Similar presentations:
4. Water and life
1. Water and life
Uria AlcolombriCampbell biology – chapter 3
1
2. Water is the biological medium on earth
• 75% of the earth’s surface is covered by water.• Water is the only common substance to exit in the natural environment
in all three physical states of matter (solid, liquid and gas).
• Life began in water. Most cells are surrounded by water, and cells
themselves are about 70–95% water
• The abundance of water is the main reason the Earth is habitable
What makes water so amazing?
2
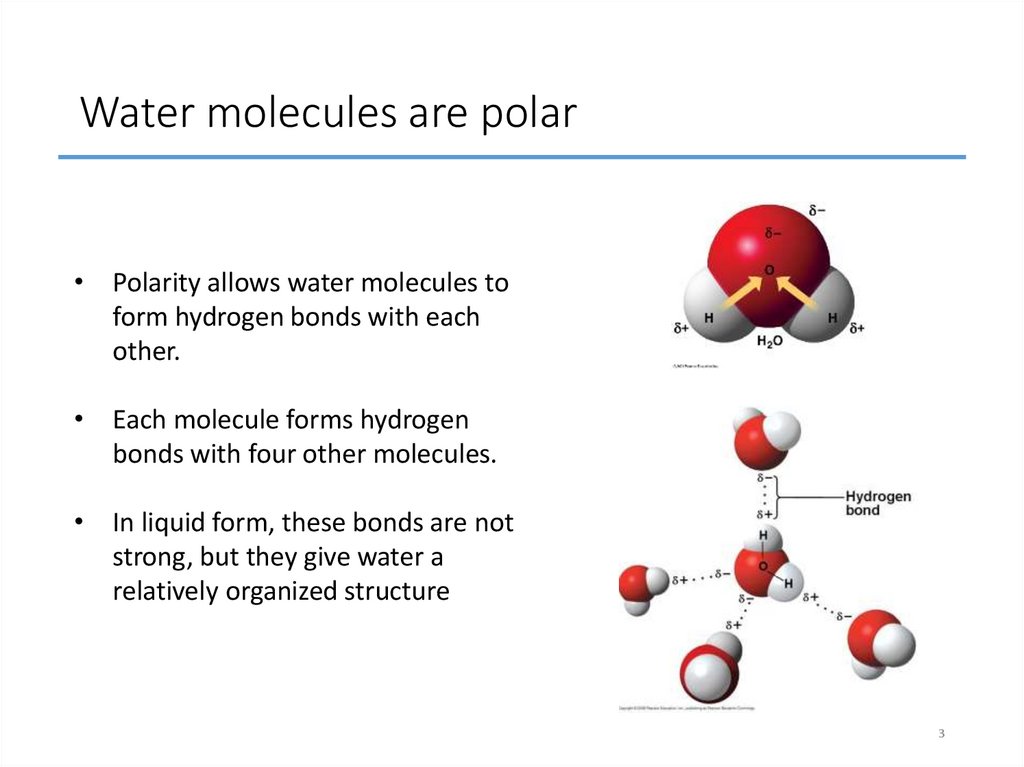
3. Water molecules are polar
• Polarity allows water molecules toform hydrogen bonds with each
other.
• Each molecule forms hydrogen
bonds with four other molecules.
• In liquid form, these bonds are not
strong, but they give water a
relatively organized structure
3
4. The qualities of water that make it a special molecule
Cohesive behaviourAbility to moderate temperature
Expansion upon freezing
Versatility as a solvent
4
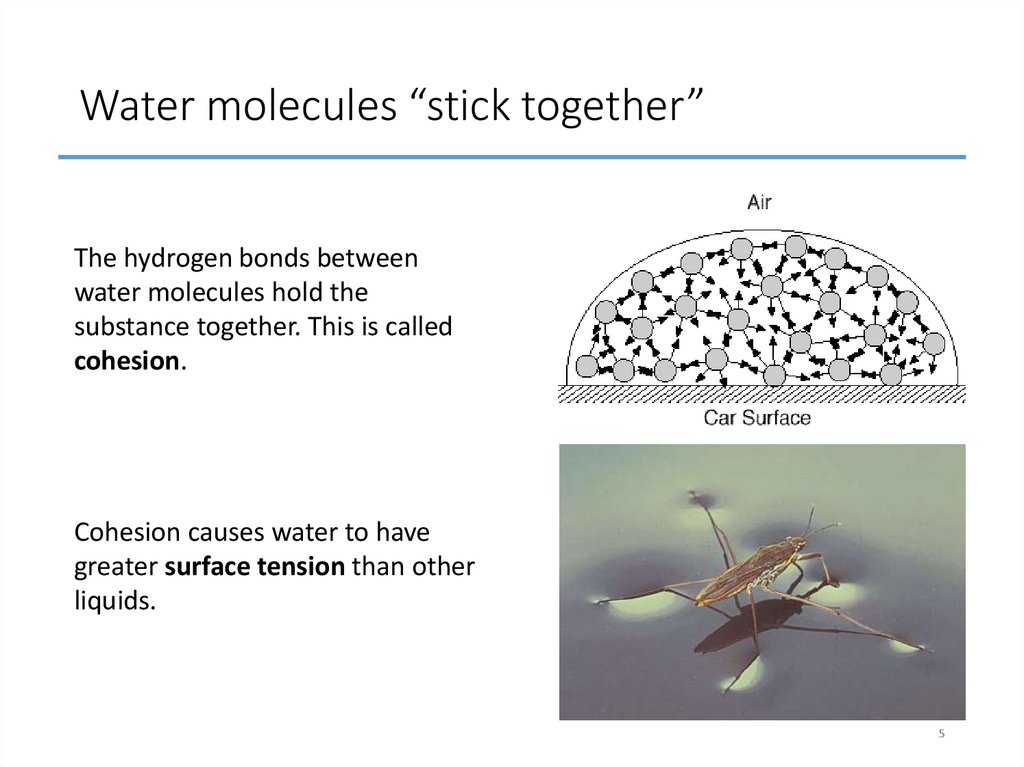
5. Water molecules “stick together”
The hydrogen bonds betweenwater molecules hold the
substance together. This is called
cohesion.
Cohesion causes water to have
greater surface tension than other
liquids.
5
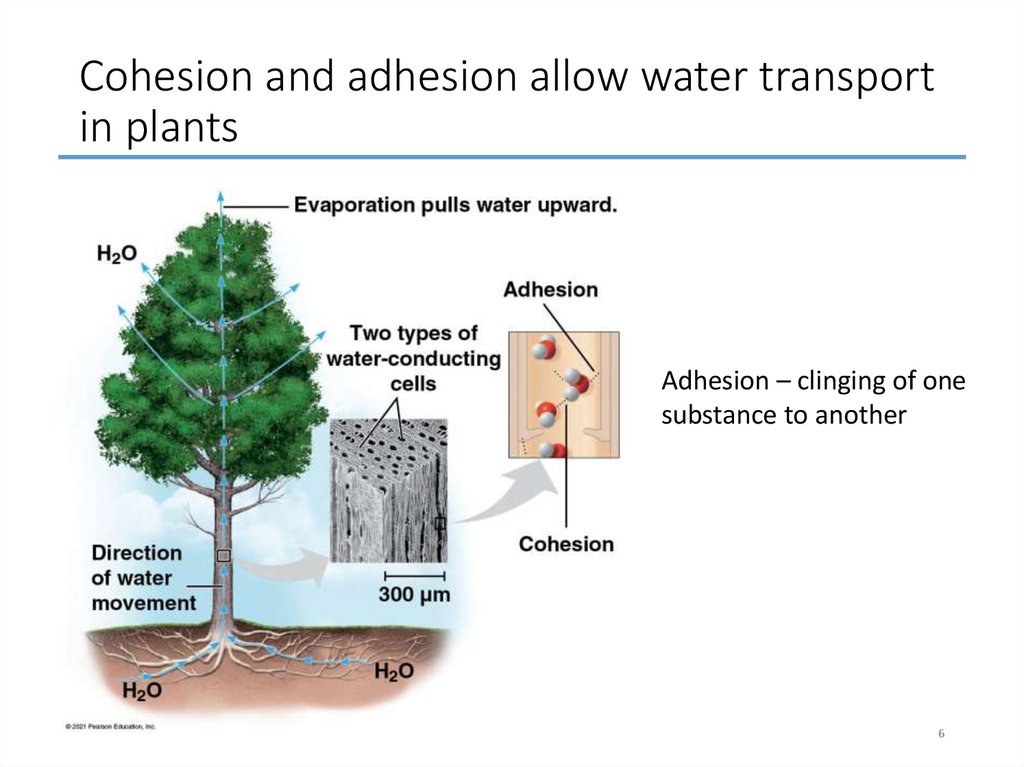
6. Cohesion and adhesion allow water transport in plants
Adhesion – clinging of onesubstance to another
6
7. Water is a temperature moderator
Water can absorb and release a large amount of heat with only a slightchange in its own temperature.
• Heat is a measure of the total amount of kinetic energy due to molecular motion
• Temperature measures the intensity of heat due to the average kinetic energy of
molecule.
• A calorie (cal) is the amount of heat required to raise the temperature of 1 g of
water by 1°C
7
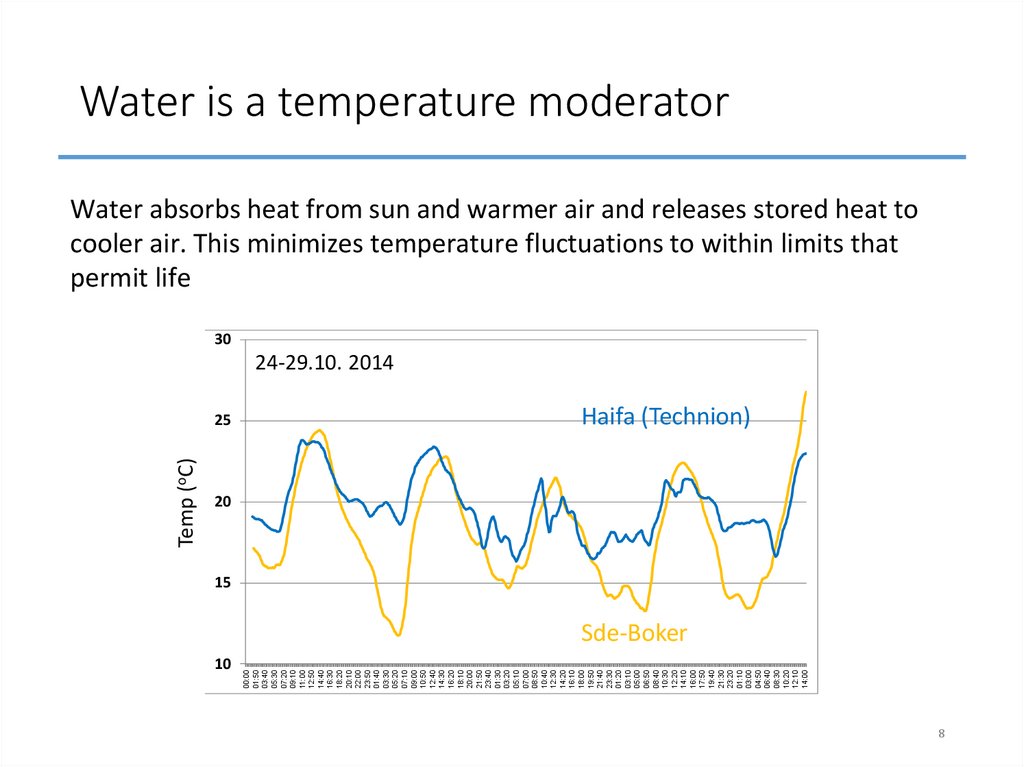
8. Water is a temperature moderator
Water absorbs heat from sun and warmer air and releases stored heat tocooler air. This minimizes temperature fluctuations to within limits that
permit life
30
24-29.10. 2014
Temp (oC)
25
Haifa (Technion)
20
15
Sde-Boker
10
8
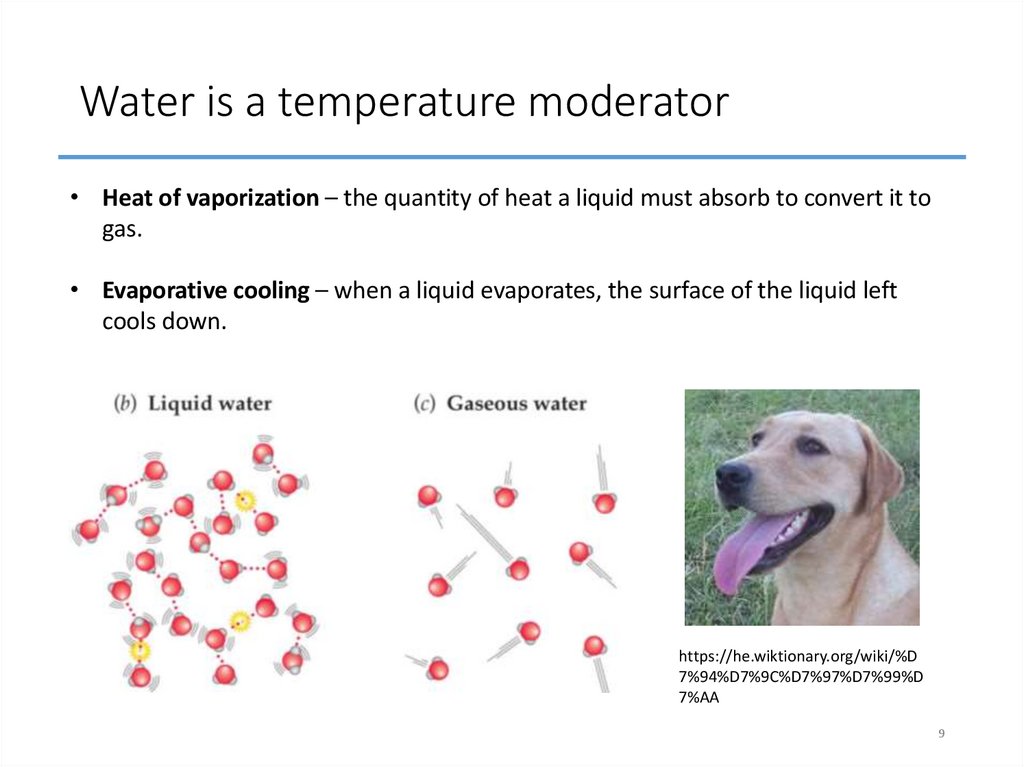
9. Water is a temperature moderator
• Heat of vaporization – the quantity of heat a liquid must absorb to convert it togas.
• Evaporative cooling – when a liquid evaporates, the surface of the liquid left
cools down.
https://he.wiktionary.org/wiki/%D
7%94%D7%9C%D7%97%D7%99%D
7%AA
9
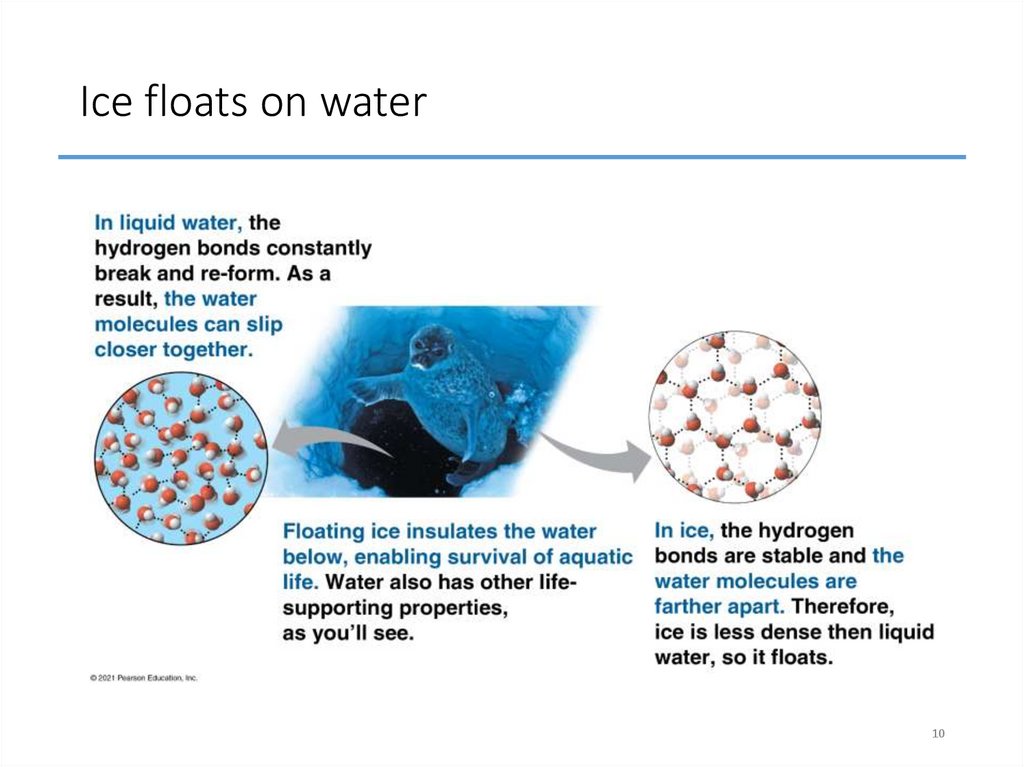
10. Ice floats on water
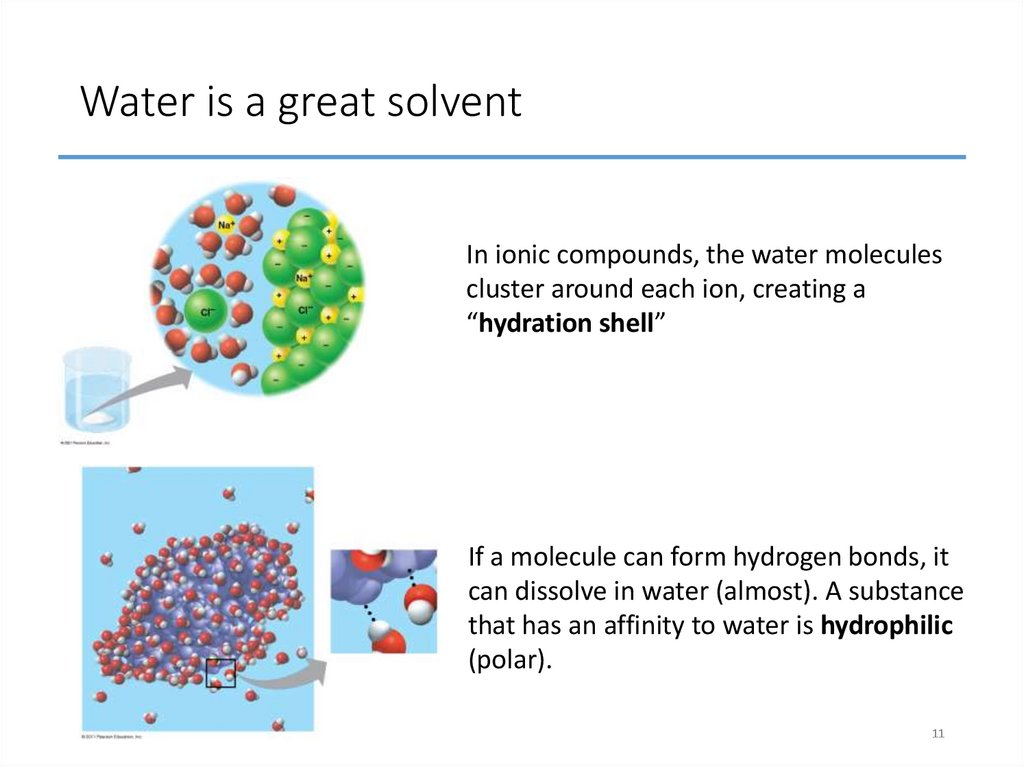
1011. Water is a great solvent
In ionic compounds, the water moleculescluster around each ion, creating a
“hydration shell”
If a molecule can form hydrogen bonds, it
can dissolve in water (almost). A substance
that has an affinity to water is hydrophilic
(polar).
11
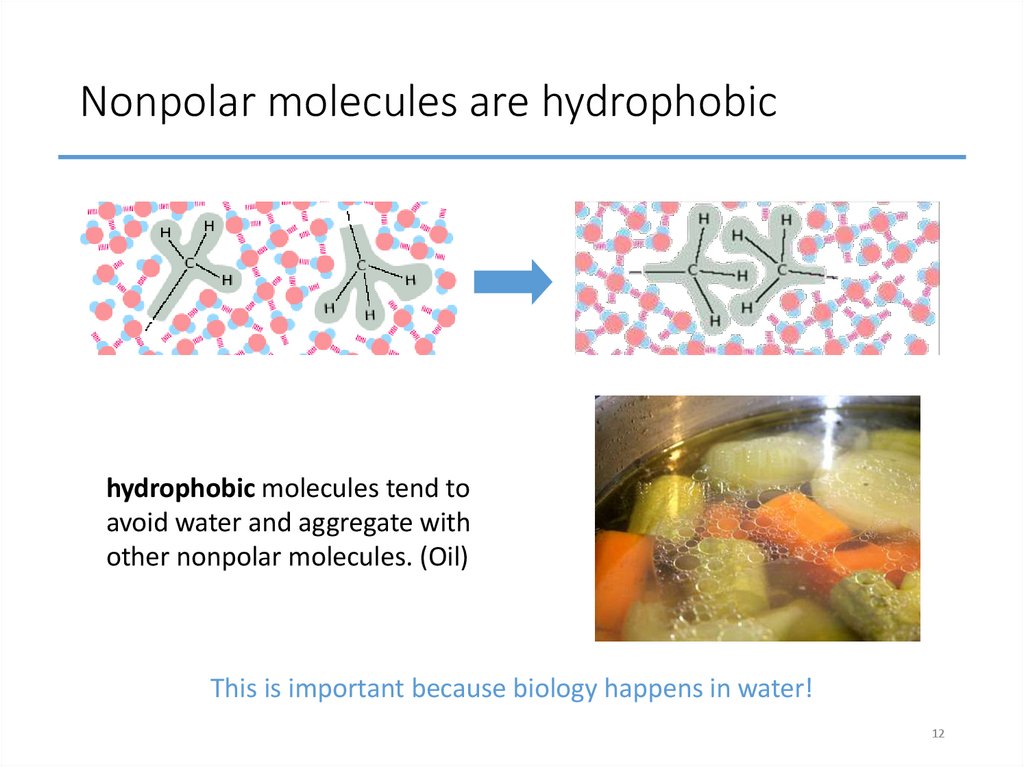
12. Nonpolar molecules are hydrophobic
hydrophobic molecules tend toavoid water and aggregate with
other nonpolar molecules. (Oil)
This is important because biology happens in water!
12
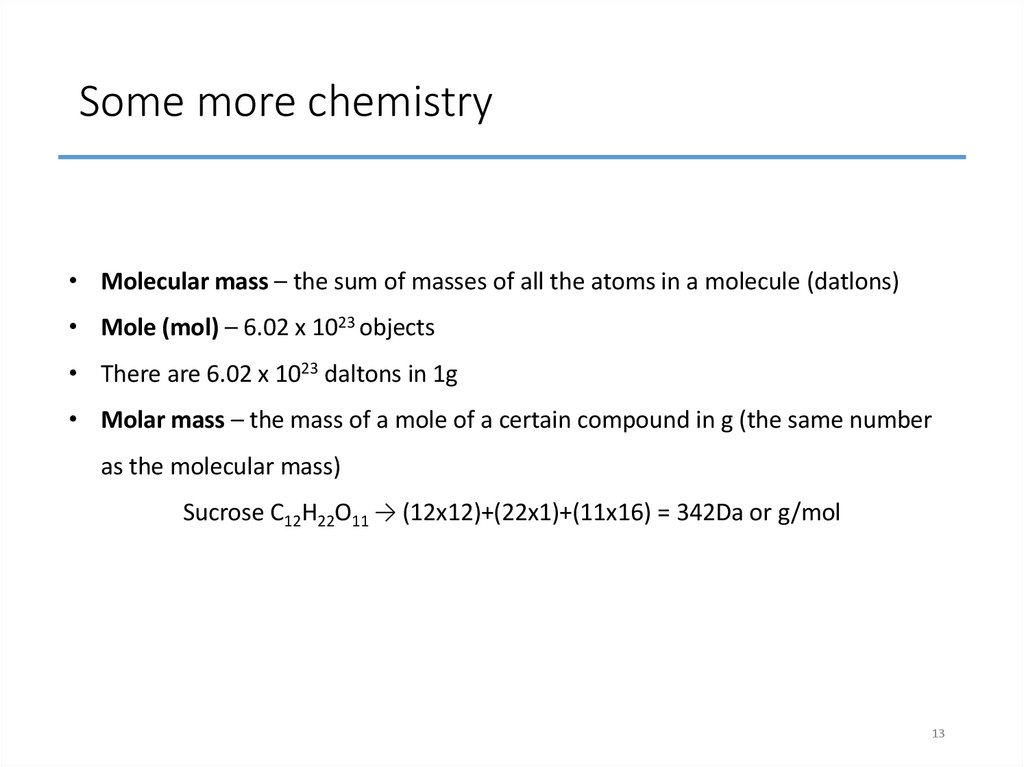
13. Some more chemistry
• Molecular mass – the sum of masses of all the atoms in a molecule (datlons)• Mole (mol) – 6.02 x 1023 objects
• There are 6.02 x 1023 daltons in 1g
• Molar mass – the mass of a mole of a certain compound in g (the same number
as the molecular mass)
Sucrose C12H22O11 → (12x12)+(22x1)+(11x16) = 342Da or g/mol
13
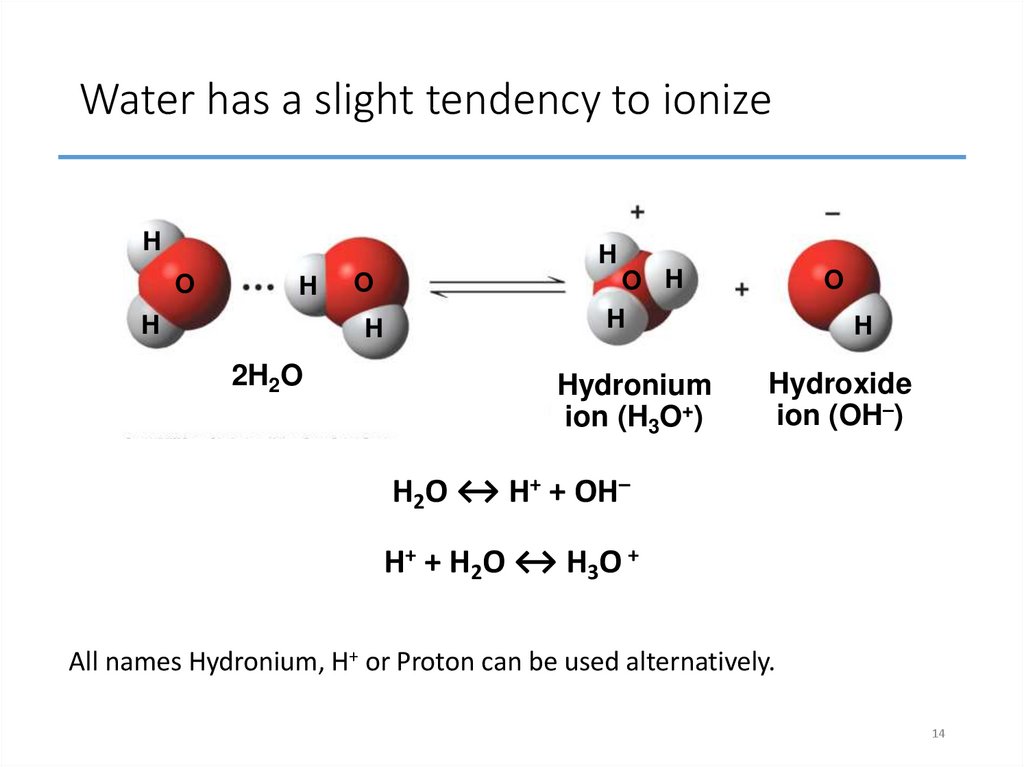
14. Water has a slight tendency to ionize
Fig. 3-UN2Water has a slight tendency to ionize
H
H
O
H
H
2H2O
O
O H
H
H
Hydronium
ion (H3O+)
O
H
Hydroxide
ion (OH–)
H2O ↔ H+ + OH–
H+ + H2O ↔ H3O +
All names Hydronium, H+ or Proton can be used alternatively.
14
15. Acids and bases
The concentration of H+ in pure water is equal to that of OH- and is very low (Onein 554,000,000 molecules). The concentration of H+ in pure water in 25°C is 10-7M.
H2O ↔ H+ + OH–
Some substances can be shift this balance when dissolved in water:
Acid – a substance that increases the H+ concentration of a solution
HCl → H+ + Clstrong acid
H2CO3↔ HCO3- + H+
weak acid
Base – a substance that reduces the H+ concentration of a solution
NaOH → Na+ + OH–
releases OHNH3 + H+ ↔ NH4+
takes H+
15
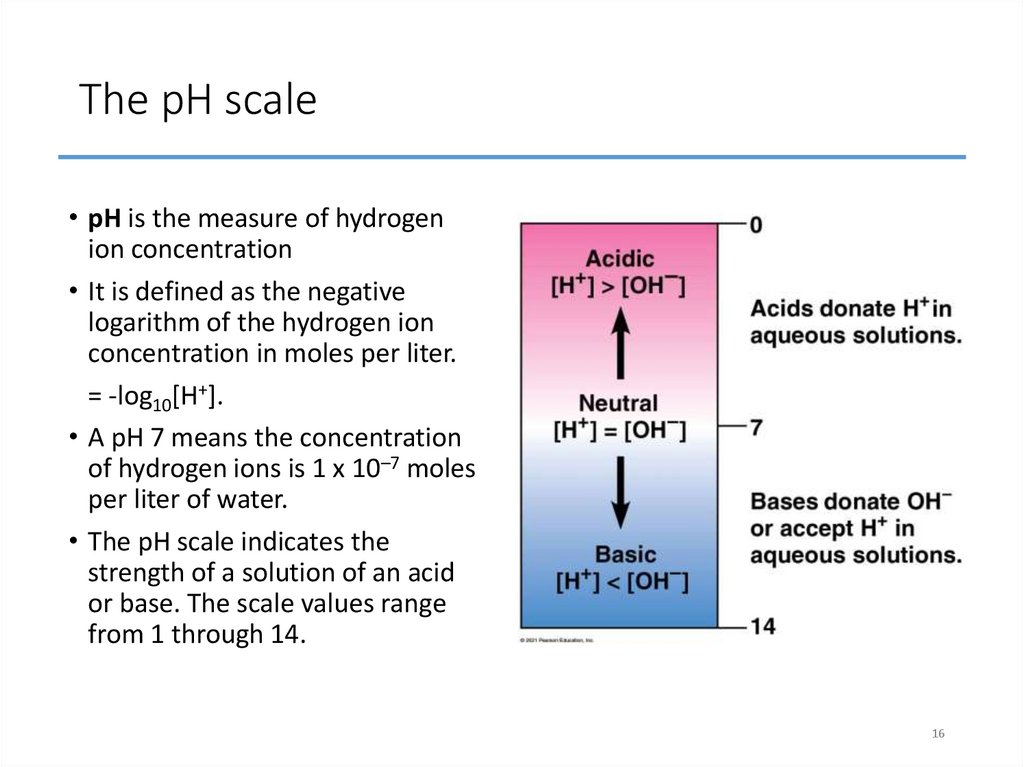
16. The pH scale
• pH is the measure of hydrogenion concentration
• It is defined as the negative
logarithm of the hydrogen ion
concentration in moles per liter.
= -log10[H+].
• A pH 7 means the concentration
of hydrogen ions is 1 x 10–7 moles
per liter of water.
• The pH scale indicates the
strength of a solution of an acid
or base. The scale values range
from 1 through 14.
16
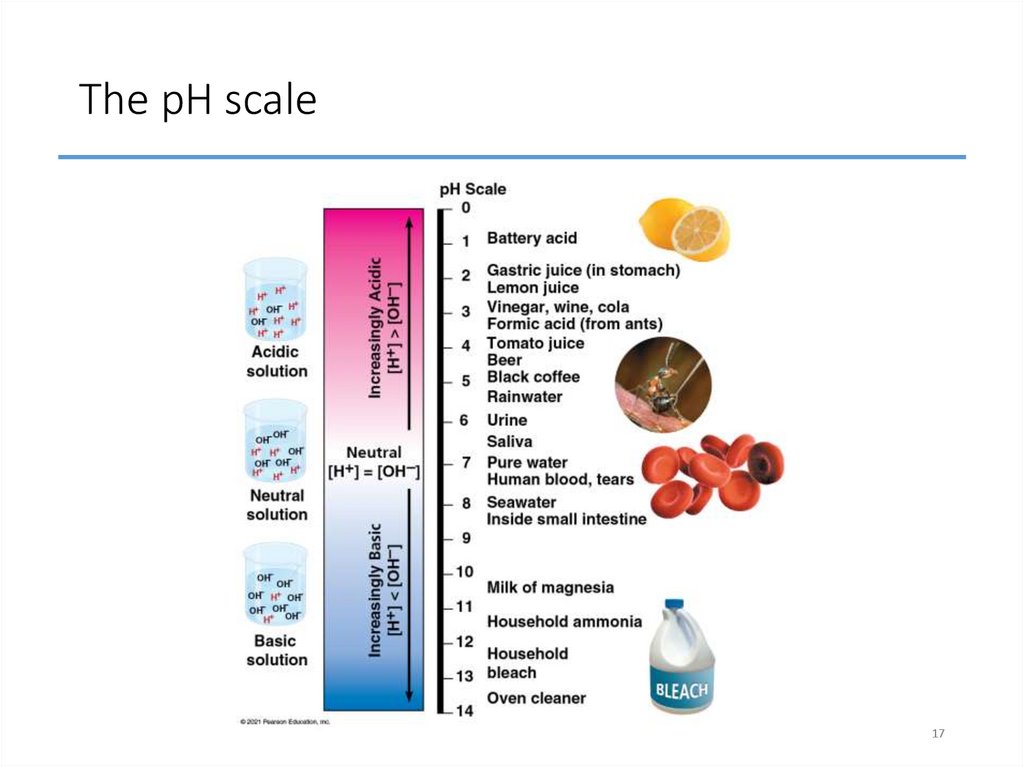
17. The pH scale
1718. Organisms are able to maintain stable pH
• The properties on the cell are very sensitive to changes in pH since theH+ ion is so reactive
• pH of blood is 7.4 and is normally very stable (pH of 7 or 7.8 means
death).
• Buffer – a substance that minimizes the changes in the concentration of
H+ and OH- in a solution. It accepts H+ ion when they are in excess and
donates them when they are depleted
H2CO3↔ HCO3- + H+
Carbonic acid
18



 chemistry
chemistry








