Similar presentations:
Born-Haber cycle spontaneity and entropy
1.
1 of 43© Boardworks Ltd 2010
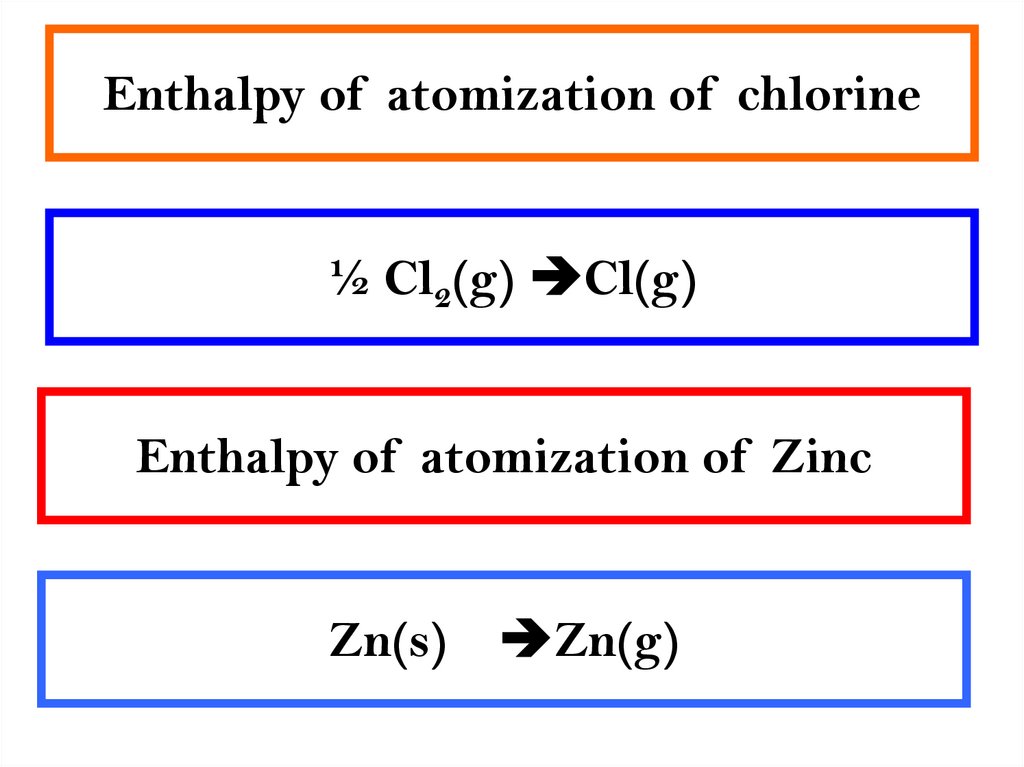
2. Enthalpy of atomization of chlorine
½ Cl2(g) Cl(g)Enthalpy of atomization of Zinc
Zn(s)
Zn(g)
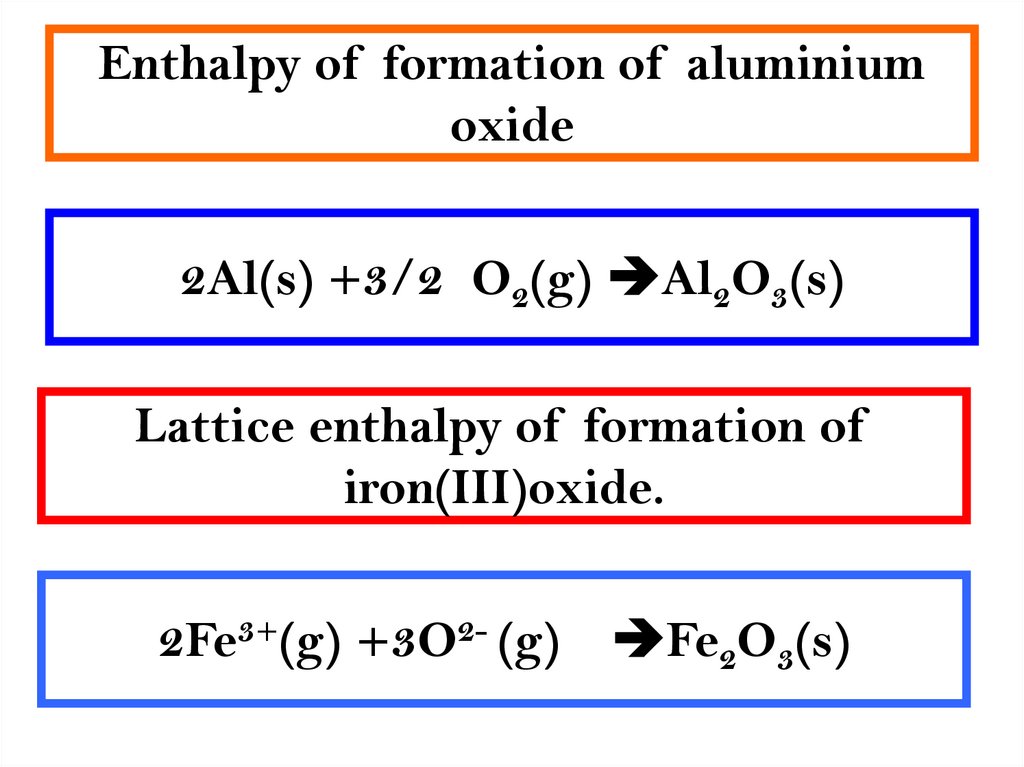
3. Enthalpy of formation of aluminium oxide
2Al(s) +3/2 O2(g) Al2O3(s)Lattice enthalpy of formation of
iron(III)oxide.
2Fe3+(g) +3O2- (g)
Fe2O3(s)
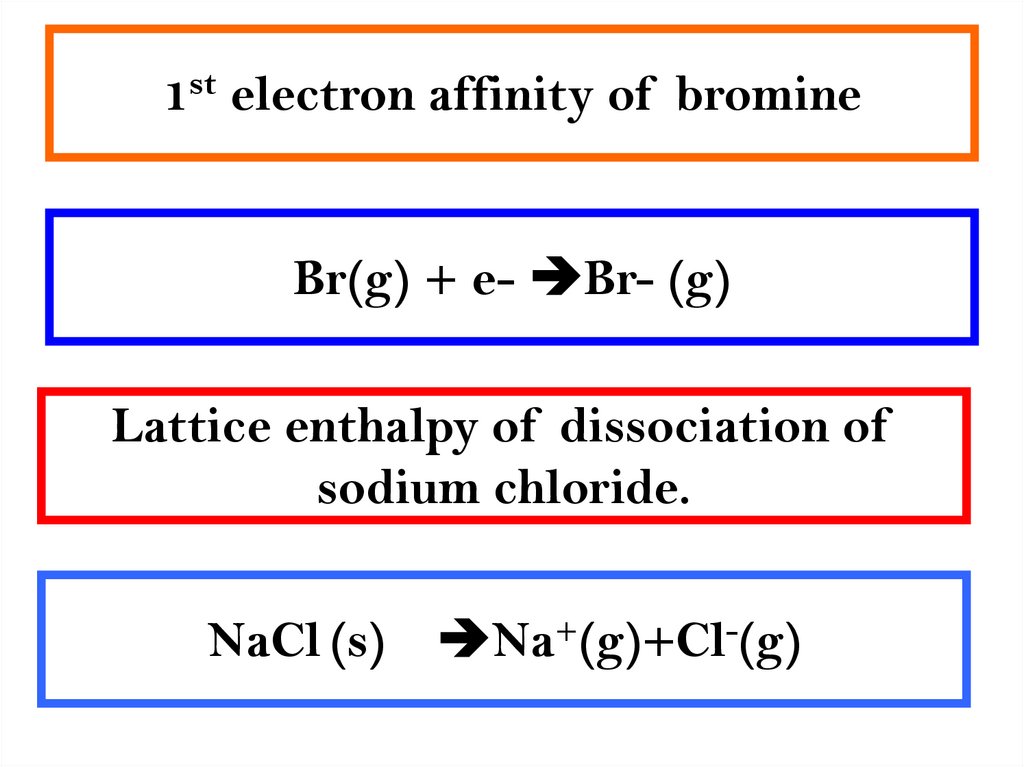
4. 1st electron affinity of bromine
Br(g) + e- Br- (g)Lattice enthalpy of dissociation of
sodium chloride.
NaCl (s)
Na+(g)+Cl-(g)
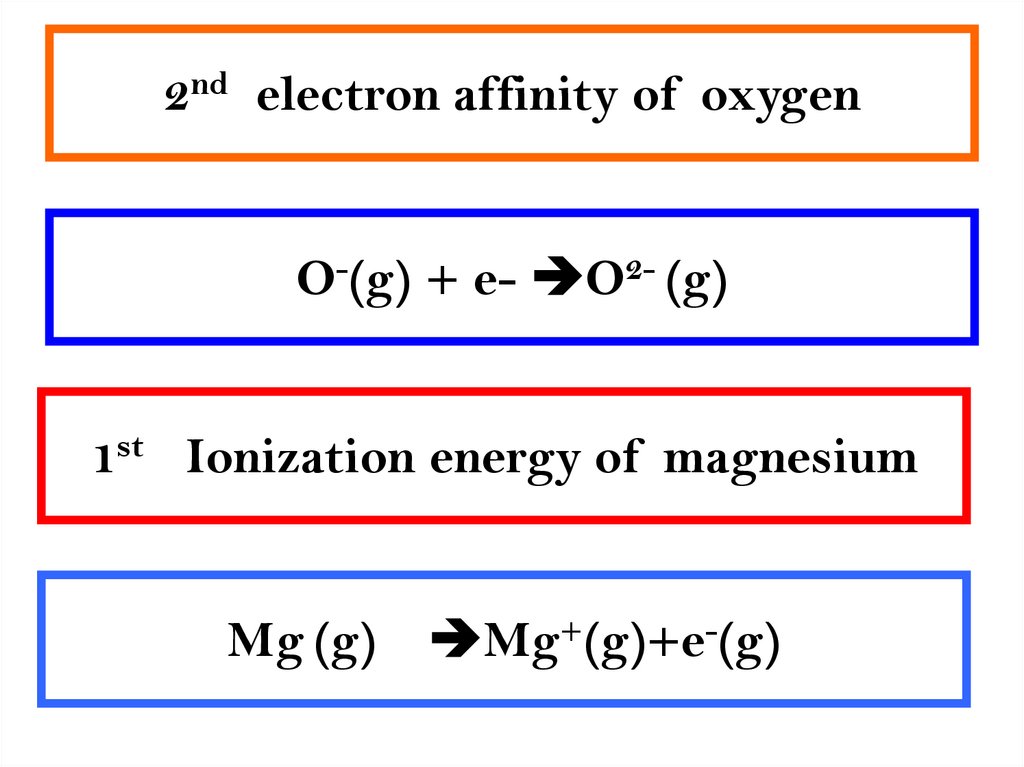
5. 2nd electron affinity of oxygen
O-(g) + e- O2- (g)1st Ionization energy of magnesium
Mg (g)
Mg+(g)+e-(g)
6. Enthalpy changes using bond enthalpies
• Enthalpy changes using bondenthalpies
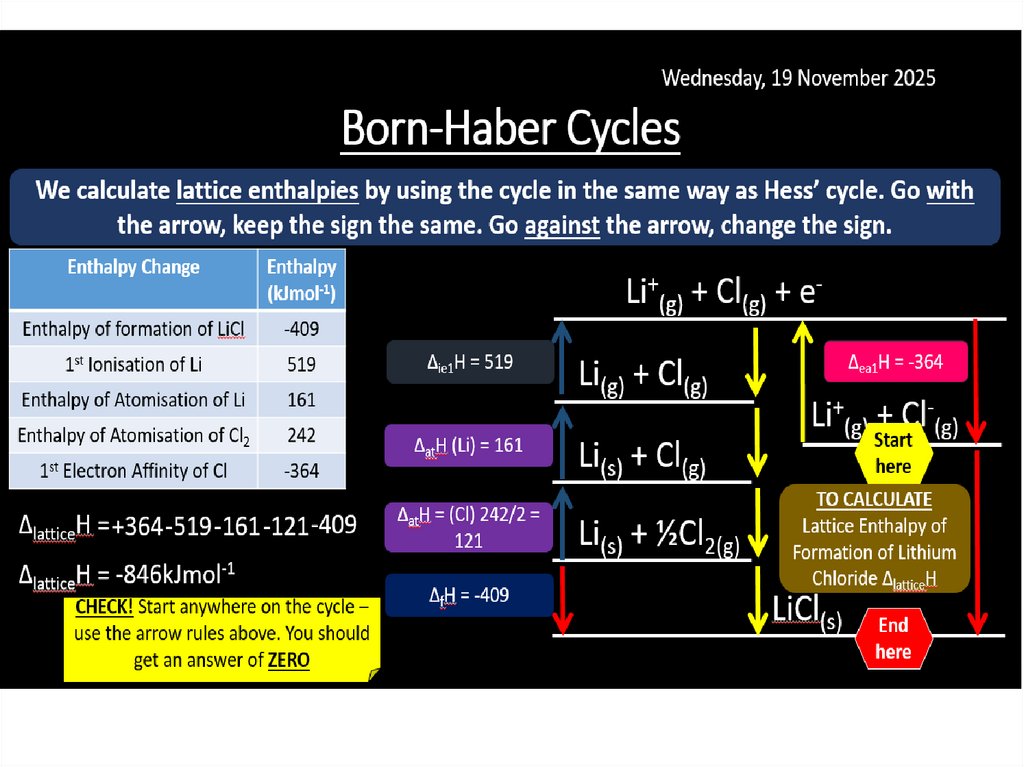
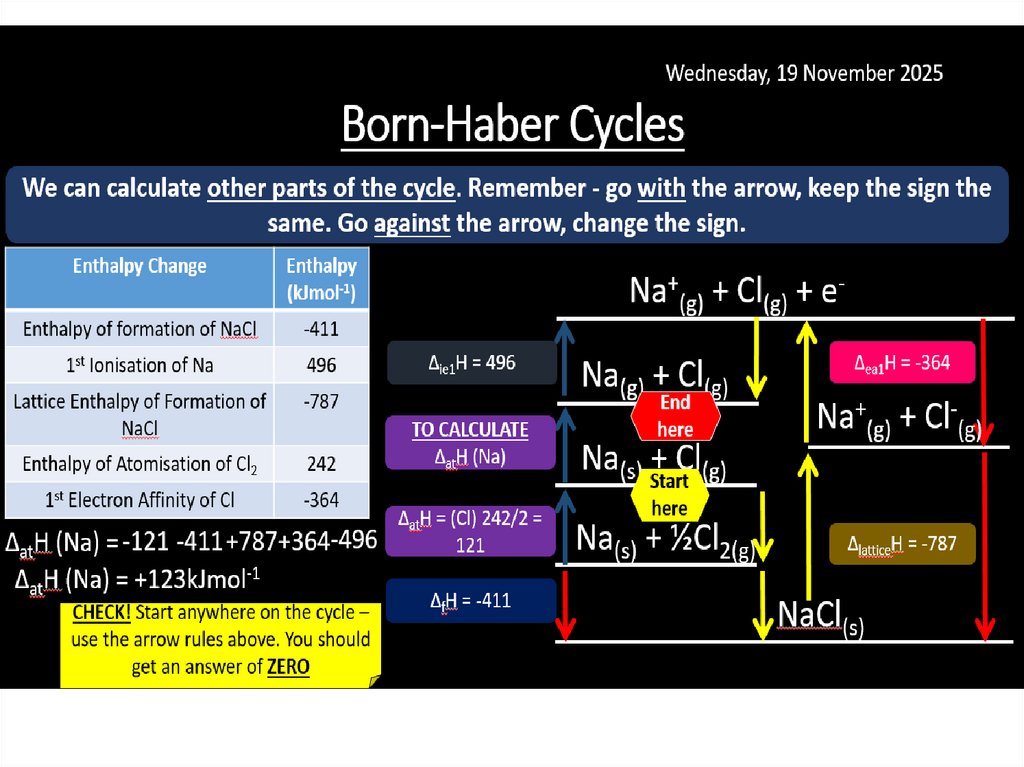
Born-Haber cycles
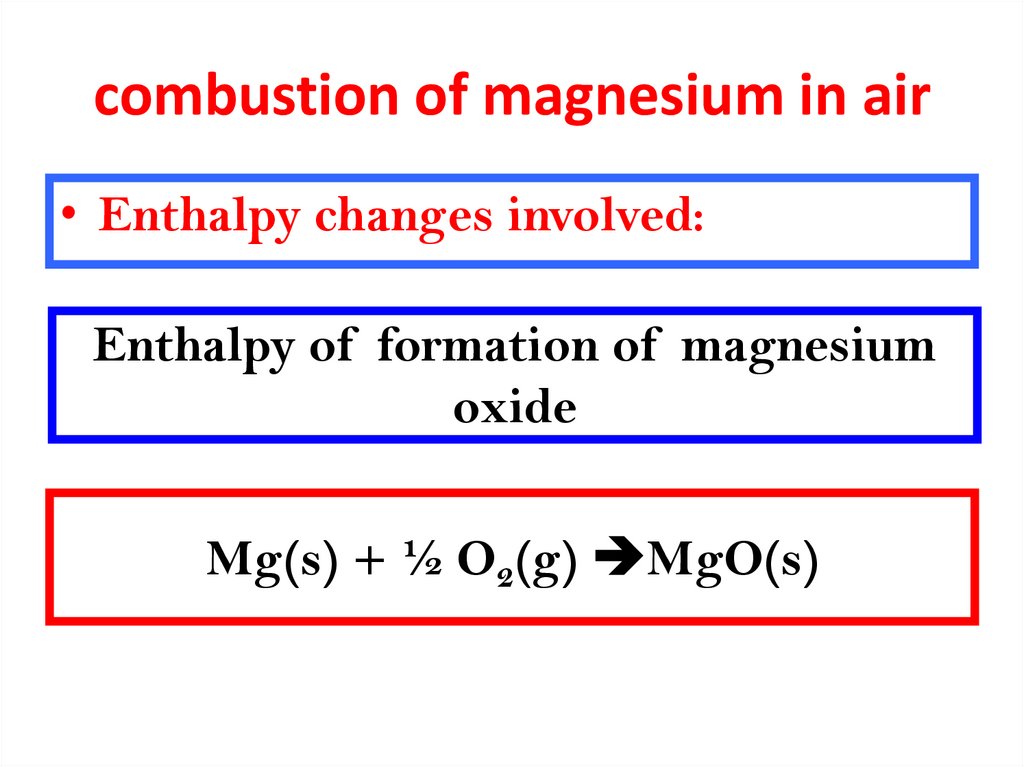
7. combustion of magnesium in air
• Enthalpy changes involved:Enthalpy of formation of magnesium
oxide
Mg(s) + ½ O2(g) MgO(s)
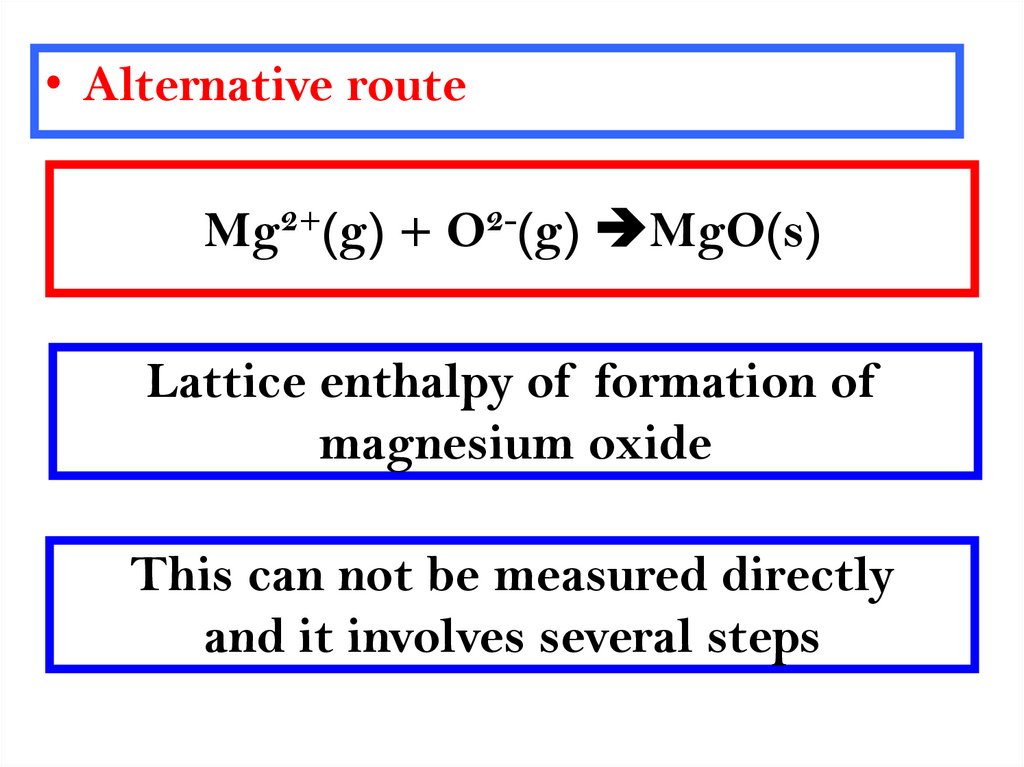
8.
• Alternative routeMg2+(g) + O2-(g) MgO(s)
Lattice enthalpy of formation of
magnesium oxide
This can not be measured directly
and it involves several steps
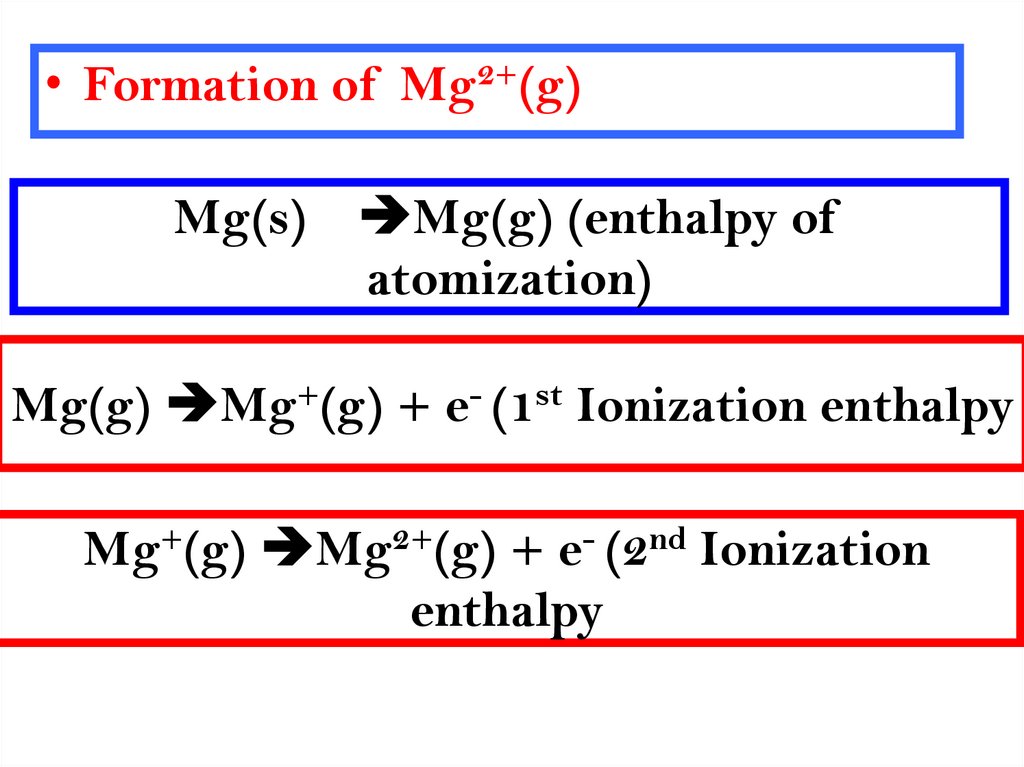
9.
• Formation of Mg2+(g)Mg(s)
Mg(g) (enthalpy of
atomization)
Mg(g) Mg+(g) + e- (1st Ionization enthalpy
Mg+(g) Mg2+(g) + e- (2nd Ionization
enthalpy
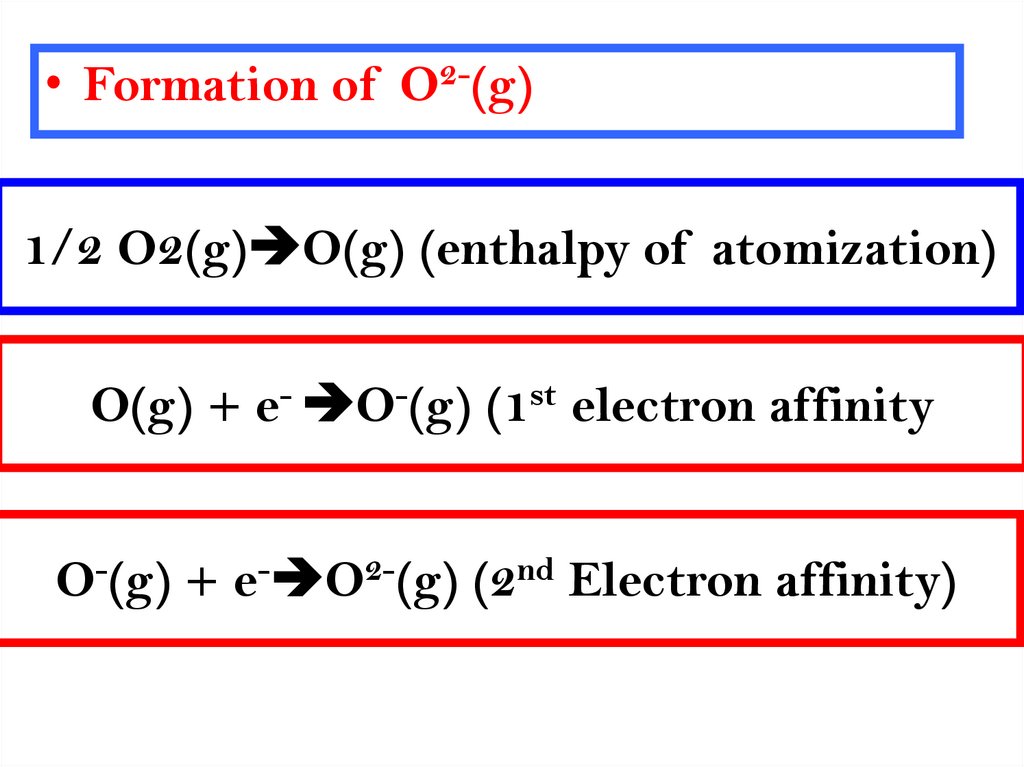
10.
• Formation of O2-(g)1/2 O2(g) O(g) (enthalpy of atomization)
O(g) + e- O-(g) (1st electron affinity
O-(g) + e- O2-(g) (2nd Electron affinity)
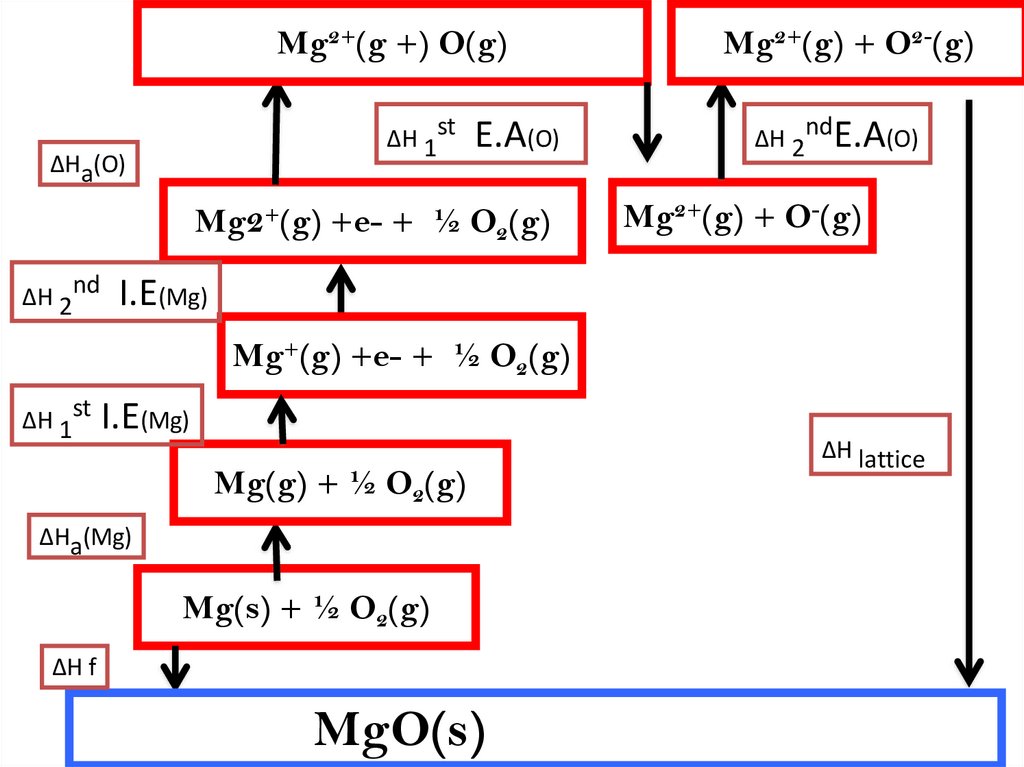
11.
Mg2+(g +) O(g)ΔH 1st
ΔHa(O)
E.A(O)
Mg2+(g) +e- + ½ O2(g)
Mg2+(g) + O2-(g)
ΔH 2ndE.A(O)
Mg2+(g) + O-(g)
ΔH 2nd I.E(Mg)
Mg+(g) +e- + ½ O2(g)
ΔH 1st I.E(Mg)
Mg(g) + ½ O2(g)
ΔHa(Mg)
Mg(s) + ½ O2(g)
ΔH f
MgO(s)
ΔH lattice
12.
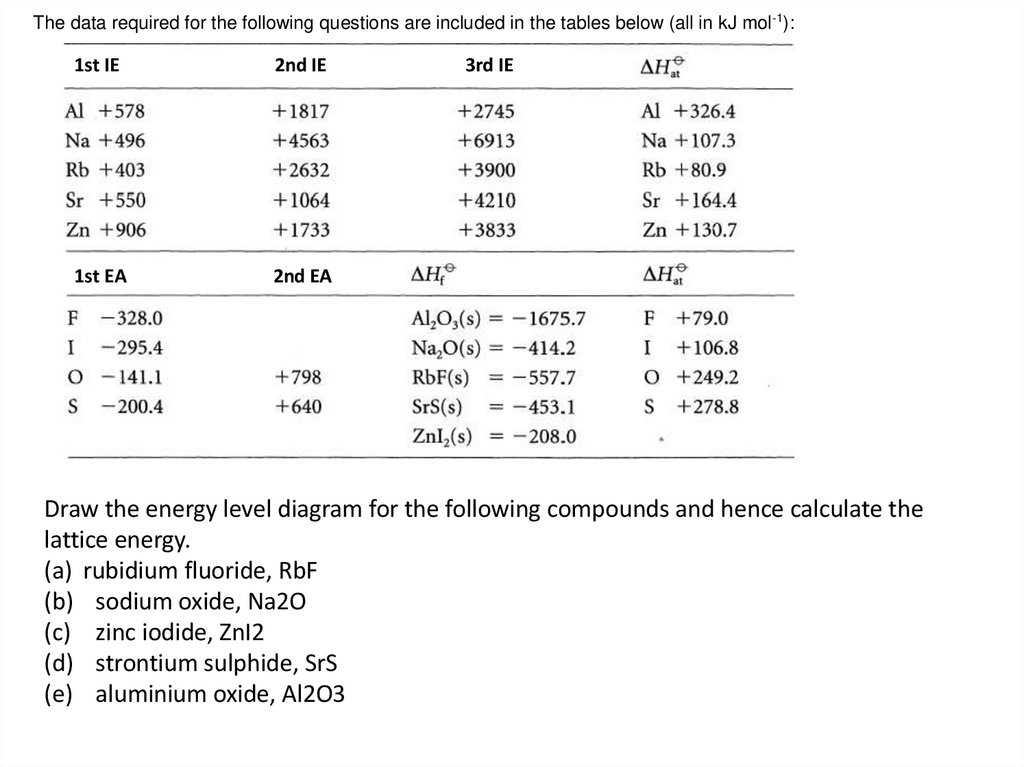
The data required for the following questions are included in the tables below (all in kJ mol-1):1st IE
2nd IE
1st EA
2nd EA
3rd IE
Draw the energy level diagram for the following compounds and hence calculate the
lattice energy.
(a) rubidium fluoride, RbF
(b) sodium oxide, Na2O
(c) zinc iodide, ZnI2
(d) strontium sulphide, SrS
(e) aluminium oxide, Al2O3
13.
14.
15. Spontaneity and entropy
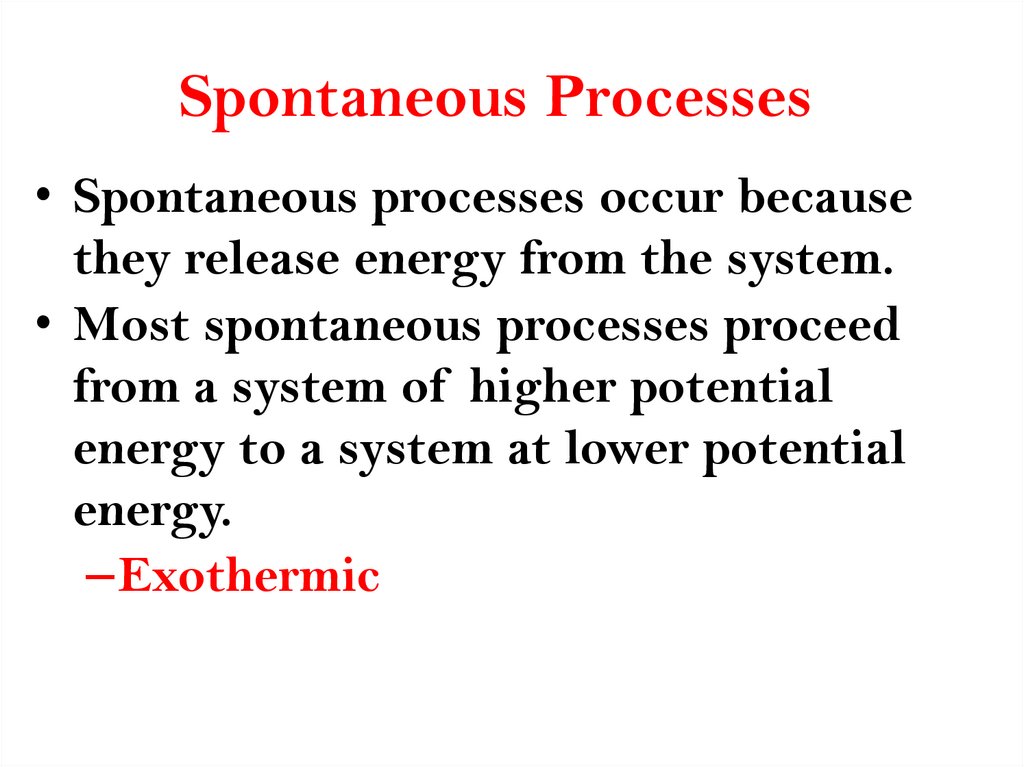
16. Spontaneous Processes
• Spontaneous processes occur becausethey release energy from the system.
• Most spontaneous processes proceed
from a system of higher potential
energy to a system at lower potential
energy.
–Exothermic
17.
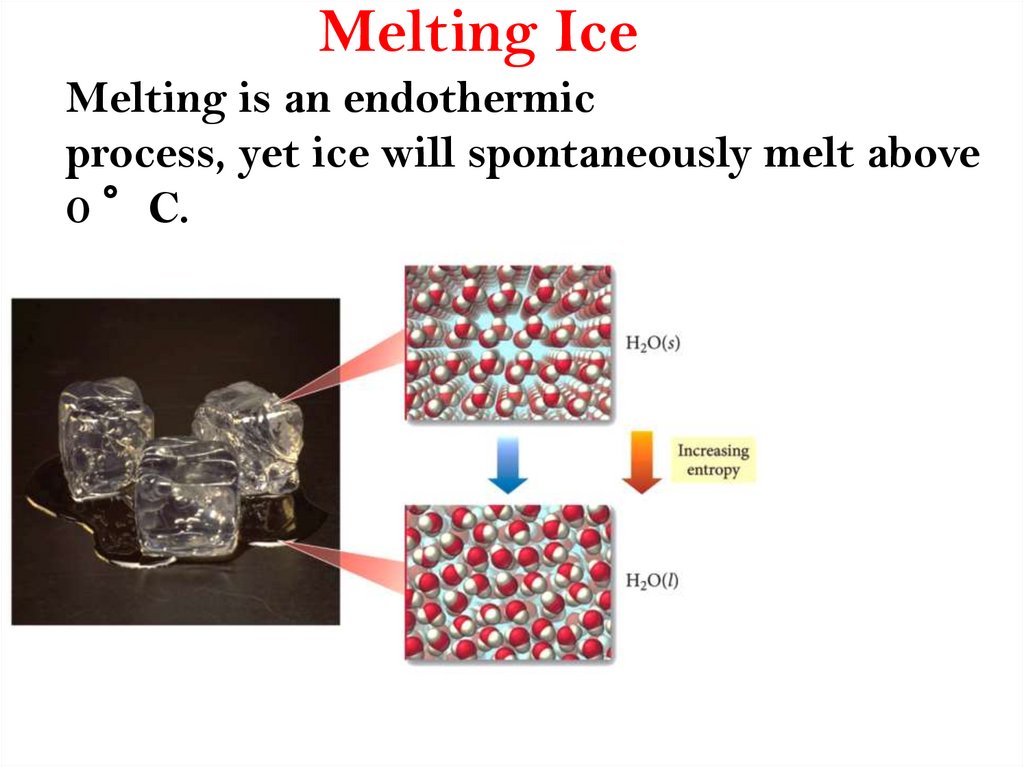
https://bilimland.kz/en/courses/chemistryen/general-chemistry/chemical-kineticsthermodynamics-and-equilibrium/chemicalthermodynamics/lesson/entropy18. Melting Ice
Melting is an endothermicprocess, yet ice will spontaneously melt above
0 °C.
19.
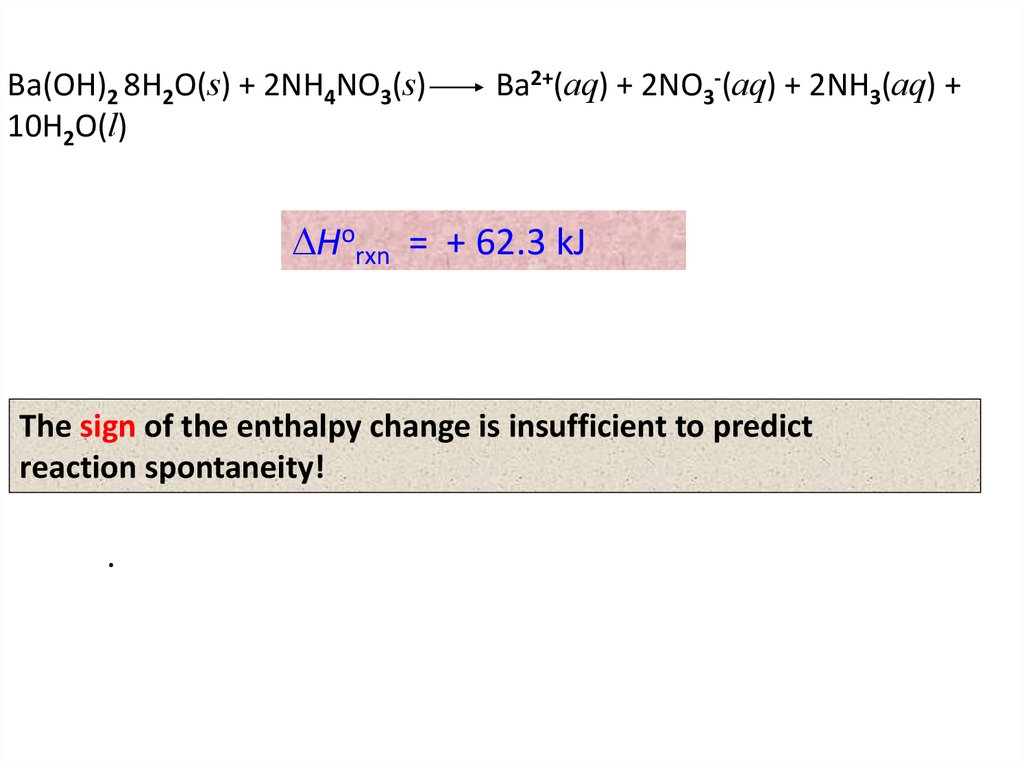
Ba(OH)2 8H2O(s) + 2NH4NO3(s)10H2O(l)
Ba2+(aq) + 2NO3-(aq) + 2NH3(aq) +
DHorxn = + 62.3 kJ
The sign of the enthalpy change is insufficient to predict
reaction spontaneity!
.
20. Factors That determines Whether Reaction Is Spontaneous
• There are two factors that determine whether areaction is spontaneous. They are the enthalpy
change and the entropy change of the system.
• The enthalpy change, ΔH the size and direction
• The entropy change, ΔS
21. Entropy
Entropy is a measure of the disorderof a system.
Reactions in which entropy increases as
reactants form products tend to be
favored. Law of disorder
22. Factors that affect entropy
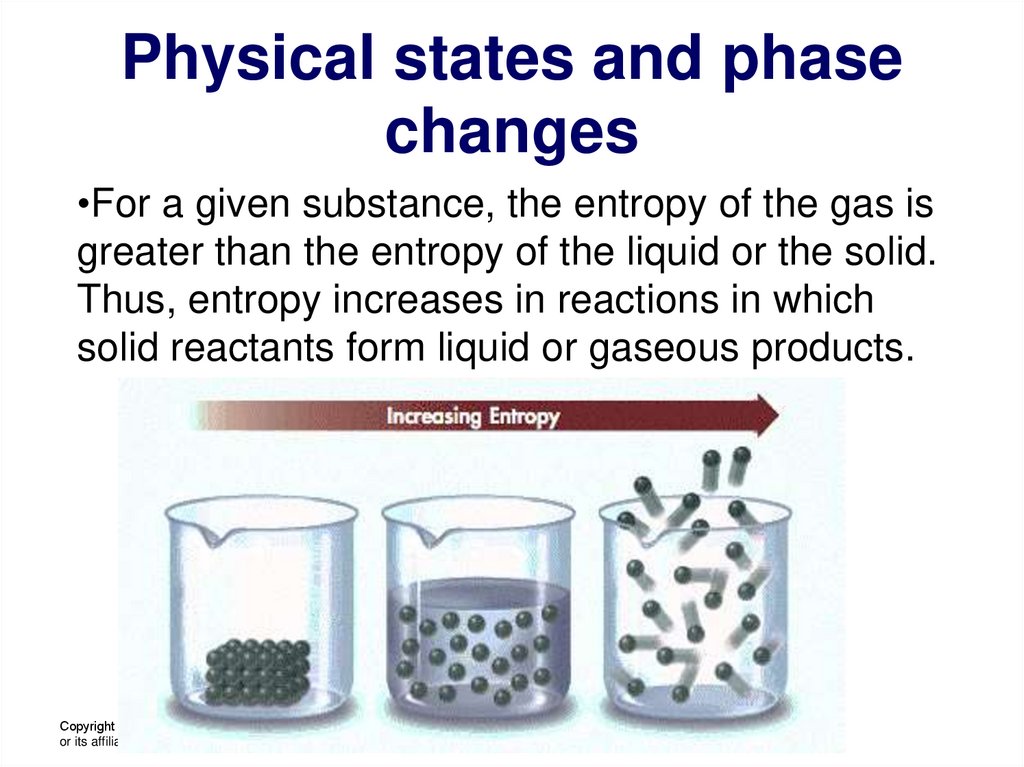
23. Physical states and phase changes
•For a given substance, the entropy of the gas isgreater than the entropy of the liquid or the solid.
Thus, entropy increases in reactions in which
solid reactants form liquid or gaseous products.
Copyright © Pearson Education, Inc.,
or its affiliates. All Rights Reserved.
24. Dissolution of a solid
•Entropy increases when a substance isdivided into parts.
• For instance, entropy increases when
an ionic compound dissolves in water.
Copyright © Pearson Education, Inc.,
or its affiliates. All Rights Reserved.
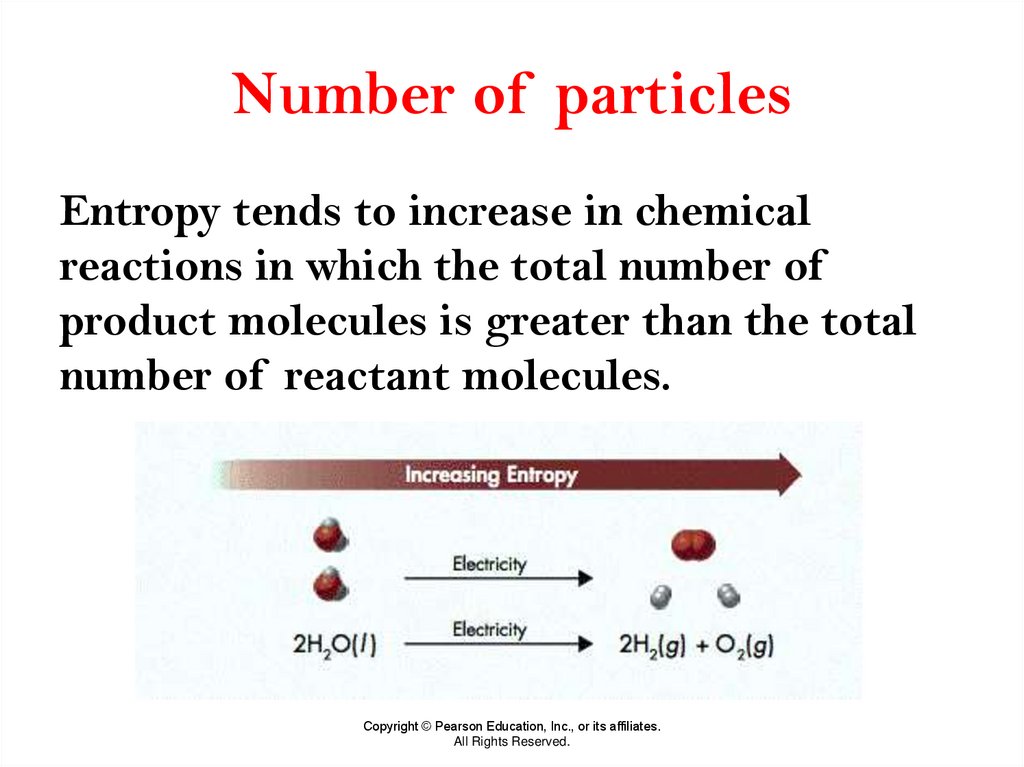
25. Number of particles
Entropy tends to increase in chemicalreactions in which the total number of
product molecules is greater than the total
number of reactant molecules.
Copyright © Pearson Education, Inc., or its affiliates.
All Rights Reserved.
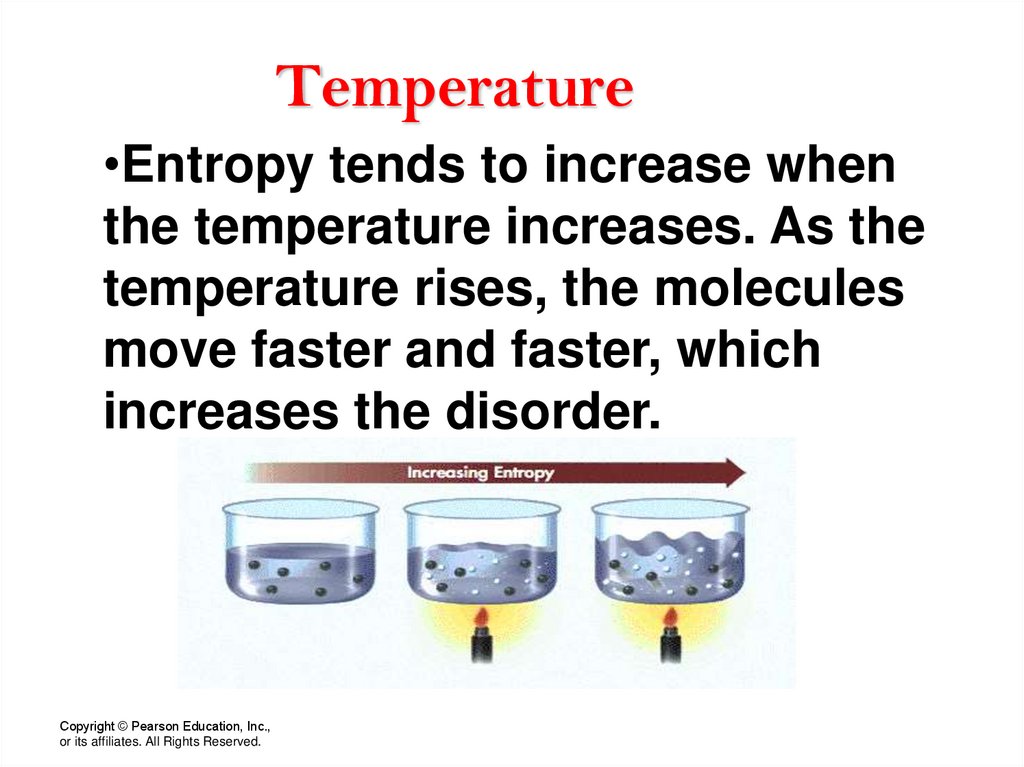
26. Temperature
•Entropy tends to increase whenthe temperature increases. As the
temperature rises, the molecules
move faster and faster, which
increases the disorder.
Copyright © Pearson Education, Inc.,
or its affiliates. All Rights Reserved.
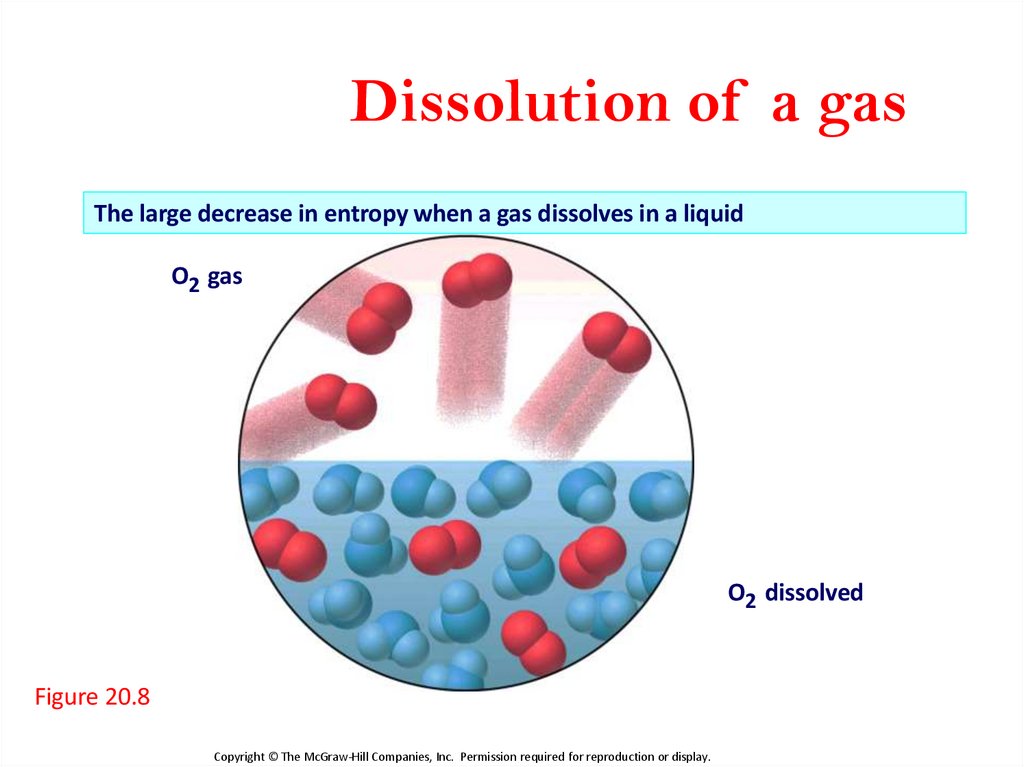
27.
Dissolution of a gasThe large decrease in entropy when a gas dissolves in a liquid
O2 gas
O2 dissolved
Figure 20.8
Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
28. Predicting entropy changes
28 of 43© Boardworks Ltd 2010
29. Atomic size and molecular complexity
• In similar substances, increases in massrelate directly to entropy e.g CI4 has higher
entropy than CF4
• In allotropic substances, increases in
complexity (e.g., bond flexibility) relate
directly to entropy. e.g Carbon(diamond)
has less entropy than graphite
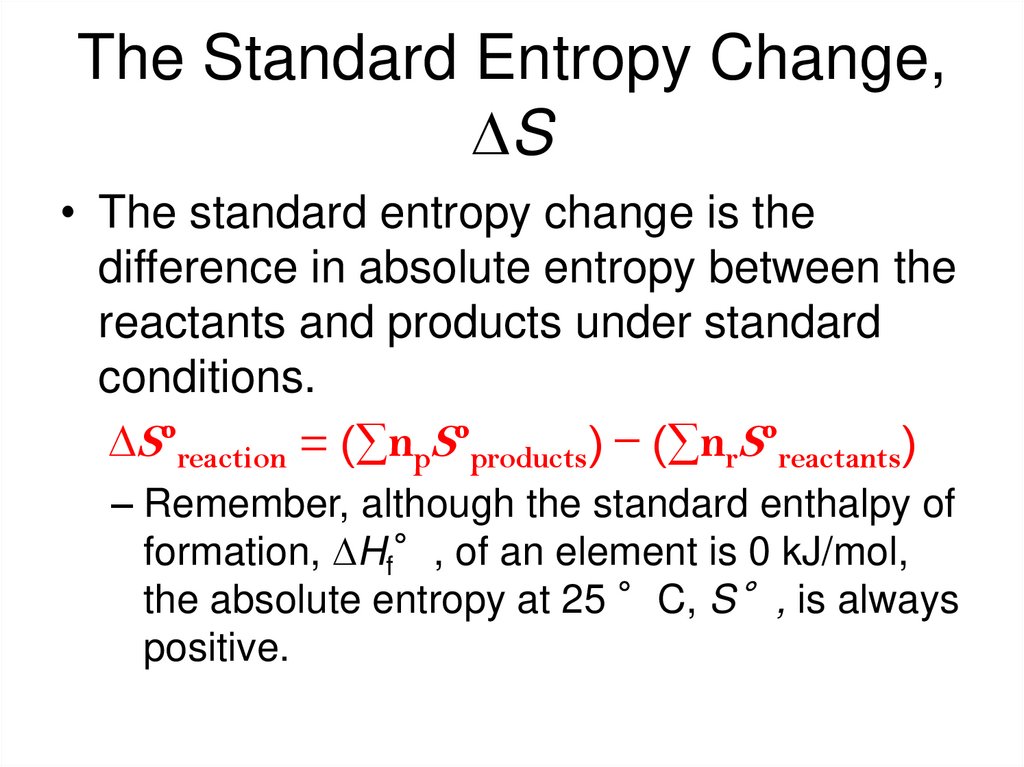
30. The Standard Entropy Change, DS
• The standard entropy change is thedifference in absolute entropy between the
reactants and products under standard
conditions.
DSºreaction = (∑npSºproducts) − (∑nrSºreactants)
– Remember, although the standard enthalpy of
formation, DHf°, of an element is 0 kJ/mol,
the absolute entropy at 25 °C, S°, is always
positive.
31.
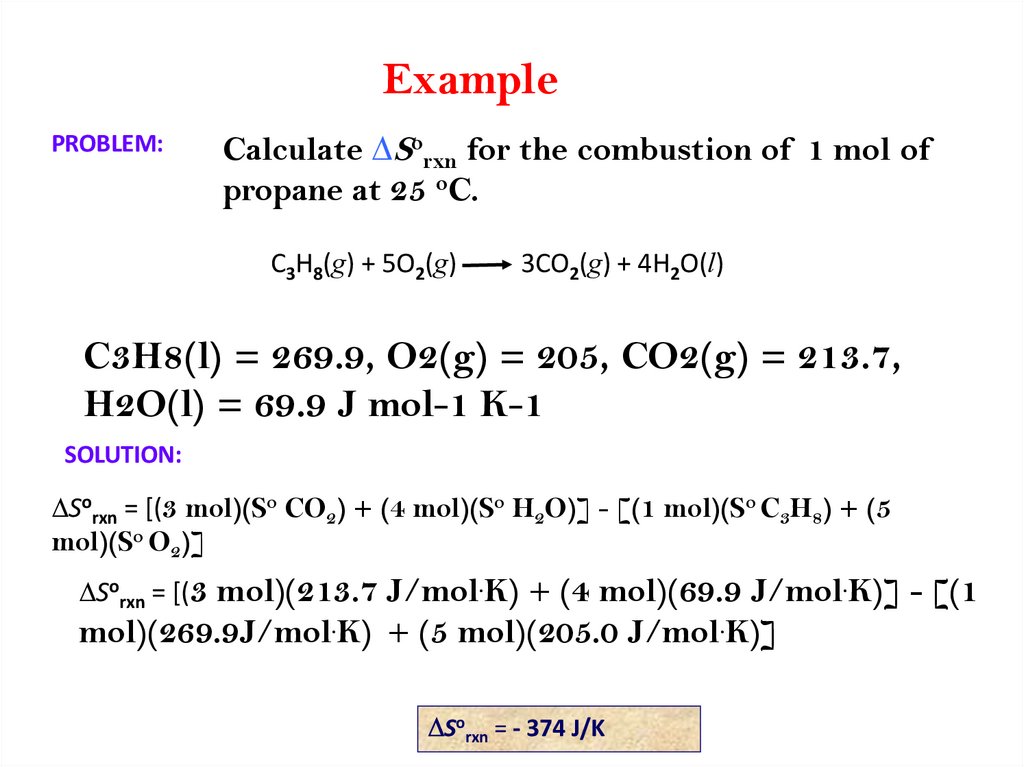
ExamplePROBLEM:
Calculate DSorxn for the combustion of 1 mol of
propane at 25 oC.
C3H8(g) + 5O2(g)
3CO2(g) + 4H2O(l)
C3H8(l) = 269.9, O2(g) = 205, CO2(g) = 213.7,
H2O(l) = 69.9 J mol-1 K-1
SOLUTION:
DSorxn = [(3 mol)(So CO2) + (4 mol)(So H2O)] - [(1 mol)(So C3H8) + (5
mol)(So O2)]
DSorxn = [(3 mol)(213.7 J/mol.K) + (4 mol)(69.9 J/mol.K)] - [(1
mol)(269.9J/mol.K) + (5 mol)(205.0 J/mol.K)]
DSorxn = - 374 J/K
32. Checkup
Calculate the entropy change for this reaction: C2H5OH(l) + 3O2(g) → 2 CO2(g) + 3 H2O(l)
Entropy: C2H5OH(l) = 161, O2(g) = 205, CO2(g) = 214, H2O(l)
= 70 J mol-1 K-1
33. Activity (10 minutes)
1. Predict the sign of entropy change for the following reactions,where possible, giving your reasoning for each one.
i) H2O(g) → H2O(l)
ii) H2(g) + Cl2(g) → 2 HCl(g)
b) Calculate entropy change for the above reactions.
S (JK-1mol-1): H2O(l) = 70, H2O(g) = 189. H2(g) = 131, Cl2(g) =223,
HCl(g)= 187
2. Given the reaction below:
2NaHCO3(s) → Na2CO3(s)+ CO2(g) + H2O(g)
Use the following entropies to calculate the entropy change for the
reaction: NaHCO3(s) 102, Na2CO3(s) 135, CO2(g) 214, H2O(g) 189 (all
JK-1mol-1)
34. Gibbs free energy
Whether a reaction is spontaneous depends on:the entropy change of the system
the enthalpy change of the system
the temperature.
The change in a quantity called the Gibbs free energy
provides a measure of whether a reaction is spontaneous.
The Gibbs free energy change is given the symbol DG and
can be calculated for a reaction using the expression:
DG = DH – TDS
34 of 43
A reaction will be spontaneous if DG < 0.
© Boardworks Ltd 2010
35. How to calculate ∆G
35 of 43© Boardworks Ltd 2010
36. Calculating ∆G
36 of 43© Boardworks Ltd 2010
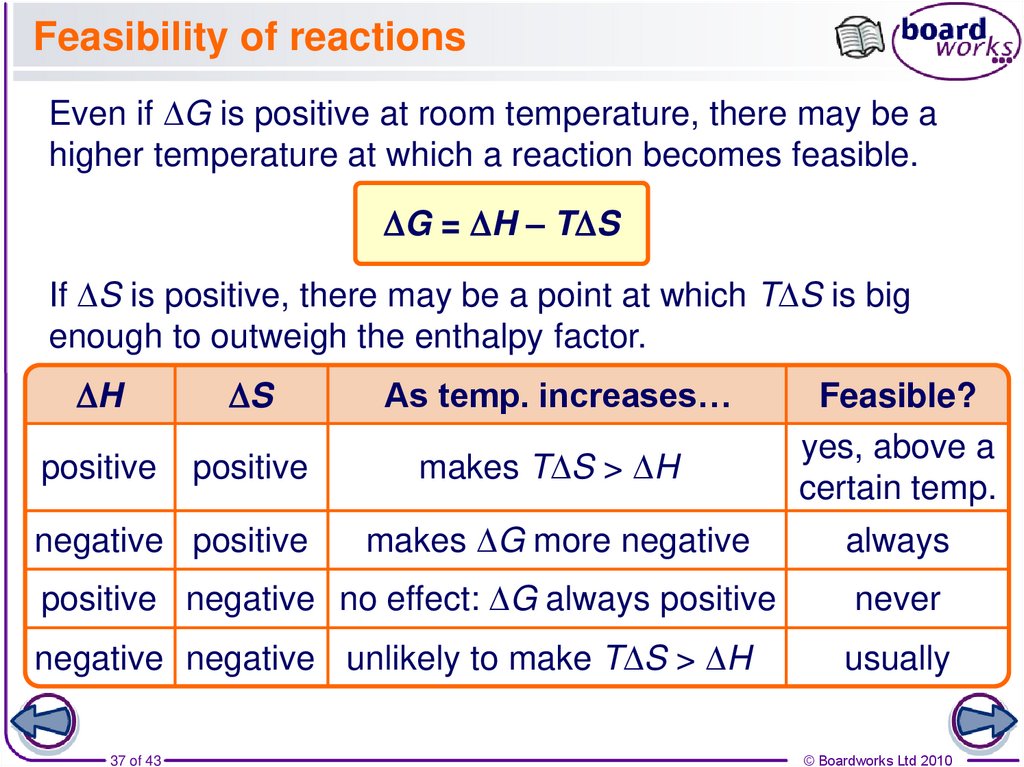
37. Feasibility of reactions
Even if DG is positive at room temperature, there may be ahigher temperature at which a reaction becomes feasible.
DG = DH – TDS
If DS is positive, there may be a point at which TDS is big
enough to outweigh the enthalpy factor.
DH
DS
As temp. increases…
positive
positive
makes TDS > DH
negative positive
makes DG more negative
Feasible?
yes, above a
certain temp.
always
positive negative no effect: DG always positive
never
negative negative unlikely to make TDS > DH
usually
37 of 43
© Boardworks Ltd 2010
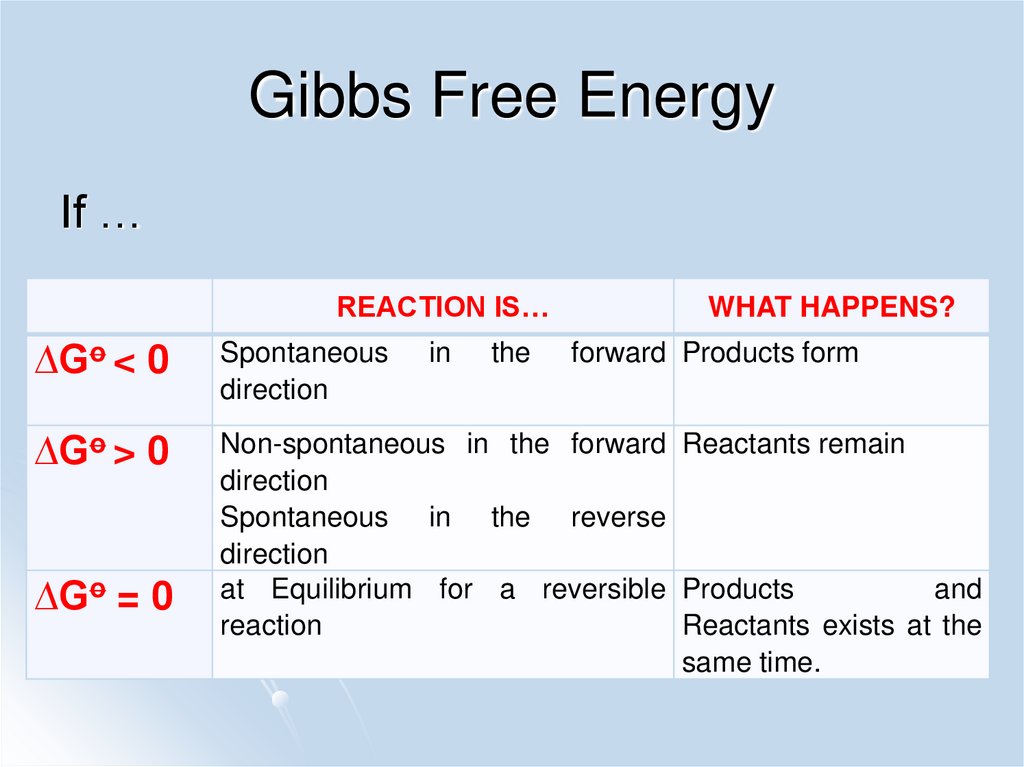
38. Gibbs Free Energy
If …REACTION IS…
WHAT HAPPENS?
∆Go < 0
Spontaneous
direction
∆Go > 0
Non-spontaneous in the forward Reactants remain
direction
Spontaneous in the reverse
direction
at Equilibrium for a reversible Products
and
reaction
Reactants exists at the
same time.
∆Go = 0
in
the
forward Products form
39.
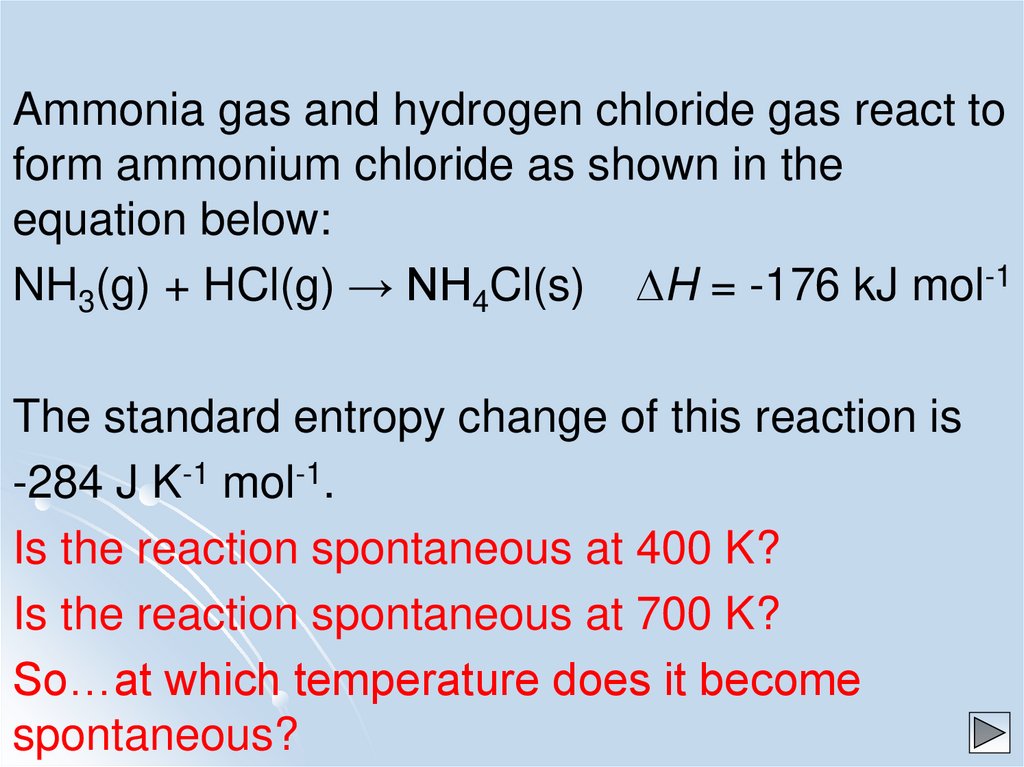
Ammonia gas and hydrogen chloride gas react toform ammonium chloride as shown in the
equation below:
NH3(g) + HCl(g) → NH4Cl(s) DH = -176 kJ mol-1
The standard entropy change of this reaction is
-284 J K-1 mol-1.
Is the reaction spontaneous at 400 K?
Is the reaction spontaneous at 700 K?
So…at which temperature does it become
spontaneous?
40. How temperature affects ∆G?
41.
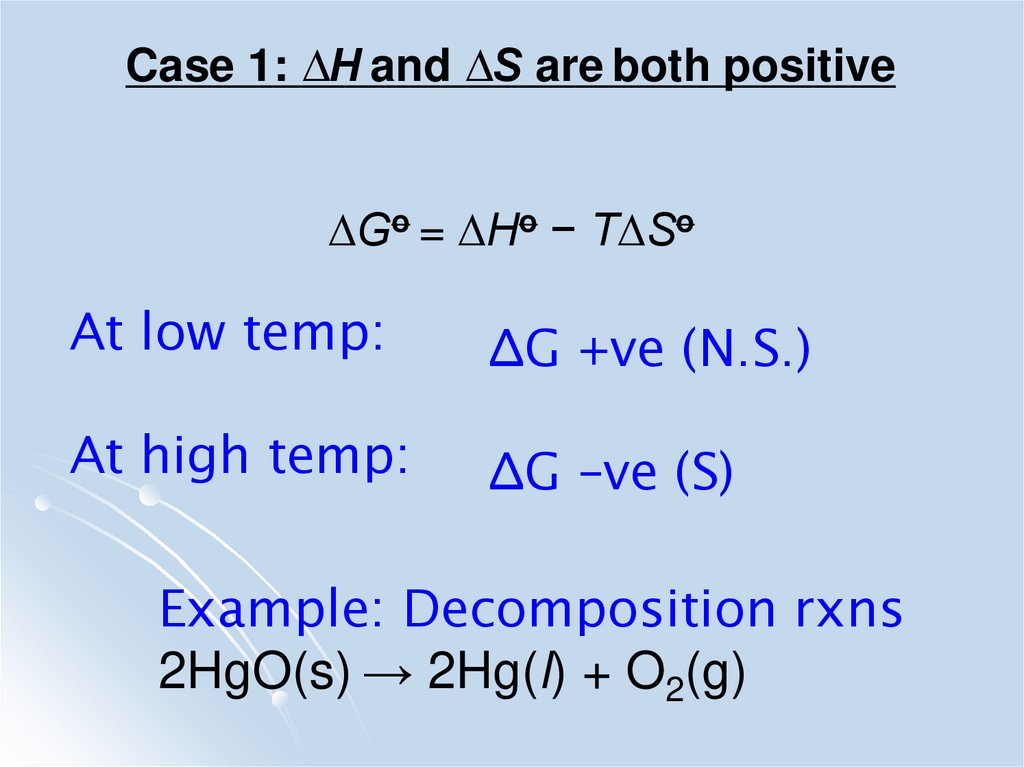
Case 1: ∆H and ∆S are both positive∆Go = ∆Ho − T∆So
At low temp:
∆G +ve (N.S.)
At high temp:
∆G –ve (S)
Example: Decomposition rxns
2HgO(s) → 2Hg(l) + O2(g)
42.
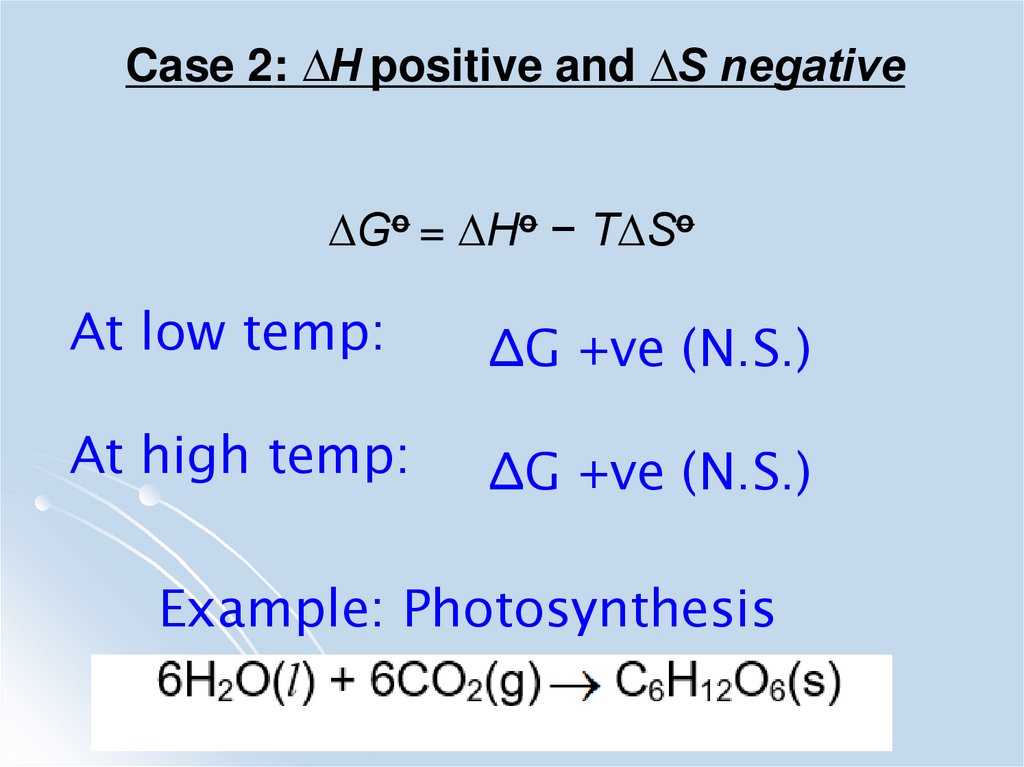
Case 2: ∆H positive and ∆S negative∆Go = ∆Ho − T∆So
At low temp:
∆G +ve (N.S.)
At high temp:
∆G +ve (N.S.)
Example: Photosynthesis
43.
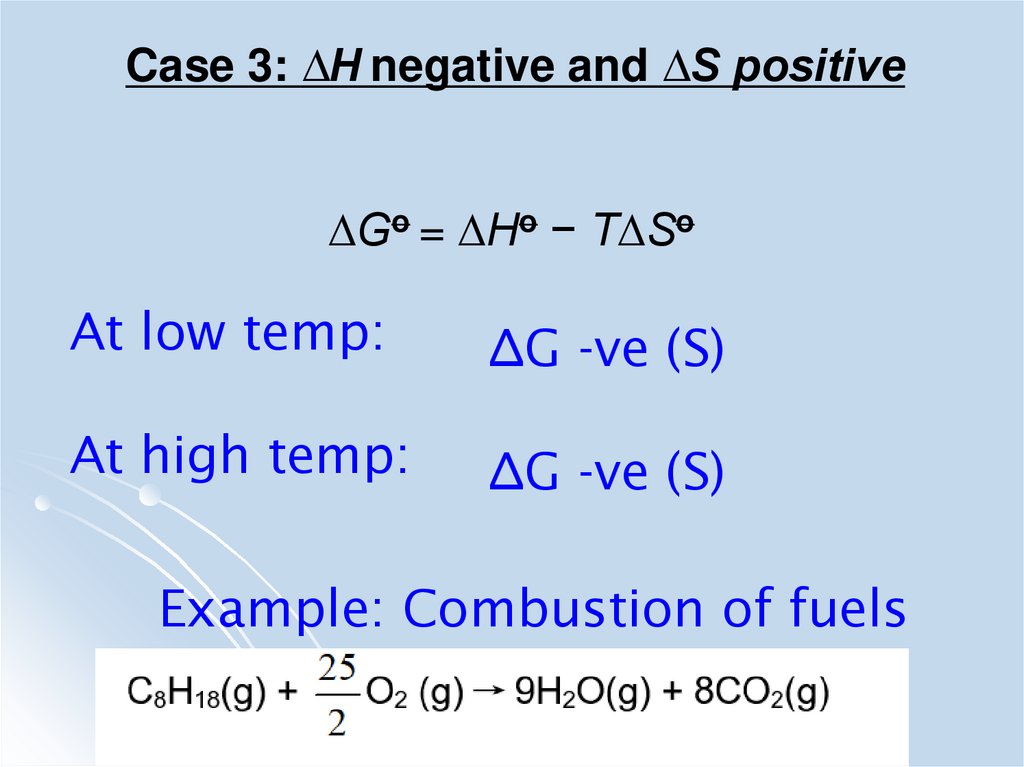
Case 3: ∆H negative and ∆S positive∆Go = ∆Ho − T∆So
At low temp:
∆G -ve (S)
At high temp:
∆G -ve (S)
Example: Combustion of fuels
44.
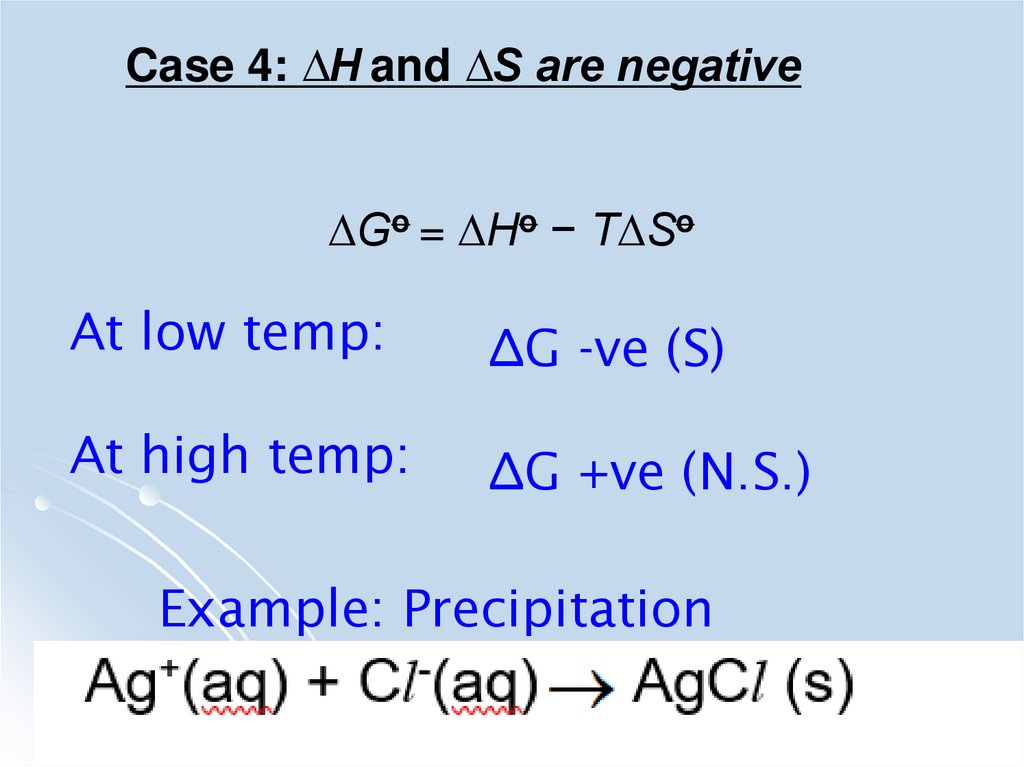
Case 4: ∆H and ∆S are negative∆Go = ∆Ho − T∆So
At low temp:
∆G -ve (S)
At high temp:
∆G +ve (N.S.)
Example: Precipitation
45.
WHAT HAVE WE LEARNED?:1. S measures disorder in JK-1mol-1
2. ΔS gets more positive in spontaneous changes
3. A reaction is spontaneous if if DG = -ve.
4. Endothermic reactions
can be feasible, provided
.
T X ΔS is big enough to be greater than DH.
Extension: Give an example of a spontaneous
reaction that does not occur readily when the
reactants are mixed
46.
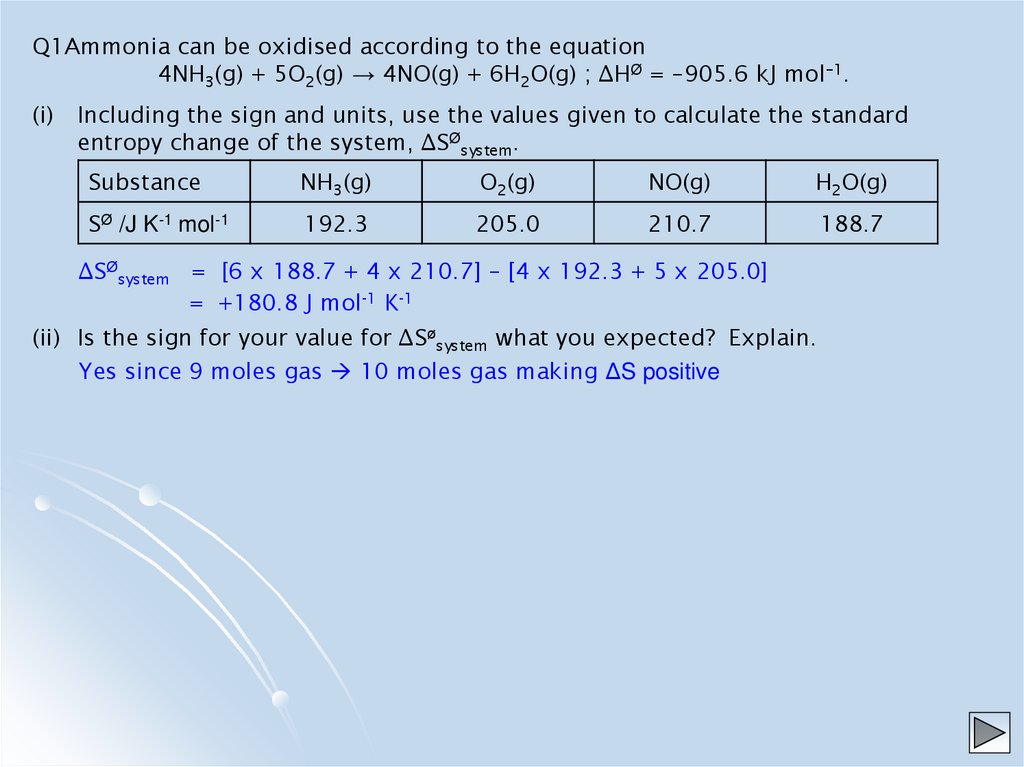
Q1Ammonia can be oxidised according to the equation4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g) ; ∆HØ = –905.6 kJ mol–1.
(i)
Including the sign and units, use the values given to calculate the standard
entropy change of the system, ∆SØsystem.
Substance
NH3(g)
O2(g)
NO(g)
H2O(g)
SØ /J K-1 mol-1
192.3
205.0
210.7
188.7
∆SØsystem = [6 x 188.7 + 4 x 210.7] – [4 x 192.3 + 5 x 205.0]
= +180.8 J mol-1 K-1
(ii) Is the sign for your value for ∆Søsystem what you expected? Explain.
Yes since 9 moles gas 10 moles gas making ΔS positive
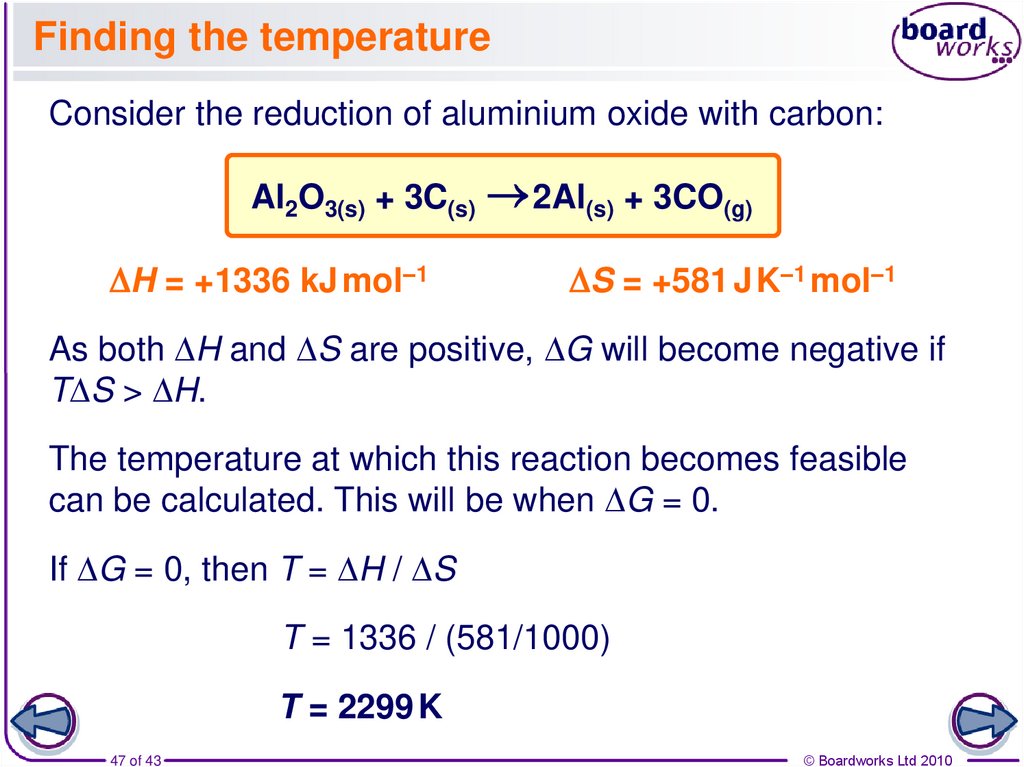
47. Finding the temperature
Consider the reduction of aluminium oxide with carbon:Al2O3(s) + 3C(s)
DH = +1336 kJ mol–1
2Al(s) + 3CO(g)
DS = +581 J K–1 mol–1
As both DH and DS are positive, DG will become negative if
TDS > DH.
The temperature at which this reaction becomes feasible
can be calculated. This will be when DG = 0.
If DG = 0, then T = DH / DS
T = 1336 / (581/1000)
T = 2299 K
47 of 43
© Boardworks Ltd 2010
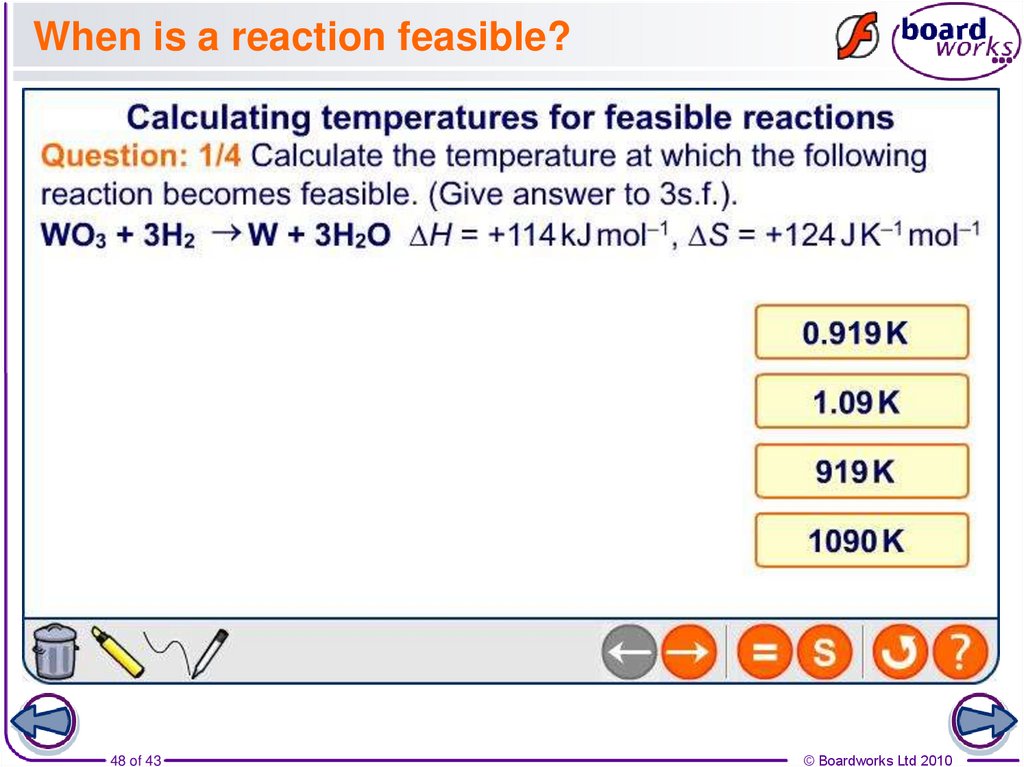
48. When is a reaction feasible?
48 of 43© Boardworks Ltd 2010
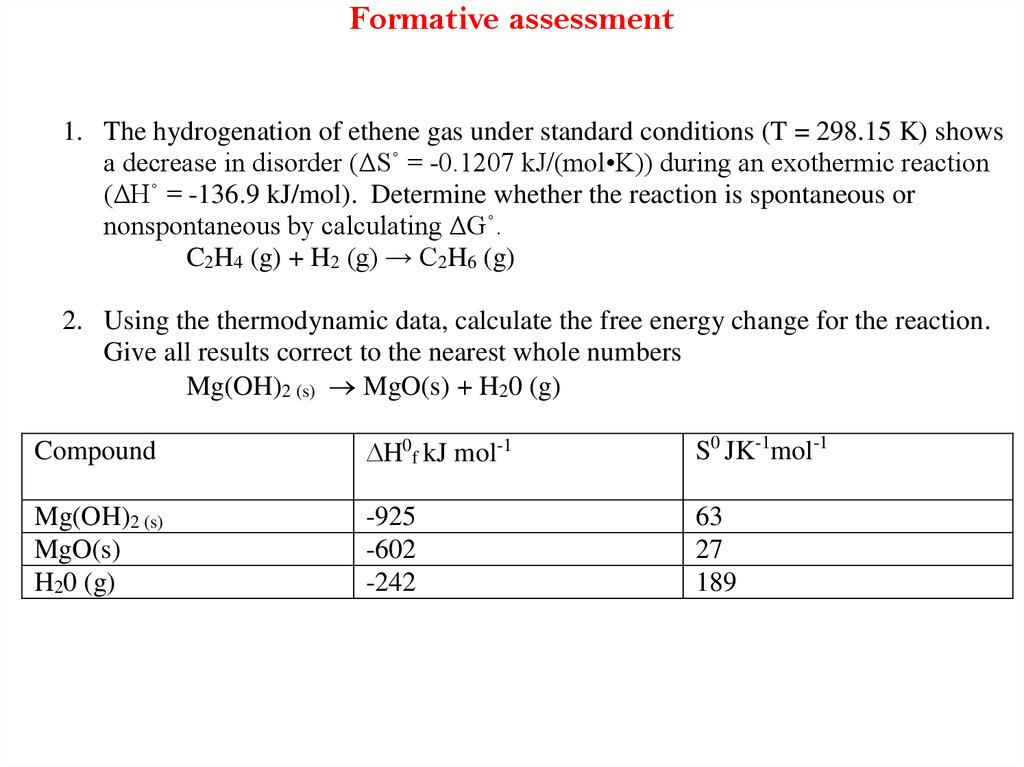
49. Formative assessment
1. The hydrogenation of ethene gas under standard conditions (T = 298.15 K) showsa decrease in disorder (ΔS˚ = -0.1207 kJ/(mol•K)) during an exothermic reaction
(ΔH˚ = -136.9 kJ/mol). Determine whether the reaction is spontaneous or
nonspontaneous by calculating ΔG˚.
C2H4 (g) + H2 (g) → C2H6 (g)
2. Using the thermodynamic data, calculate the free energy change for the reaction.
Give all results correct to the nearest whole numbers
Mg(OH)2 (s) MgO(s) + H20 (g)
Compound
DH0f kJ mol-1
S0 JK-1mol-1
Mg(OH)2 (s)
MgO(s)
H20 (g)
-925
-602
-242
63
27
189












 chemistry
chemistry