Similar presentations:
Table of Content. Neurotech company introduction
1. Table of Content
• Neurotech company introduction• Neurotech history
• Neurotech product overview
• Russian MD market objectives
• Competitors’ analysis
• Demand estimation
• Questions to be discussed
2. Neurotech company introduction
• We establish core technologies in instrumentation and software,which are subsequently integrated to produce final solutions
• We manage the full equipment development cycle, from idea
generation to mass production
• We operate in the open market, without external clients or
contracts
• We perform high-tech serial production in compliance with ISO
13485 quality standards
• We prototype technical solutions in circuit design, engineering,
and industrial design
• We develop software for desktop and mobile devices
Mission: creation of innovative medical devices and systems that
3. Neurotech History
• 1992 Neurotech was founded in Pyatigorsk• 1993 first electroencephalograph and echoencephalograph were introduced
to the market
• 1994 echoencephalograph by S.N. Bulanov introduced to the market,
biofeedback device prototype
• 1996 first electromyograph introduced to the market
• 2002 company relocation to Taganrog, consolidating its operations.
• 2004 "Synapsis" EMG
• 2009 "MIST" electromyograph
• 2012 products registration in the Uzbekistan market and the foundation
of a company in Poland.
• 2016 foundation of BrainBit Inc. in the USA
• 2017 development of dry-electrode neuro-interfaces,
• 2022 IT company "ProSoftLab“ foundation
• 2024 integration with the biotechnical laboratory Neiry for
intensification of innovation path.
4. Neurotech product overview
Functional diagnostics:• electroencephalographs,
• Electromyographs,
• echoencephalographs
Rehabilitation products:
• multifunctional EMG-based system MIST for nerve localization,
biofeedback sessions for paralyzed and weakened muscles and
physiotherapeutic neuromuscular stimulation.
• «Kolibri» biofeedback system for musculoskeletal correction
• «Kolibri» biofeedback system for psycho-emotional correction
Dentistry products:
• Dental muscle stimulator «MIST TENS»
• Dental electromyograph «Synapsis»
• Wireless dental system «Kolibri»
Urogynecology/proctology:
• Biofeedback system «Callibri BeFit PRO»
5. Russian MD market objectives
Any Medical device in the Russian market is classified according to:• Risk Class (1, 2a, 2b, 3)
• Medical Device Nomenclature Code – a 6-digit code standing to denote a
generalized group of products united by intended use, operation
principle, supposed appearance, sterility/ non-sterility.
• All-Russian Product Classification by Economic Activity
NB: The following information about a Medical Device is public for all
participants of the market:
• Manufacturer name & manufacturer’s address
• Instructions for Use
• Photos of the Medical Device, individual and group packaging included
Federal Law No. 394-FZ of July 31, 2023 "On the Ratification of the
Protocol on Amending the Agreement on Common Principles and Rules for
the Circulation of Medical Devices (Medical Equipment and Medical
Appliances) within the Eurasian Economic Union dated December 23, 2014"
A MD registered after 2026 will be simultaneously admitted to
circulation in the markets of Russia, Belorus, Armenia, Kyrgyzstan,
Kazakstan
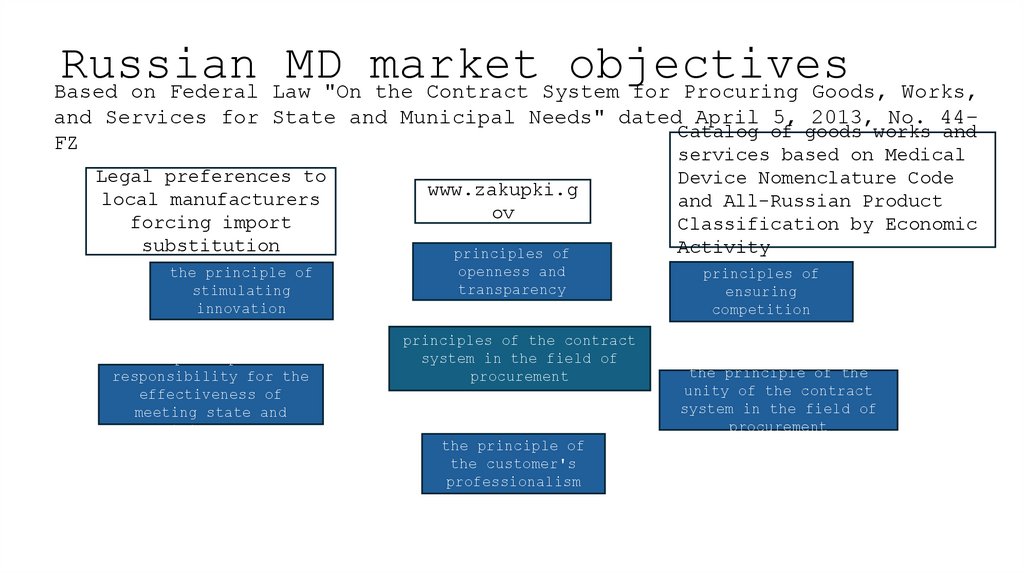
6. Russian MD market objectives
Based on Federal Law "On the Contract System for Procuring Goods, Works,and Services for State and Municipal Needs" dated April 5, 2013, No. 44Catalog of goods works and
FZ
Legal preferences to
local manufacturers
forcing import
substitution
the principle of
stimulating
innovation
the principle of
responsibility for the
effectiveness of
meeting state and
municipal needs
www.zakupki.g
ov
principles of
openness and
transparency
principles of the contract
system in the field of
procurement
the principle of
the customer's
professionalism
services based on Medical
Device Nomenclature Code
and All-Russian Product
Classification by Economic
Activity
principles of
ensuring
competition
the principle of the
unity of the contract
system in the field of
procurement
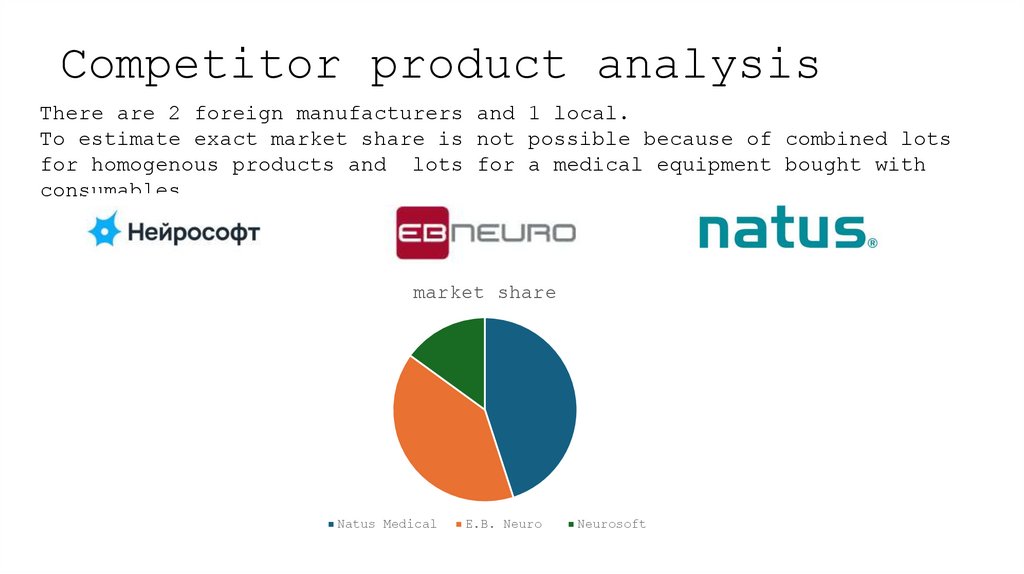
7. Competitor product analysis
There are 2 foreign manufacturers and 1 local.To estimate exact market share is not possible because of combined lots
for homogenous products and lots for a medical equipment bought with
consumables.
market share
Natus Medical
E.B. Neuro
Neurosoft
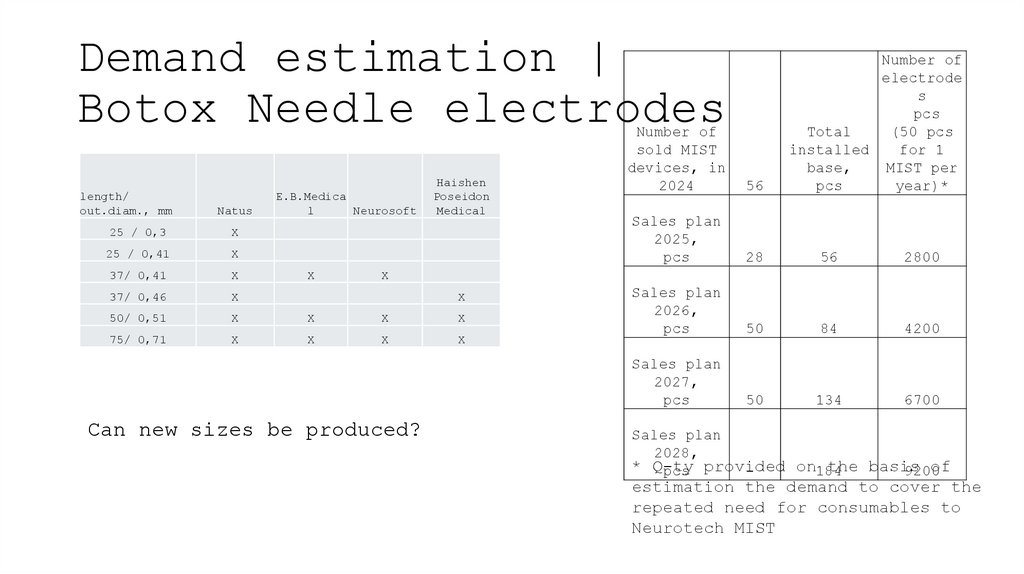
8. Demand estimation | Botox Needle electrodes
length/out.diam., mm
Natus
E.B.Medica
l
Neurosoft
Haishen
Poseidon
Medical
25 / 0,3
X
25 / 0,41
X
37/ 0,41
X
37/ 0,46
X
50/ 0,51
X
X
X
X
75/ 0,71
X
X
X
X
X
Number of
electrode
s
pcs
Total
(50 pcs
installed
for 1
base,
MIST per
pcs
year)*
Number of
sold MIST
devices, in
2024
56
Sales plan
2025,
pcs
28
56
2800
Sales plan
2026,
pcs
50
84
4200
Sales plan
2027,
pcs
50
134
6700
X
X
Can new sizes be produced?
Sales plan
2028,
* Q-ty
on184
the basis
of
pcs provided
9200
estimation the demand to cover the
repeated need for consumables to
Neurotech MIST
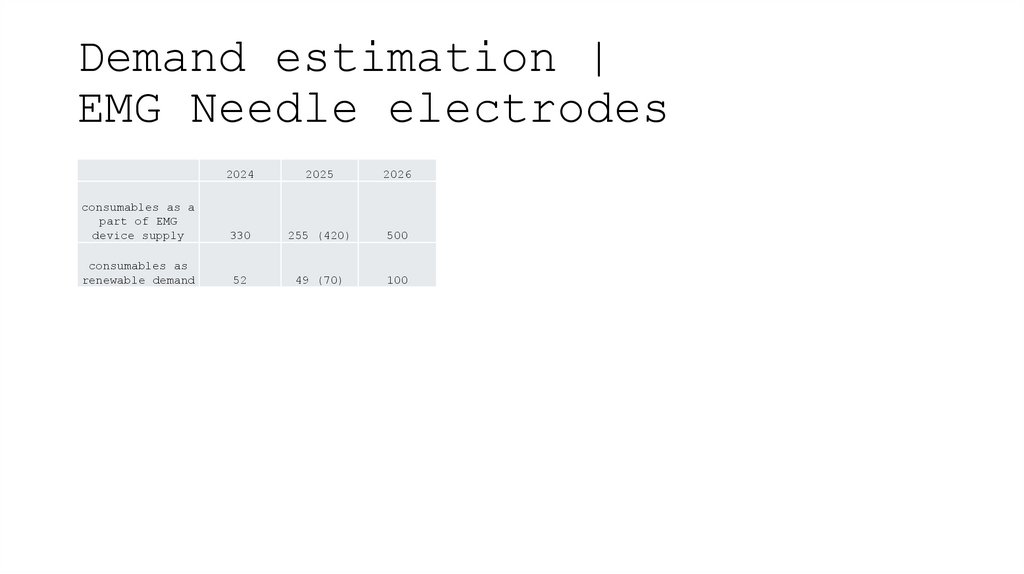
9. Demand estimation | EMG Needle electrodes
20242025
2026
consumables as a
part of EMG
device supply
330
255 (420)
500
consumables as
renewable demand
52
49 (70)
100
10. Questions to be discussed
1.Exclusive rights.At present the cost of the registration procedure is around USD 95.000
– 98.000. Investments shall be guaranteed by an expected investment
return period.
2. OEM Manufacturing under the brand name Neurotech
The current path to forcing import substitution will further more and
more limit the share of foreign brands in the medical device market.
3. Territory for exclusive representation
The products of Neurotech have registration in Kazakhstan (a jointventure company) and in Uzbekistan. Taking into consideration the
registration novelties we ask for the exclusivity for the territory of 6
countries – Russia, Kazakhstan, Uzbekistan, Belorus, Armenia, Kyrgizstan.
4. Expected annual volumes per product groups
To be discussed for the agreed territories individually.





 programming
programming