Similar presentations:
Remote Testing of the Laboratory Samples
1.
FSBI "SCEEMP"of the Ministry of Health of the Russian Federation
RegLek
Remote Testing of the
Laboratory Samples
Olga Aleksandrovna Vaganova, Head of the
Testing Centre for Medicinal Products Quality
Control of the FSBI "SCEEMP"
of the Ministry of Health of the Russian
Federation
April 24, 2023
Federal State Budgetary Institution
"Scientific Center for Expert Evaluation of Medicinal Products"
of the Ministry of Healthcare of the Russian Federation
2.
DOCUMENTS REGULATING THEFSBI "SCEEMP"
REMOTE EXPERTISE
of the Ministry of Health of the Russian Federation
RegLek
Decree of the Government of the Russian Federation No. 440 dated March 23, 2020
"On adoption of specific requirements for variations to registration dossier documents of the
authorized medicinal product for human use in case of shortage or risk of shortage of medicinal
products due to restrictive economic measures imposed against the Russian Federation"
Decree of the Government of the Russian Federation No. 593
dated April 5, 2022
"On special considerations relating to medicinal products circulation in
case of shortage or risk of shortage of medicinal products due to
restrictive economic measures imposed against the Russian Federation"
Decision of the Council of the Eurasian
Economic Commission No. 78 dated
November 3, 2016
"On the Rules for Registration and Expert Evaluation of
Medicinal Products for Human Use"
3.
PROCEDURE OF THE REMOTEEXPERTISE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
The remote expertise is
possible for the procedures
according to
the Federal Law No. 61 "ON
CIRCULATION OF MEDICINES"
(national procedure)
Decision of the Council of
the Eurasian Economic
Commission No. 78 dated
November 3, 2016 (EAEU
procedure)
There are peculiarities in each case
4.
PROCEDURE OF THE REMOTE EXPERTISEAUTHORIZATION PURSUANT TO THE NATIONAL
PROCEDURE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
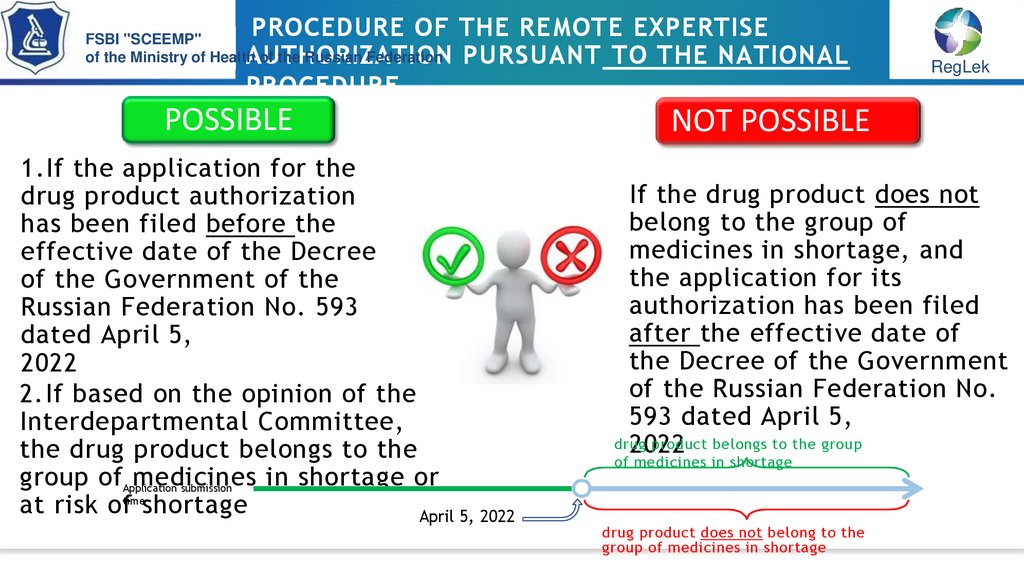
POSSIBLE
1.If the application for the
drug product authorization
has been filed before the
effective date of the Decree
of the Government of the
Russian Federation No. 593
dated April 5,
2022
2.If based on the opinion of the
Interdepartmental Committee,
the drug product belongs to the
group ofApplication
medicines
in shortage or
submission
at risk oftimeshortage
April 5, 2022
RegLek
NOT POSSIBLE
If the drug product does not
belong to the group of
medicines in shortage, and
the application for its
authorization has been filed
after the effective date of
the Decree of the Government
of the Russian Federation No.
593 dated April 5,
drug
product belongs to the group
2022
of medicines in shortage
drug product does not belong to the
group of medicines in shortage
5.
PROCEDURE OF THE REMOTE EXPERTISEAUTHORIZATION PURSUANT TO THE NATIONAL
PROCEDURE
BASED ON THE CLAUSE 17 (PARAGRAPH 5) OF SECTION II AND CLAUSE 45
(PARAGRAPH 2) OF SECTION V OF THE DECREE NO. 593, THE DECISION ON
THE REMOTE EXPERTISE MAY BE TAKEN WHEN THE
LABORATORY EXPERTISE BY THE EXPERT INSTITUTION IS IMPOSSIBLE,
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
I N C L U DI N G OWI N G TO THE L AC K OF THE FOL L OWI N G :
- equipment and/or accessories to it;
- expendable materials;
- samples of a drug substance;
- test strains of microorganisms;
- cell cultures;
- samples of substances used for drug product quality control by
comparing the study drug product with them;
The procedure and forms of the remote expertise shall be defined
by
the expert institution based on the agreement with the Applicant
6.
PROCEDURE OF THE REMOTE EXPERTISE AMENDINGFSBI "SCEEMP"
TO THE NATIONAL PROCEDURE
of the Ministry of HealthACCORDING
of the Russian Federation
POSSIBLE
1.If the application for
amendments has been filed
before the effective date of the
Decree of the Government of the
Russian Federation No. 440 dated
March 23,
2022
2. If the drug product belongs to
the group of medicines in shortage,
and the application for its
authorization has been filed after
the effective
date of the Decree of
Application submission
time
the Government
of the Russian
March 23,
Federation No. 440 dated March2022
23,
2022
RegLek
NOT POSSIBLE
If the drug product does not
belong to the group of
medicines in shortage, and
the application for its
authorization has been filed
after the effective date of
the Decree of the Government
of the Russian Federation No.
440 dated March 23, 2022
drug product belongs to the group
of medicines in shortage
drug product does not belong to the
group of medicines in shortage
7.
PROCEDURE OF THE REMOTE EXPERTISE AMENDINGFSBI "SCEEMP"
TO THE NATIONAL PROCEDURE
of the Ministry of HealthACCORDING
of the Russian Federation
BASED ON CLAUSE 7 (PARAGRAPH 2) OF THE DECREE NO.
440, THE PROCEDURE AND FORMS OF THE REMOTE
EXPERTISE
SHALL BE DEFINED BY THE EXPERT INSTITUTION
THE PROCEDURE AND FORMS OF THE REMOTE EXPERTISE SHALL BE AGREED
WITH THE APPLICANT
RegLek
8.
PROCEDURE OF THE REMOTE EXPERTISE AMENDINGFSBI "SCEEMP"
TO THE NATIONAL PROCEDURE
of the Ministry of HealthACCORDING
of the Russian Federation
RegLek
Peculiarities of the expertise outside the expert institution
pursuant to the Decrees of the Government No. 593 and No. 440:
The decrees do not stipulate a lack of DP samples in the
institution as the possible reasons.
• Pay close attention to the timing of expertises! The
expertise period is very limited in both cases.
9.
PROCEDURE OF THE REMOTE EXPERTISE AMENDINGFSBI "SCEEMP"
TO THE NATIONAL PROCEDURE
of the Ministry of HealthACCORDING
of the Russian Federation
RegLek
BASED ON THE DECREE OF THE GOVERNMENT OF THE RUSSIAN FEDERATION NO. 440 DATED
MARCH 23 2022,
THE REMOTE EXPERTISE SHOULD BE PERFORMED WITHIN THE PERIOD
ESTABLISHED FOR THE QUALITY EXPERTISE OF THE SAMPLES — 15 WORKING
DAYS FROM THE DATE OF THE SAMPLES SUBMISSION TO THE EXPERT
INSTITUTION
THE SAMPLES SHALL BE SUBMITTED TO THE EXPERT INSTITUTION
WITHIN
10 WORKING
DAYS
FROM
DATE
OF RECEIPT
OF THE
DECISION
ONFederation
THE on the conduct of
10. Within
10 working days
from the
date ofTHE
receipt
of the decision
of the Ministry
of Health
of the Russian
the expertises indicated in subclause "b" of clause 3 of the present document, the Applicant shall submit the following to the expert
institution for the quality expertise of the drug product:
samples of the drug product manufactured according to the requirements of the manufacturing formula and/or master formula approved by the
head officer of the drugs manufacturing company, as well as (in the corresponding cases) samples of the drug substance, test strain of
microorganisms, cell cultures, samples of substances used for drug product quality control by comparing the study drug product with them; in
quantities necessary to reproduce the quality control methods,
and in the volume established by the commission of experts of the expert institution based on the Article 16 of the Federal
Law "On Circulation of Medicines", taking into account the type (nature) of the changes made and the drug product.
10.
PROCEDURE OF THE REMOTE EXPERTISEAUTHORIZATION PURSUANT TO THE EAEU
PROCEDURE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
TO BE
PERFORMED:
1. When samples
are difficult to
obtain;
2. If it is impossible to comply with
the conditions of transportation of
these samples to the territory of
the Russian Federation and (or)
their storage
3. In absence of special
equipment at the expert
institution;
4 . Due to other reasons
according to the decision of
the authorized body (expert
institution) , including owing to
the peculiarities of the
manufacturing and quality
RegLek
NOT POSSIBLE
If the applicant cannot
provide
the required
samples to the expert
institution within the
established period.
11.
PROCEDURE OF THE REMOTE EXPERTISEAUTHORIZATION PURSUANT TO THE EAEU
PROCEDURE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
THE CONCEPT OF DIFFICULTY OF OBTAINING MAY APPLY TO THE
SAMPLES OF THE DPs BELONGING TO THE FOLLOWING GROUPS OF
MEDICINES:
1. NARCOTICS
2. PSYCHOTROPICS
3. DRUGS INDICATED FOR TREATMENT OF
EXPENSIVE NOSOLOGIES STATED IN THE LIST APPROVED
BY THE DECREE
OF THE GOVERNMENT OF THE RUSSIAN FEDERATION NO. 1416 DATED
NOVEMBER 26, 2018
4. RADIOPHARMACEUTICALS
5. ORPHAN DRUGS
12.
REMOTE EXPERTISE DIFFICULT TOOBTAIN=SHORT-LIVING DPs
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
THE DRUG PRODUCTS THAT ARE DIFFICULT TO OBTAIN (SHORTLIVING) MAY INCLUDE RADIOPHARMACEUTICALS, BIOMEDICAL
CELL PRODUCTS.
THE REMOTE EXPERTISE IS POSSIBLE IN TERMS OF ALL QUALITY ATTRIBUTES
FOR EXAMPLE, FOR BIOMEDICAL CELL PRODUCTS (BCP) WITH THE
PERIOD OF STORAGE/USE OF LESS THAN 15 DAYS*
Order of the Ministry of Health of the Russian Federation No. 195n
dated April 28, 2017 "On approval of the procedure of quality
expertise of a biomedical cell product at the place of manufacture of
the biomedical cell product using the manufacturer's equipment"
* - Pursuant to the Federal Law No. 180 dated June 23, 2016 "On biomedical cell products" (clause 4, art. 15)
13.
PROCEDURE OF THE REMOTE EXPERTISEUNAVAILABLE SAMPLES
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
IF THE QUALITY EXPERTISE
REQUIRES A SAMPLE OR MATERIAL,
THE IMPORT OF WHICH INTO THE
TERRITORY OF THE RUSSIAN
FEDERATION IS CURRENTLY
PROHIBITED,
THE EXPERTISE IN TERMS OF THE PARAMETER, FOR WHICH THIS MATERIAL OR
SAMPLE IS REQUIRED, MAY BE CARRIED OUT REMOTELY
14.
PROCEDURE OF THE REMOTE EXPERTISEAUTHORIZATION PURSUANT TO THE EAEU
PROCEDURE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
A JUSTIFICATION IS REQUIRED TO
DESIGNATE THE DP AND THE SAMPLES AS "DIFFICULT TO
OBTAIN"
"... including the cases when they are classified
as orphan drugs ... or indicated for treatment of
expensive nosologies due to their high cost..."
Individual considerations:
A small batch of generic DP?
High cost?
When conducting a remote expertise, the DP samples,
special reagents, materials, RS, etc. will be spent
THE JUSTIFICATION AS "DUE TO THE DIFFICULT
GEOPOLITICAL SITUATION IT IS IMPOSSIBLE TO PROVIDE
THE SAMPLES" IS NOT ENOUGH.
RegLek
15.
PROCEDURE OF THE REMOTE EXPERTISEAUTHORIZATION PURSUANT TO THE EAEU
RegLek
PROCEDURE
IN CASES WHEN THE DRUG PRODUCT EXPERTISE PURSUANT TO THE
ASSIGNMENT OF THE MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION
INCLUDES A QUALITY EXPERTISE OF THE PROVIDED SAMPLES, THE DECISION
TO CARRY OUT SUCH EXPERTISE BASED EXCLUSIVELY ON THE
MANUFACTURER'S DOCUMENTATION
WITHIN THE REGISTRATION DOSSIER SHALL NOT BELONG TO THE COMPETENCE
OF ofTHE
Decision
the INSTITUTION
Council of the Eurasian Economic Commission No. 78 dated
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
November 3, 2016 "On the Rules for Registration and Expert Evaluation of Medicinal
Products for Human Use" 48. In the cases specified in paragraphs 8-11 of clause 47 of
the present Rules, laboratory tests shall be carried out in the quality control laboratory
of the drug product manufacturer in presence of the representatives of the expert
institution or in the contract laboratory used by the manufacturer in presence of the
representatives of the expert institution (as amended by the Decision of the Council of
the Eurasian Economic Commission No. 36 dated March 17, 2022).
16.
AGREEMENT ON THE REMOTE EXPERTISEPROCEDURE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
PRACTICE
THE REMOTE QUALITY EXPERTISE SHALL BE CARRIED OUT
IN TERMS OF SEPARATE AGREED PARAMETERS,
WHICH CANNOT BE TESTED IN THE INSTITUTION.
IN TERMS OF OTHER PARAMETERS, THE EXPERTISE SHALL BE CARRIED OUT AT THE EXPERT
INSTITUTION, RESPECTIVELY. FOR THIS PURPOSE, THE SAMPLES,
REAGENTS AND MATERIALS SHOULD BE SUBMITTED BASED ON THE ESTIMATIONS OF THE EXPERT INSTITUTION
17.
AGREEMENT ON THE REMOTEEXPERTISE PROCEDURE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
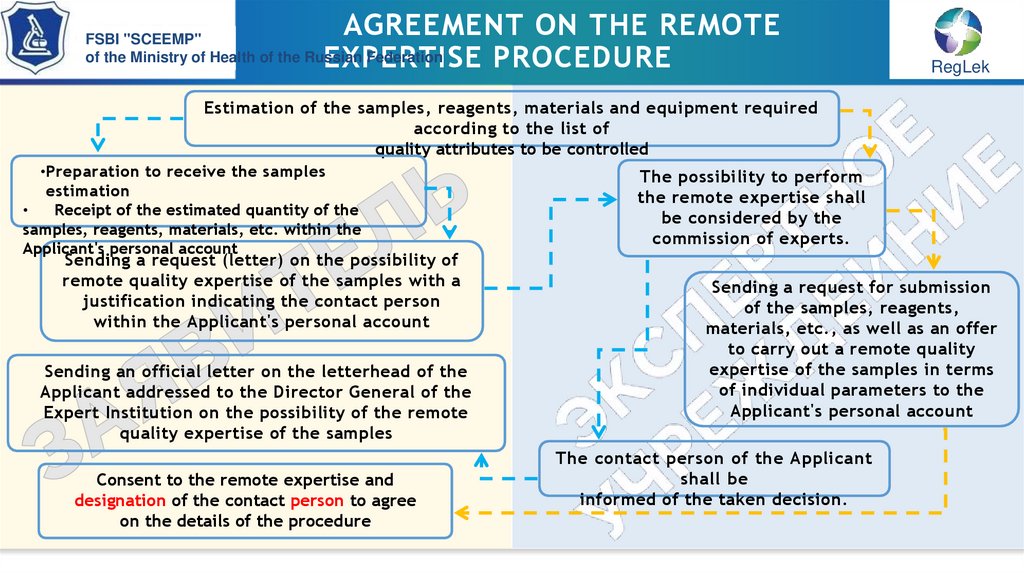
Estimation of the samples, reagents, materials and equipment required
according to the list of
quality attributes to be controlled
•Preparation to receive the samples
estimation
Receipt of the estimated quantity of the
samples, reagents, materials, etc. within the
Applicant's personal account
Sending a request (letter) on the possibility of
remote quality expertise of the samples with a
justification indicating the contact person
within the Applicant's personal account
Sending an official letter on the letterhead of the
Applicant addressed to the Director General of the
Expert Institution on the possibility of the remote
quality expertise of the samples
Consent to the remote expertise and
designation of the contact person to agree
on the details of the procedure
The possibility to perform
the remote expertise shall
be considered by the
commission of experts.
Sending a request for submission
of the samples, reagents,
materials, etc., as well as an offer
to carry out a remote quality
expertise of the samples in terms
of individual parameters to the
Applicant's personal account
The contact person of the Applicant
shall be
informed of the taken decision.
18.
AGREEMENT ON THE REMOTEEXPERTISE PROCEDURE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
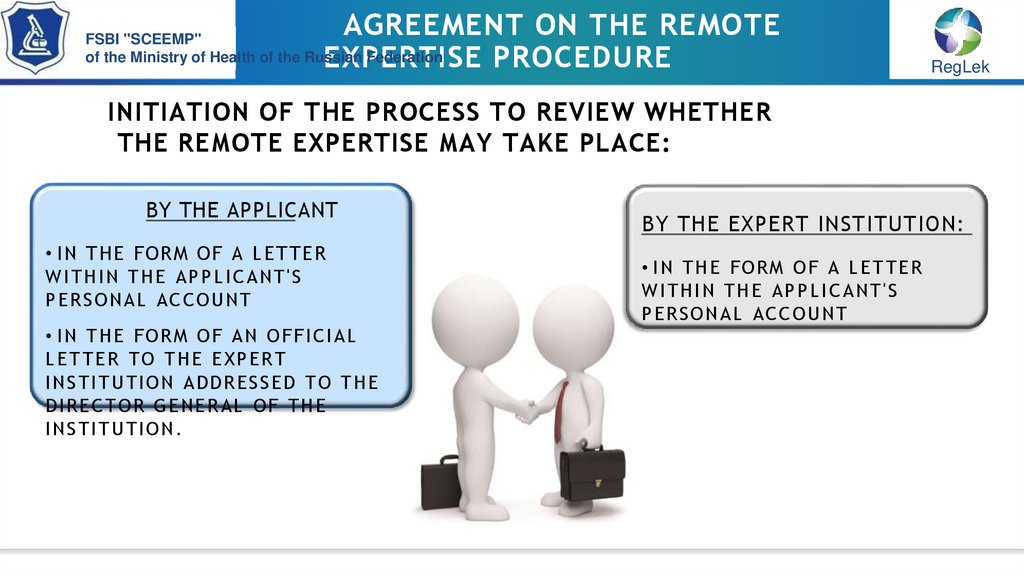
INITIATION OF THE PROCESS TO REVIEW WHETHER
THE REMOTE EXPERTISE MAY TAKE PLACE:
BY THE APPLICANT
• IN THE F ORM OF A LETTER
WI THI N THE AP P L I C AN T'S
P ERSON AL AC C OUN T
• I N THE F ORM OF AN OF F I C I AL
LETTER TO THE EXP ERT
I N STI TUTI ON ADDRESSED TO THE
DI REC TOR GEN ERAL OF THE
I N STI TUTI ON .
BY THE EXPERT INSTITUTION:
• I N THE F ORM OF A LETTER
WI THI N THE AP P LI C AN T'S
P ERSON AL AC C OUN T
19.
AGREEMENT ON THE REMOTEEXPERTISE PROCEDURE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
THE INFORMATION FILE WITH A
DETAILED DESCRIPTION OF THE
PROCESS OF INTERACTION ON
THE ISSUE OF A REMOTE
EXPERTISE IS AVAILABLE ON THE
WEBSITE OF THE INSTITUTION
WWW.REGMED.RU
THIS MEMO CAN ALSO BE READ IN THE APPLICANT'S PERSONAL ACCOUNT
20.
AGREEMENT ON THE REMOTEEXPERTISE PROCEDURE PRACTICE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
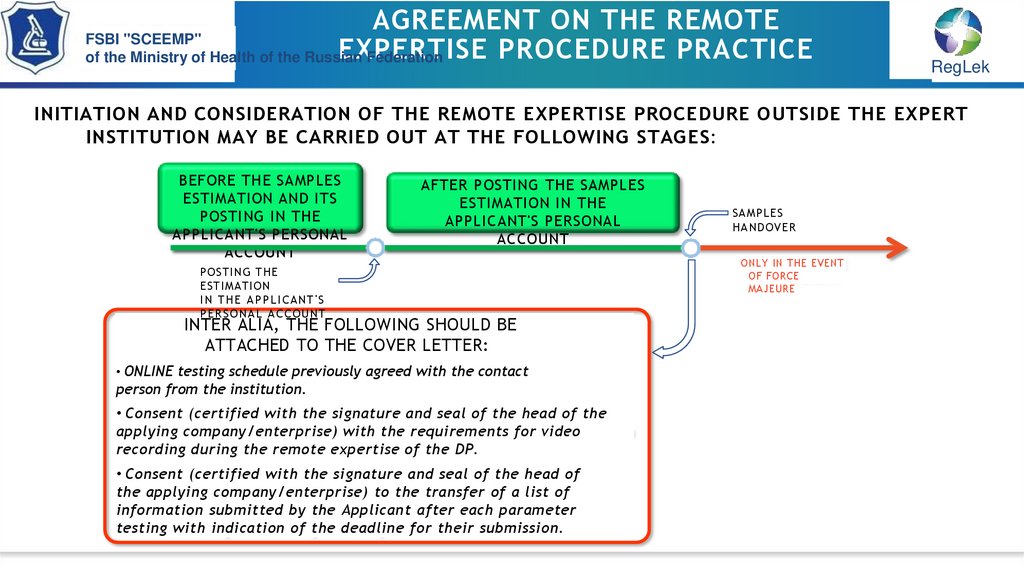
INITIATION AND CONSIDERATION OF THE REMOTE EXPERTISE PROCEDURE OUTSIDE THE EXPERT
INSTITUTION MAY BE CARRIED OUT AT THE FOLLOWING STAGES:
BEFORE THE SAMPLES
ESTIMATION AND ITS
POSTING IN THE
APPLICANT'S PERSONAL
ACCOUNT
AFTER POSTING THE SAMPLES
ESTIMATION IN THE
APPLICANT'S PERSONAL
ACCOUNT
POSTING THE
ESTIMATION
I N THE APPL I CANT'S
PER SON AL ACCOU NT
INTER ALIA, THE FOLLOWING SHOULD BE
ATTACHED TO THE COVER LETTER:
• ONLINE testing schedule previously agreed with the contact
person from the institution.
• Consent (certified with the signature and seal of the head of the
applying company/enterprise) with the requirements for video
recording during the remote expertise of the DP.
• Consent (certified with the signature and seal of the head of
the applying company/enterprise) to the transfer of a list of
information submitted by the Applicant after each parameter
testing with indication of the deadline for their submission.
SAMPLES
HANDOVER
ONLY IN THE EVENT
OF FORCE
MAJEURE
21.
AGREEMENT ON THE REMOTEFSBI "SCEEMP"
EXPERTISE PROCEDURE PRACTICE
of the Ministry of Health of the Russian Federation
RegLek
REQUIREMENTS FOR THE PROCEDURE AND FORMAT OF THE REMOTE
EXPERTISE, EXECUTION OF APPLICATIONS, LETTERS, PRESENTATION
OF INFORMATION ABOUT THE REMOTE TESTING TO BE
COMMUNICATED TO THE APPLICANTS VIA PERSONAL ACCOUNT
WITH A PURPOSE TO AGREE ON THE ORGANIZATIONAL
AND PRACTICAL ISSUES RELATED TO THE REMOTE
EXPERTISE PROCESS,
TO FAMILIARIZE THE APPLICANT WITH
THE REQUIREMENTS FOR VIDEO RECORDING
AND WITH THE LIST OF INFORMATION PROVIDED,
IT IS POSSIBLE TO ARRANGE A TECHNICAL MEETING
VIA VIDEO CONNECTION,
TELEPHONE COMMUNICATION WITH THE CONTACT
PERSON
OF THE APPLICANT, INCLUDING THE CASES
WITH THE INVOLVEMENT OF SPECIALISTS OF THE
INFORMATION TECHNOLOGIES DEPARTMENT
22.
AGREEMENT ON THE REMOTEFSBI "SCEEMP"
EXPERTISE PROCEDURE PRACTICE
of the Ministry of Health of the Russian Federation
RegLek
AFTER THE AGREEMENT ON THE REMOTE
EXPERTISE IN THE APPLICANT'S PERSONAL ACCOUNT:
• ESTIMATION IS CARRIED OUT WITH RESPECT TO THE SAMPLES AND MATERIALS
REQUIRED FOR THE EXPERTISE IN TERMS OF THE PARAMETERS EVALUATED AT THE
EXPERT INSTITUTION (EXCEPT FOR THOSE APPROVED FOR THE REMOTE EXPERTISE)
• A LETTER WITH THE INFORMATION SHALL BE SENT TO THE PERSONAL ACCOUNT:
WITH RESPECT TO A NECESSITY TO CHOOSE AND AGREE ON THE DATES OF THE REMOTE EXPERTISE;
WITH RESPECT TO NECESSITY FOR THE REMOTE EXPERTISE USING THE SAME DRUG PRODUCT
BATCHES, WHICH WILL BE SUBMITTED TO THE EXPERT INSTITUTION FOR THE QUALITY EXPERTISE IN
TERMS OF OTHER PARAMETERS;
WITH RESPECT TO CONDITIONS OF THE DEMONSTRATION EXAM AND A LIST OF PRESENTED INFORMATION,
ETC.
• A LIST OF REQUIREMENTS FOR VIDEO RECORDING AND A LIST OF DOCUMENTS, WHICH
THE APPLICANT IS REQUIRED TO PROVIDE TO THE EXPERT INSTITUTION, WILL BE
SUBMITTED FOR APPROVAL AFTER THE ONLINE EXPERTISE IS COMPLETED.
23.
AGREEMENT ON THE REMOTE EXPERTISE PROCEDUREFSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
PRACTICE
AT THE SAMPLES HANDOVER:
1. The Applicant shall submit a cover letter, which, inter alia, should state that the
sample(s) (in case of separate testing as a part of the quality expertise, a portion of
the samples with indication of their quantity) are stored by the manufacturer at the
manufacturing site with indication of their location;
2. In addition to the talon (for quality expertise of the DP according to the Decision
No. 78), copy of the Decision of the Ministry of Health of the Russian Federation,
and other documents (the list of them is given in the memo for the Applicant), the
Applicant should attach to the cover letter a schedule of online testing, which has
been agreed on with the contact person of the Institution in advance.
3. The Applicant should provide a Consent (certified with the signature and seal of
the head of the applying company/enterprise) to the requirements for video recording
during the remote expertise of the DP and for the transfer of a list of information
submitted by the Applicant after the testing of each parameter (indicating the
deadline for their submission).
RegLek
24.
AGREEMENT ON THE REMOTEFSBI "SCEEMP"
EXPERTISE PROCEDURE
of the Ministry of Health of the Russian Federation
PRACTICE
RegLek
THE REMOTE EXPERTISE:
TRICKIER DUE TO:
IS EASIER DUE TO:
• LACK OF NECESSITY FOR
IMPORTATION/PRESENTATION OF SOME
SAMPLES AND MATERIALS.
• HYPOTHETICALLY POSSIBLE REDUCTION OF THE
PROPOSED CONSUMPTION OF SAMPLES AND
MATERIALS.
• NECESSITY TO AGREE ON THE PROCESS WITH ALL
PARTICIPANTS (INCLUDING CONTRACT LABORATORIES IN OTHER
COUNTRIES).
• ADDITIONAL STRESS AND INCREASED POSSIBILITY OF MISTAKES WHILE
WORKING "UNDER VIDEO CAMERA" IN CASE OF ONLINE EXPERTISE.
• THE TESTING SHOULD BE CARRIED OUT IN STRICT COMPLIANCE WITH
THE DRAFT ND. IN CASES WHEN, DURING THE EXPERTISE OF THE
SAMPLES OR DOCUMENTS, IT IS CLEAR THAT A CORRECTION OF THE
METHODOLOGY AND A RE-TEST IS REQUIRED, EITHER THE AGREED
DATES SHOULD BE POSTPONED, OR A RE-TEST SHALL BE REQUIRED
AFTER RECEIVING THE ANSWER TO THE REQUEST. THIS LEADS TO
NEW AGREEMENTS OF DATES, INCL. THOSE WITH THE CONTRACT
LABORATORIES, IF SUCH ARE INVOLVED
• IT IS NECESSARY TO ENSURE FULL COMPLIANCE WITH THE
TECHNICAL REQUIREMENTS FOR ONLINE COMMUNICATION AND
RECORDING.
25.
QUALITY EXPERTISE OF THEFSBI "SCEEMP"
SAMPLES
of the Ministry of Health of the Russian Federation
OUTSIDE THE EXPERT
INSTITUTION PRACTICE.
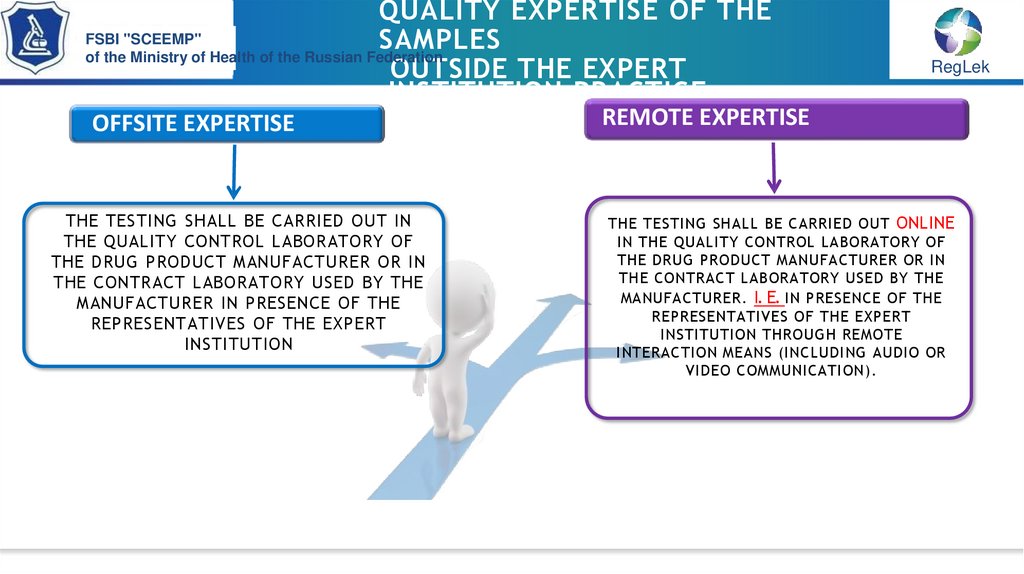
OFFSITE EXPERTISE
THE TESTING SHALL BE CARRIED OUT IN
THE QUALITY CONTROL LABORATORY OF
THE DRUG PRODUCT MANUFACTURER OR IN
THE CONTRACT LABORATORY USED BY THE
MANUFACTURER IN PRESENCE OF THE
REPRESENTATIVES OF THE EXPERT
INSTITUTION
RegLek
REMOTE EXPERTISE
THE TESTING SHALL BE CARRIED OUT ONLINE
IN THE QUALITY CONTROL LABORATORY OF
THE DRUG PRODUCT MANUFACTURER OR IN
THE CONTRACT LABORATORY USED BY THE
MANUFACTURER. I. E. IN PRESENCE OF THE
REPRESENTATIVES OF THE EXPERT
INSTITUTION THROUGH REMOTE
INTERACTION MEANS (INCLUDING AUDIO OR
VIDEO COMMUNICATION).
26.
PROCEDURE OF THE REMOTEEXPERTISE PRACTICE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
OFFSITE EXPERTISE
REMOTE EXPERTISE
MORE OFTEN, IT IS CARRIED
OUT AT THE
LABORATORIES OF
THE DOMESTIC
MANUFACTURERS
MANUFACTURERS, THE LABORATORIES OF
WHICH ARE LOCATED OUTSIDE THE
RUSSIAN FEDERATION OR AT A SIGNIFICANT
DISTANCE FROM THE EXPERT INSTITUTION,
THAT MAKES IT DIFFICULT TO
CARRY OUT AN OFFSITE EXPERTISE, OR
IN CASES DESCRIBED IN CLAUSE 48,
PARAGRAPH 2
27.
ONLINE REMOTE EXPERTISEPRACTICE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
According to the security policy of the expert institution,
the online expertise is possible only on the official platform
of TrueConf.
It is necessary to agree on the possibility of using the
TrueConf platform with the legal department,
information technology department, and other
interested departments of the applying company in
advance. Especially if you are the representatives of a
global company with headquarters abroad.
If there are other organizations involved in the process of online expertise, such as contract laboratories, the
use of the TrueConf platform should be agreed on with them as well.
When holding a video conference, the expert institution shall appoint this meeting, acting as an organizer, and will send the
Applicant a link to join. The broadcast will be recorded by the expert institution.
28.
PROCEDURE OF THE ONLINEREMOTE EXPERTISE PRACTICE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
REQUIREMENTS FOR VIDEO RECORDING:
Minimum quality of 1920x1080 60Hz (Full HD format at 60 FPS)
AT THE SAME TIME, THE APPLICANT SHALL ENSURE THE FOLLOWING:
• Provision of the operator (full name and position) fulfilling a particular test;
• Indication of the date and time;
• Indication of information about the place of testing and exact name of the site in Russian; Indication of
information about the sample name, primary and secondary packaging, batch number, quantity of the sample
collected for testing;
• Indication of information about the parameter name, method;
• Clear identification of all used samples, reagents, materials, and instruments, as well as required data on them.
• Precise fixation of all necessary readings/values of the instruments, including those used to control the
environmental conditions in the premise, where testing is carried out;
• Clear capturing of all stages of the sample preparation and equipment operation;
• Accurate recording of the obtained primary data and their analysis.
The Applicant also should give a consent to video recording for the remote expertise. The consent shall
be executed on the applicant's letterhead addressed to the Director General of the FSBI "SCEEMP" of
the Ministry of Health of the Russian Federation, certified with the signature and seal of the head of the
applying company/enterprise.
29.
ONLINE REMOTE EXPERTISEPRACTICE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
Due to arising questions and discussions, the ONLINE
expertise can take much more time than a routine
analysis, and also require the efforts of more than
usual number of employees. This is especially
important to be taken into account for the testing, in
which time intervals must be observed!
It can be difficult for the operator (analyst), and
therefore the samples and materials should be
estimated for at least three test replications.
RegLek
30.
PROCEDURE OF THE ONLINEREMOTE EXPERTISE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
1.The tests within the online expertise shall be carried out
strictly on the same batch of the drug product, which will be
submitted/submitted for testing to the expert Institution.
2.During the analysis in real time, it is necessary to
comment on the actions and answers of the analyst and
make simultaneous interpretation into Russian.
3.The remote expertise cannot be carried out on the
basis of video recordings from the Applicant. Testing
shall be carried out only online.
4.In cases, when the testing of the parameter takes more
time than one working day, the connection of the experts to
the video broadcasting shall occur according to the schedule
proactively approved by the expert institution.
RegLek
31.
PROCEDURE OF THE ONLINEREMOTE EXPERTISE PRACTICE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
PLEASE NOTE!
Due to arising questions and discussions, the ONLINE expertise can take much
more time than a routine analysis, and also require the efforts of more than usual
number of employees. This is especially important to be taken into account for
the testing, in which time intervals must be observed!
This can be difficult for the operator (analyst), and
result in various mistakes, so therefore the samples
and materials should be estimated for at least three
test replications.
32.
PROCEDURE OF THE ONLINEREMOTE EXPERTISE
FSBI "SCEEMP"
of the Ministry of Health of the Russian Federation
RegLek
AFTER THE END OF THE ONLINE EXPERTISE, THE APPLICANT
SHOULD SUBMIT THE FOLLOWING TO THE EXPERT INSTITUTION:
Copies of original forms to be filled in, laboratory records, all other materials with primary data and
calculations (printouts/screenshots/photos from instrument screens, instrument reports, receipts,
chromatograms, spectra, photographs of thin layer chromatography plates, etc.) and their translation into
Russian;
Copies of certificates/passports of quality for raw materials, reagents, kits of specific reagents, materials,
reference standards (name, manufacturer, cat no., batch no., shelf life) used in testing;
Copies of documents containing the information about qualification/attestation of the equipment used in
the testing (type/brand, manufacturer, serial No., protocols/certificates/other qualifications/attestations with
indicating the verification period);
Copies of accompanying documentation (passport, certificate, warrant, etc.) for the in-house company
standards used in the testing.
The applicant should submit the required volume of information
within 2 days after the testing.
33.
RegLekTHANK YOU FOR YOUR ATTENTION!
FSBI "SCEEMP"
of the Ministry of Health o

























 medicine
medicine law
law








