Similar presentations:
Carbohydrates and lipids
1.
TransformationKey concept: Change
Related concept: Transformation
Global context: Identities and relationships
Statement of inquiry:
Small chemical changes can transform our identity.
Topic: Carbohydrates and lipids
2.
Terminology• Carbohydrates Сn(H2O)m:
• Simple:
• Pentoses (С5H10O5);
• Hexoses (С6H12O6).
• Monosaccharides:
• Glucose;
• Fructose;
• Disaccharides :
• Sucrose;
• Lactose;
• Oligosaccharides:
• Sucrose (Disaccharide)
• Complex:
• Polysaccharides:
• Starch;
• Glycogen;
• Cellulose.
• Structure of molecule:
• Trioses (С3H6O3);
• Tetroses (С4H8O4);
• Lipids:
• Simple:
• Fats (HFA + glycerin);
• Waxes (HFA + monohydric alcohol);
• Compound:
• Phospholipids (HFA + alcohol + phosphate);
• Glycolipids (HFA + alcohol + carbohydrate);
• Structure of molecule :
• Glycerol;
• Fatty acid:
• Saturated;
• Unsaturated.
3.
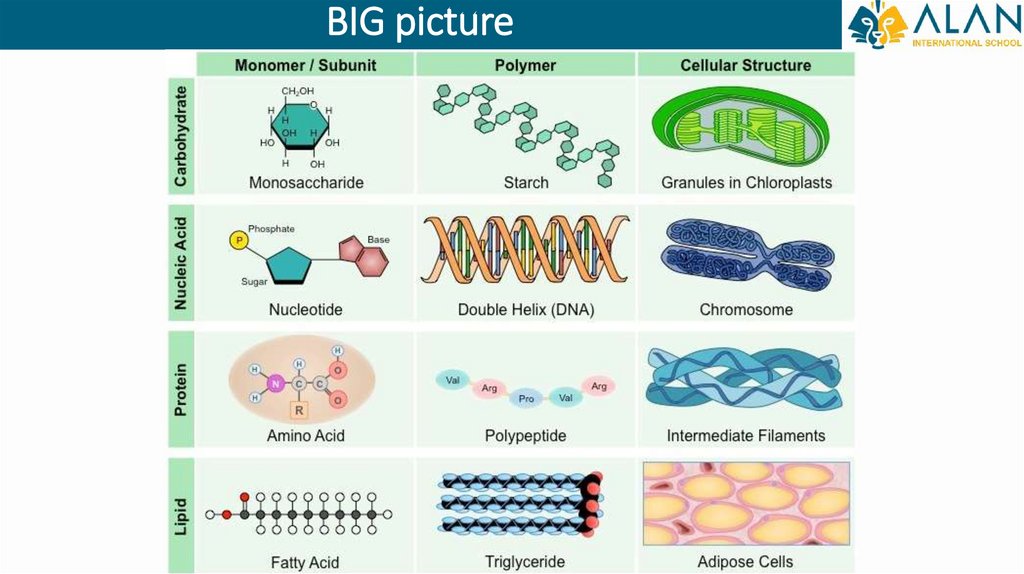
BIG picture4.
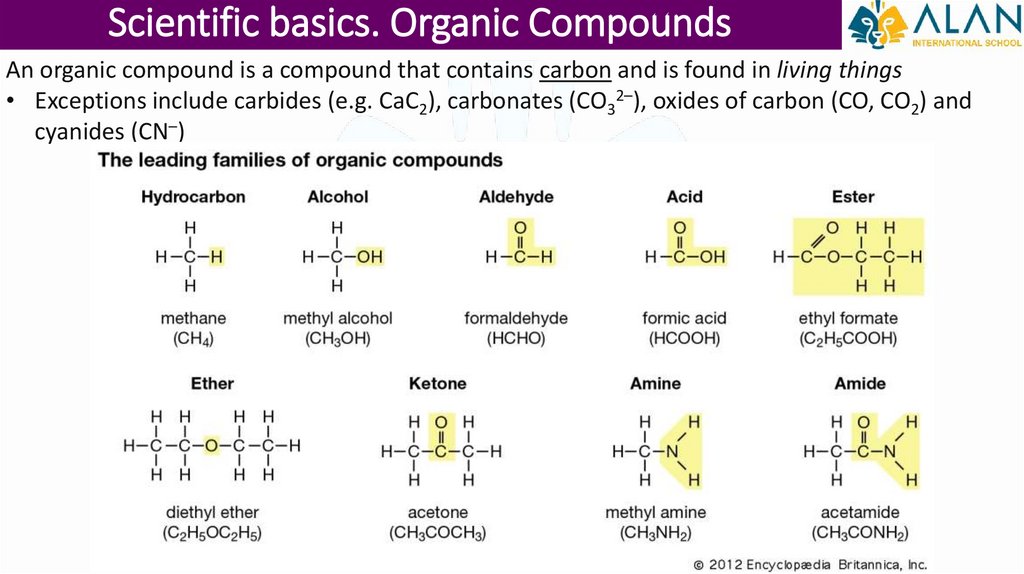
Scientific basics. Organic CompoundsAn organic compound is a compound that contains carbon and is found in living things
• Exceptions include carbides (e.g. CaC2), carbonates (CO32–), oxides of carbon (CO, CO2) and
cyanides (CN–)
5.
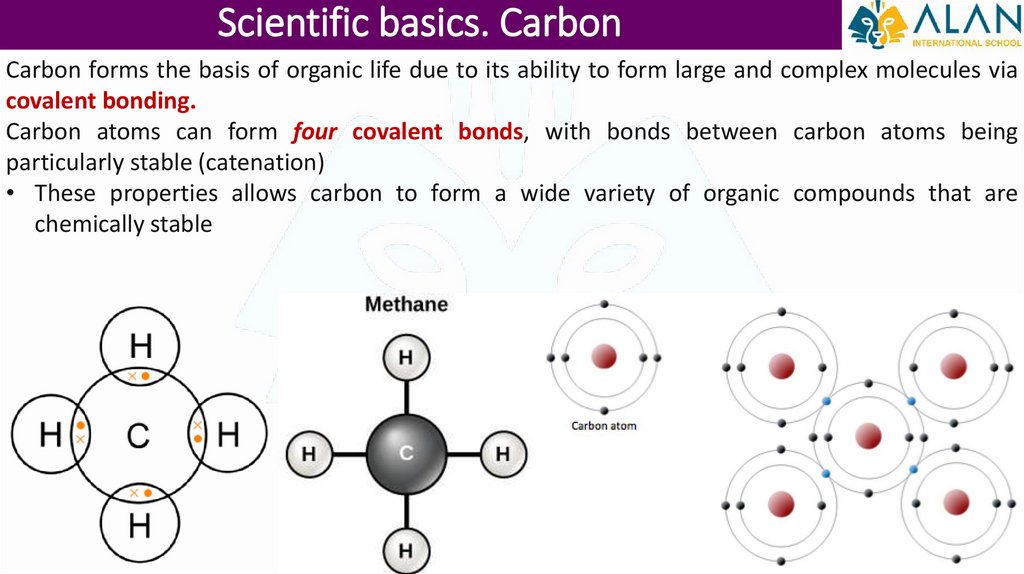
Scientific basics. CarbonCarbon forms the basis of organic life due to its ability to form large and complex molecules via
covalent bonding.
Carbon atoms can form four covalent bonds, with bonds between carbon atoms being
particularly stable (catenation)
• These properties allows carbon to form a wide variety of organic compounds that are
chemically stable
6.
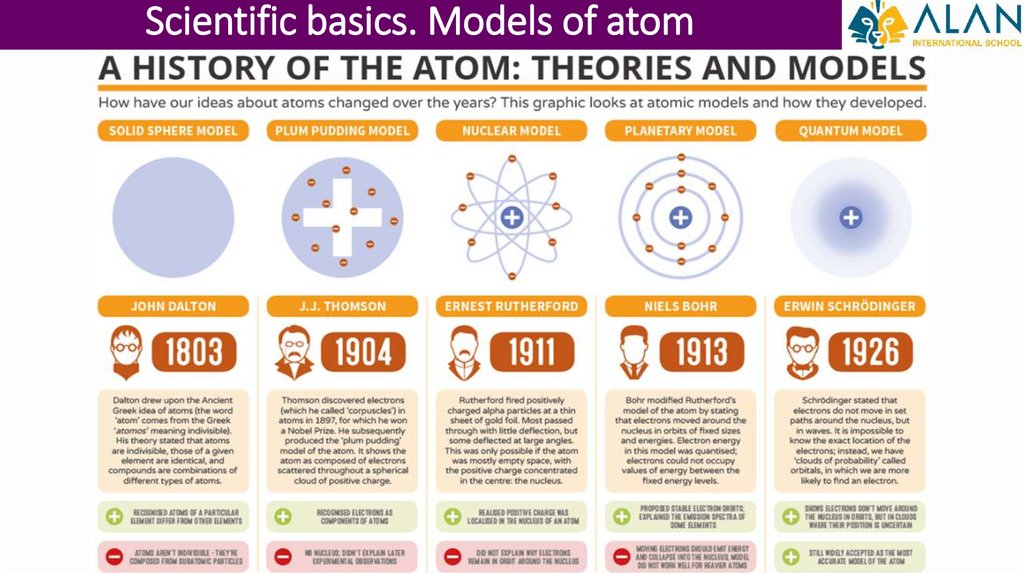
Scientific basics. Models of atom7.
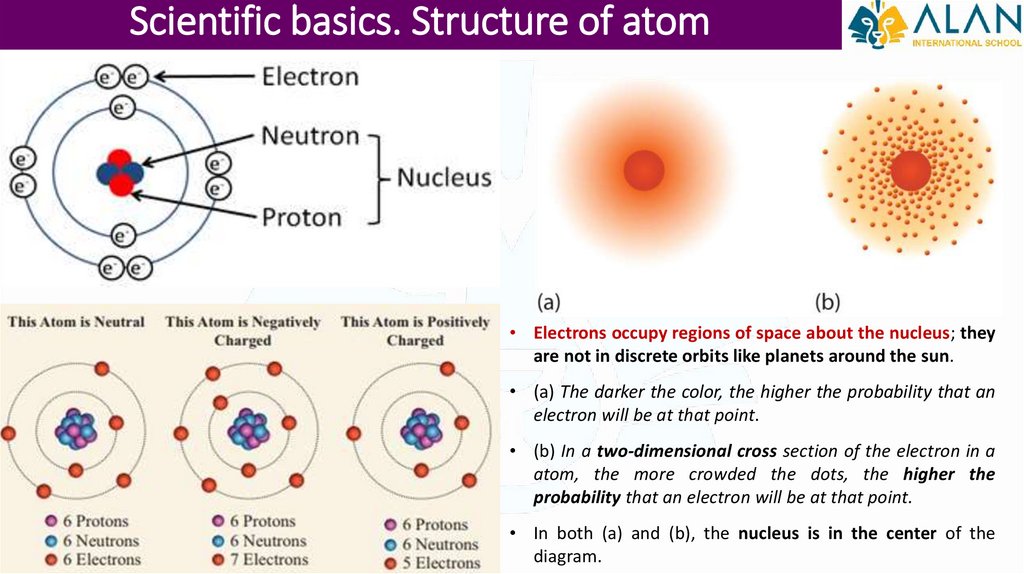
Scientific basics. Structure of atom• Electrons occupy regions of space about the nucleus; they
are not in discrete orbits like planets around the sun.
• (a) The darker the color, the higher the probability that an
electron will be at that point.
• (b) In a two-dimensional cross section of the electron in a
atom, the more crowded the dots, the higher the
probability that an electron will be at that point.
• In both (a) and (b), the nucleus is in the center of the
diagram.
8.
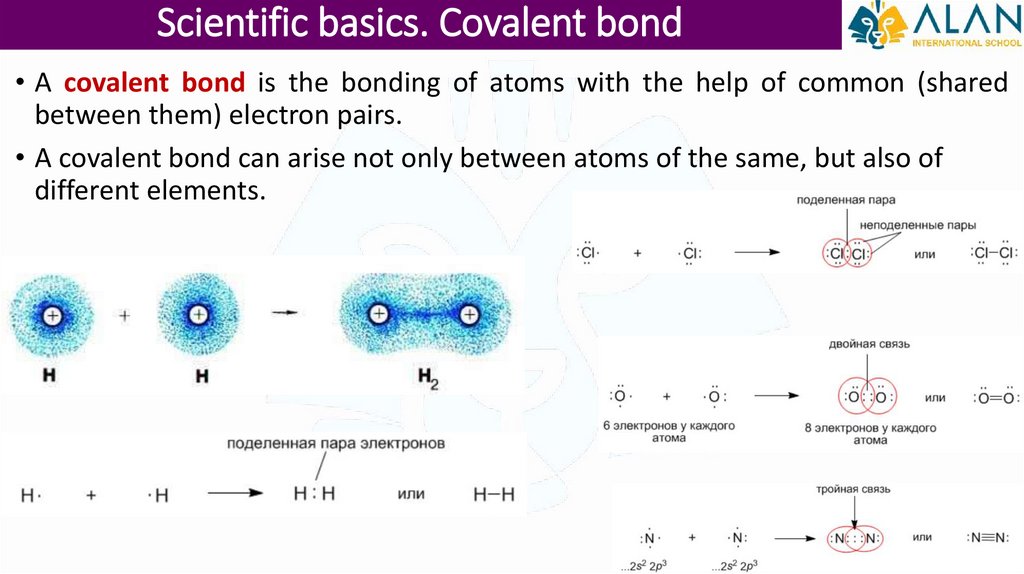
Scientific basics. Covalent bond• A covalent bond is the bonding of atoms with the help of common (shared
between them) electron pairs.
• A covalent bond can arise not only between atoms of the same, but also of
different elements.
9.
Scientific basics. Covalent bond• The fluorine atom on the outer electronic level contains electrons – electron pairs
and an unpaired electron.
• Before the completion of the electronic level, the fluorine atom lacks one
electron.
• Each fluorine atom, when forming a bond, provides for the common use of one
unpaired electron.
• This leads to the formation of a common electron pair, as a result, the fluorine
atoms acquire a stable eight-electron configuration of the inert neon gas:
10.
Glucose and sucrose are carbohydrates that taste sweet tohumans. Starch and cellulose are also carbohydrates, but
they are tasteless to humans.
Explain why some carbohydrates taste good to humans and
others don't.
11.
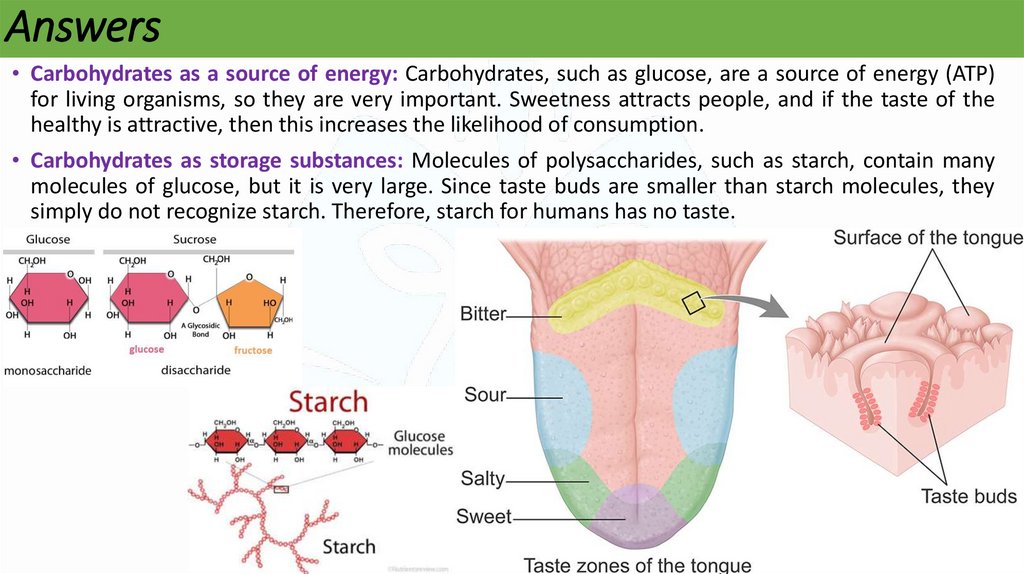
Answers• Carbohydrates as a source of energy: Carbohydrates, such as glucose, are a source of energy (ATP)
for living organisms, so they are very important. Sweetness attracts people, and if the taste of the
healthy is attractive, then this increases the likelihood of consumption.
• Carbohydrates as storage substances: Molecules of polysaccharides, such as starch, contain many
molecules of glucose, but it is very large. Since taste buds are smaller than starch molecules, they
simply do not recognize starch. Therefore, starch for humans has no taste.
12.
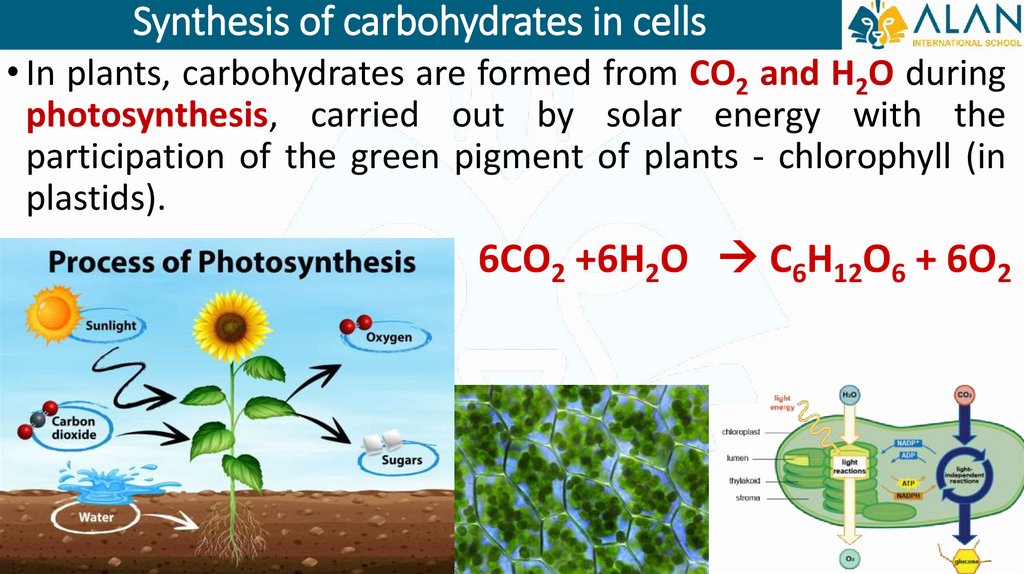
Synthesis of carbohydrates in cells• In plants, carbohydrates are formed from CO2 and H2O during
photosynthesis, carried out by solar energy with the
participation of the green pigment of plants - chlorophyll (in
plastids).
6СО2 +6Н2О С6Н12О6 + 6О2
13.
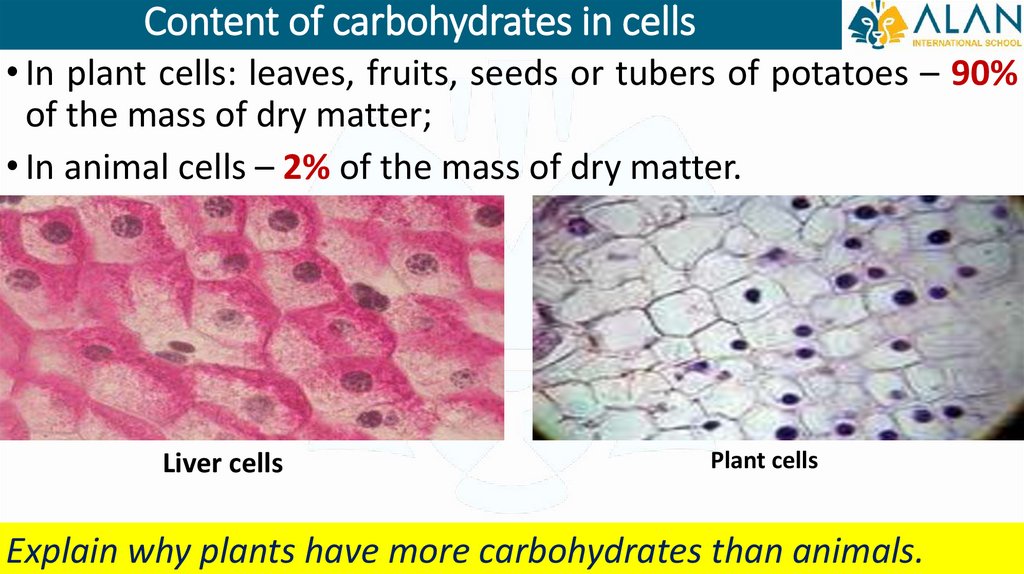
Content of carbohydrates in cells• In plant cells: leaves, fruits, seeds or tubers of potatoes – 90%
of the mass of dry matter;
• In animal cells – 2% of the mass of dry matter.
Liver cells
Plant cells
Explain why plants have more carbohydrates than animals.
14.
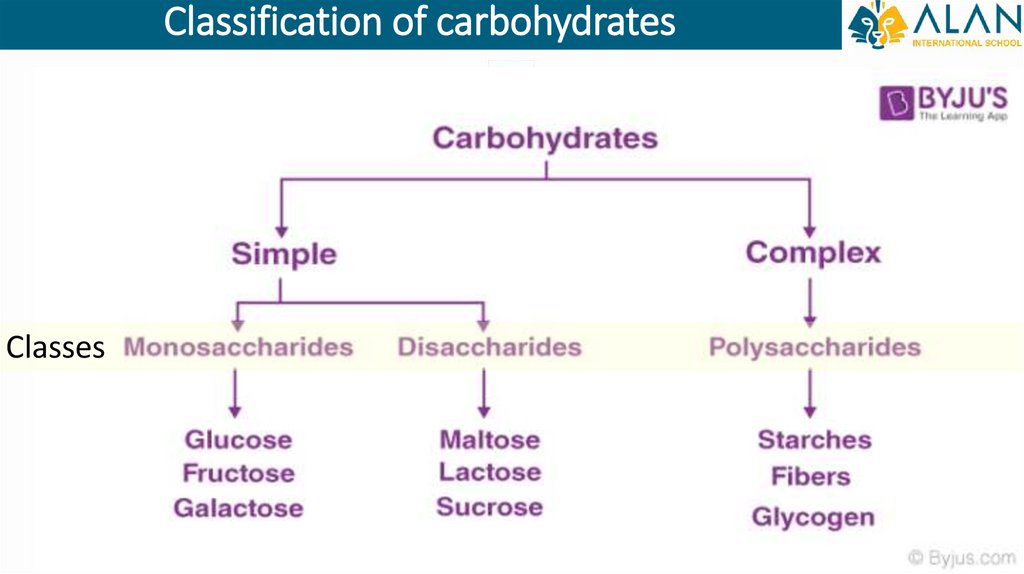
Classification of carbohydratesClasses
15.
The general structure of the monosaccharide molecule• General formula for simple carbohydrates – (СН20)n
or Сn(H2O)m.
• The name of carbohydrates depends on how many
carbon atoms they contain:
• Trioses (С3H6O3);
• Tetroses (С4H8O4);
• Pentoses (С5H10O5);
• Hexoses (С6H12O6).
16.
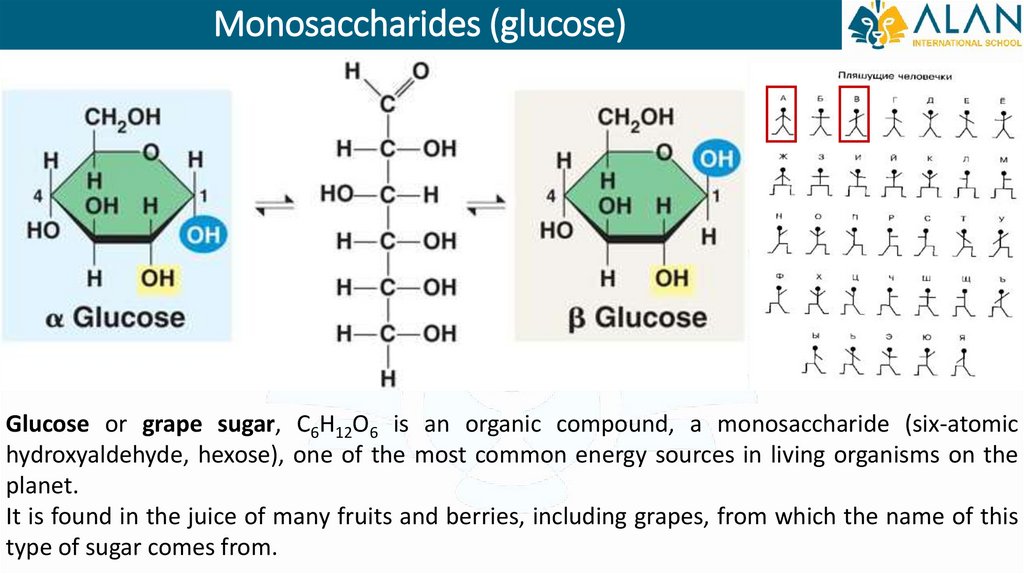
Monosaccharides (glucose)Glucose or grape sugar, C6H12O6 is an organic compound, a monosaccharide (six-atomic
hydroxyaldehyde, hexose), one of the most common energy sources in living organisms on the
planet.
It is found in the juice of many fruits and berries, including grapes, from which the name of this
type of sugar comes from.
17.
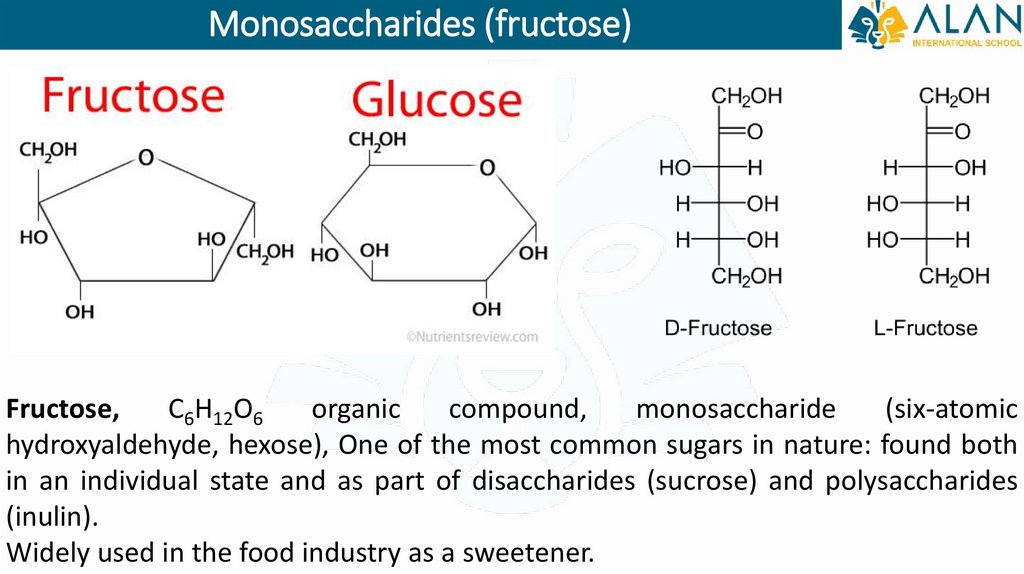
Monosaccharides (fructose)Fructose,
C6H12O6
organic
compound,
monosaccharide
(six-atomic
hydroxyaldehyde, hexose), One of the most common sugars in nature: found both
in an individual state and as part of disaccharides (sucrose) and polysaccharides
(inulin).
Widely used in the food industry as a sweetener.
18.
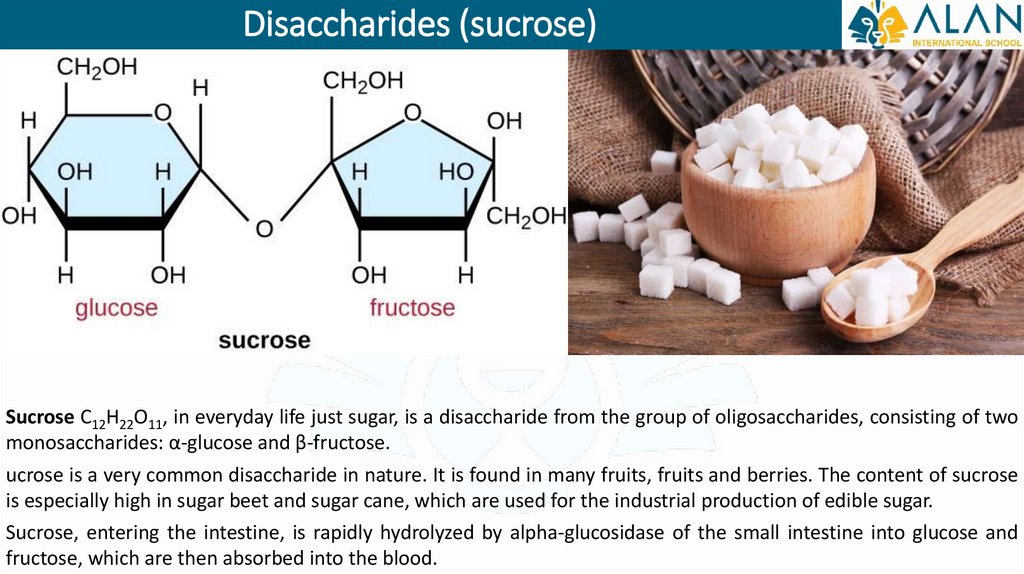
Disaccharides (sucrose)Sucrose C12H22O11, in everyday life just sugar, is a disaccharide from the group of oligosaccharides, consisting of two
monosaccharides: α-glucose and β-fructose.
ucrose is a very common disaccharide in nature. It is found in many fruits, fruits and berries. The content of sucrose
is especially high in sugar beet and sugar cane, which are used for the industrial production of edible sugar.
Sucrose, entering the intestine, is rapidly hydrolyzed by alpha-glucosidase of the small intestine into glucose and
fructose, which are then absorbed into the blood.
19.
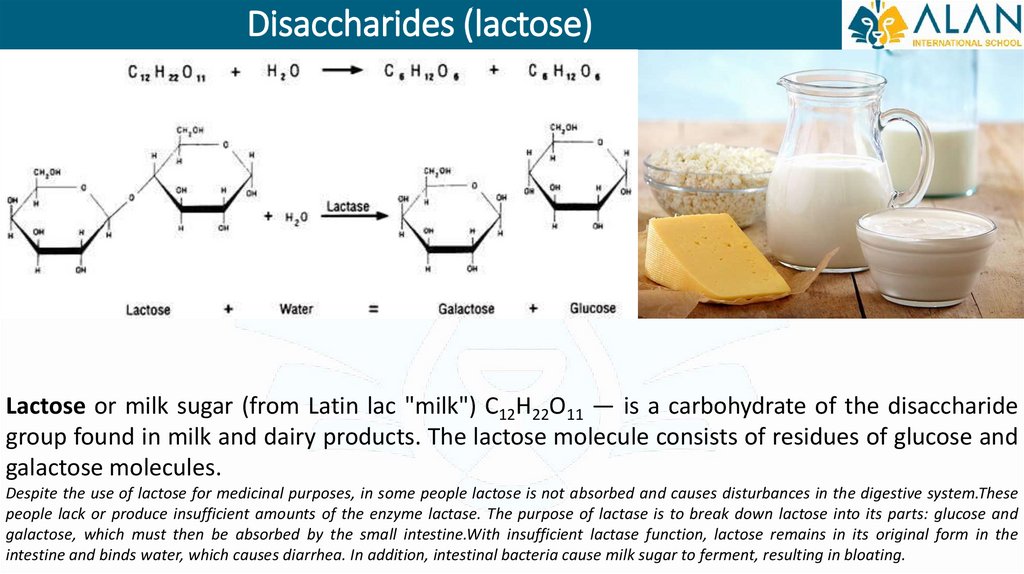
Disaccharides (lactose)Lactose or milk sugar (from Latin lac "milk") С12H22O11 — is a carbohydrate of the disaccharide
group found in milk and dairy products. The lactose molecule consists of residues of glucose and
galactose molecules.
Despite the use of lactose for medicinal purposes, in some people lactose is not absorbed and causes disturbances in the digestive system.These
people lack or produce insufficient amounts of the enzyme lactase. The purpose of lactase is to break down lactose into its parts: glucose and
galactose, which must then be absorbed by the small intestine.With insufficient lactase function, lactose remains in its original form in the
intestine and binds water, which causes diarrhea. In addition, intestinal bacteria cause milk sugar to ferment, resulting in bloating.
20.
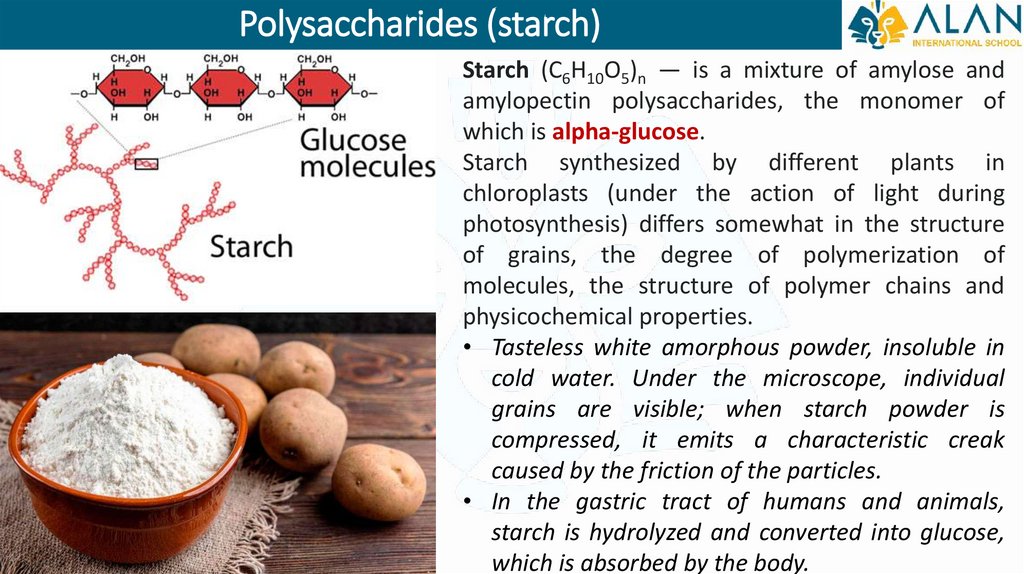
Polysaccharides (starch)Starch (C6H10O5)n — is a mixture of amylose and
amylopectin polysaccharides, the monomer of
which is alpha-glucose.
Starch synthesized by different plants in
chloroplasts (under the action of light during
photosynthesis) differs somewhat in the structure
of grains, the degree of polymerization of
molecules, the structure of polymer chains and
physicochemical properties.
• Tasteless white amorphous powder, insoluble in
cold water. Under the microscope, individual
grains are visible; when starch powder is
compressed, it emits a characteristic creak
caused by the friction of the particles.
• In the gastric tract of humans and animals,
starch is hydrolyzed and converted into glucose,
which is absorbed by the body.
21.
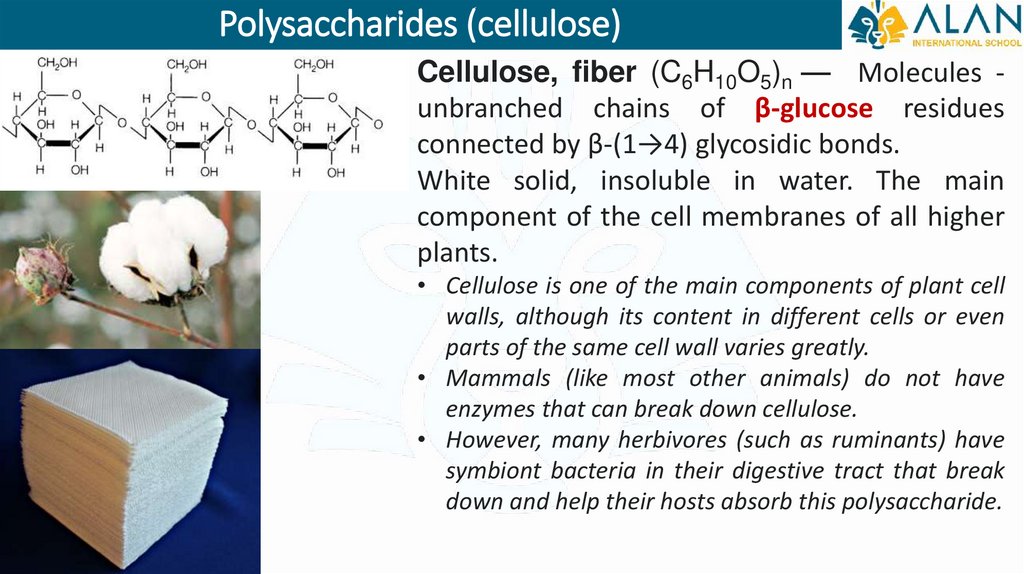
Polysaccharides (cellulose)Cellulose, fiber (C6H10O5)n — Molecules unbranched chains of β-glucose residues
connected by β-(1→4) glycosidic bonds.
White solid, insoluble in water. The main
component of the cell membranes of all higher
plants.
• Cellulose is one of the main components of plant cell
walls, although its content in different cells or even
parts of the same cell wall varies greatly.
• Mammals (like most other animals) do not have
enzymes that can break down cellulose.
• However, many herbivores (such as ruminants) have
symbiont bacteria in their digestive tract that break
down and help their hosts absorb this polysaccharide.
22.
Polysaccharides (glycogen)Glycogen
Glycogen is a polysaccharide of composition
(C6H10O5)n, formed by glucose residues.
• In animal cells, it serves as the main storage carbohydrate
and the main form of glucose storage.It is deposited in the
form of granules in the cytoplasm in many types of cells
(mainly in liver and muscle cells).
• Glycogen is sometimes called animal starch. It differs from
starch in a more branched and compact structure.
• Glycogen forms an energy reserve that can be quickly
mobilized if necessary to make up for a sudden lack of
glucose.
• Only the glycogen stored in the liver cells (hepatocytes)
can be converted into glucose to feed the entire body.
23.
Vegetable oils and fish oils are liquid at room temperature (25⁰C).At -16°C, most oils solidify. Animal fats are solid at 25⁰С, but melt
at 35⁰С and above.
Explain why vegetable oils and animal fats have different states of
aggregation under completely different temperature conditions.
24.
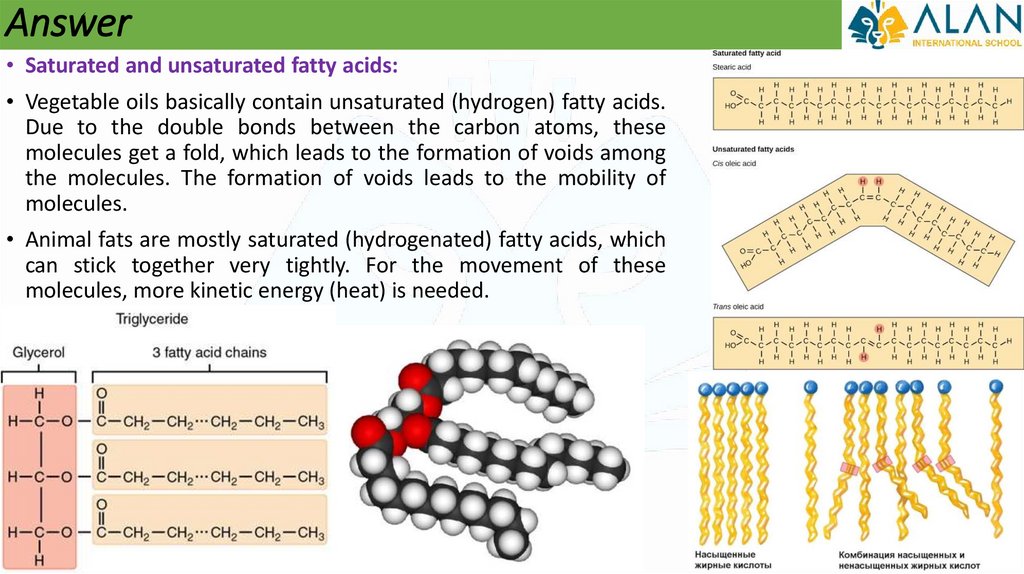
Answer• Saturated and unsaturated fatty acids:
• Vegetable oils basically contain unsaturated (hydrogen) fatty acids.
Due to the double bonds between the carbon atoms, these
molecules get a fold, which leads to the formation of voids among
the molecules. The formation of voids leads to the mobility of
molecules.
• Animal fats are mostly saturated (hydrogenated) fatty acids, which
can stick together very tightly. For the movement of these
molecules, more kinetic energy (heat) is needed.
25.
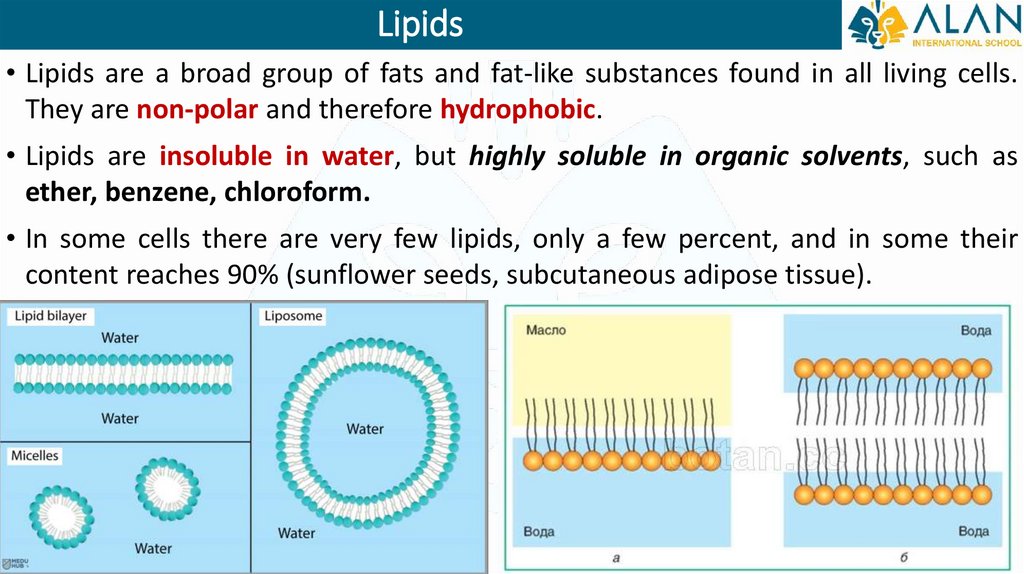
Lipids• Lipids are a broad group of fats and fat-like substances found in all living cells.
They are non-polar and therefore hydrophobic.
• Lipids are insoluble in water, but highly soluble in organic solvents, such as
ether, benzene, chloroform.
• In some cells there are very few lipids, only a few percent, and in some their
content reaches 90% (sunflower seeds, subcutaneous adipose tissue).
26.
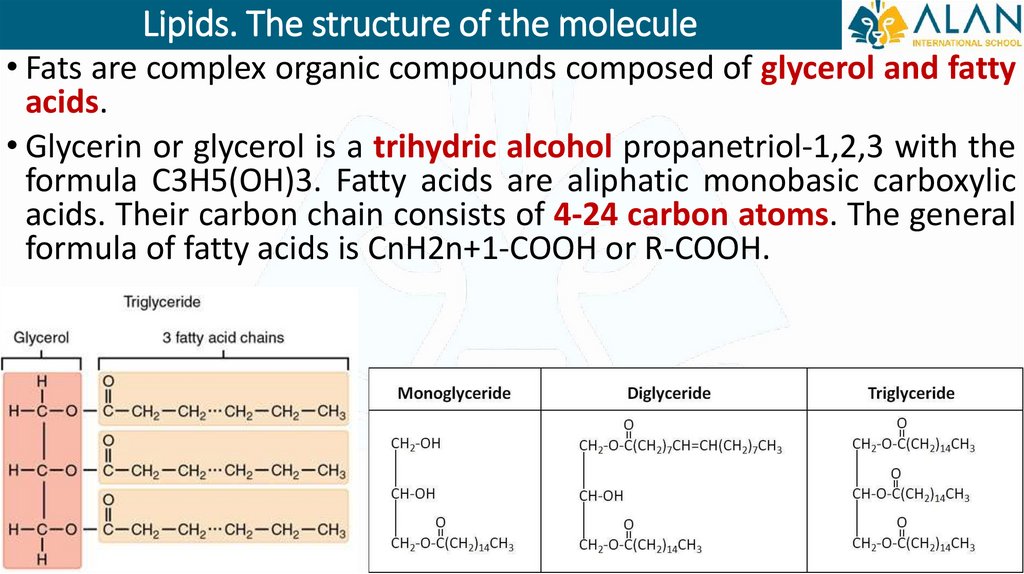
Lipids. The structure of the molecule• Fats are complex organic compounds composed of glycerol and fatty
acids.
• Glycerin or glycerol is a trihydric alcohol propanetriol-1,2,3 with the
formula C3H5(OH)3. Fatty acids are aliphatic monobasic carboxylic
acids. Their carbon chain consists of 4-24 carbon atoms. The general
formula of fatty acids is CnH2n+1-COOH or R-COOH.
27.
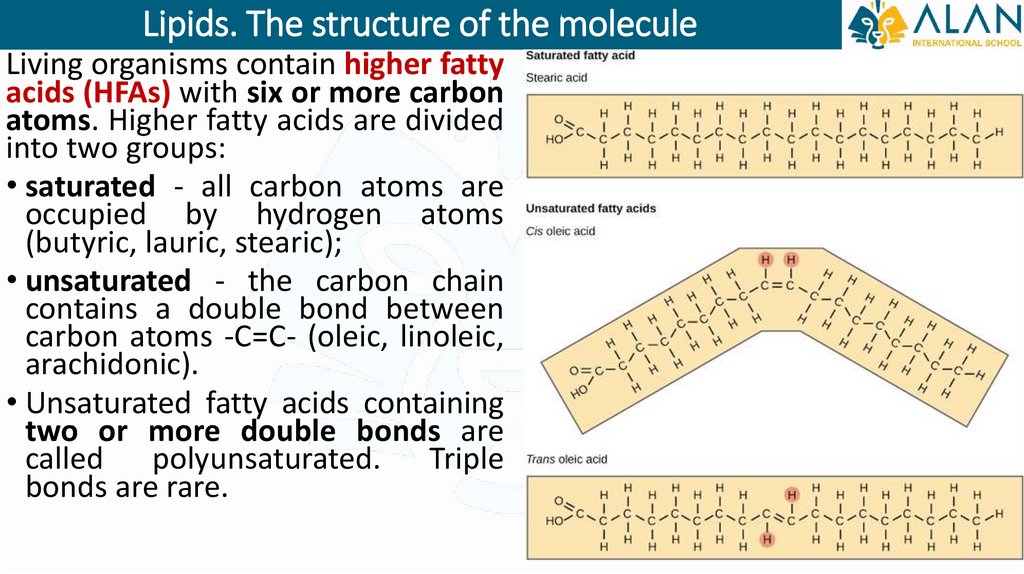
Lipids. The structure of the moleculeLiving organisms contain higher fatty
acids (HFAs) with six or more carbon
atoms. Higher fatty acids are divided
into two groups:
• saturated - all carbon atoms are
occupied by hydrogen atoms
(butyric, lauric, stearic);
• unsaturated - the carbon chain
contains a double bond between
carbon atoms -С=С- (oleic, linoleic,
arachidonic).
• Unsaturated fatty acids containing
two or more double bonds are
called polyunsaturated. Triple
bonds are rare.
28.
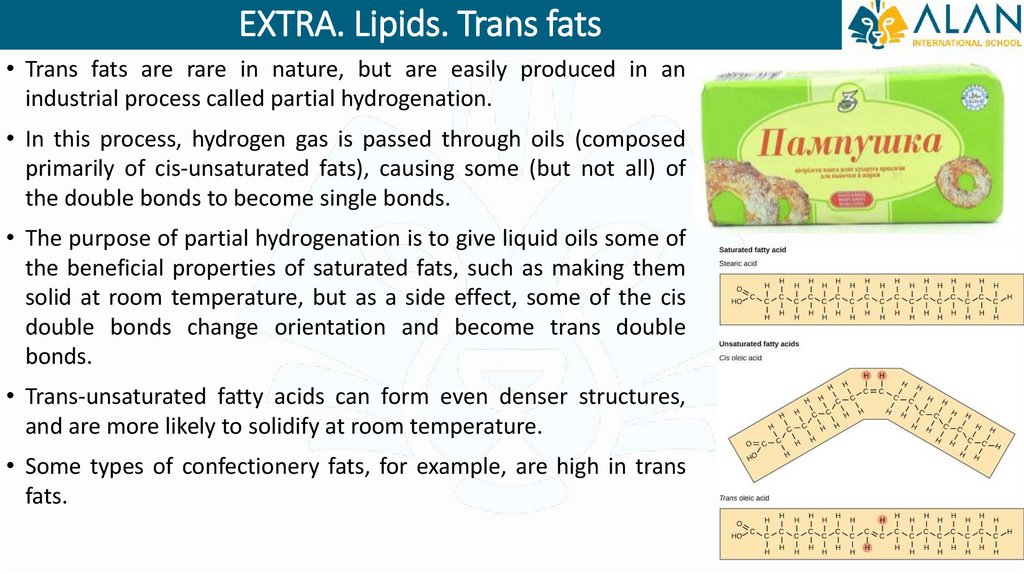
EXTRA. Lipids. Trans fats• Trans fats are rare in nature, but are easily produced in an
industrial process called partial hydrogenation.
• In this process, hydrogen gas is passed through oils (composed
primarily of cis-unsaturated fats), causing some (but not all) of
the double bonds to become single bonds.
• The purpose of partial hydrogenation is to give liquid oils some of
the beneficial properties of saturated fats, such as making them
solid at room temperature, but as a side effect, some of the cis
double bonds change orientation and become trans double
bonds.
• Trans-unsaturated fatty acids can form even denser structures,
and are more likely to solidify at room temperature.
• Some types of confectionery fats, for example, are high in trans
fats.
29.
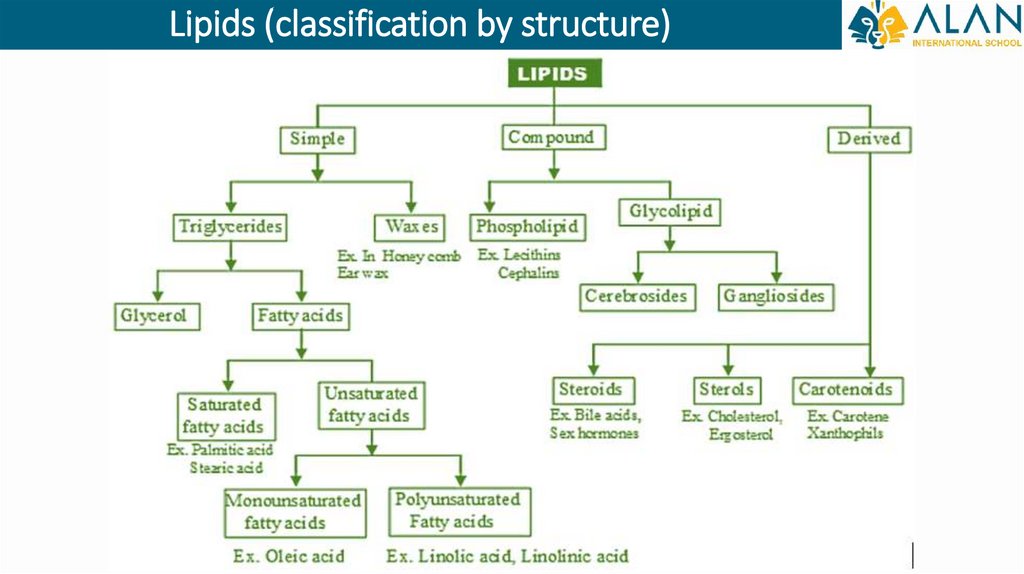
Lipids (classification by structure)30.
Simple lipids• Simple lipids include triacylglycerols (neutral fats) and waxes.
1. Neutral fats are the most common lipids found in nature. Their molecules
are formed as a result of the addition of three residues of high molecular
weight fatty acids to one molecule of the trihydric alcohol glycerol.
• Among the compounds of this group, fats are distinguished, which remain solid at a
temperature of 20 ° C, and oils, which become liquid under these conditions.
2.
Waxes are esters formed from fatty acids and polyhydric alcohols. They
cover the skin, wool, feathers of animals, softening them and protecting
them from water. Also, bees build honeycombs from waxes.
31.
Simple lipids (biological significance)• In the body of hibernating animals, a large amount of fat
accumulates, which is consumed during hibernation.
• In vertebrates, fat also accumulates in the subcutaneous
adipose tissue and serves as thermal insulation. The
subcutaneous layer is especially pronounced in mammals living
in cold climates.
• Plants tend to store oils rather than fats. Seeds, fruits,
chloroplasts are rich in oils. And some seeds, such as seeds of
coconut palm, castor bean, soybean, sunflower, serve as raw
materials for industrial production of oil.
32.
Wax (biological significance)In both plants and animals, waxes are used primarily as a waterrepellent coating:
1. waxes form an additional water-repellent protective layer
on the cuticle of the epidermis of some plant organs (leaves,
fruits, seeds);
2. waxes cover the skin, wool and feathers of animals,
protecting them from getting wet and, therefore,
hypothermia (for example, during diving, rain);
3. waxes are part of the chitin - the outer skeleton of insects,
also thanks to them, many insects do not get wet and do
not drown.
• Wax is used by insects (bees) as a building material.
33.
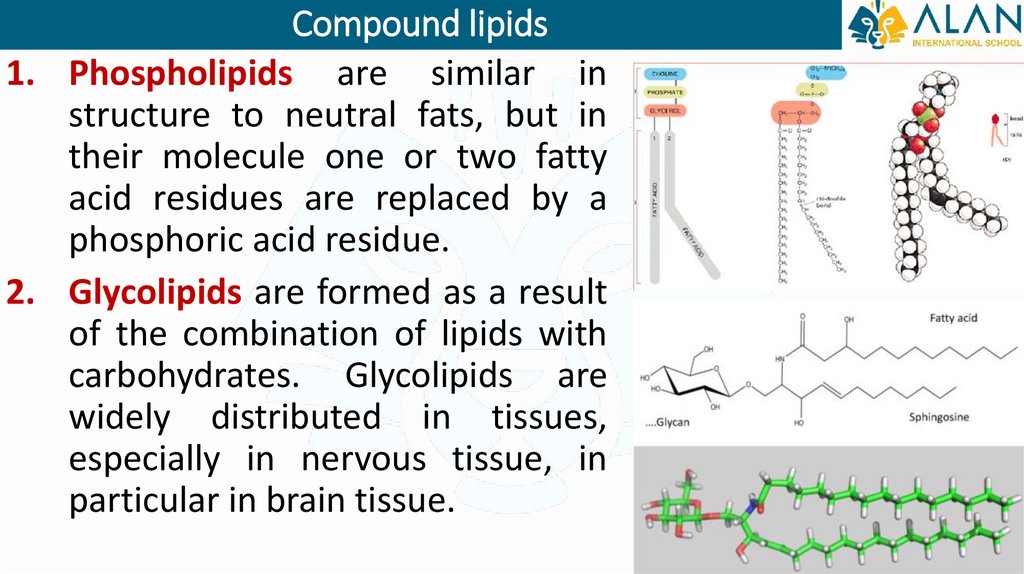
Compound lipids1. Phospholipids are similar in
structure to neutral fats, but in
their molecule one or two fatty
acid residues are replaced by a
phosphoric acid residue.
2. Glycolipids are formed as a result
of the combination of lipids with
carbohydrates. Glycolipids are
widely distributed in tissues,
especially in nervous tissue, in
particular in brain tissue.
34.
Compound lipids (main function)• The cell membrane is a double layer (bilayer) of molecules of the lipid class, most of which are
the so-called complex lipids - phospholipids.
• Lipid molecules have a hydrophilic (“head”) and a hydrophobic (“tail”) part. When membranes
are formed, the hydrophobic portions of the molecules turn inward, while the hydrophilic
portions turn outward.
35.
Steroids and terpenes• Steroids and terpenes are lipids
that do not have fatty acids and
have a special structure.
• Steroids include sex hormones,
such as progesterone and estrogen
(female
sex
hormones),
testosterone (male sex hormone)
• Steroids also include vitamin D,
the lack of which causes a disease
called rickets.
• Terpenes are substances on which
the aroma of essential oils of
plants depends, for example:
menthol, mint, camphor.
36.
Biological functions of lipids:1. Energy (main function of triglycerides)
• The main function of triglycerides is to serve as an energy depot. The
calorie content of lipids is higher than the calorie content of
carbohydrates, i.e. a given mass of lipid releases more energy during
oxidation than an equal mass of carbohydrate. This is explained by
the fact that in comparison with carbohydrates, lipids contain more
hydrogen and very little oxygen.
• With the complete oxidation of 1 g of lipids, 38.9 kJ of energy is
released, that is, 2 times more than with the oxidation of 1 g of
carbohydrates.
37.
Biological functions of lipids:2. In the body of hibernating animals, before hibernation, excess fat
accumulates and is also deposited under the skin - in the so-called
subcutaneous tissue, where it serves for thermal insulation.
3. Protective
Possessing pronounced thermal insulation properties, lipids protect our body
from temperature extremes. Lipids also protect the body from mechanical and
physical influences.
Waxes that cover the body of plants protect them from excessive evaporation of
water. This is very important for those plants that live in arid regions in
conditions of moisture deficiency.
38.
Biological functions of lipids:4. Structural
In combination with proteins, lipids are structural components of all
biological membranes.
5. Regulatory
Lipids are involved in the regulation of the physiological functions of the
body, as some of them are hormones.











 chemistry
chemistry








