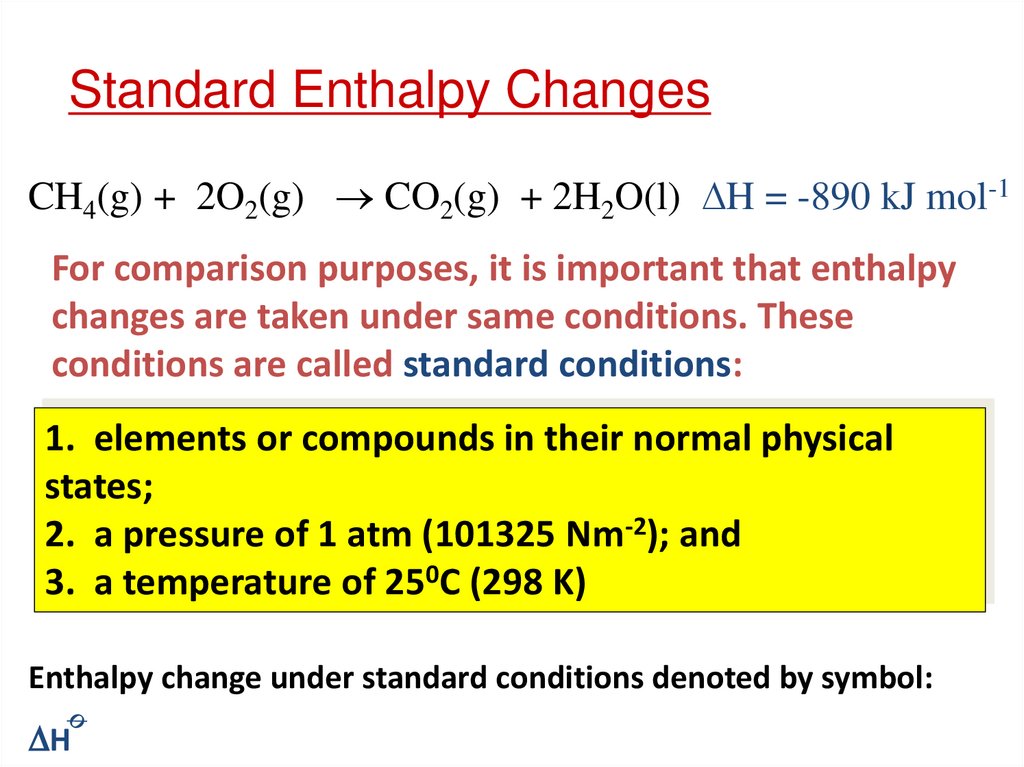
11. Standard Enthalpy Changes
CH4(g) + 2O2(g) CO2(g) + 2H2O(l) H = -890 kJ mol-1For comparison purposes, it is important that enthalpy
changes are taken under same conditions. These
conditions are called standard conditions:
1. elements or compounds in their normal physical
states;
2. a pressure of 1 atm (101325 Nm-2); and
3. a temperature of 250C (298 K)
Enthalpy change under standard conditions denoted by symbol:
H
12.
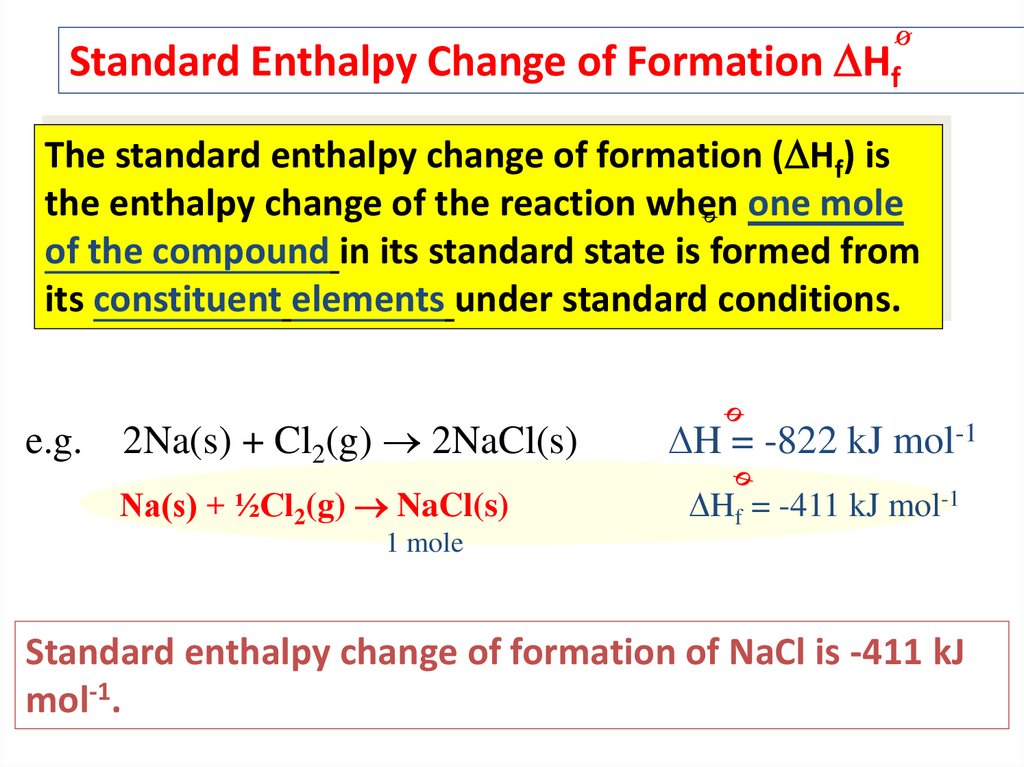
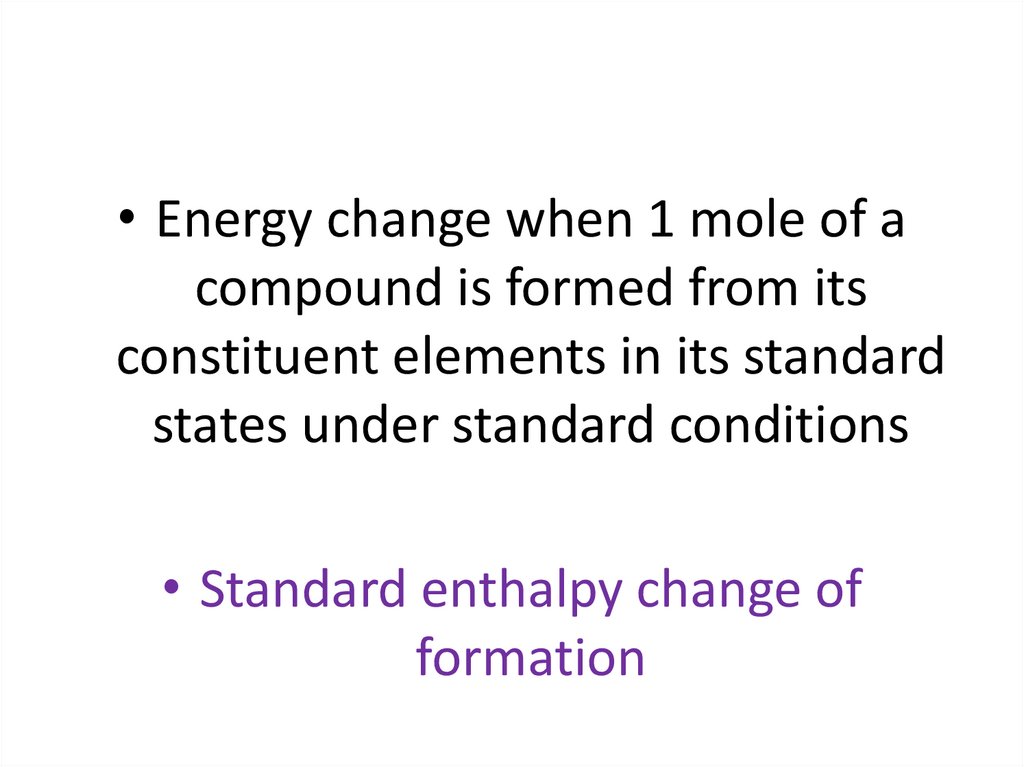
Standard Enthalpy Change of Formation HfThe standard enthalpy change of formation ( Hf) is
the enthalpy change of the reaction when one mole
of the compound in its standard state is formed from
its constituent elements under standard conditions.
e.g.
2Na(s) + Cl2(g) 2NaCl(s)
H = -822 kJ mol-1
Na(s) + ½Cl2(g) NaCl(s)
Hf = -411 kJ mol-1
1 mole
Standard enthalpy change of formation of NaCl is -411 kJ
mol-1.
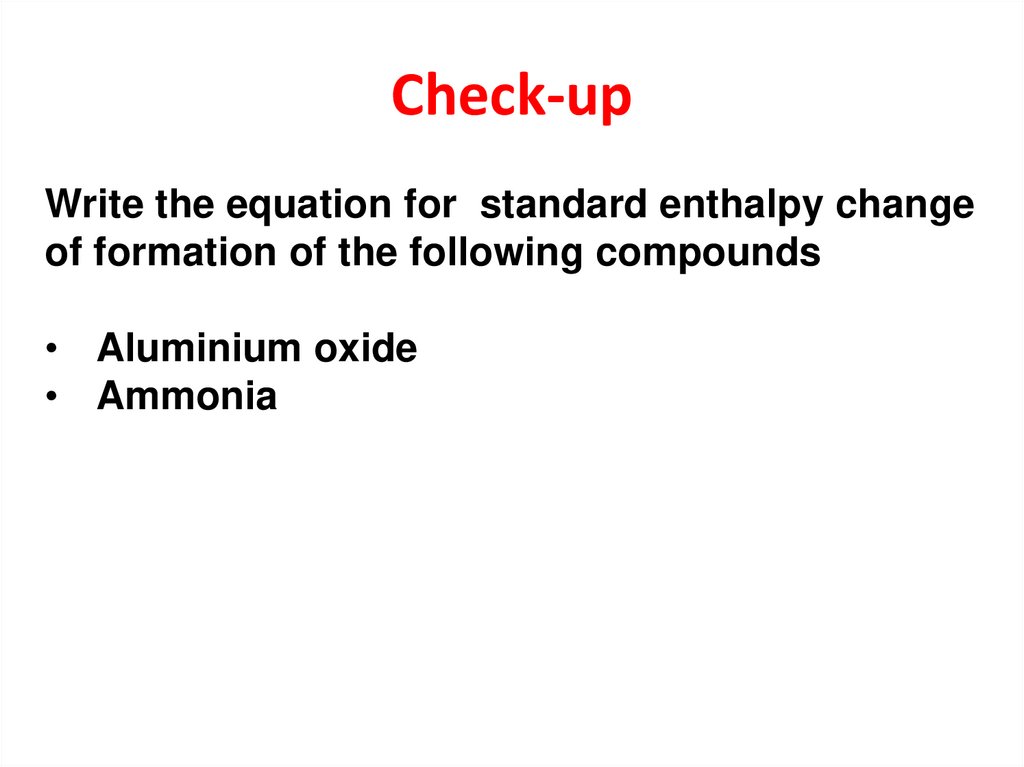
13. Check-up
Write the equation for standard enthalpy changeof formation of the following compounds
• Aluminium oxide
• Ammonia
14.
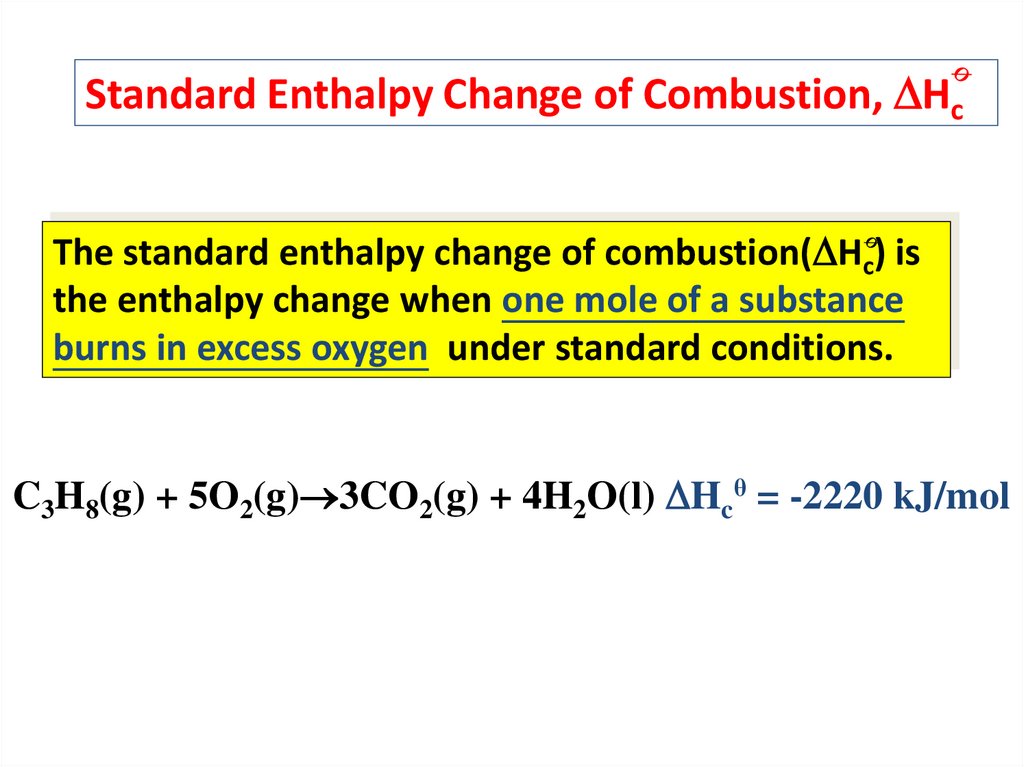
Standard Enthalpy Change of Combustion, HcThe standard enthalpy change of combustion( Hc) is
the enthalpy change when one mole of a substance
burns in excess oxygen under standard conditions.
C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(l) Hcθ = -2220 kJ/mol
15. Check-up
Write the equation for standard enthalpy changeOf combustion of the following substances
Ethane
Magnesium
16.
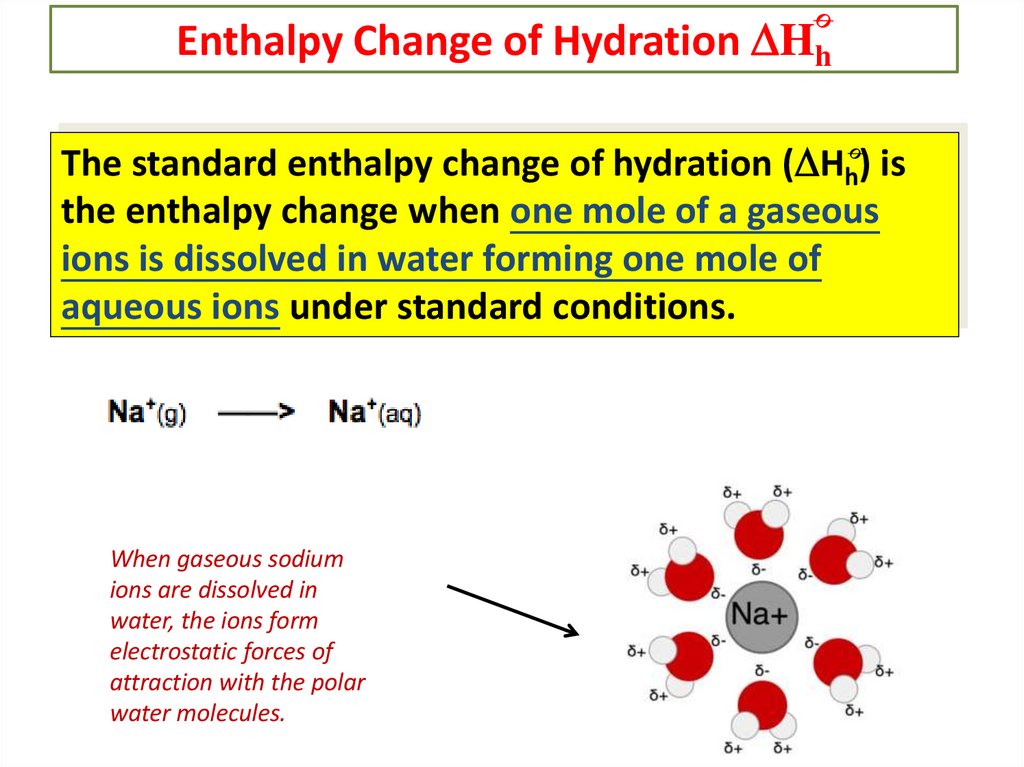
Enthalpy Change of Hydration HhThe standard enthalpy change of hydration ( Hh) is
the enthalpy change when one mole of a gaseous
ions is dissolved in water forming one mole of
aqueous ions under standard conditions.
When gaseous sodium
ions are dissolved in
water, the ions form
electrostatic forces of
attraction with the polar
water molecules.
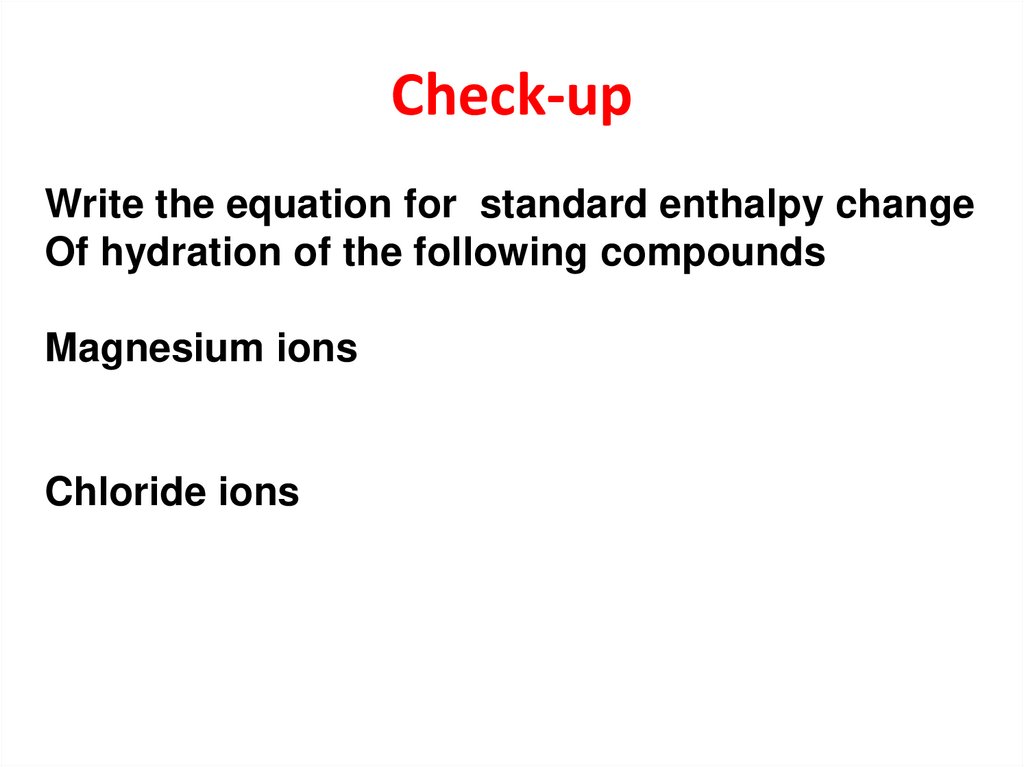
17. Check-up
Write the equation for standard enthalpy changeOf hydration of the following compounds
Magnesium ions
Chloride ions
18.
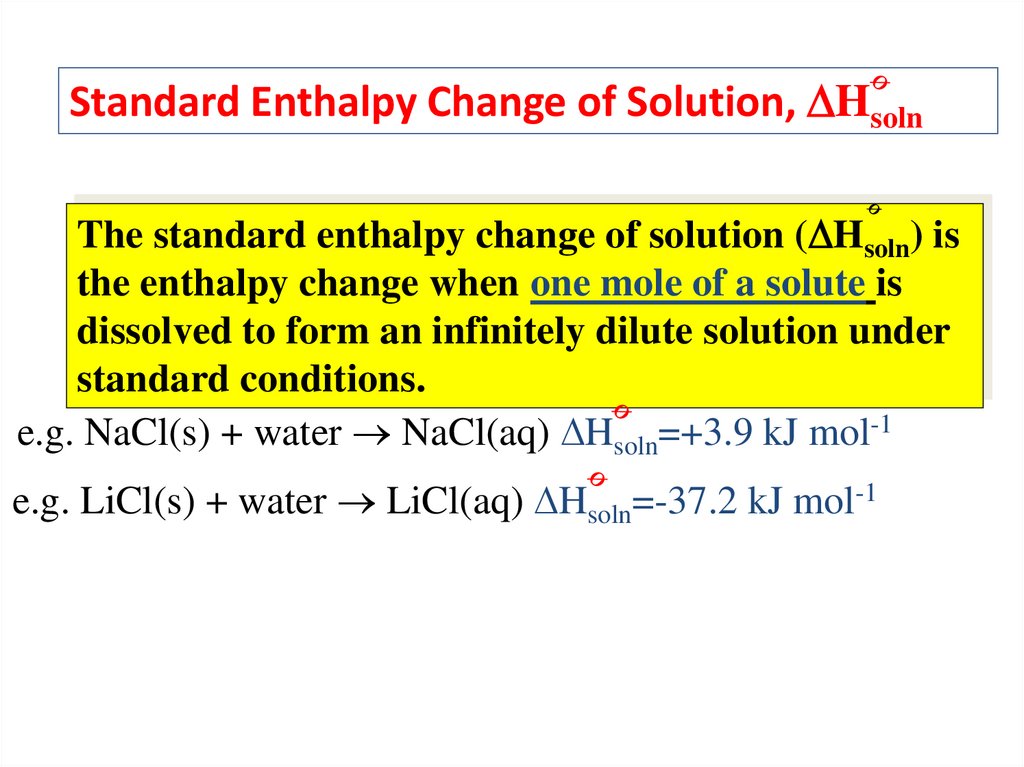
Standard Enthalpy Change of Solution, HsolnThe standard enthalpy change of solution ( Hsoln) is
the enthalpy change when one mole of a solute is
dissolved to form an infinitely dilute solution under
standard conditions.
e.g. NaCl(s) + water NaCl(aq) Hsoln=+3.9 kJ mol-1
e.g. LiCl(s) + water LiCl(aq) Hsoln=-37.2 kJ mol-1
19.
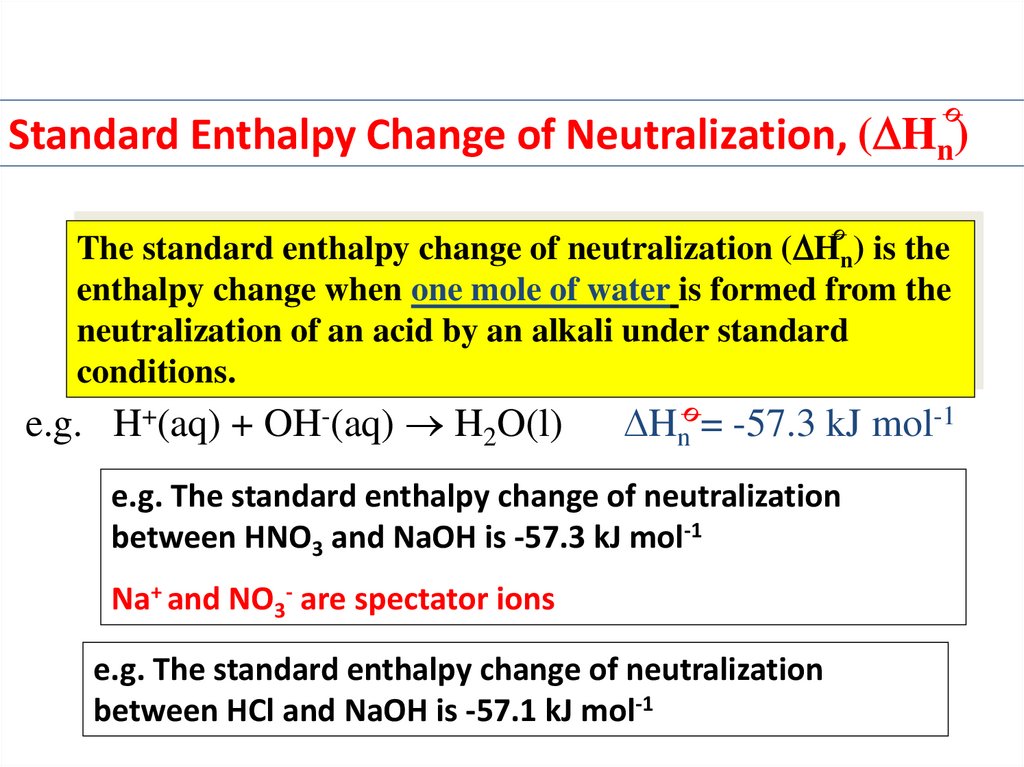
Standard Enthalpy Change of Neutralization, ( Hn)The standard enthalpy change of neutralization ( Hn) is the
enthalpy change when one mole of water is formed from the
neutralization of an acid by an alkali under standard
conditions.
e.g. H+(aq) + OH-(aq) H2O(l)
Hn = -57.3 kJ mol-1
e.g. The standard enthalpy change of neutralization
between HNO3 and NaOH is -57.3 kJ mol-1
Na+ and NO3- are spectator ions
e.g. The standard enthalpy change of neutralization
between HCl and NaOH is -57.1 kJ mol-1
20.
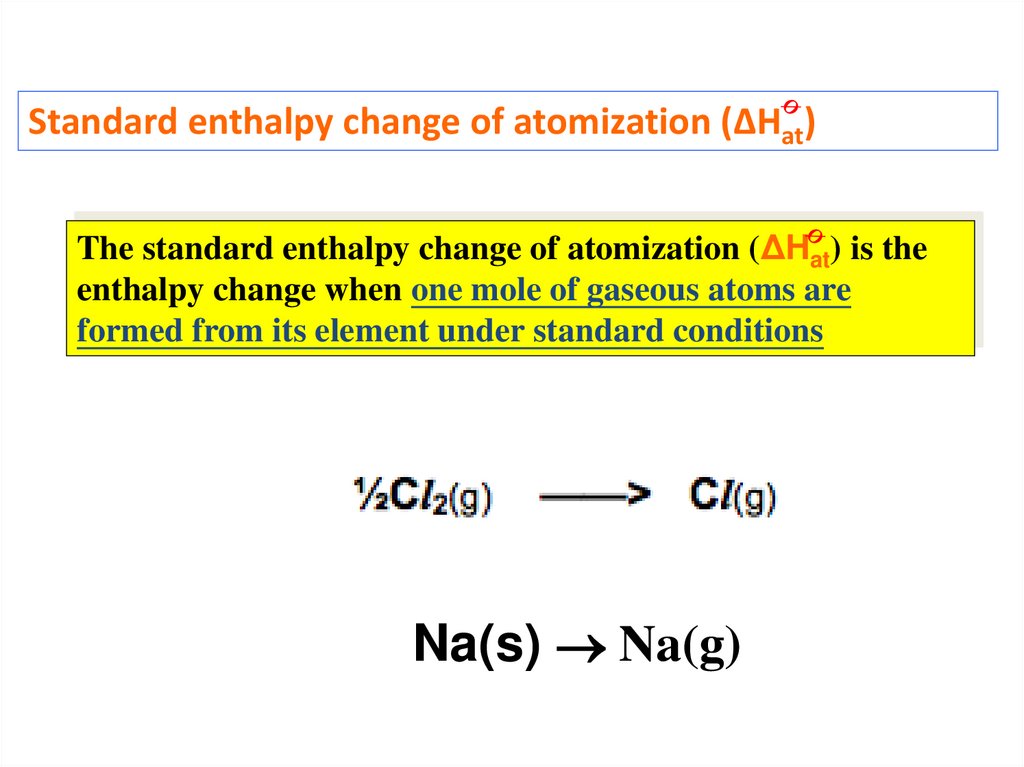
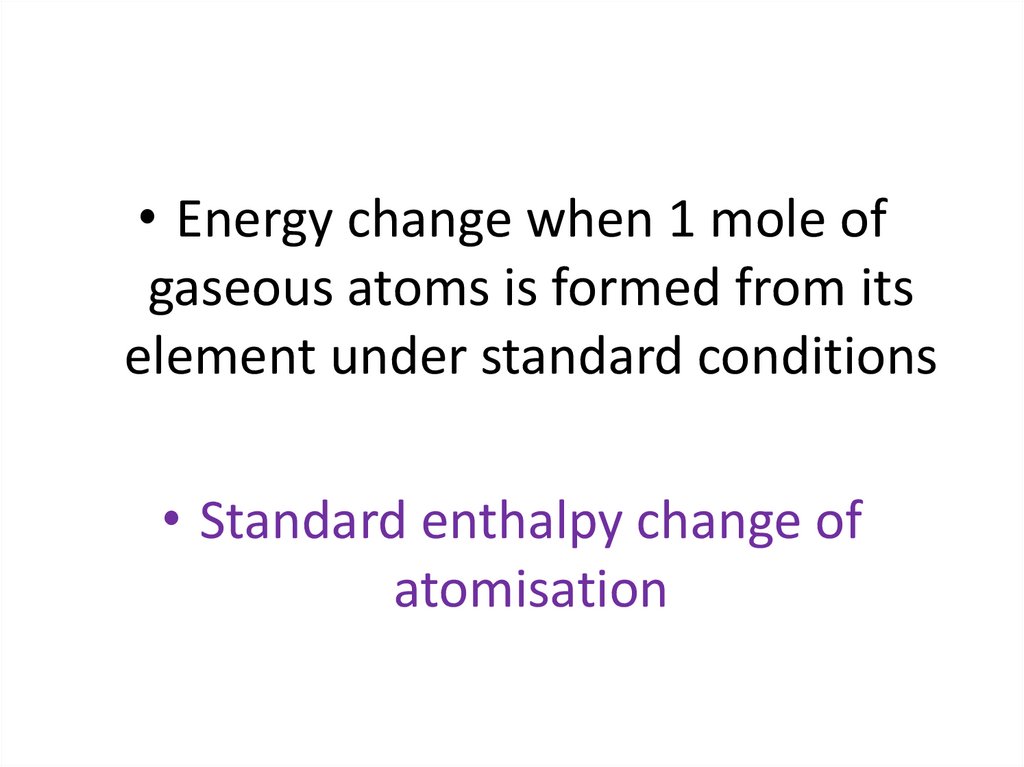
Standard enthalpy change of atomization (ΔHat)The standard enthalpy change of atomization (ΔHat) is the
enthalpy change when one mole of gaseous atoms are
formed from its element under standard conditions
Na(s) Na(g)
21.
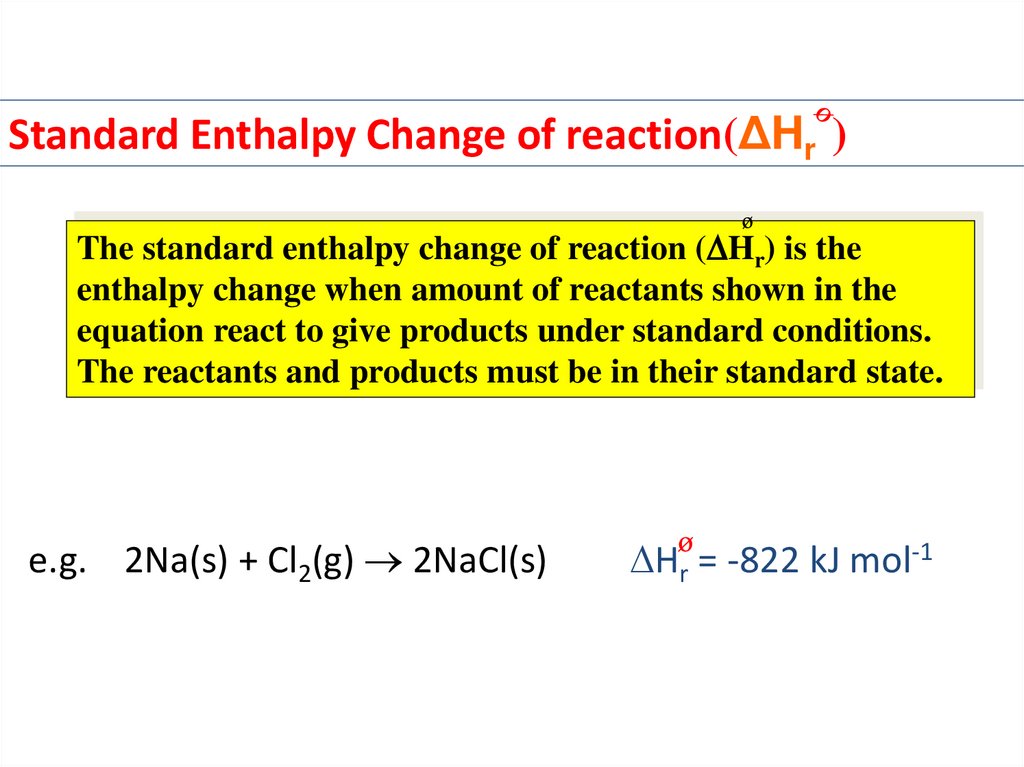
Standard Enthalpy Change of reaction(ΔHr )The standard enthalpy change of reaction ( Hr) is the
enthalpy change when amount of reactants shown in the
equation react to give products under standard conditions.
The reactants and products must be in their standard state.
e.g. 2Na(s) + Cl2(g) 2NaCl(s)
Hr = -822 kJ mol-1
22.
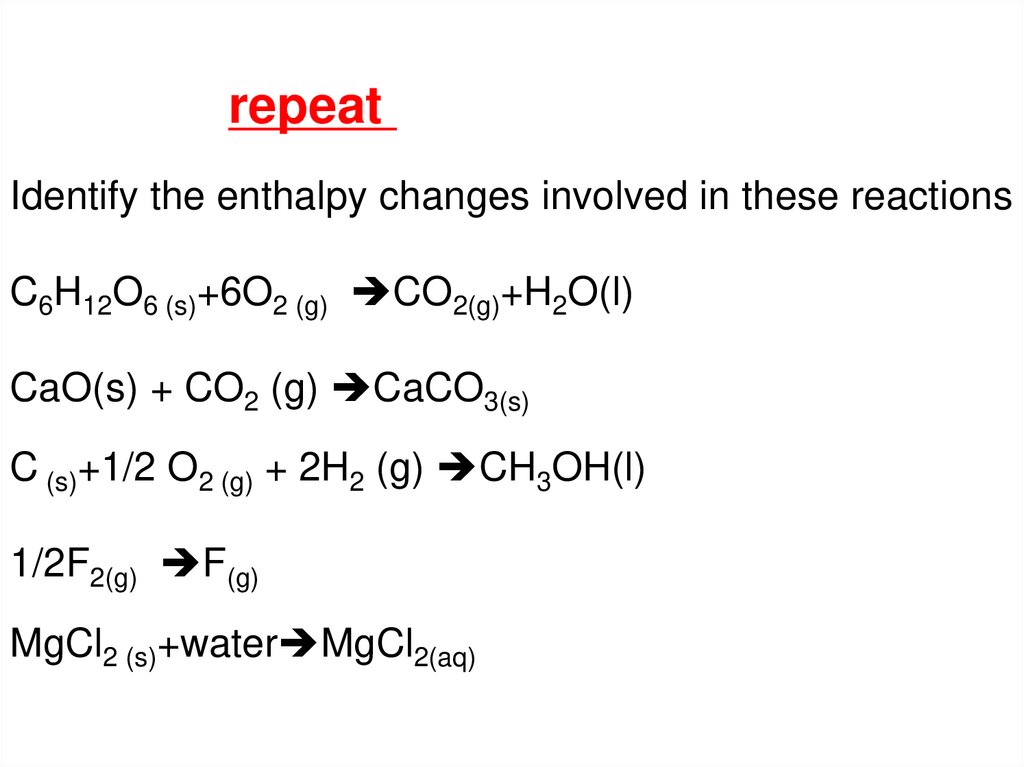
repeatIdentify the enthalpy changes involved in these reactions
C6H12O6 (s)+6O2 (g) CO2(g)+H2O(l)
CaO(s) + CO2 (g) CaCO3(s)
C (s)+1/2 O2 (g) + 2H2 (g) CH3OH(l)
1/2F2(g) F(g)
MgCl2 (s)+water MgCl2(aq)
23.
HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l)CH4(g) + 2O2(g) CO2(g) + 2H2O(l)
Na(s) + ½ Cl2(g) NaCl(s)
Na(s) Na(g)
Na+(g) Na+(aq)
24.
Energy change when 1 mole of a substanceis burnt in excess oxygen under standard
conditions
Standard enthalpy change of combustion
25.
• Energy change when 1 mole ofsolute is dissolved in a solvent to
form an infinitely dilute solution
under standard conditions
• Standard enthalpy change of solution
26.
• Energy change when 1 mole ofgaseous ion dissolves in water to
form 1 mole of hydrated ion
• Standard enthalpy change of
hydration
27.
• Energy change when 1 mole of acompound is formed from its
constituent elements in its standard
states under standard conditions
• Standard enthalpy change of
formation
28.
• Energy change when 1 mole of wateris formed by the reaction of an acid
with an alkali under standard
conditions
• Standard enthalpy change of
neutralisation
29.
• Energy change when 1 mole ofgaseous atoms is formed from its
element under standard conditions
• Standard enthalpy change of
atomisation
30. Bond energy
• It is the energy required tobreak 1 mole of gaseous
covalent bond to form
gaseous atoms
31.
KEY STATEMENT!Breaking bonds is endothermic (energy in)
Making bonds is exothermic (energy out)
32.
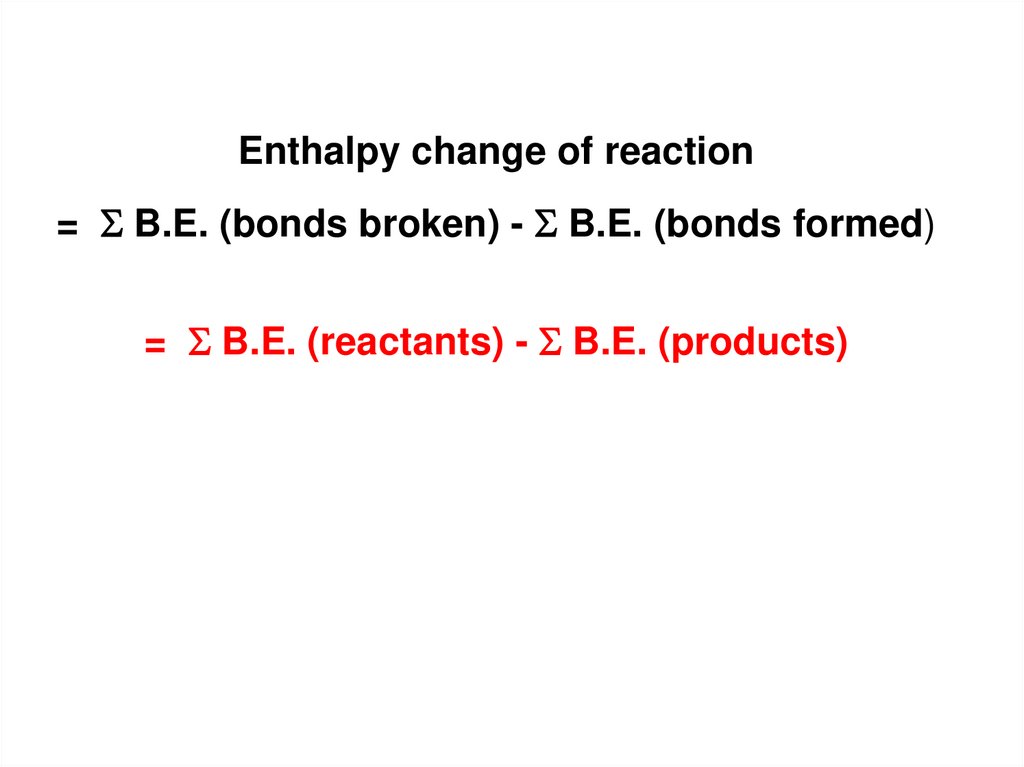
Enthalpy change of reaction= B.E. (bonds broken) - B.E. (bonds formed)
= B.E. (reactants) - B.E. (products)
33.
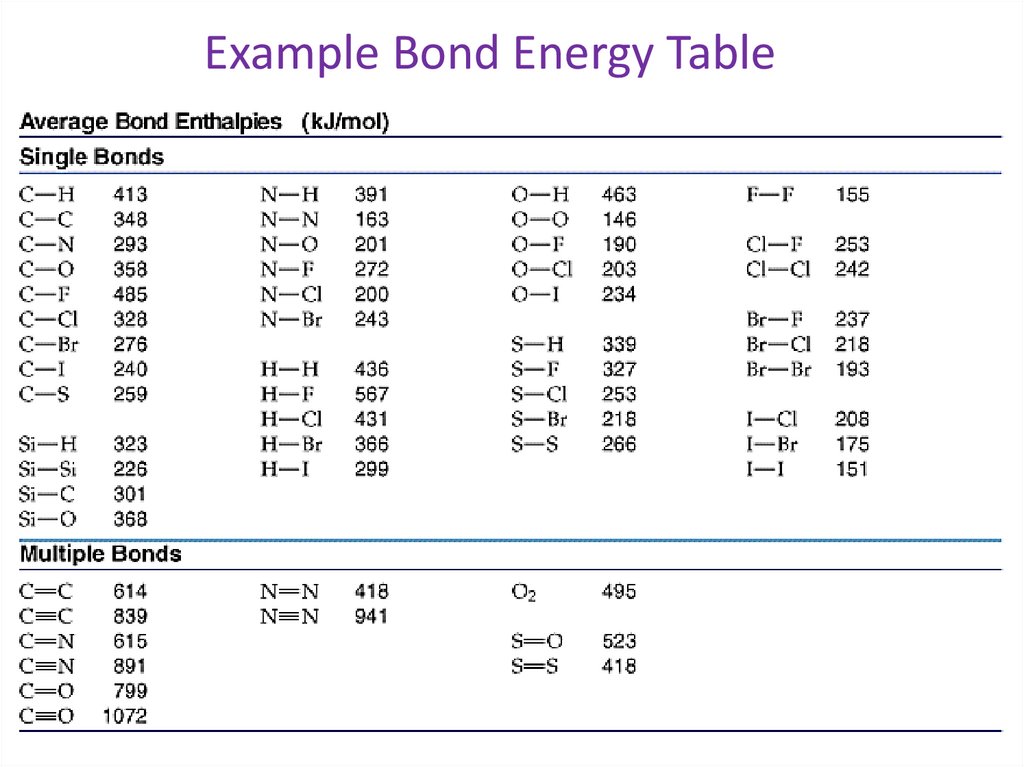
Example Bond Energy Table34.
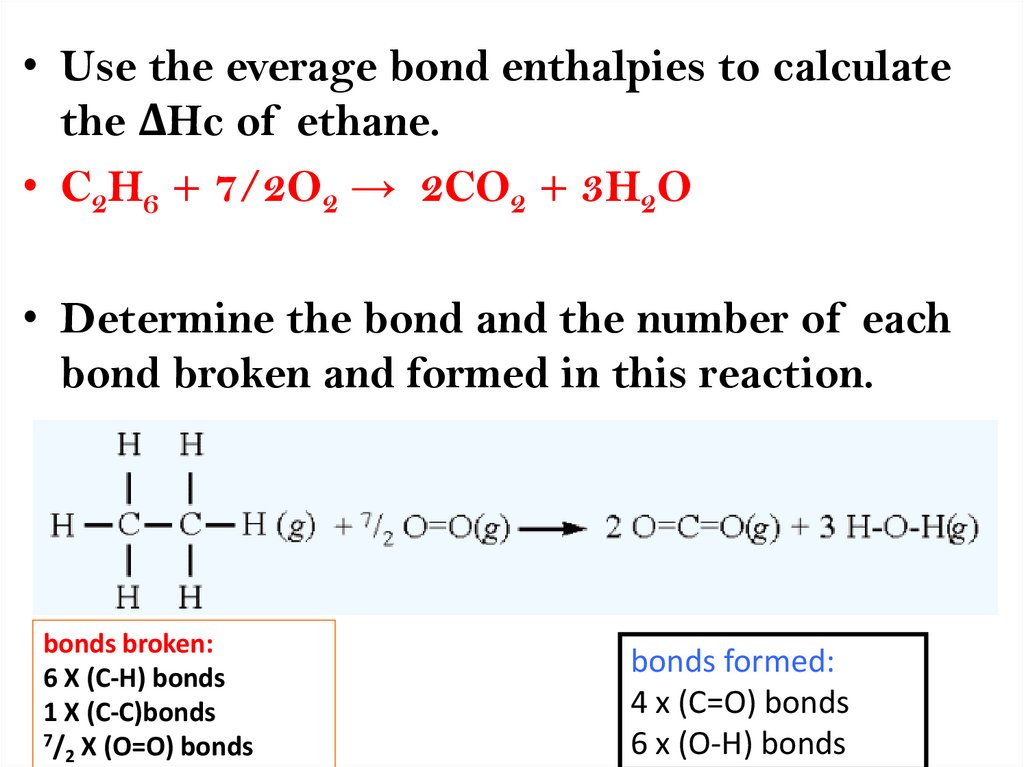
• Use the everage bond enthalpies to calculatethe ΔHc of ethane.
• C2H6 + 7/2O2 → 2CO2 + 3H2O
• Determine the bond and the number of each
bond broken and formed in this reaction.
bonds broken:
6 X (C-H) bonds
1 X (C-C)bonds
7/ X (O=O) bonds
2
bonds formed:
4 x (C=O) bonds
6 x (O-H) bonds
35.
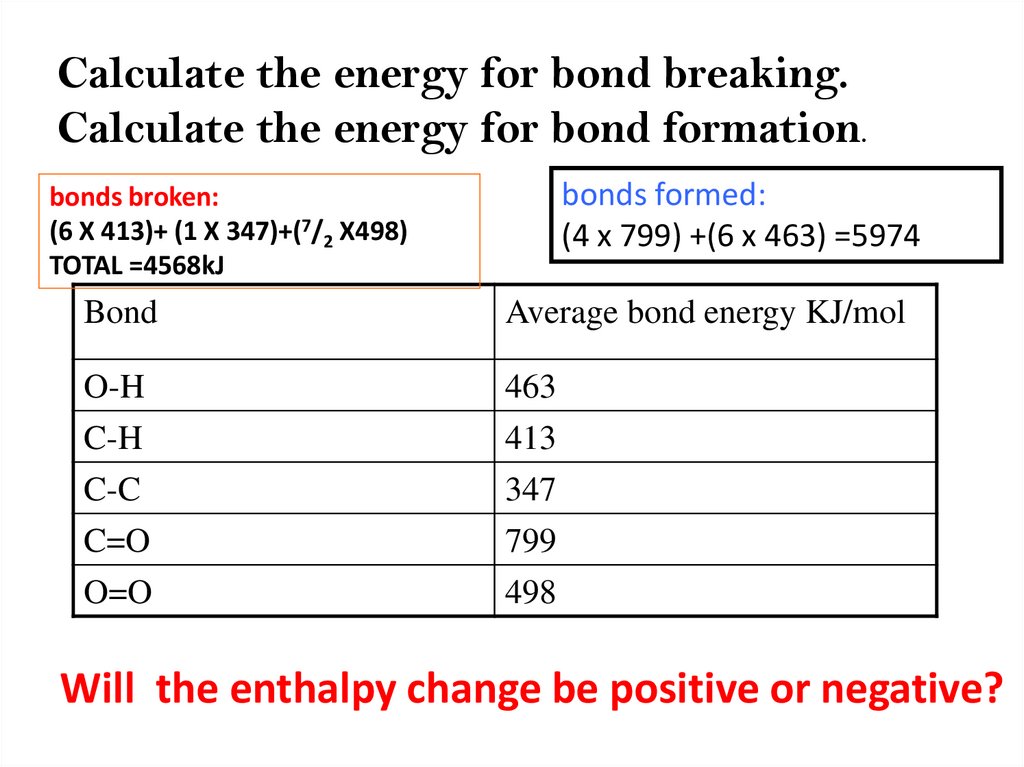
Calculate the energy for bond breaking.Calculate the energy for bond formation.
bonds formed:
(4 x 799) +(6 x 463) =5974
bonds broken:
(6 X 413)+ (1 X 347)+(7/2 X498)
TOTAL =4568kJ
Bond
Average bond energy KJ/mol
O-H
C-H
C-C
C=O
O=O
463
413
347
799
498
Will the enthalpy change be positive or negative?
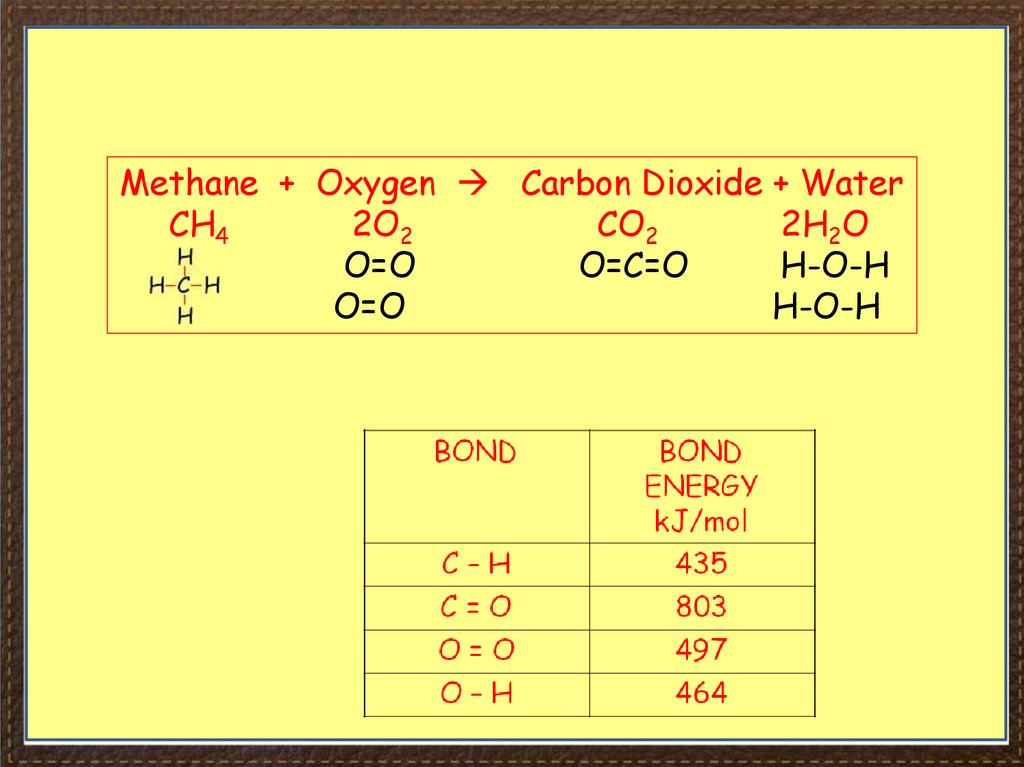
36.
Methane + Oxygen Carbon Dioxide + WaterCH4
2O2
CO2
2H2O
O=O
O=C=O
H-O-H
O=O
H-O-H
37.
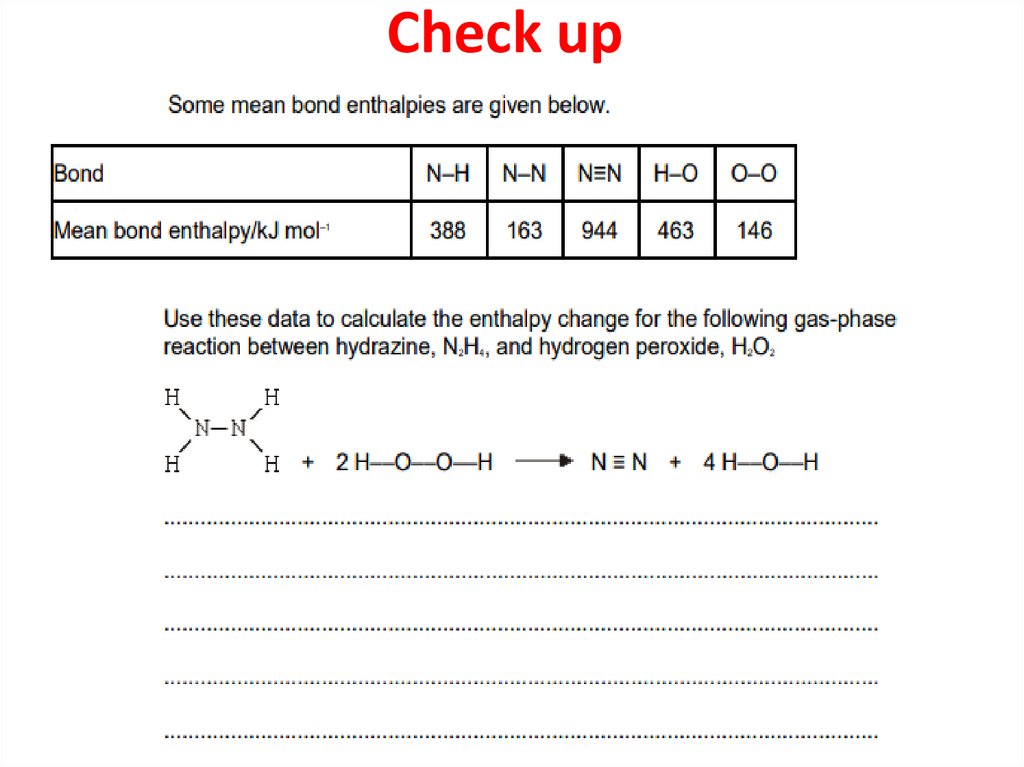
38. Check up
39.
40.
© www.chemsheets.co.ukAS1048
30-Jun-2015
41.
q = m c ∆T© www.chemsheets.co.uk
AS1048
30-Jun-2015
42.
q∆H =
moles in reaction
© www.chemsheets.co.uk
AS1048
30-Jun-2015
43.
© www.chemsheets.co.ukAS1048
30-Jun-2015
44.
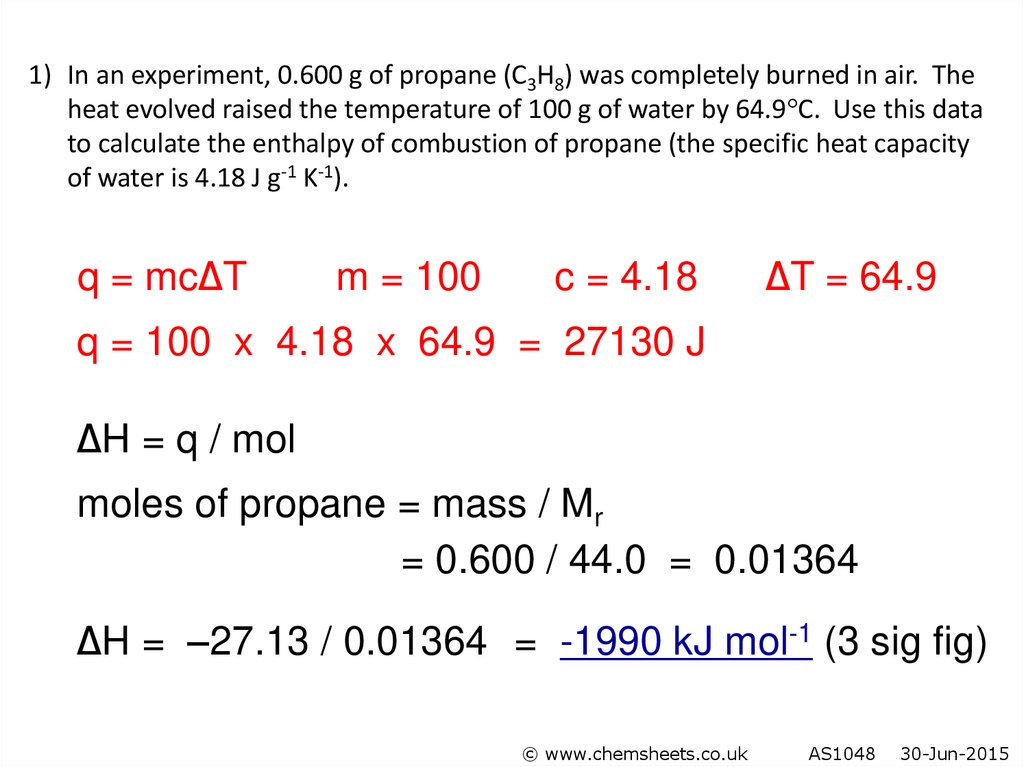
1) In an experiment, 0.600 g of propane (C3H8) was completely burned in air. Theheat evolved raised the temperature of 100 g of water by 64.9 C. Use this data
to calculate the enthalpy of combustion of propane (the specific heat capacity
of water is 4.18 J g-1 K-1).
q = mc∆T
m = 100
c = 4.18
∆T = 64.9
q = 100 x 4.18 x 64.9 = 27130 J
∆H = q / mol
moles of propane = mass / Mr
= 0.600 / 44.0 = 0.01364
∆H = –27.13 / 0.01364 = -1990 kJ mol-1 (3 sig fig)
© www.chemsheets.co.uk
AS1048
30-Jun-2015
45.
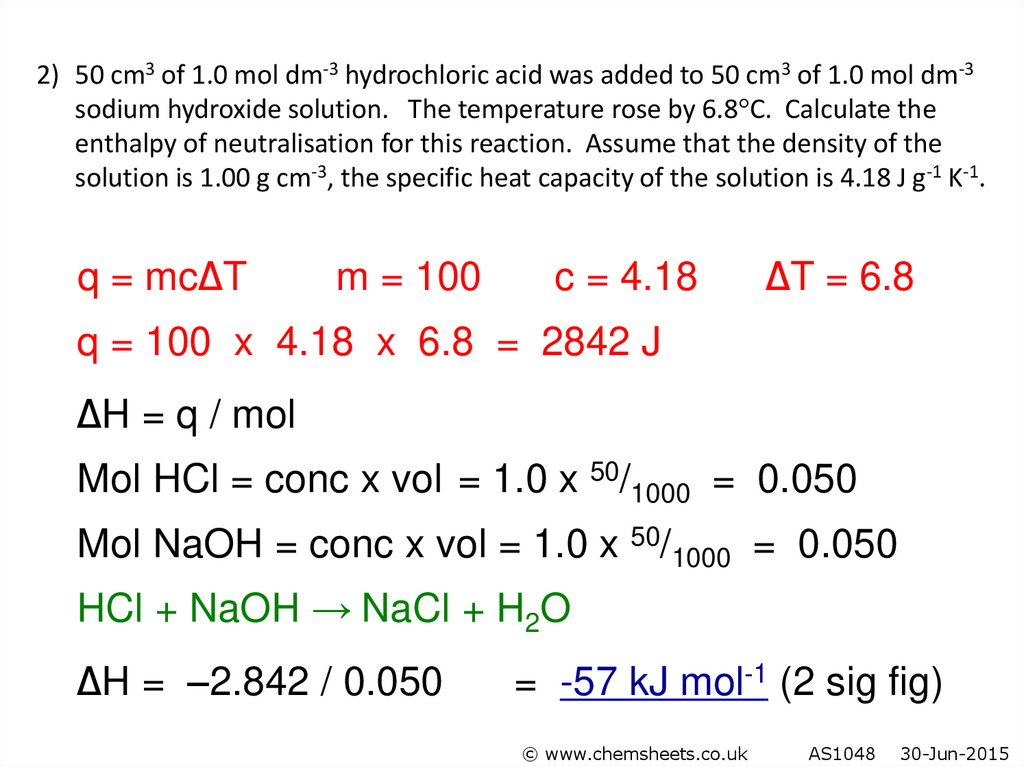
2) 50 cm3 of 1.0 mol dm-3 hydrochloric acid was added to 50 cm3 of 1.0 mol dm-3sodium hydroxide solution. The temperature rose by 6.8 C. Calculate the
enthalpy of neutralisation for this reaction. Assume that the density of the
solution is 1.00 g cm-3, the specific heat capacity of the solution is 4.18 J g-1 K-1.
q = mc∆T
m = 100
c = 4.18
∆T = 6.8
q = 100 x 4.18 x 6.8 = 2842 J
∆H = q / mol
Mol HCl = conc x vol = 1.0 x 50/1000 = 0.050
Mol NaOH = conc x vol = 1.0 x 50/1000 = 0.050
HCl + NaOH → NaCl + H2O
∆H = –2.842 / 0.050
= -57 kJ mol-1 (2 sig fig)
© www.chemsheets.co.uk
AS1048
30-Jun-2015
46.
3) 100 cm3 of 0.200 mol dm-3 copper sulphate solution was put in a calorimeter and 2.00 gof magnesium powder added. The temperature of the solution rose by 25.1 C. Work
out which reagent was in excess and then calculate the enthalpy change for the
reaction. Assume that the density of the solution is 1.00 g cm-3, the specific heat
capacity of the solution is 4.18 J g-1 K-1. Ignore the heat capacity of the metals.
q = mc∆T
m = 100
c = 4.18
∆T = 25.1
q = 100 x 4.18 x 25.1 = 10490 J
∆H = q / mol
Mol CuSO4 = conc x vol = 0.200 x 100/1000 = 0.020
Mol Mg = mass / Mr
= 2.00 / 24.3 = 0.0823 XS
CuSO4 + Mg → MgSO4 + Cu
∆H = –10.49 / 0.020
= -525 kJ mol-1 (3 sig fig)
© www.chemsheets.co.uk
AS1048
30-Jun-2015
47.
1) In an experiment, 1.00 g of propanone (CH3COCH3) was completely burned inair. The heat evolved raised the temperature of 150 g of water from 18.8 C to
64.3 C. Use this data to calculate the enthalpy of combustion of propanone
(the specific heat capacity of water is 4.18 J g-1 K-1).
-1650 kJ mol-1 (3 sig fig)
© www.chemsheets.co.uk
AS1048
30-Jun-2015
48.
6) 50 cm3 of 1.0 mol dm-3 nitric acid was added to 20 cm3 of 1.0 mol dm-3 bariumhydroxide solution. The temperature rose by 7.9 C. Calculate the enthalpy of
neutralisation for this reaction (per mole of nitric acid reacting). Assume that
the density of the solution is 1.00 g cm-3, the specific heat capacity of the
solution is 4.18 J g-1 K-1.
-57.8 kJ mol-1 (3 sig fig)
© www.chemsheets.co.uk
AS1048
30-Jun-2015
49.
7) 25 cm3 of 1.00 mol dm-3 copper sulphate solution was put in a calorimeter and 6.0 g ofzinc powder added. The temperature of the solution rose by 50.6 C. Work out which
reagent was in excess and then calculate the enthalpy change for the reaction. Assume
that the density of the solution is 1.00 g cm-3, the specific heat capacity of the solution
is 4.18 J g-1 K-1. Ignore the heat capacity of the metals.
-212 kJ mol-1 (3 sig fig)
© www.chemsheets.co.uk
AS1048
30-Jun-2015
50.
HESS’S LAW states that:The total enthalpy change of a
reaction is independent of the route
taken provided the initial and final
states of the reactants and products
remain the same
51. Enthalpy change of reaction using combustion data
The enthalpy changes of combustion of reactants andproducts is a useful way to complete the cycle in a
way that can be experimentally determined.
Reactants
Products
Combustion products
ΔHr = Σ ΔHc(reactants) – Σ ΔHc(products)
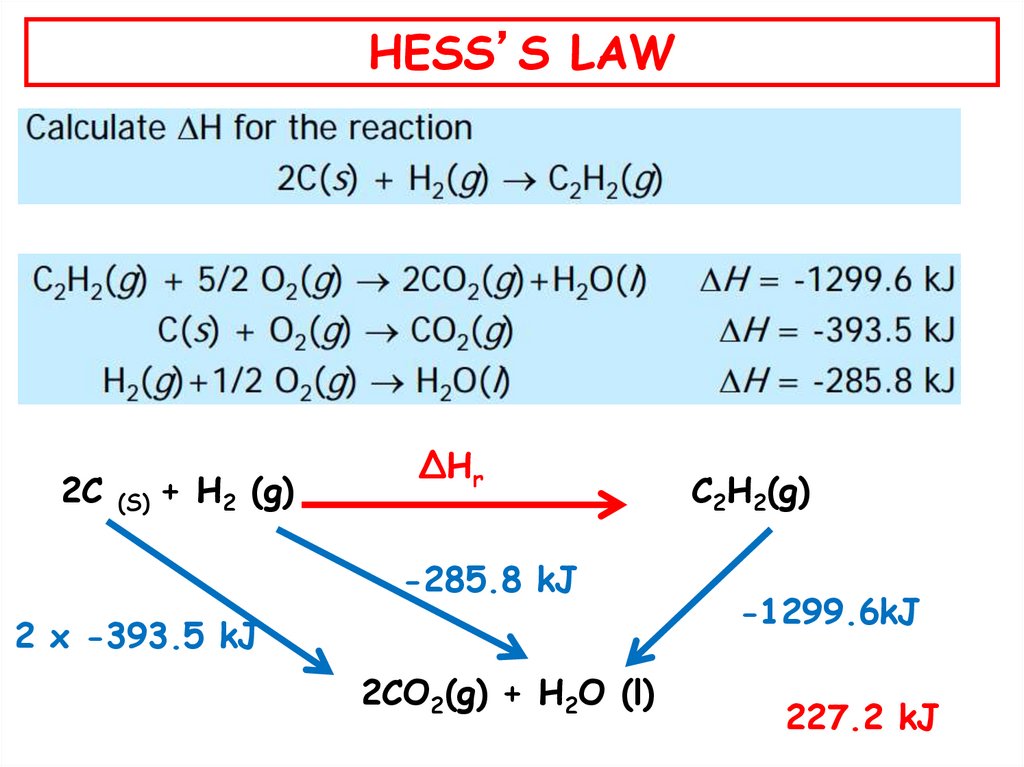
52.
HESS’S LAW2C (S) + H2 (g)
ΔHr
-285.8 kJ
2 x -393.5 kJ
2CO2(g) + H2O (l)
C2H2(g)
-1299.6kJ
227.2 kJ
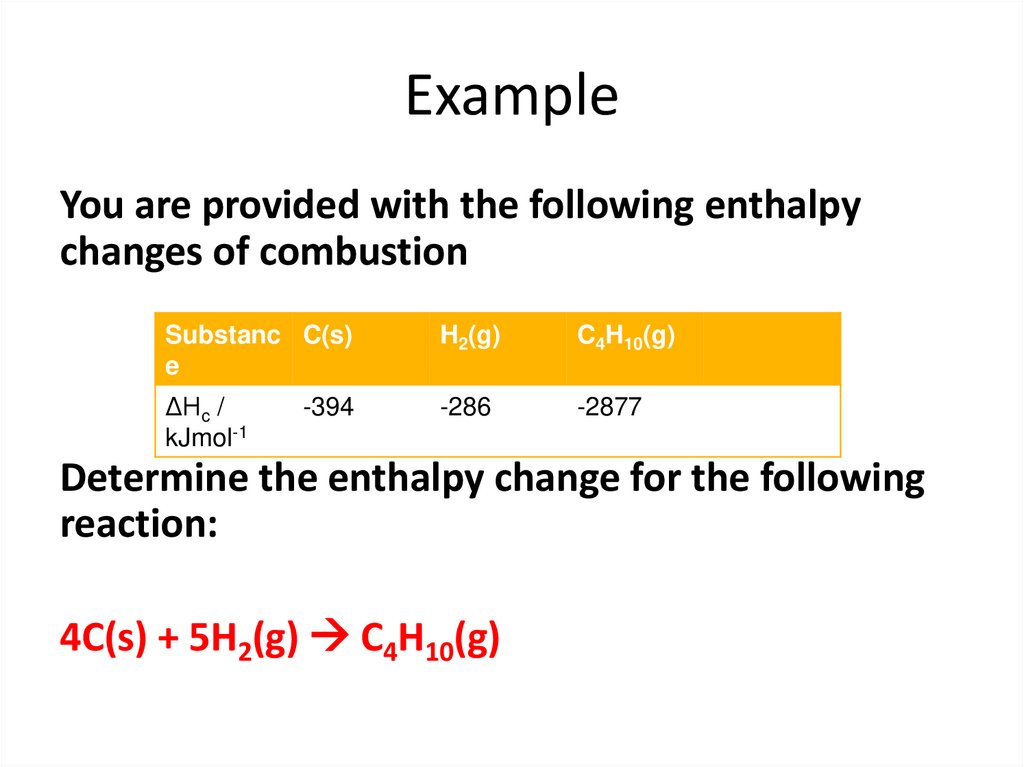
53. Example
You are provided with the following enthalpychanges of combustion
Substanc C(s)
e
H2(g)
C4H10(g)
ΔHc /
kJmol-1
-286
-2877
-394
Determine the enthalpy change for the following
reaction:
4C(s) + 5H2(g) C4H10(g)
54.
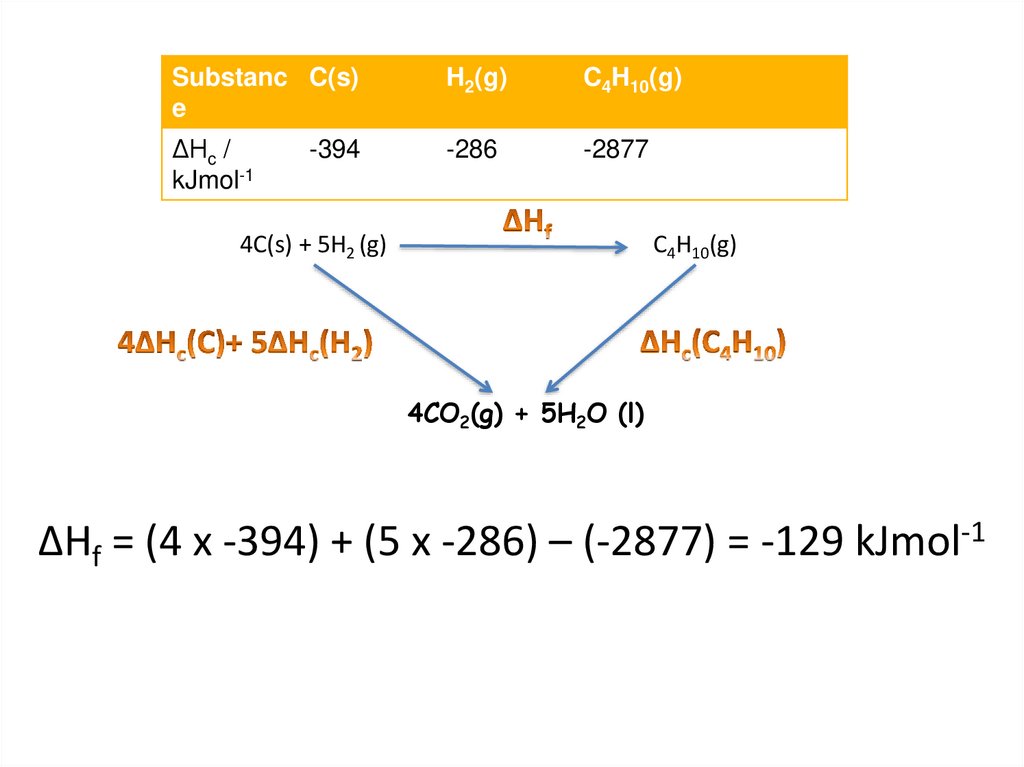
Substanc C(s)e
H2(g)
C4H10(g)
ΔHc /
kJmol-1
-286
-2877
-394
4C(s) + 5H2 (g)
C4H10(g)
4CO2(g) + 5H2O (l)
ΔHf = (4 x -394) + (5 x -286) – (-2877) = -129 kJmol-1
55.
Calculate the enthalpy of formation of pentane,C5H12(l), given the following enthalpies of
combustion.
∆H oc : H2(g) -286; C(s) -393; C5H12(l) -3509 kJ mol-1
-172kJ/mol
56.
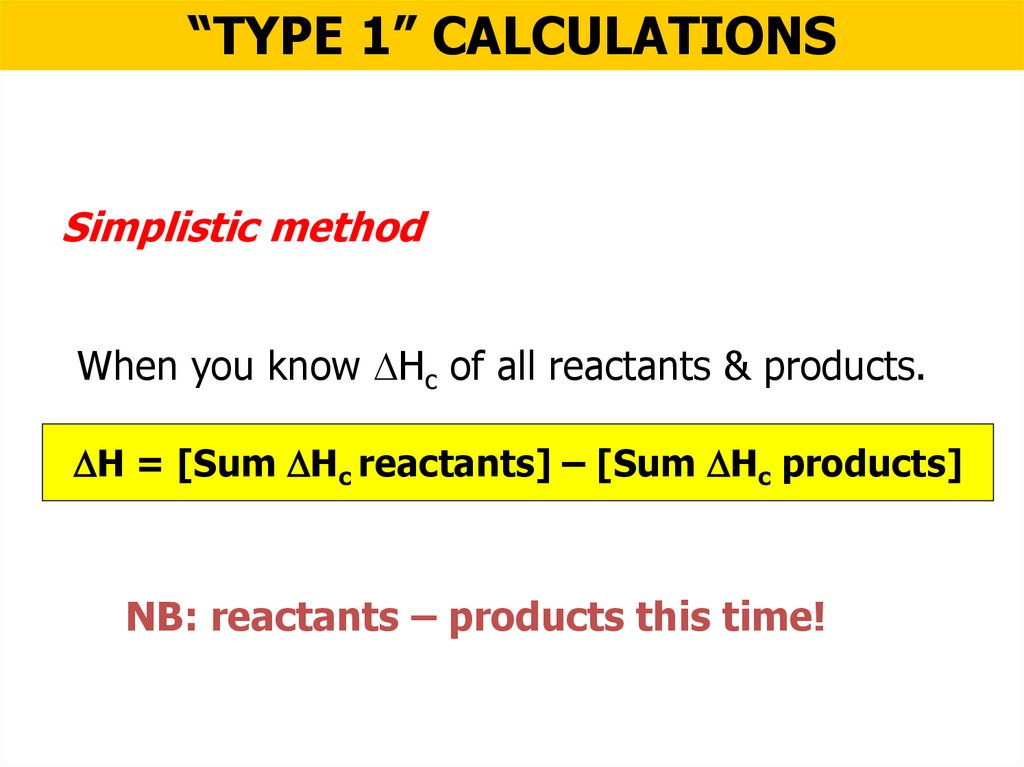
“TYPE 1” CALCULATIONSSimplistic method
When you know Hc of all reactants & products.
H = [Sum Hc reactants] – [Sum Hc products]
NB: reactants – products this time!
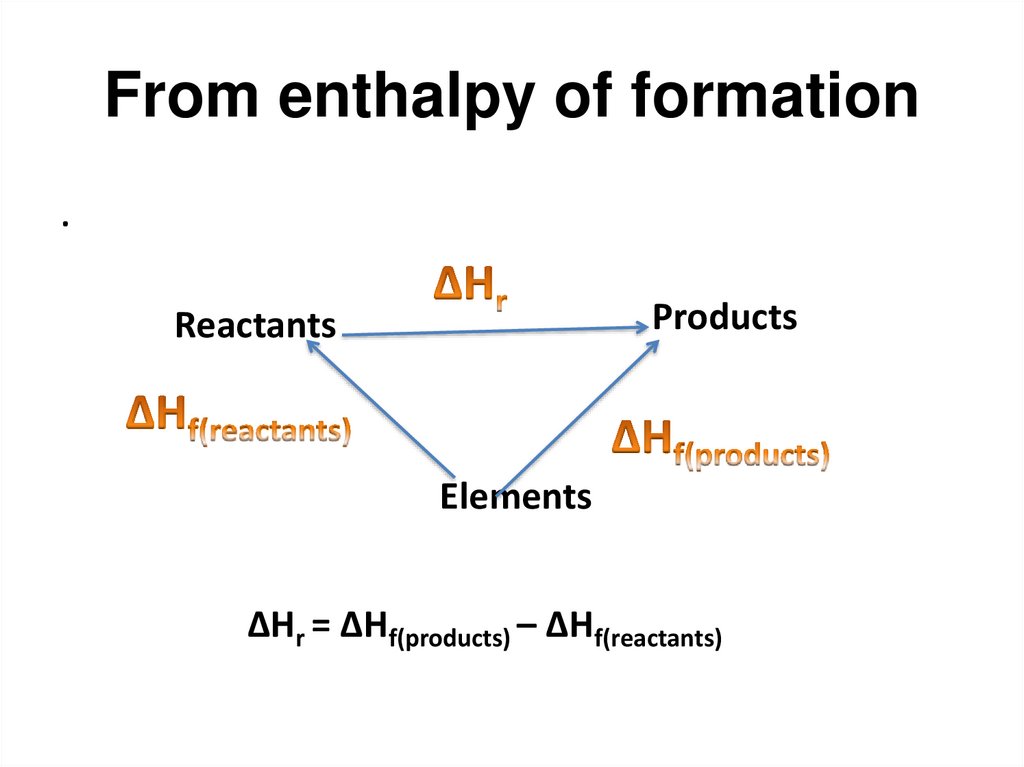
57. From enthalpy of formation
.Products
Reactants
Elements
ΔHr = ΔHf(products) – ΔHf(reactants)
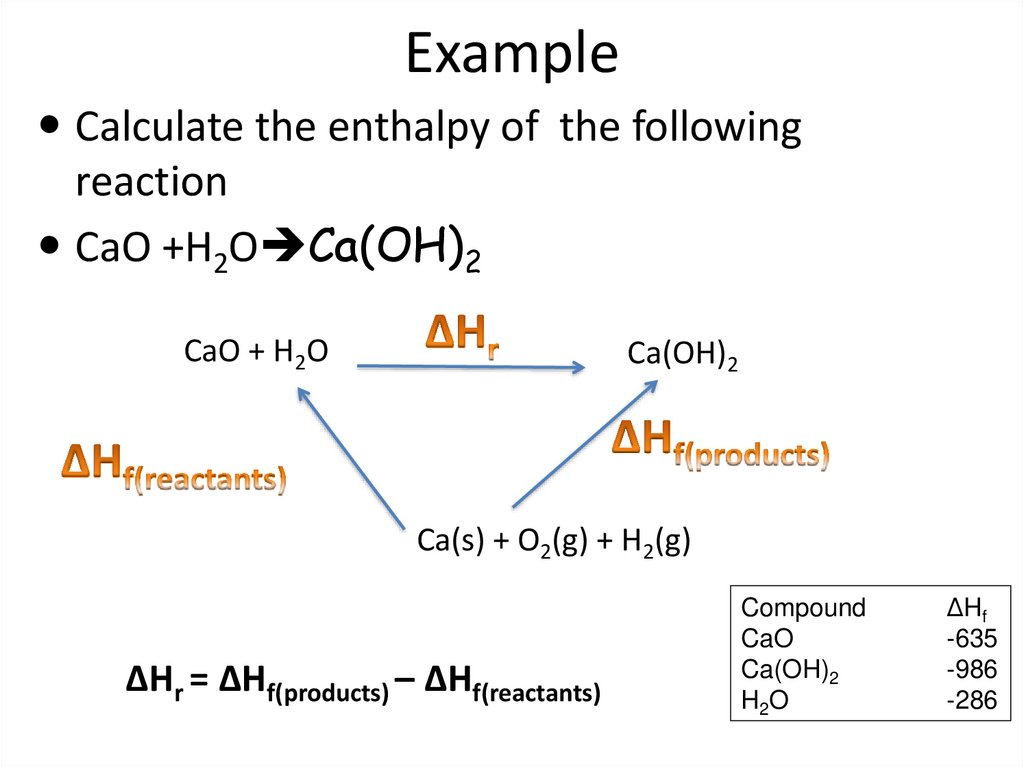
58. Example
Calculate the enthalpy of the followingreaction
CaO +H2O Ca(OH)2
CaO + H2O
Ca(OH)2
Ca(s) + O2(g) + H2(g)
ΔHr = ΔHf(products) – ΔHf(reactants)
Compound
CaO
Ca(OH)2
H2O
ΔHf
-635
-986
-286
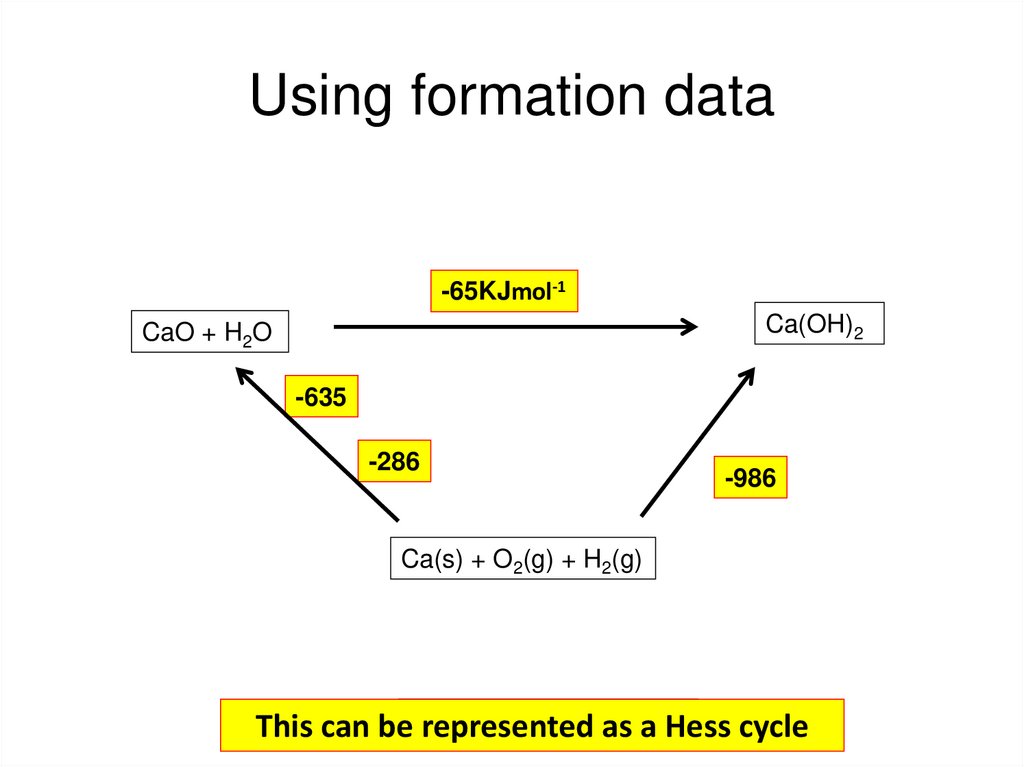
59. Using formation data
-65KJmol-1Ca(OH)2
CaO + H2O
-635
-286
-986
Ca(s) + O2(g) + H2(g)
+ H2O → Ca(OH)
2
This can beCaO
represented
as a Hess
cycle
60.
ActivityCalculate the enthalpy of the following reaction
C2H4(g) + 2 O2(g) → 2 CO(g) + 2 H2O(l)
∆Hf : H2O(g)= -286; CO(g) = -111;
C2H4(g)= +52 kJ mol-1
-852kJ/mol
61. Activity
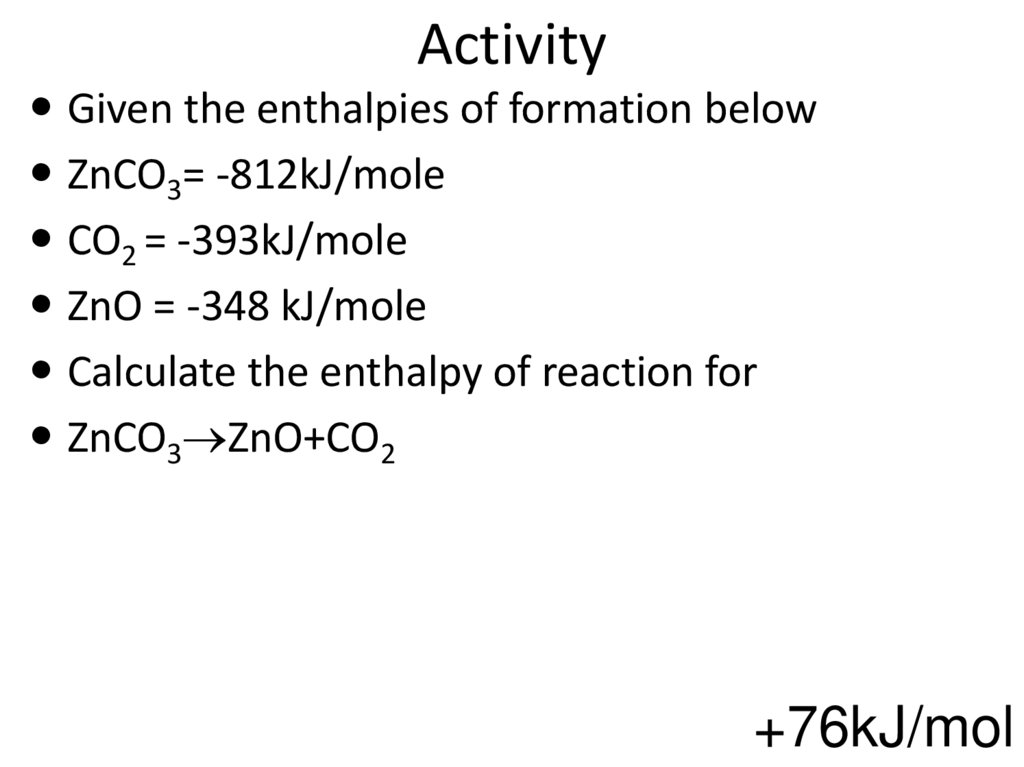
Given the enthalpies of formation belowZnCO3= -812kJ/mole
CO2 = -393kJ/mole
ZnO = -348 kJ/mole
Calculate the enthalpy of reaction for
ZnCO3 ZnO+CO2
+76kJ/mol
62. Check up
• Calculate the enthalpy of combustion of propane(C3H8) given the following enthalpy changes.
• ∆Hc: C(s) -393; H2(g) -286 kJ mol-1, ∆Hf: C3H8(l) 103 kJ mol-1
-2220kJ/mol
63. “TYPE 2” CALCULATIONS
• Simplistic methodH = [Sum Hf products] – [Sum Hf reactants]



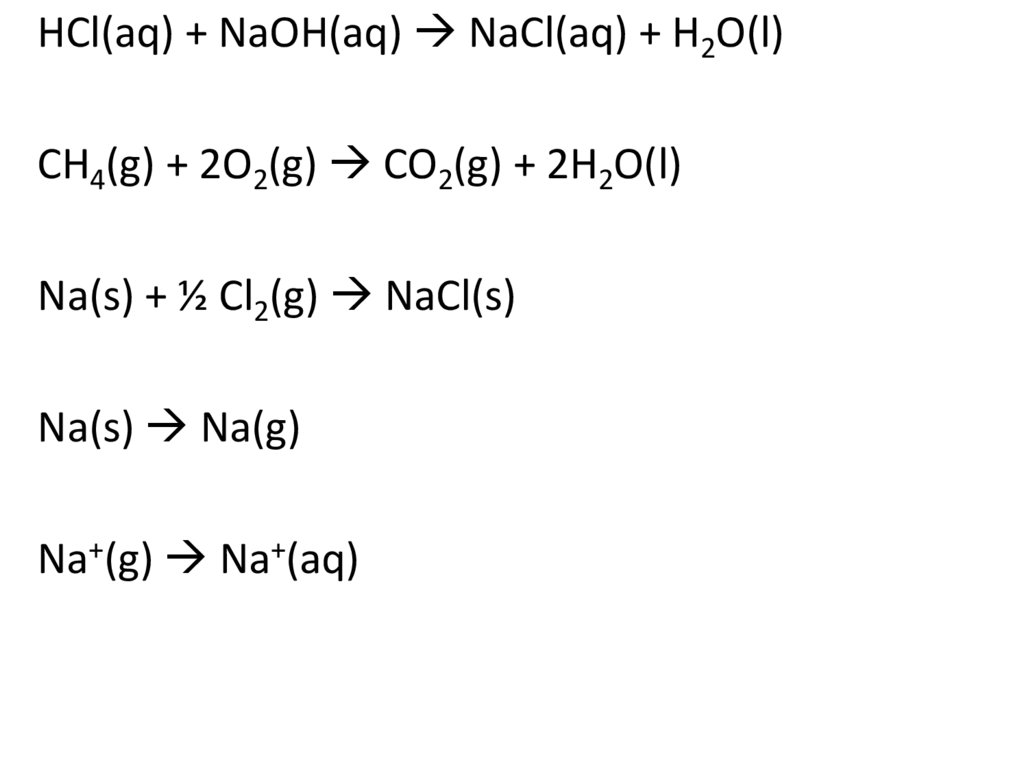

























 chemistry
chemistry