Similar presentations:
Introduction of lepu medical
1.
Introduction ofLEPU MEDICAL
Copyright © Lepu Medical Technology(Beijing)Co.,Ltd. All Rights Reserved.
2.
Outline of PresentationIntroduction of Lepu Medical Group
Product Portfolio
Prosthesis Implant
Trauma
Spine
Sports Medicine
Exhibition
3.
Introduction ofLepu Medical
Group
4.
Now, We have80+ subsidiaries globally
Establish in 1999
11000+ Employees globally
24 years experiences in
manufacturing medical devices
6.3 Billion USD Market
Value
HeadquarterLocatedinBeijing,China
Copyright © Lepu Medical Technology(Beijing)Co.,Ltd. All Rights Reserved.
* Statistics by March 21, 2023
5.
2022TOP 100
presented by
Medical Device
Companies by
Sales
NO. 62
Copyright © Lepu Medical Technology(Beijing)Co.,Ltd. All Rights Reserved.
6.
2023MTI 100
presented by
Medical Device Manufacturingb
Companies
NO. 58
Copyright © Lepu Medical Technology(Beijing)Co.,Ltd. All Rights Reserved.
7.
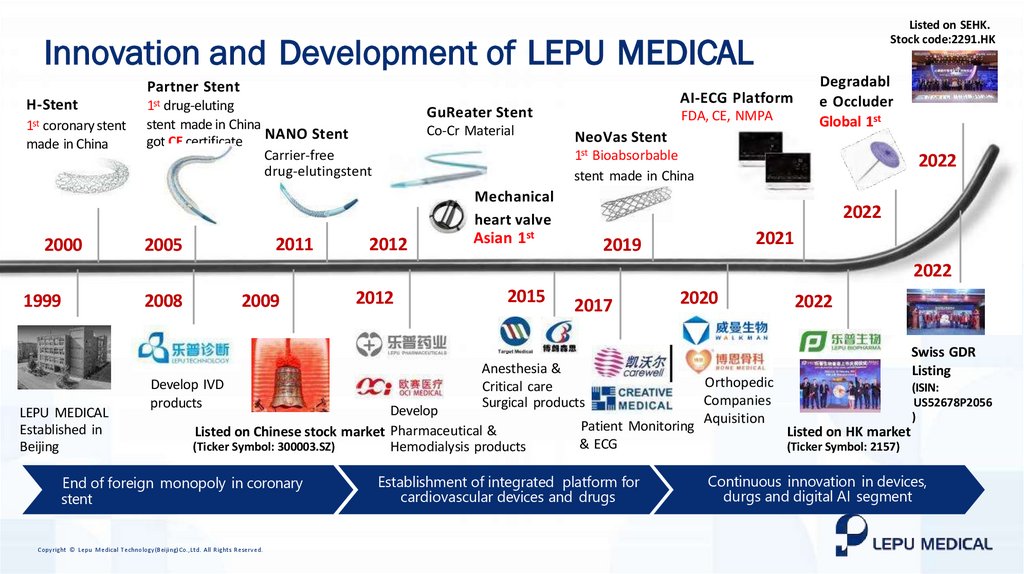
Listed on SEHK.Stock code:2291.HK
Innovation and Development of LEPU MEDICAL
Partner Stent
H-Stent
1st coronary stent
made in China
1st drug-eluting
stent made in China
got CE certificate NANO Stent
Carrier-free
drug-elutingstent
AI-ECG Platform
GuReater Stent
Co-Cr Material
FDA, CE, NMPA
NeoVas Stent
Degradabl
e Occluder
Global 1st
1st Bioabsorbable
stent made in China
2022
Mechanical
heart valve
2000
2011
2005
2012
2022
Asian 1st
2021
2019
2022
1999
LEPU MEDICAL
Established in
Beijing
2008
2009
Develop IVD
products
2012
2015
Copyright © Lepu Medical Technology(Beijing)Co.,Ltd. All Rights Reserved.
2020
2022
Swiss GDR
Listing
Anesthesia &
Critical care
Surgical products
Develop
Listed on Chinese stock market Pharmaceutical &
(Ticker Symbol: 300003.SZ)
Hemodialysis products
End
foreign
monopoly
in coronaryin
stent
Endofof
foreign
monopoly
coronary
stent
2017
Orthopedic
Companies
Patient Monitoring Aquisition
& ECG
Establishment of integrated platform for
cardiovascular devices and drugs
(ISIN:
US52678P2056
)
Listed on HK market
(Ticker Symbol: 2157)
Continuous innovation in devices, drugs and digital AI
Continuous innovation
segment in devices,
durgs and digital AI segment
8.
Sales Revenue Review and R&D INVESTMENTS2016-2023 Sales Revenue(Million USD)
1523 1516
1114 1148
1140
908
Proportion of Revenue in 2023
Main Business +6.7 %
(year on year)
Service & Healthcare
15.81%
Medical Device
46.04%
651
495
2016
Medicine
38.15%
2017
2018
2019
2020
2021
2022
2023
R&D input from 2020-2023(Million USD)
183
153
159
The total R&D input of medical device and
115
service & healthcare accounts for almost 80%.
Patents until 2023
2020
2021
2022
Copyright © Lepu Medical Technology(Beijing)Co.,Ltd. All Rights Reserved.
Notes: Exchange rates adopted: $/CNY=7
2023
Total: 2069 (+419)
9.
CertificateYY/T0287-2017 idtISO 13485:2016
MDD 93/42/EEC
FDA 21 CFR part 820
Compliance to USA, Brazil, Japan Regulations
609 NMPA
Certificates for
Medical Device
Copyright © Lepu Medical Technology(Beijing)Co.,Ltd. All Rights Reserved.
194 NMPA
Certificates for
Medicine
Over 15,000m2 cleaning workshop under 100,000 Class
300+ professional QA& QC members support
Multiple onsite audits experience covering FDA, CE and third-party local facility review
241 CE
Certificates
33 FDA
Approvals
600+ Local
Registration
Certificates
10.
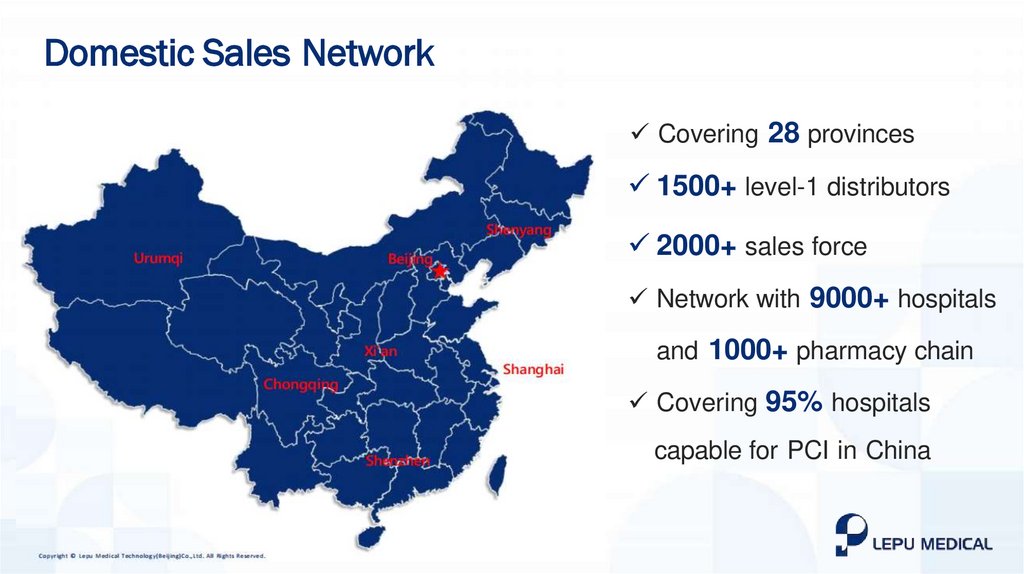
Domestic Sales NetworkCovering 28 provinces
1500+ level-1 distributors
2000+ sales force
Network with 9000+ hospitals
and 1000+ pharmacy chain
Covering 95% hospitals
capable for PCI in China
11.
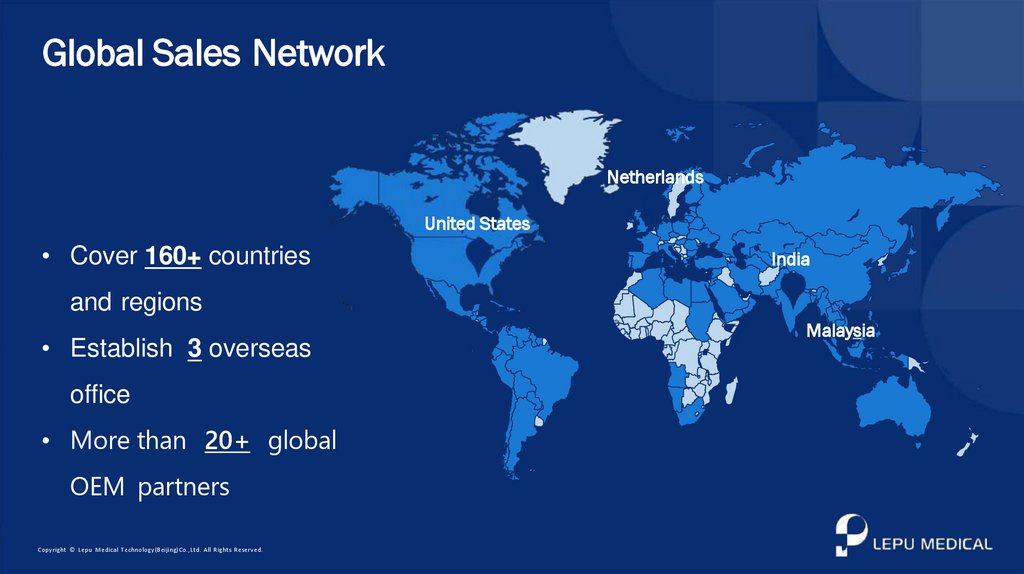
Global Sales NetworkNetherlands
United States
• Cover 160+ countries
India
and regions
• Establish 3 overseas
office
• More than 20+ global
OEM partners
Copyright © Lepu Medical Technology(Beijing)Co.,Ltd. All Rights Reserved.
7
Malaysia
12.
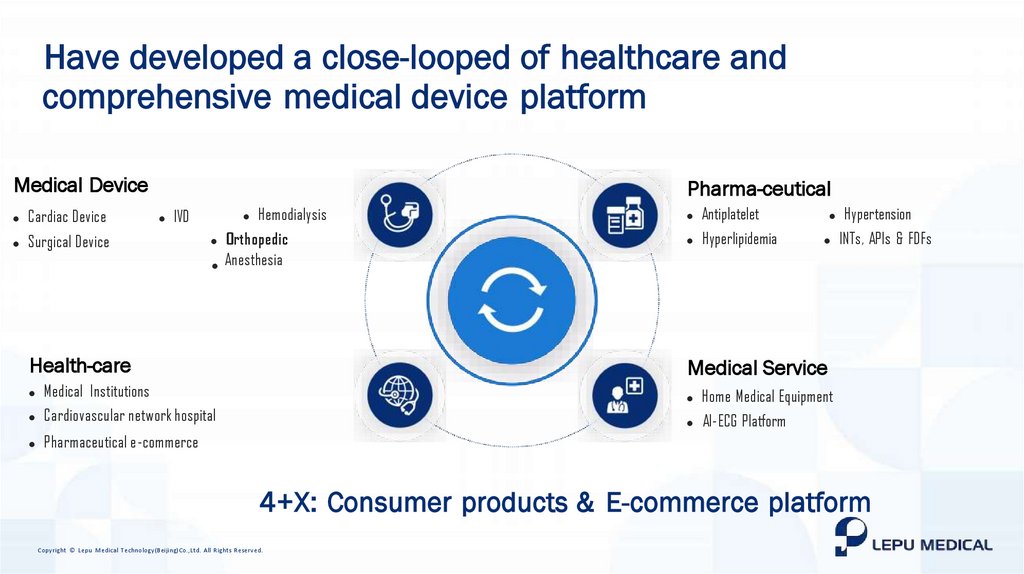
Have developed a close-looped of healthcare andcomprehensive medical device platform
Medical Device
l
Cardiac Device
l
Surgical Device
Pharma-ceutical
l
IVD
l
Hemodialysis
Orthopedic
l Anesthesia
l
Health-care
l
Medical Institutions
l
Cardiovascular network hospital
l
Pharmaceutical e-commerce
l
Antiplatelet
l
Hypertension
l
Hyperlipidemia
l
INTs, APIs & FDFs
Medical Service
l
Home Medical Equipment
l
AI-ECG Platform
4+X: Consumer products & E-commerce platform
Copyright © Lepu Medical Technology(Beijing)Co.,Ltd. All Rights Reserved.
13.
ProductPortfolio
14.
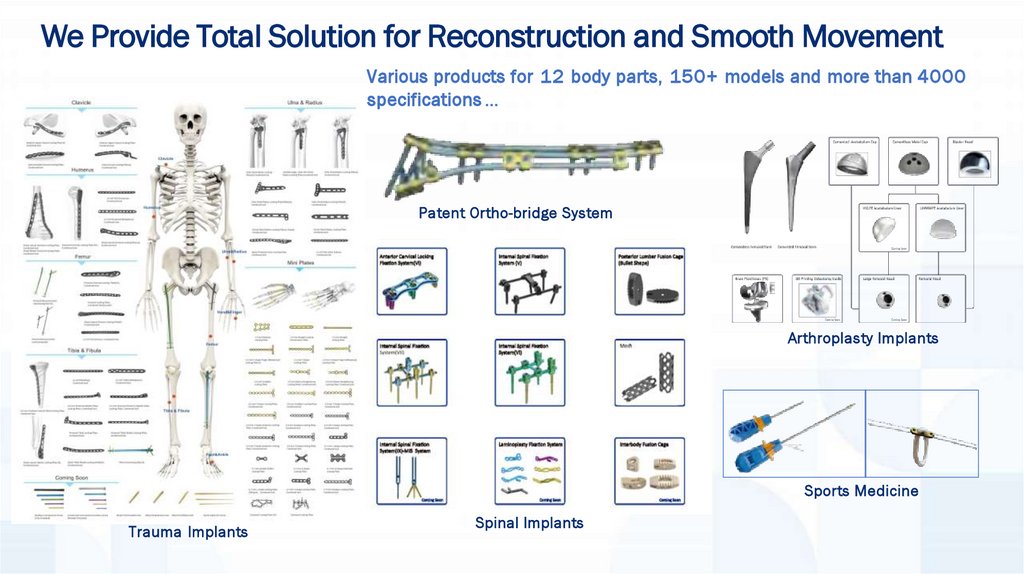
We Provide Total Solution for Reconstruction and Smooth MovementVarious products for 12 body parts, 150+ models and more than 4000
specifications …
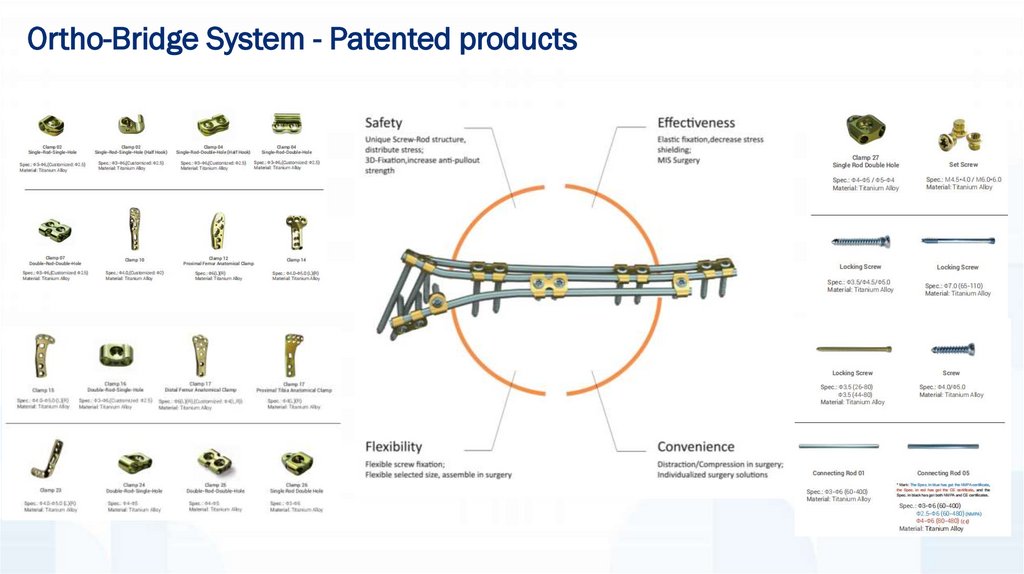
Patent Ortho-bridge System
Arthroplasty Implants
Sports Medicine
Trauma Implants
Spinal Implants
15.
ProsthesisImplant
16.
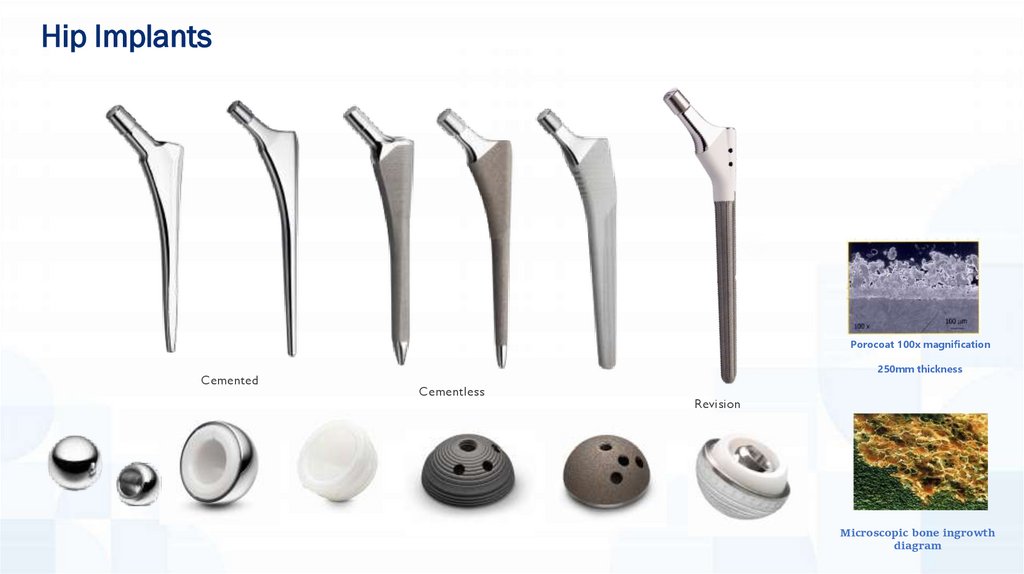
Hip ImplantsPorocoat 100x magnification
Cemented
250mm thickness
Cementless
Revision
Microscopic bone ingrowth
diagram
17.
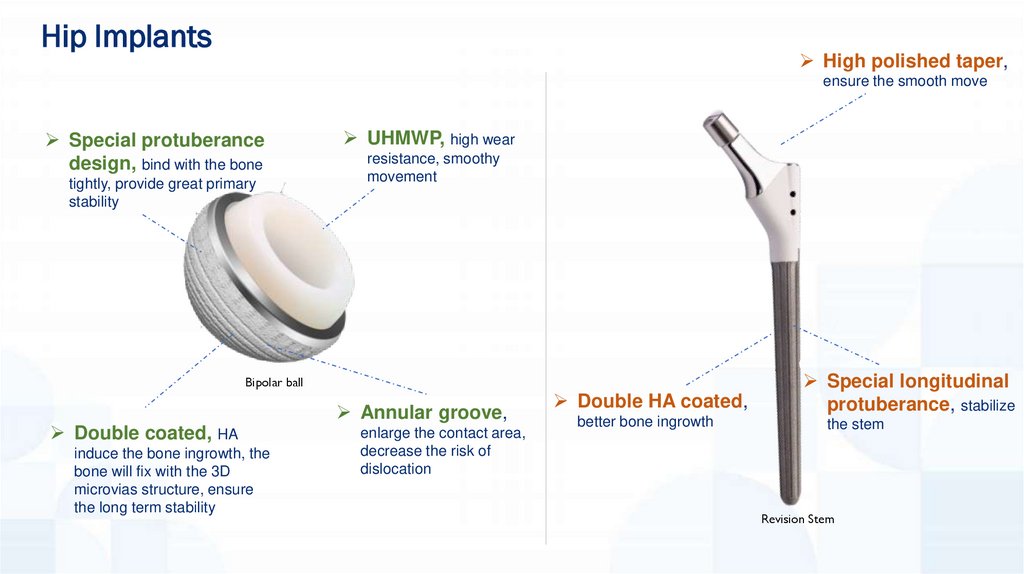
Hip ImplantsHigh polished taper,
ensure the smooth move
Special protuberance
design, bind with the bone
tightly, provide great primary
stability
UHMWP, high wear
resistance, smoothy
movement
Bipolar ball
Double coated, HA
induce the bone ingrowth, the
bone will fix with the 3D
microvias structure, ensure
the long term stability
Annular groove,
enlarge the contact area,
decrease the risk of
dislocation
Double HA coated,
better bone ingrowth
Special longitudinal
protuberance, stabilize
the stem
Revision Stem
18.
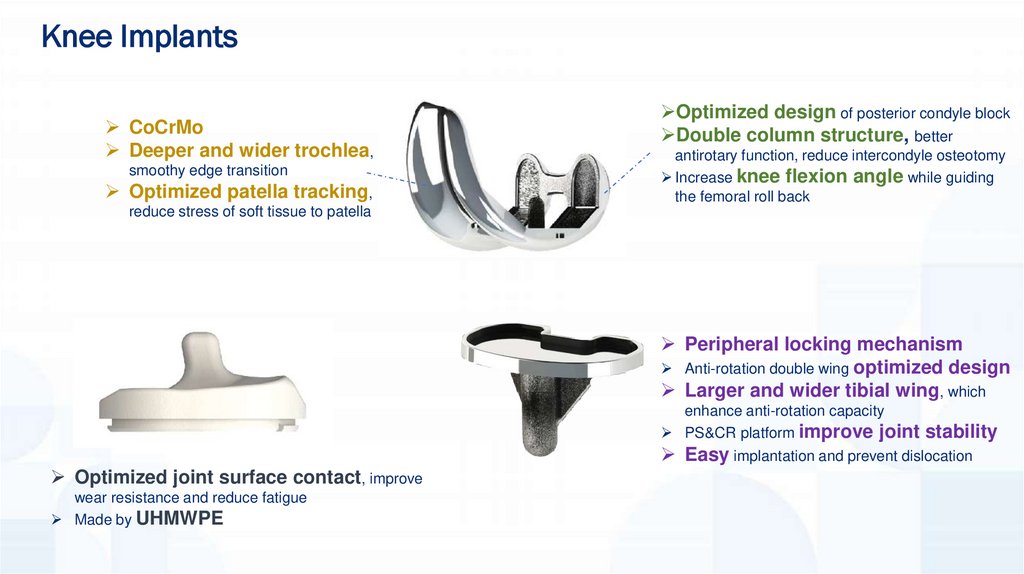
Knee ImplantsCoCrMo
Deeper and wider trochlea,
smoothy edge transition
Optimized patella tracking,
Optimized design of posterior condyle block
Double column structure, better
antirotary function, reduce intercondyle osteotomy
Increase knee flexion angle while guiding
the femoral roll back
reduce stress of soft tissue to patella
Peripheral locking mechanism
Anti-rotation double wing optimized design
Larger and wider tibial wing, which
enhance anti-rotation capacity
PS&CR platform improve joint stability
Easy implantation and prevent dislocation
Optimized joint surface contact, improve
wear resistance and reduce fatigue
Made by UHMWPE
19.
TraumaProduct
20.
Ortho-Bridge System - Patented products21.
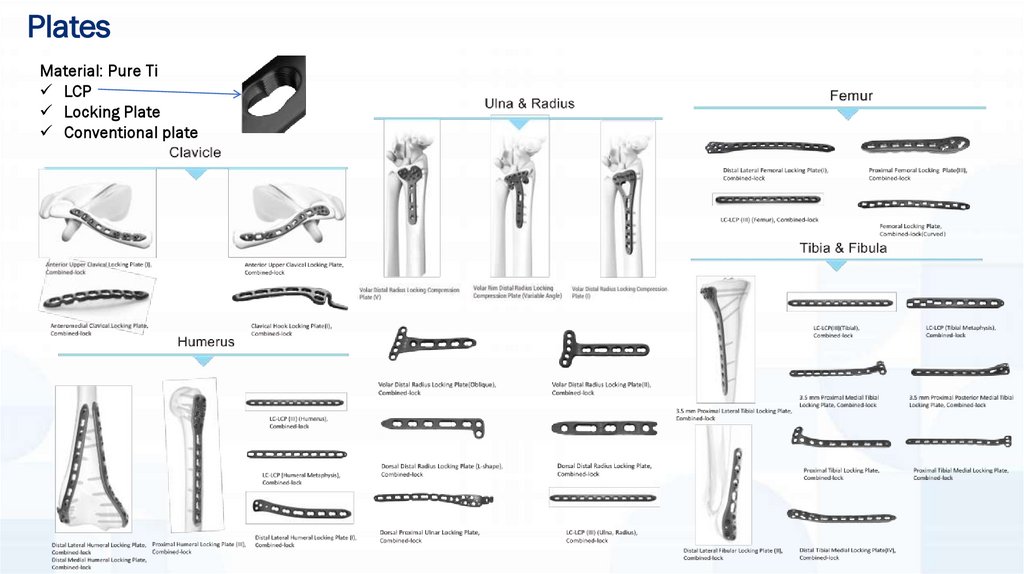
PlatesMaterial: Pure Ti
LCP
Locking Plate
Conventional plate
22.
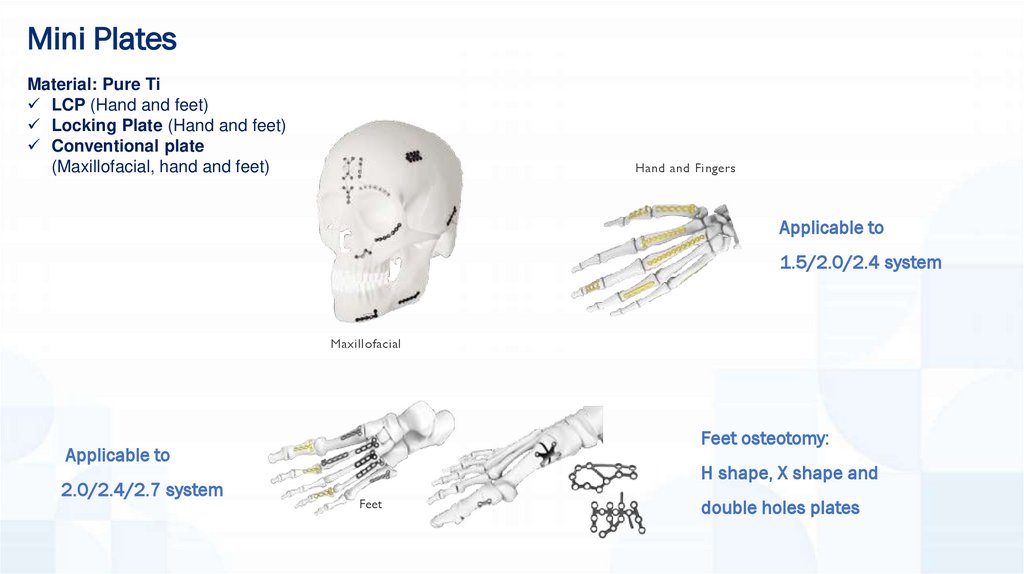
Mini PlatesMaterial: Pure Ti
LCP (Hand and feet)
Locking Plate (Hand and feet)
Conventional plate
(Maxillofacial, hand and feet)
Hand and Fingers
Applicable to
1.5/2.0/2.4 system
Maxillofacial
Feet osteotomy:
Applicable to
2.0/2.4/2.7 system
H shape, X shape and
Feet
double holes plates
23.
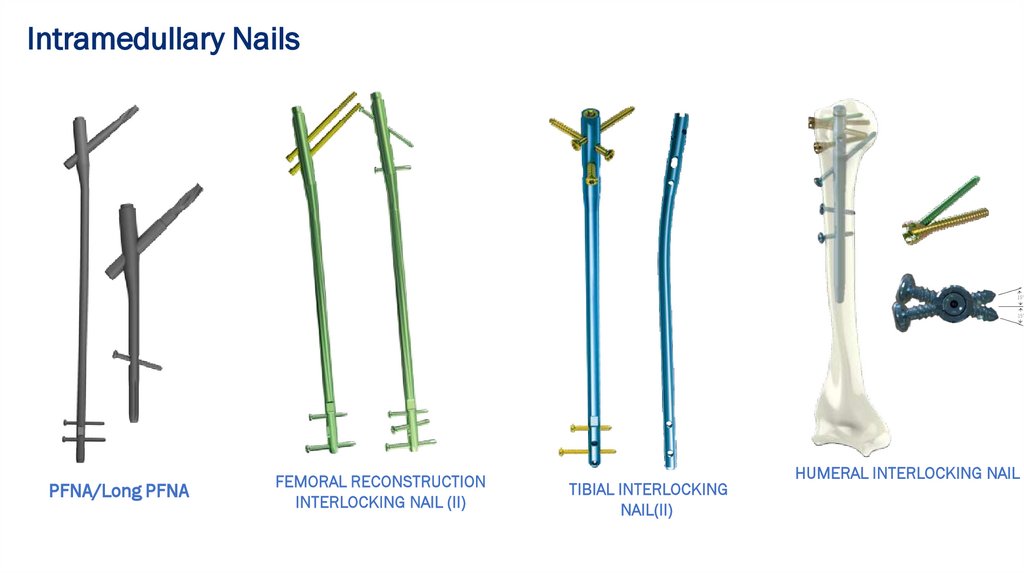
Intramedullary NailsPFNA/Long PFNA
FEMORAL RECONSTRUCTION
INTERLOCKING NAIL (II)
TIBIAL INTERLOCKING
NAIL(II)
HUMERAL INTERLOCKING NAIL
24.
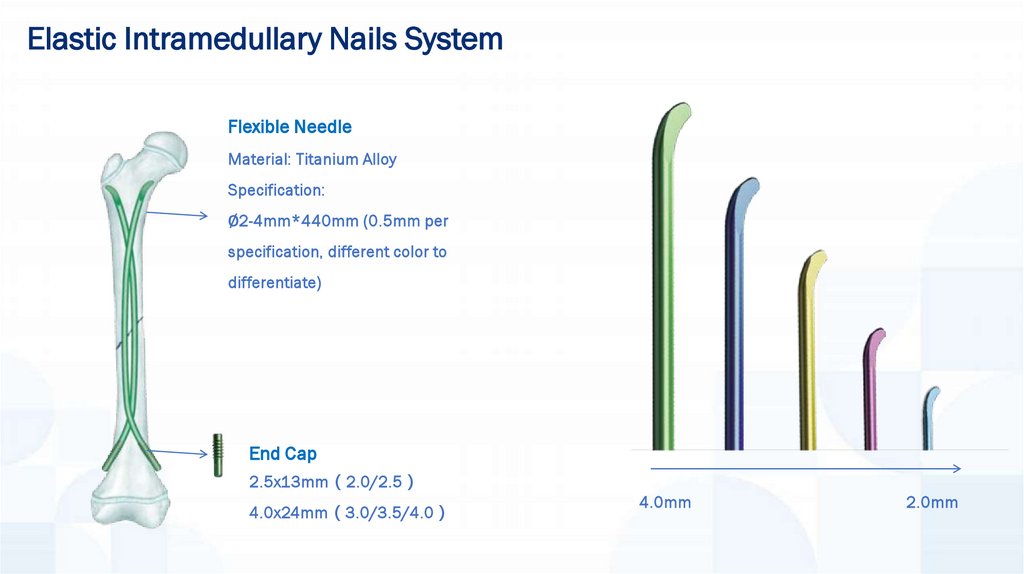
Elastic Intramedullary Nails SystemFlexible Needle
Material: Titanium Alloy
Specification:
∅2-4mm*440mm (0.5mm per
specification, different color to
differentiate)
End Cap
2.5x13mm 2.0/2.5
4.0x24mm 3.0/3.5/4.0
4.0mm
2.0mm
25.
ScrewsPure Ti
Various design
Multiple Specification
Could be used with various
trauma products
Cortical Screw
Cancellous Screw
Self-tapping screw
(Mono-axial & multi-axial)
26.
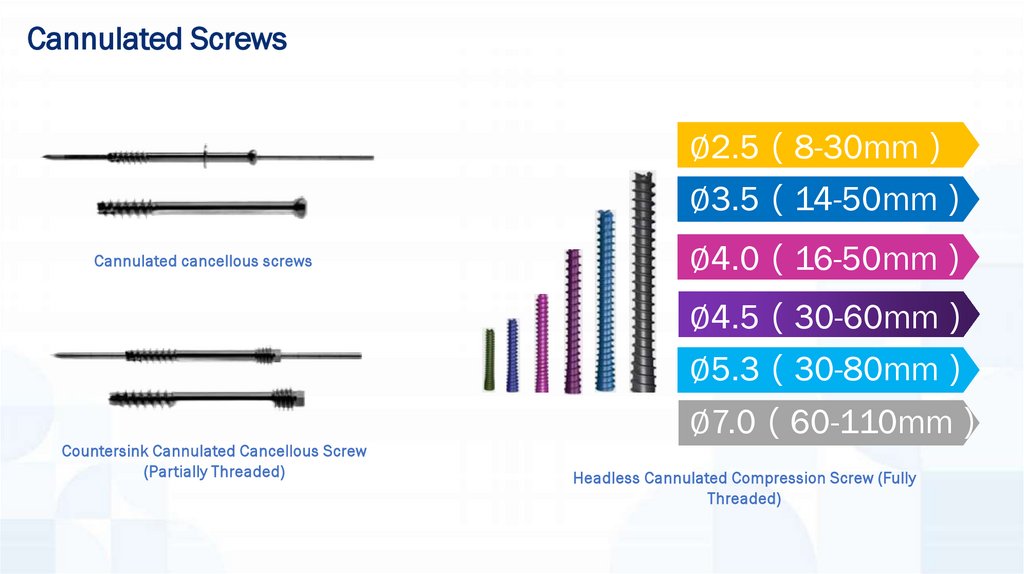
Cannulated Screws∅2.5 8-30mm
∅3.5 14-50mm
Cannulated cancellous screws
∅4.0 16-50mm
∅4.5 30-60mm
∅5.3 30-80mm
Countersink Cannulated Cancellous Screw
(Partially Threaded)
∅7.0 60-110mm
Headless Cannulated Compression Screw (Fully
Threaded)
27.
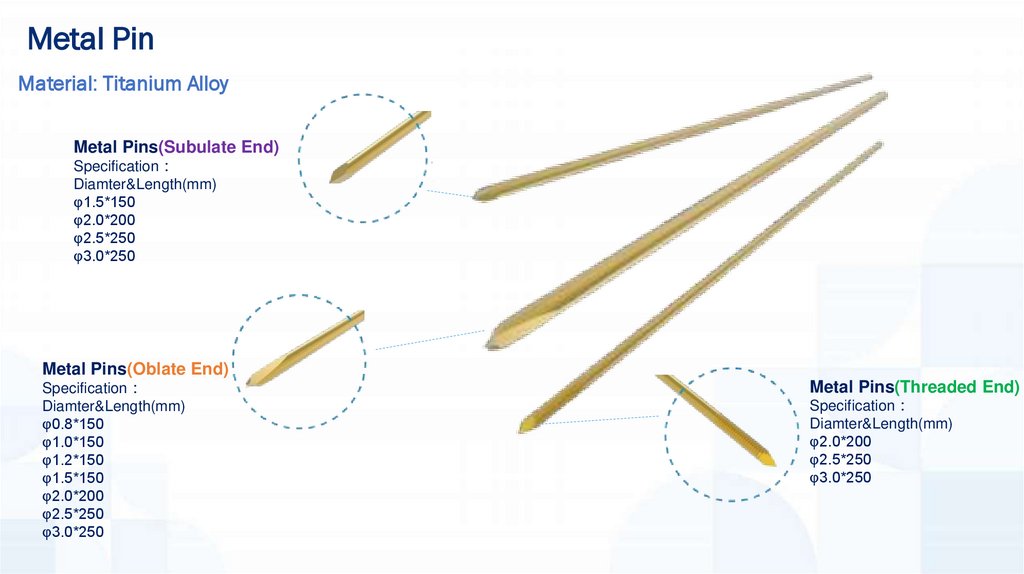
Metal PinMaterial: Titanium Alloy
Metal Pins(Subulate End)
Specification
Diamter&Length(mm)
φ1.5*150
φ2.0*200
φ2.5*250
φ3.0*250
Metal Pins(Oblate End)
Specification
Diamter&Length(mm)
φ0.8*150
φ1.0*150
φ1.2*150
φ1.5*150
φ2.0*200
φ2.5*250
φ3.0*250
Metal Pins(Threaded End)
Specification
Diamter&Length(mm)
φ2.0*200
φ2.5*250
φ3.0*250
28.
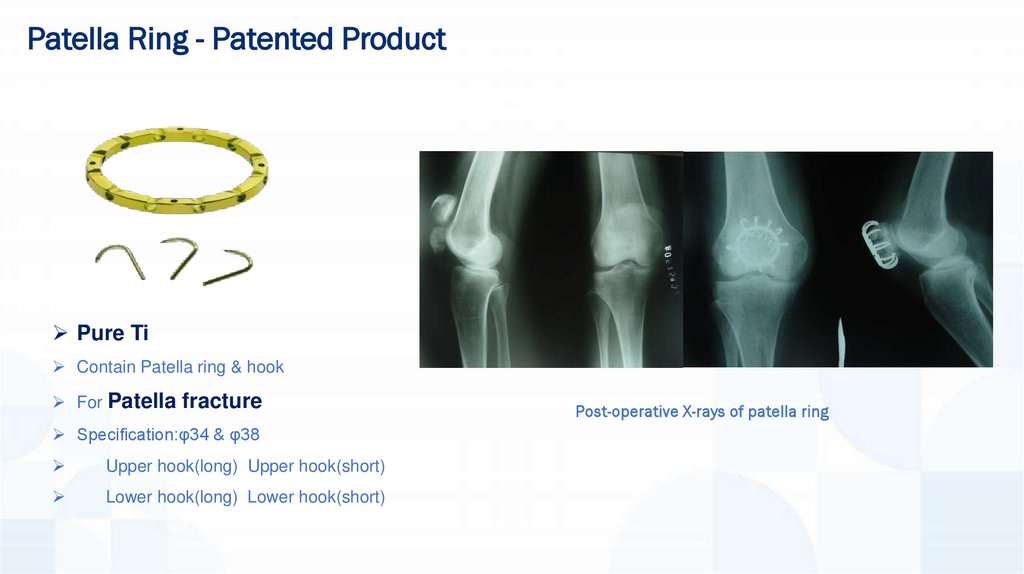
Patella Ring - Patented ProductPure Ti
Contain Patella ring & hook
For Patella fracture
Specification:φ34 & φ38
Upper hook(long) Upper hook(short)
Lower hook(long) Lower hook(short)
Post-operative X-rays of patella ring
29.
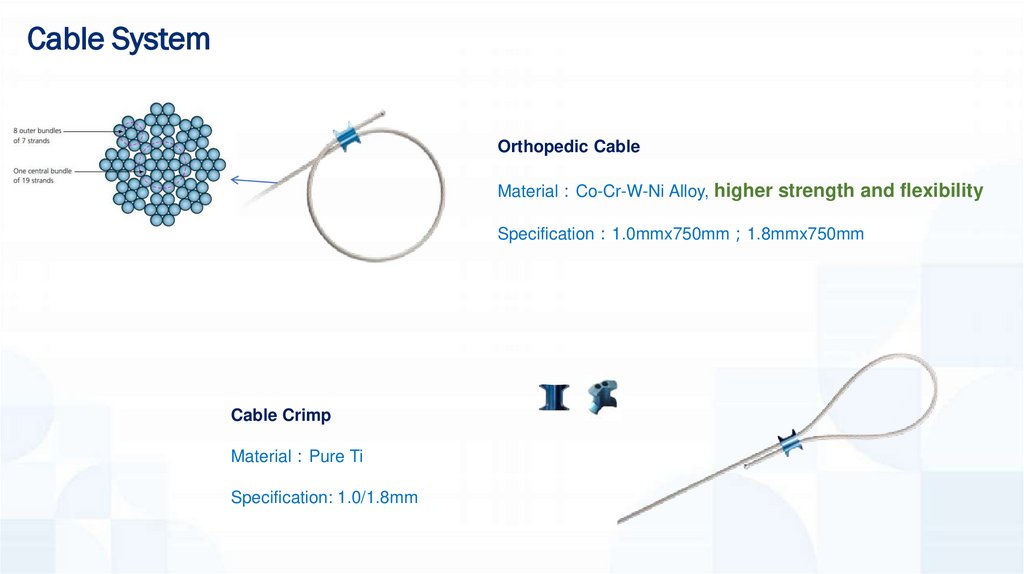
Cable SystemOrthopedic Cable
Material Co-Cr-W-Ni Alloy, higher strength and flexibility
Specification 1.0mmx750mm 1.8mmx750mm
Cable Crimp
Material Pure Ti
Specification: 1.0/1.8mm
30.
SpineProducts
31.
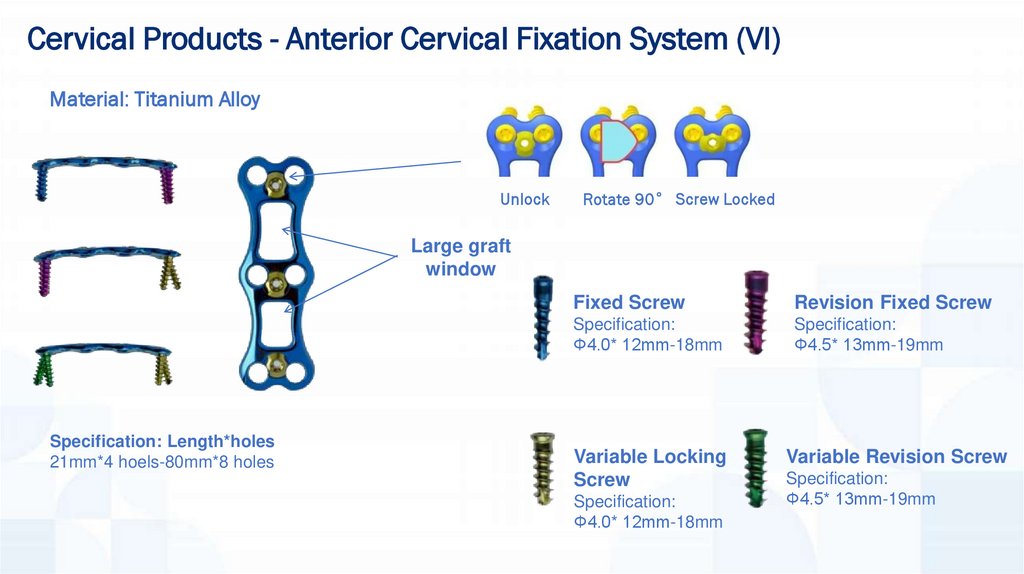
Cervical Products - Anterior Cervical Fixation System (VI)Material: Titanium Alloy
Unlock
Rotate 90° Screw Locked
Large graft
window
Specification: Length*holes
21mm*4 hoels-80mm*8 holes
Fixed Screw
Revision Fixed Screw
Specification:
Φ4.0* 12mm-18mm
Specification:
Φ4.5* 13mm-19mm
Variable Locking
Screw
Variable Revision Screw
Specification:
Φ4.0* 12mm-18mm
Specification:
Φ4.5* 13mm-19mm
32.
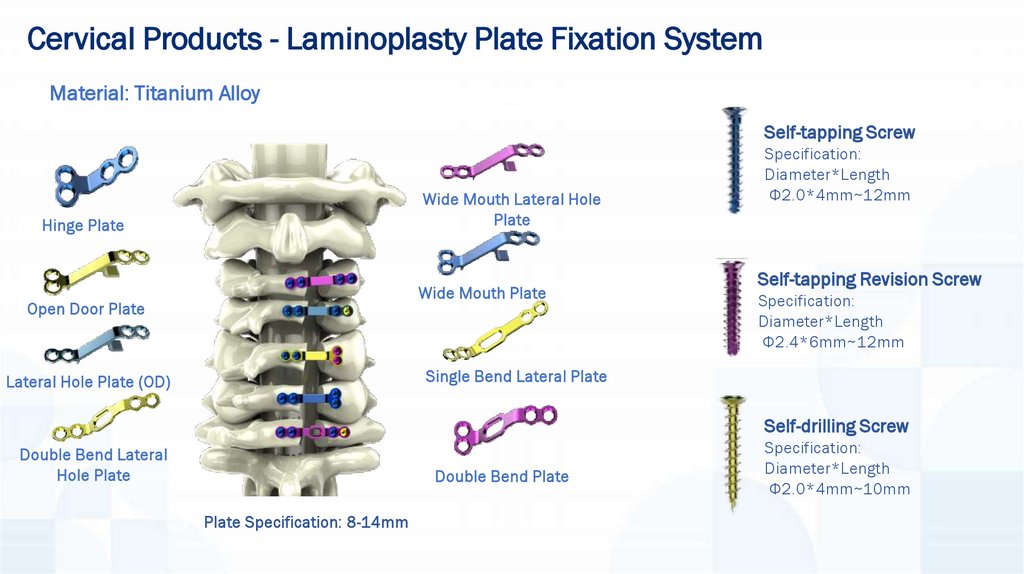
Cervical Products - Laminoplasty Plate Fixation SystemMaterial: Titanium Alloy
Self-tapping Screw
Wide Mouth Lateral Hole
Plate
Hinge Plate
Wide Mouth Plate
Open Door Plate
Specification:
Diameter*Length
Φ2.0*4mm~12mm
Self-tapping Revision Screw
Specification:
Diameter*Length
Φ2.4*6mm~12mm
Single Bend Lateral Plate
Lateral Hole Plate (OD)
Self-drilling Screw
Double Bend Lateral
Hole Plate
Double Bend Plate
Plate Specification: 8-14mm
Specification:
Diameter*Length
Φ2.0*4mm~10mm
33.
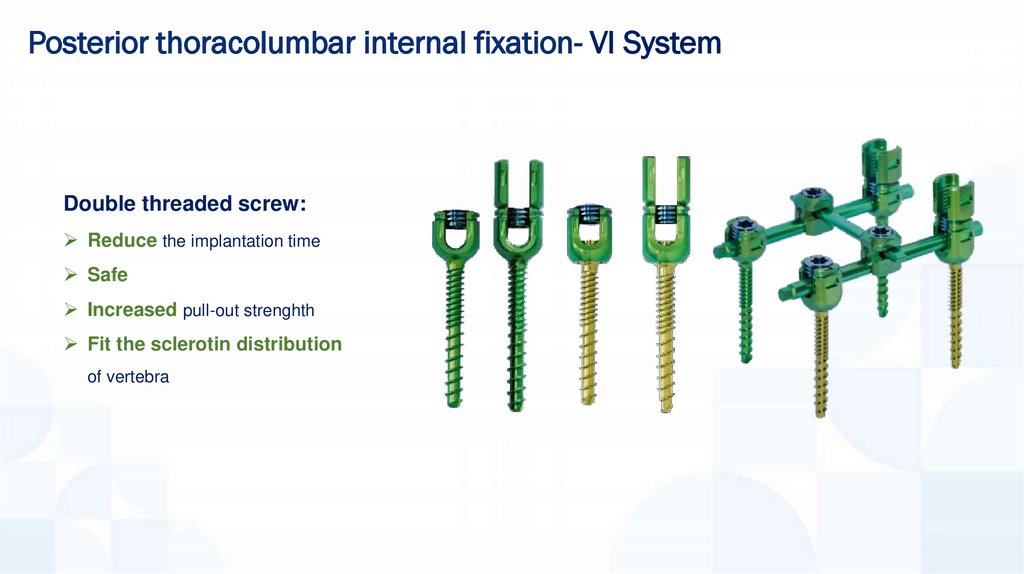
Posterior thoracolumbar internal fixation- VI SystemDouble threaded screw:
Reduce the implantation time
Safe
Increased pull-out strenghth
Fit the sclerotin distribution
of vertebra
34.
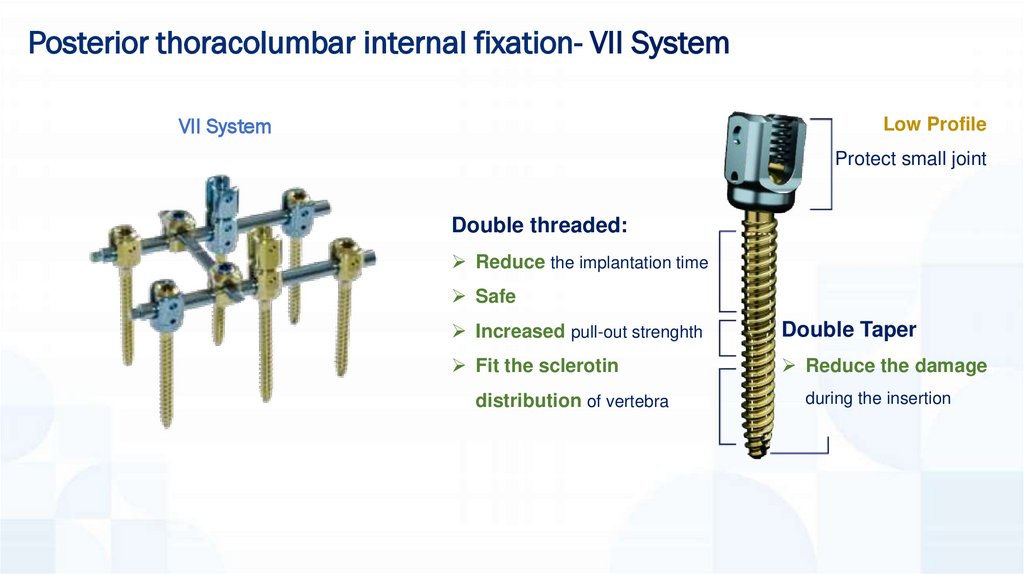
Posterior thoracolumbar internal fixation- VII SystemVII System
Low Profile
Protect small joint
Double threaded:
Reduce the implantation time
Safe
Increased pull-out strenghth
Double Taper
Fit the sclerotin
Reduce the damage
distribution of vertebra
during the insertion
35.
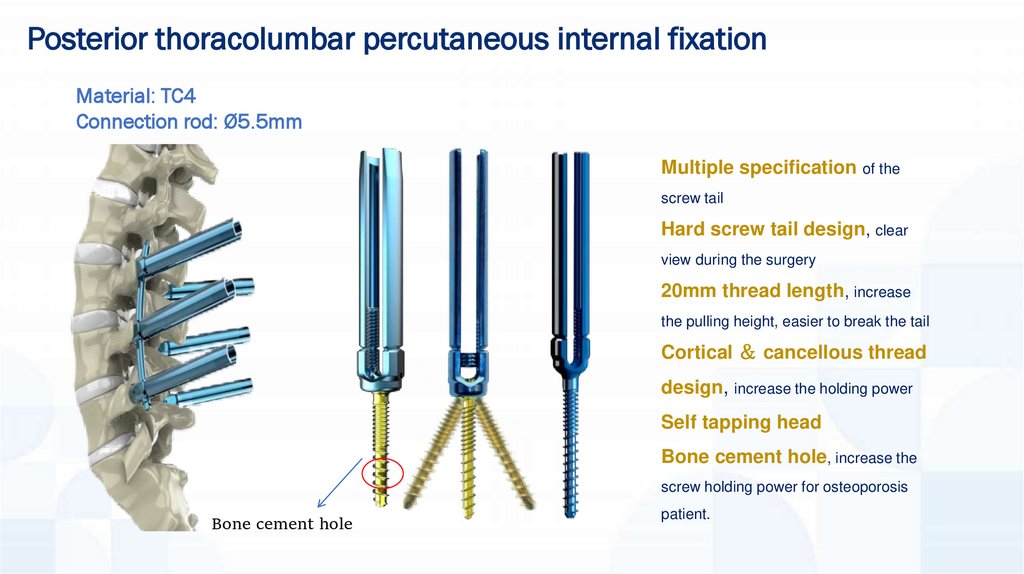
Posterior thoracolumbar percutaneous internal fixationMaterial: TC4
Connection rod: Ø5.5mm
Multiple specification of the
screw tail
Hard screw tail design, clear
view during the surgery
20mm thread length, increase
the pulling height, easier to break the tail
Cortical cancellous thread
design, increase the holding power
Self tapping head
Bone cement hole, increase the
screw holding power for osteoporosis
Bone cement hole
patient.
36.
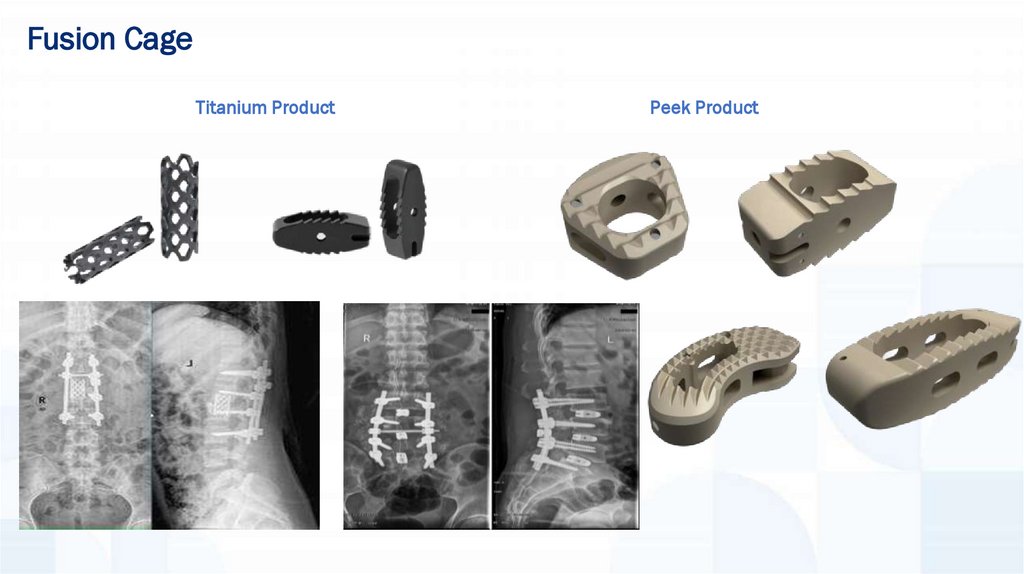
Fusion CageTitanium Product
Peek Product
37.
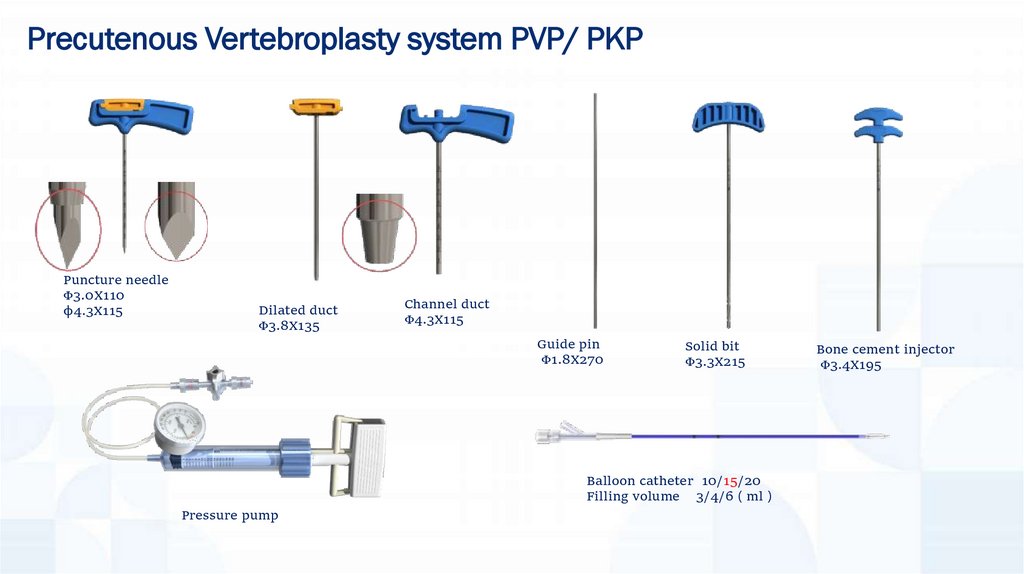
Precutenous Vertebroplasty system PVP/ PKPPuncture needle
Φ3.0X110
φ4.3X115
Dilated duct
Φ3.8X135
Channel duct
Φ4.3X115
Guide pin
Φ1.8X270
Solid bit
Φ3.3X215
Balloon catheter 10/15/20
Filling volume 3/4/6 ml
Pressure pump
Bone cement injector
Φ3.4X195
38.
SportsMedicine
39.
Suture AnchorWrist
Shoulder
Screw Material: Titanium Alloy
Knee
Single thread Screw
Φ1.8 / 2.0 / 2.8mm
Ankle
Single thread Screw
(High-low)
Φ3.5 / 4.5 / 5.0 / 5.5mm
Double thread Screw
Thread Material: UHMWPE
Φ4.5 / 5.0 / 5.5 / 6.5mm
Elbow
40.
Cortical Fixation SystemShoulder
Slotted Button
11.3x8mm
Fixed Loop
Spec. 20/25/30/35mm
Renovation Button
19.4x5mm
Knee
Slotted Renovation
Button
Adjustable Loop
Spec. one size
20x5.5mm
Ankle
41.
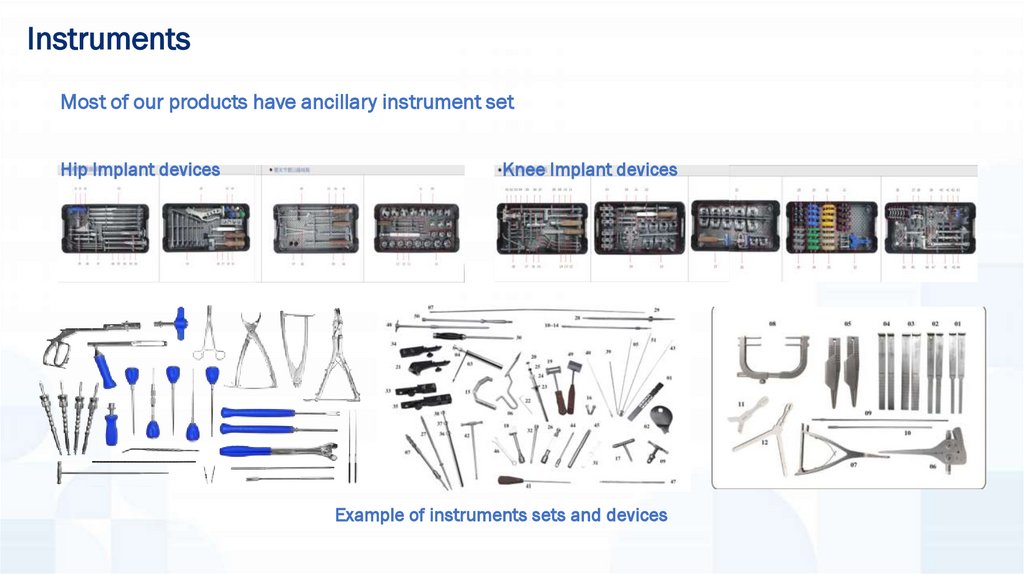
Instruments42.
InstrumentsMost of our products have ancillary instrument set
Hip Implant devices
Knee Implant devices
Example of instruments sets and devices
43.
InternationalCooperation
44.
Activities Around The World45.
Exhibition46.
Thank YouCopyright © Lepu Medical Technology(Beijing)Co.,Ltd. All Rights Reserved.





















 medicine
medicine








