Similar presentations:
Process Corrosion
1.
PROCESS CORROSIONCONTINUOUSLY DEGRADING INTEGRITY
2.
GOLDOBINALEKSANDR
Maintenance Engineer
https://dzen.ru/alexgold
asgoldobin@gmail.com
3.
CORROSION PRINCIPLESCorrosion rate is measured as weight loss per unit area and is expressed in mils per year (mpy) or mm/y.
Corrosion Rates can be affected by:
Passivity forming protective surface films (including corrosion inhibitors, paints and coatings)
Oxygen content
Flow velocity/rates
Temperature
pH effects (Low and High)
Contaminants/intermediates
4.
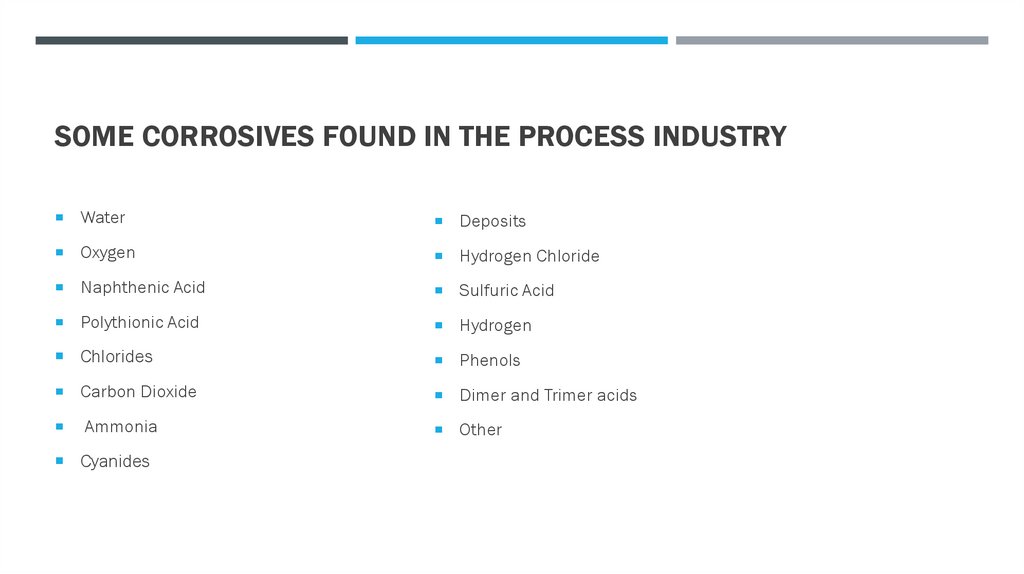
SOME CORROSIVES FOUND IN THE PROCESS INDUSTRYWater
Deposits
Oxygen
Hydrogen Chloride
Naphthenic Acid
Sulfuric Acid
Polythionic Acid
Hydrogen
Chlorides
Phenols
Carbon Dioxide
Dimer and Trimer acids
Ammonia
Cyanides
Other
5.
LOW TEMPERATURE CORROSIONBelow 500° F (<260’ C)
Occurrences:
Inorganic compounds such as water, hydrogen sulphide, hydrogen chloride, sulphuric acid, salts, etc.
Presence of water (even in very small amounts)
Electrolyte in hydrocarbon stream
Hydrocarbons in water streams creating acidic conditions
Solids.. Under deposit
Organic acids
Vapour Streams at water condensation points
Obeys electrochemical laws
Stable films can reduce or prevent corrosion
6.
LOW TEMPERATURE CORROSIONFrom Process chemicals
From Process contaminants
Not caused by clean hydrocarbons
Caused by inorganic compounds such as water, hydrogen sulphide, hydrogen chloride, sulphuric acid, salts, etc.
7.
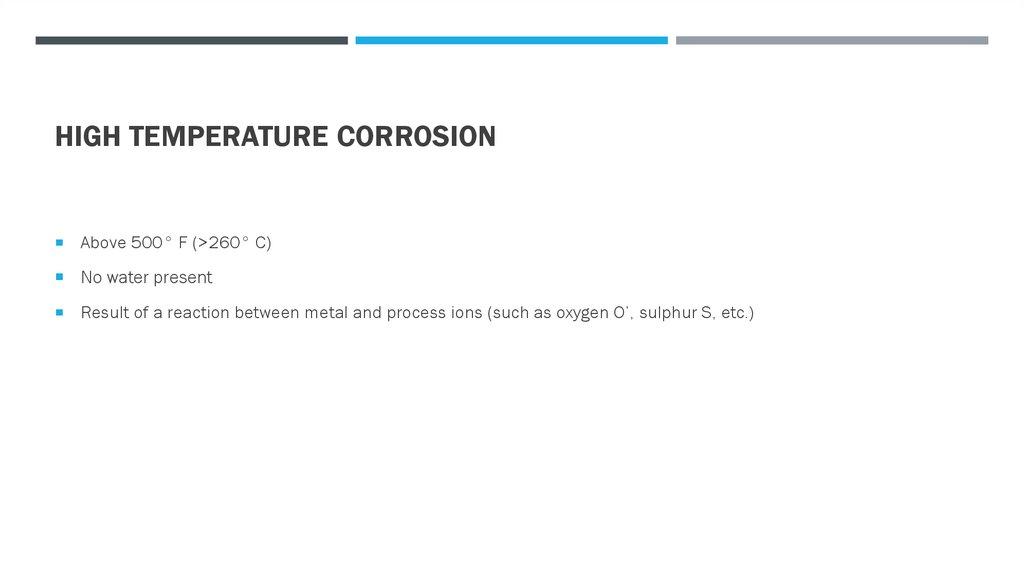
HIGH TEMPERATURE CORROSIONAbove 500° F (>260° C)
No water present
Result of a reaction between metal and process ions (such as oxygen O’, sulphur S, etc.)
8.
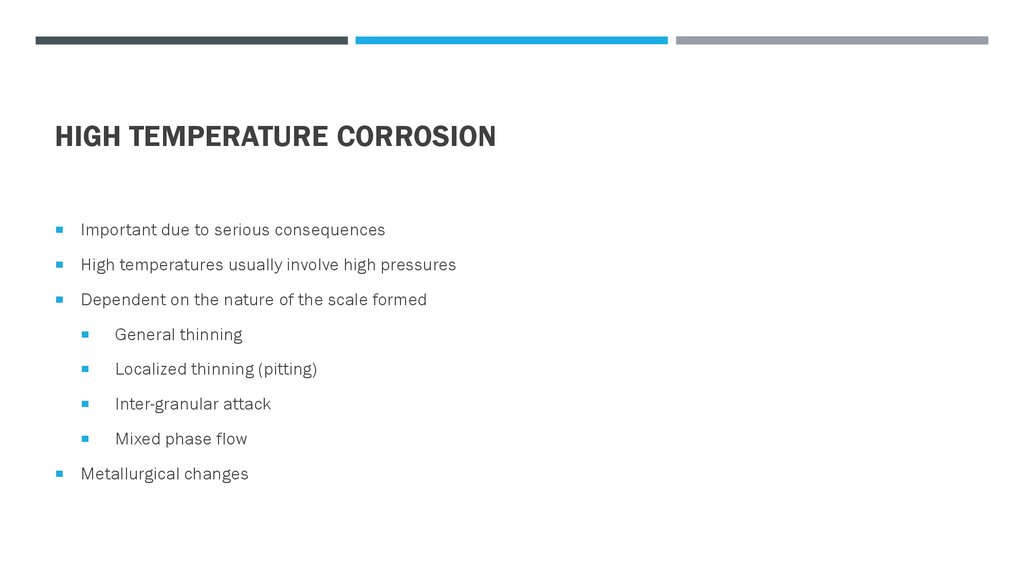
HIGH TEMPERATURE CORROSIONImportant due to serious consequences
High temperatures usually involve high pressures
Dependent on the nature of the scale formed
General thinning
Localized thinning (pitting)
Inter-granular attack
Mixed phase flow
Metallurgical changes
9.
SITUATIONS LEADING TO DETERIORATIONNormal operation, upset, startup/shutdown conditions
Material/Environment condition interactions
Many combinations of corrosive process streams and temperature/pressure conditions.
In the absence of corrosion, mechanical and metallurgical deterioration can occur
Weather effects ....
10.
FORMS OF THE DAMAGEGeneral loss due to general or localized corrosion
Pitting attack 0 Stress Corrosion Cracking (SCC)
Metallurgical Changes d Mechanical damage
High Temperature Hydrogen Attack (HTHA)
Damage types occur with specific combinations of materials and environmental/ operating conditions
11.
STRESS CORROSION CRACKING DETECTIONSOH IC in soft base metal.
Stress-Oriented Hydrogen Induced Cracking
In contrast to general corrosion, SCC is very hard to
detect visually even when it has progressed to an
extreme condition.
12.
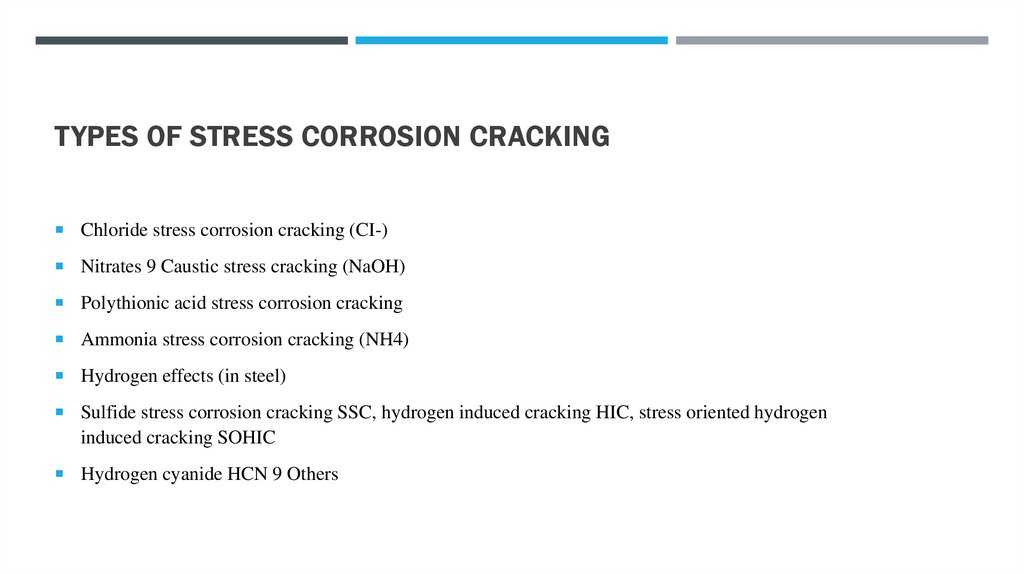
TYPES OF STRESS CORROSION CRACKINGChloride stress corrosion cracking (CI-)
Nitrates 9 Caustic stress cracking (NaOH)
Polythionic acid stress corrosion cracking
Ammonia stress corrosion cracking (NH4)
Hydrogen effects (in steel)
Sulfide stress corrosion cracking SSC, hydrogen induced cracking HIC, stress oriented hydrogen
induced cracking SOHIC
Hydrogen cyanide HCN 9 Others
13.
HIGHT TEMPERATURE HYDROGEN ATTACKLongitudinal Weld
Magnification: 500x
Etch: 2% Nital









 industry
industry








