Similar presentations:
ПР 9
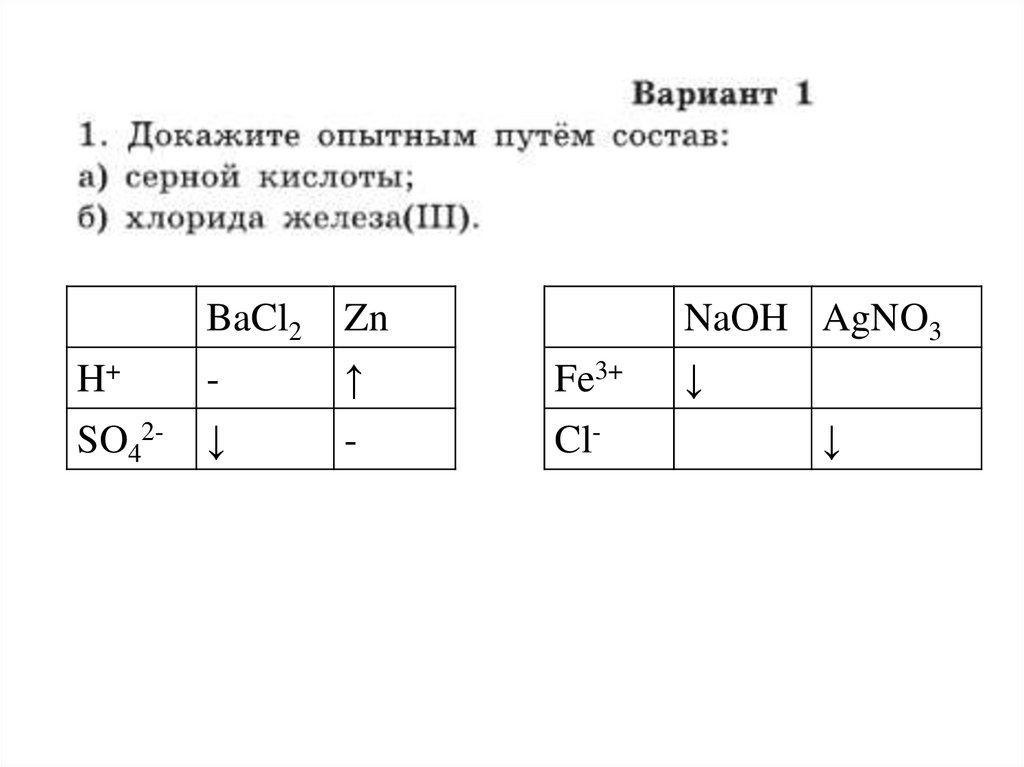
1.
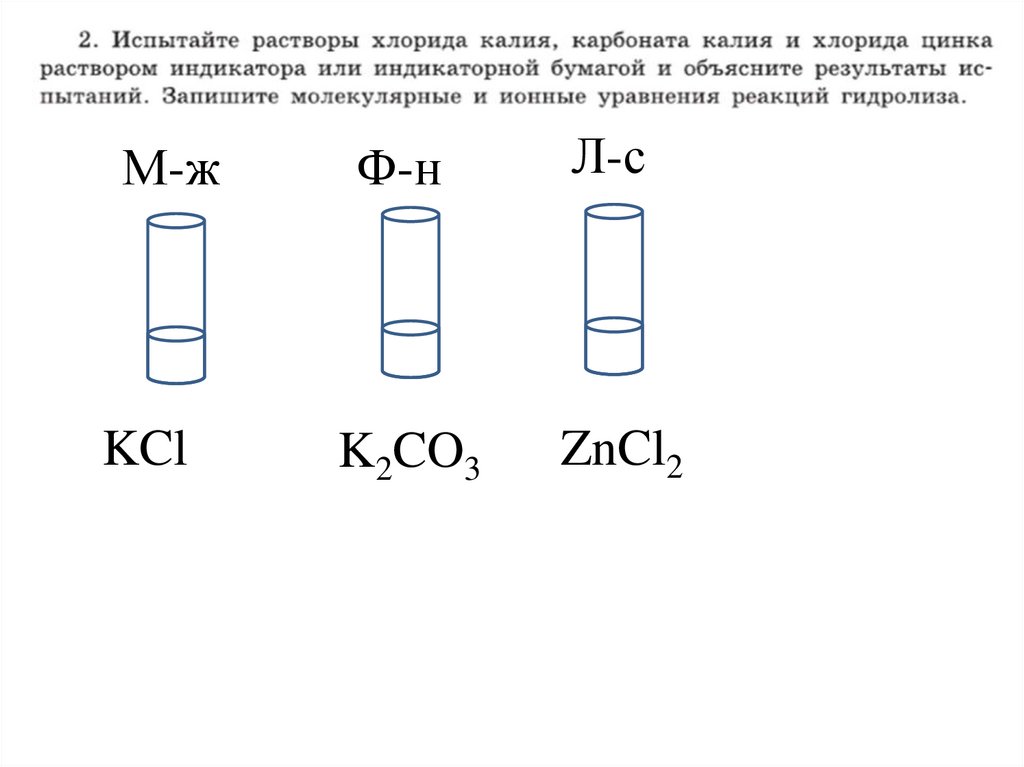
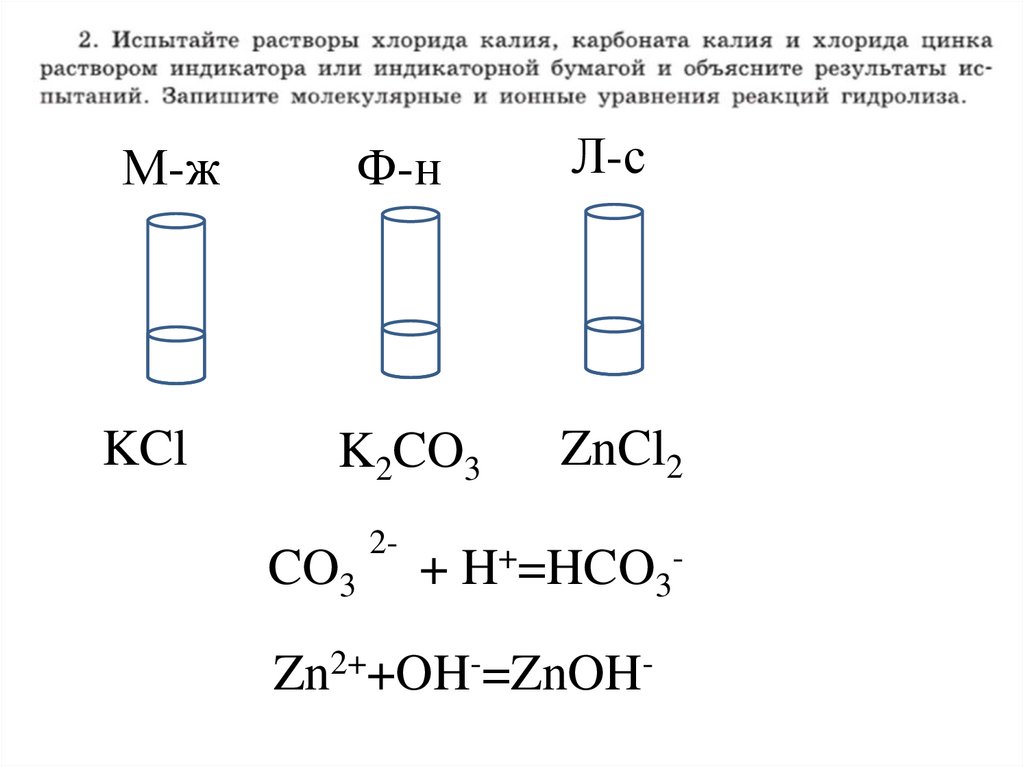
2.
BaCl2Zn
NaOH AgNO3
H+
-
↑
Fe3+
SO42-
↓
-
Cl-
↓
↓
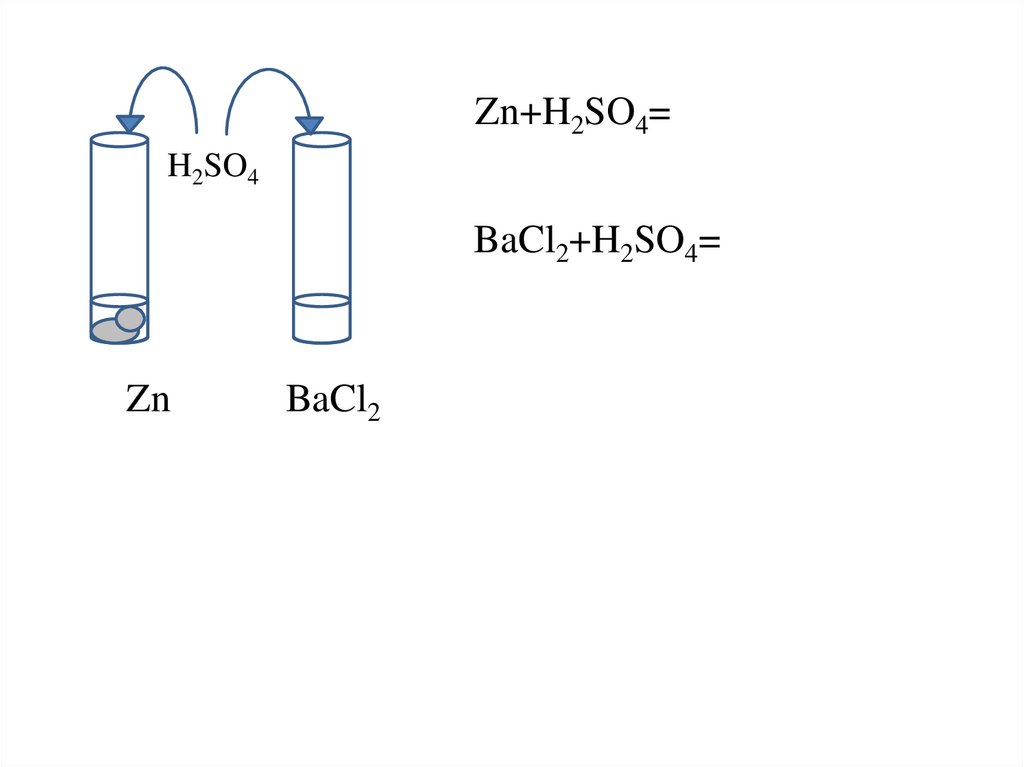
3.
Zn+H2SO4=H2SO4
BaCl2+H2SO4=
Zn
BaCl2
4.
М-жФ-н
Л-с
KCl
K2CO3
ZnCl2
5.
М-жФ-н
Л-с
KCl
K2CO3
ZnCl2
CO3
2-
+ H+=HCO3-
Zn2++OH-=ZnOH-
6.
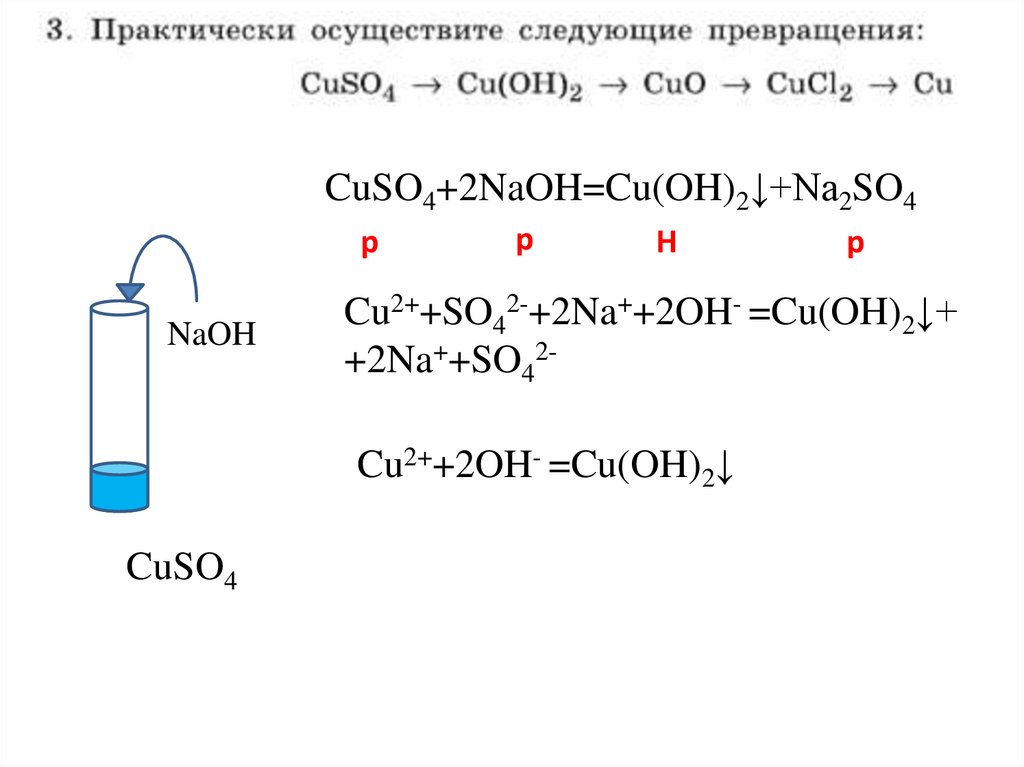
CuSO4+2NaOH=Cu(OH)2↓+Na2SO4p
NaOH
p
H
Cu2++SO42-+2Na++2OH- =Cu(OH)2↓+
+2Na++SO42Cu2++2OH- =Cu(OH)2↓
CuSO4
p
7.
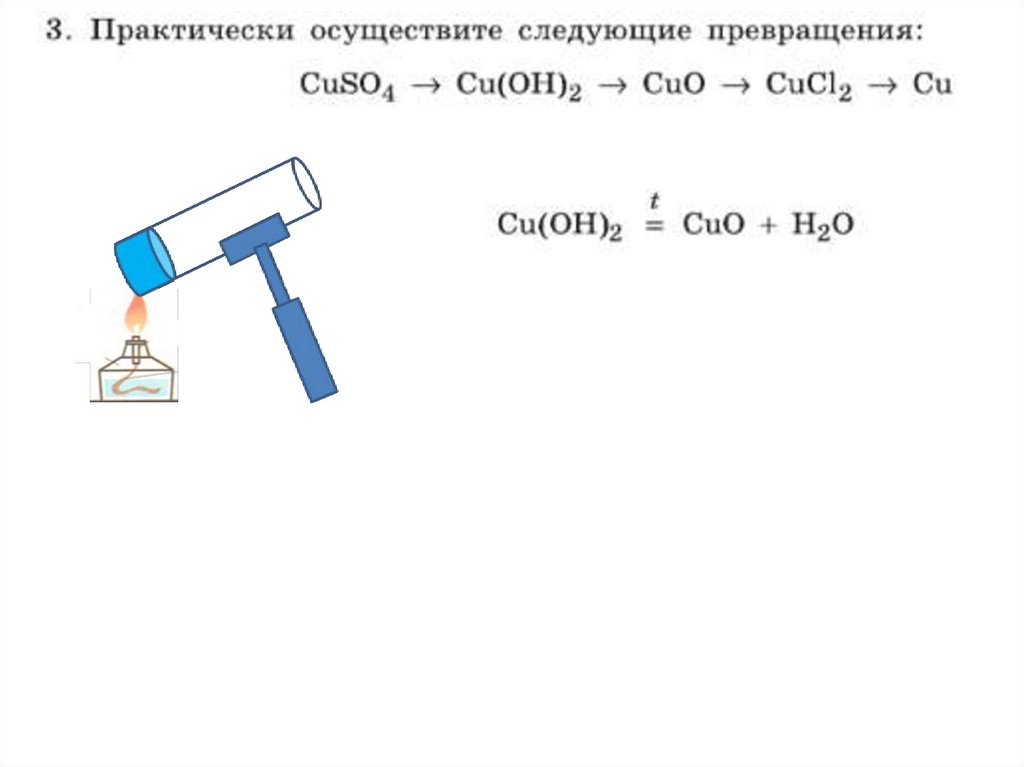
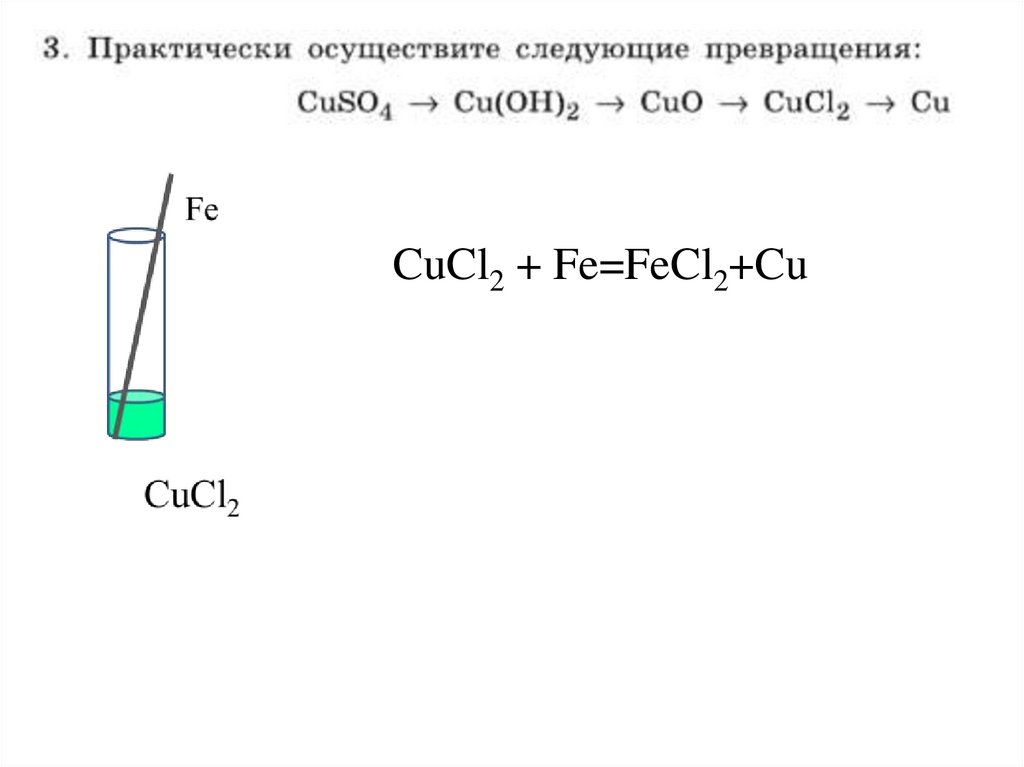
8.
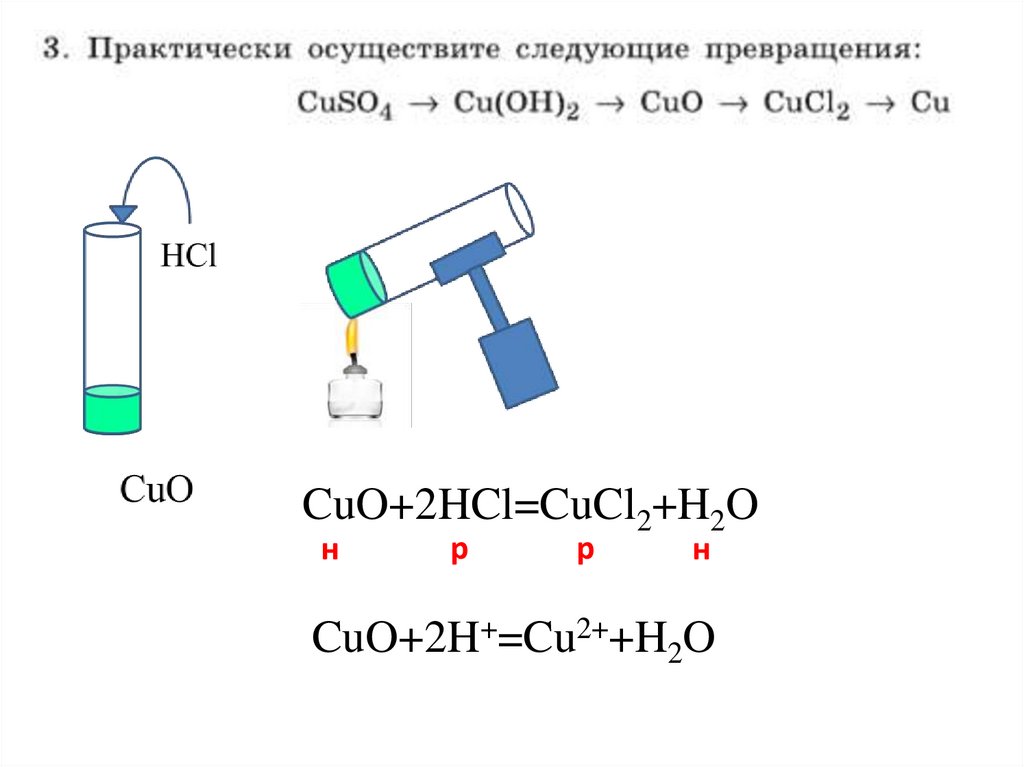
CuO+2HCl=CuCl2+H2Oн
p
p
н
CuO+2H+=Cu2++H2O